Bioproduction Optimization, Characterization, and Bioactivity of Extracellular Pigment Produced by Streptomyces parvulus
Abstract
1. Introduction
2. Results
2.1. Selected Streptomyces Strain S145 and Response Variable
2.2. Evaluation of the Main Factors That Influence Pigment Production
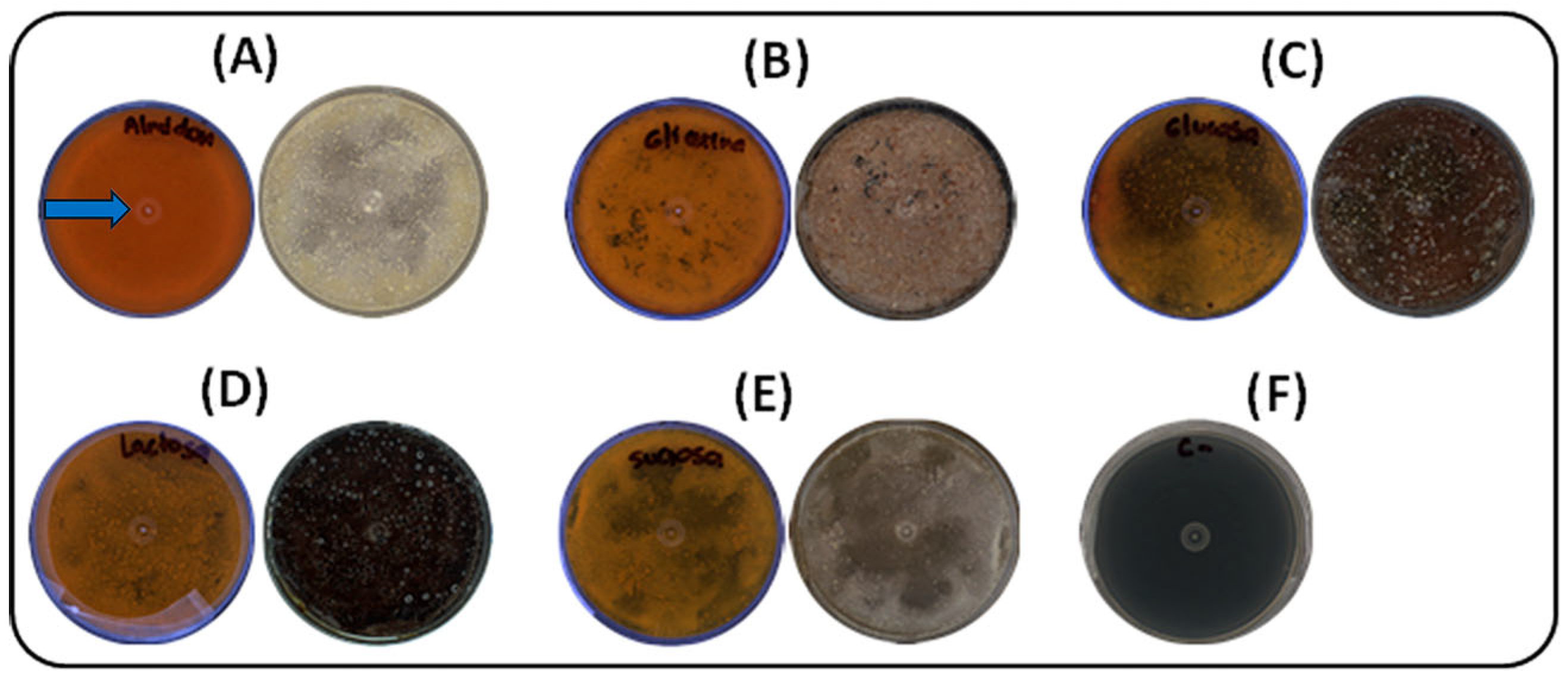
2.2.1. Carbon Source Selection
2.2.2. Nitrogen Source Selection
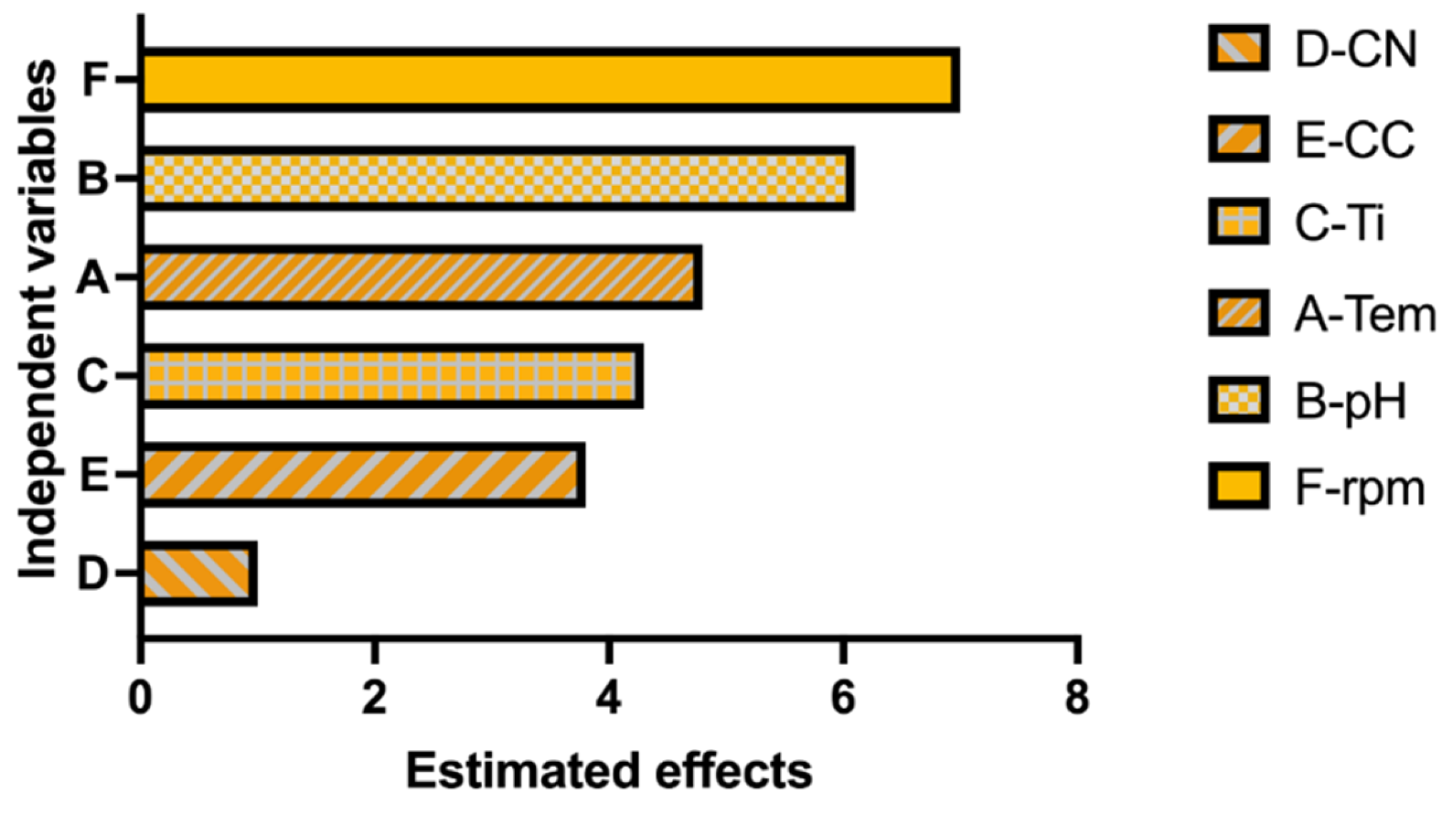
2.2.3. Plackett–Burman Experimental Design
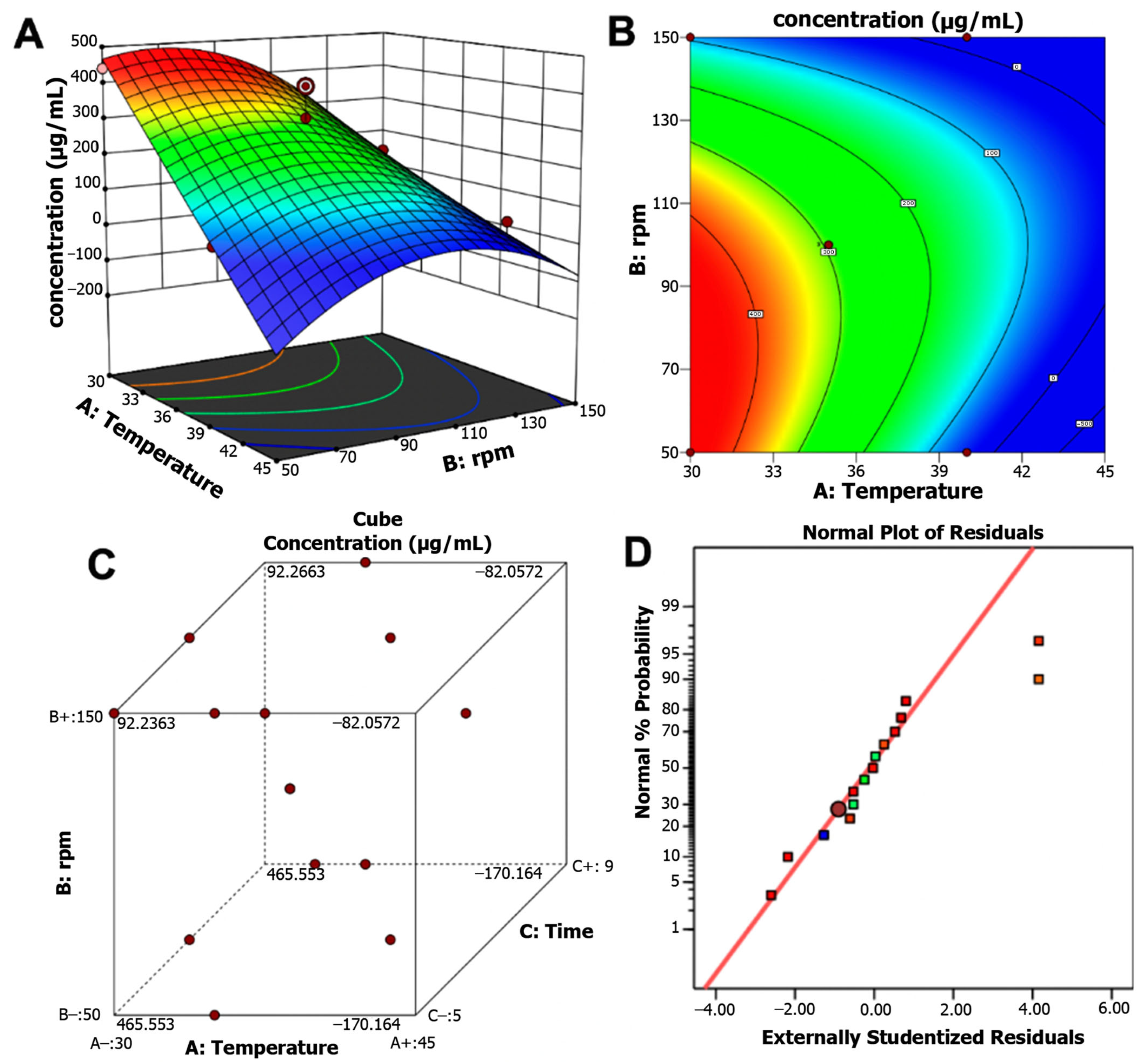
2.2.4. Response Surface Methodology (Box–Behnken Design)
2.3. Chemical Characterization of the S. parvulus S145-Derived Pigment-Rich Fraction
2.4. Bioactivity of S. parvulus S145-Derived Pigment-Rich Fraction
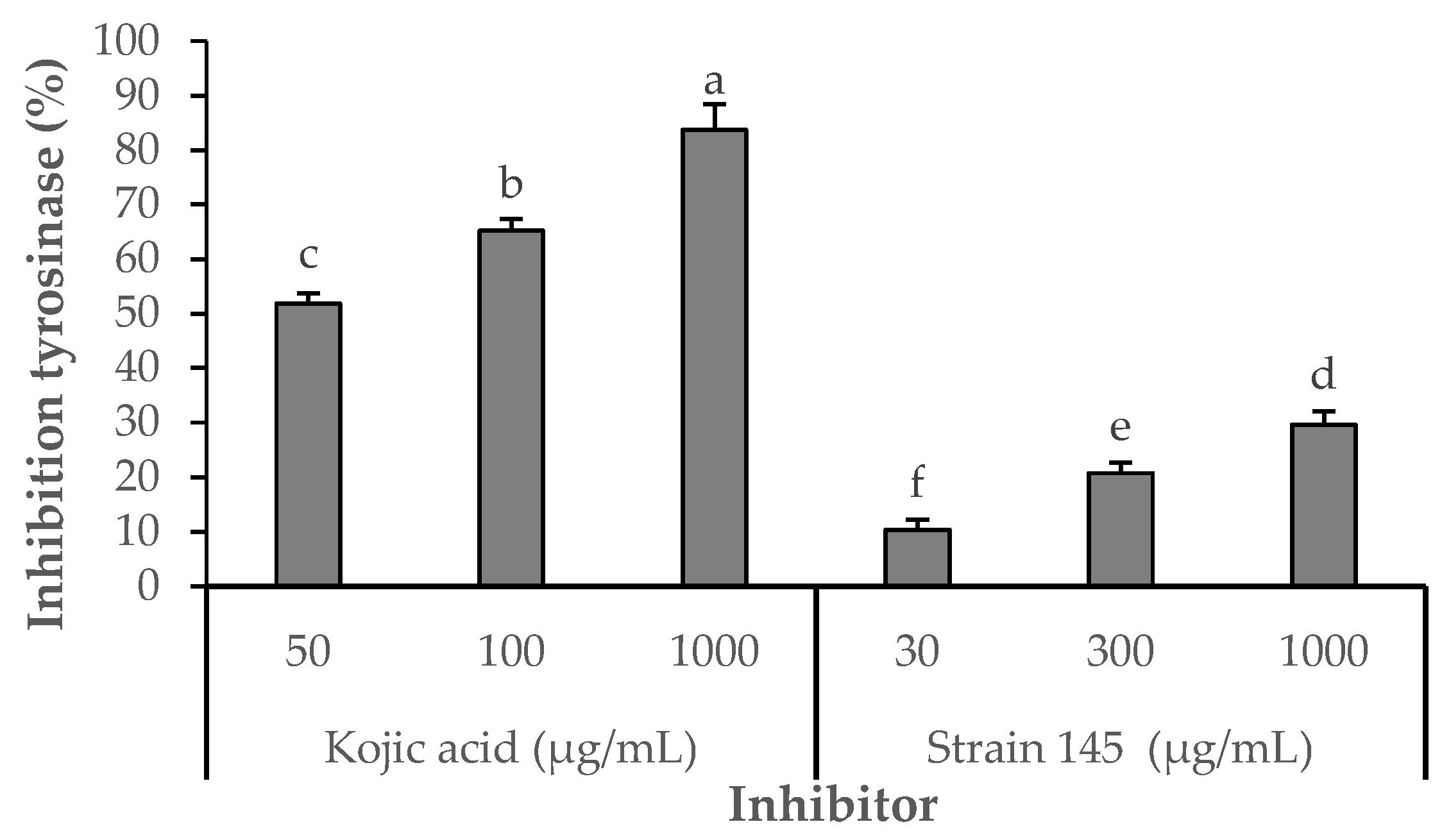
2.4.1. Tyrosinase Inhibitory Activity
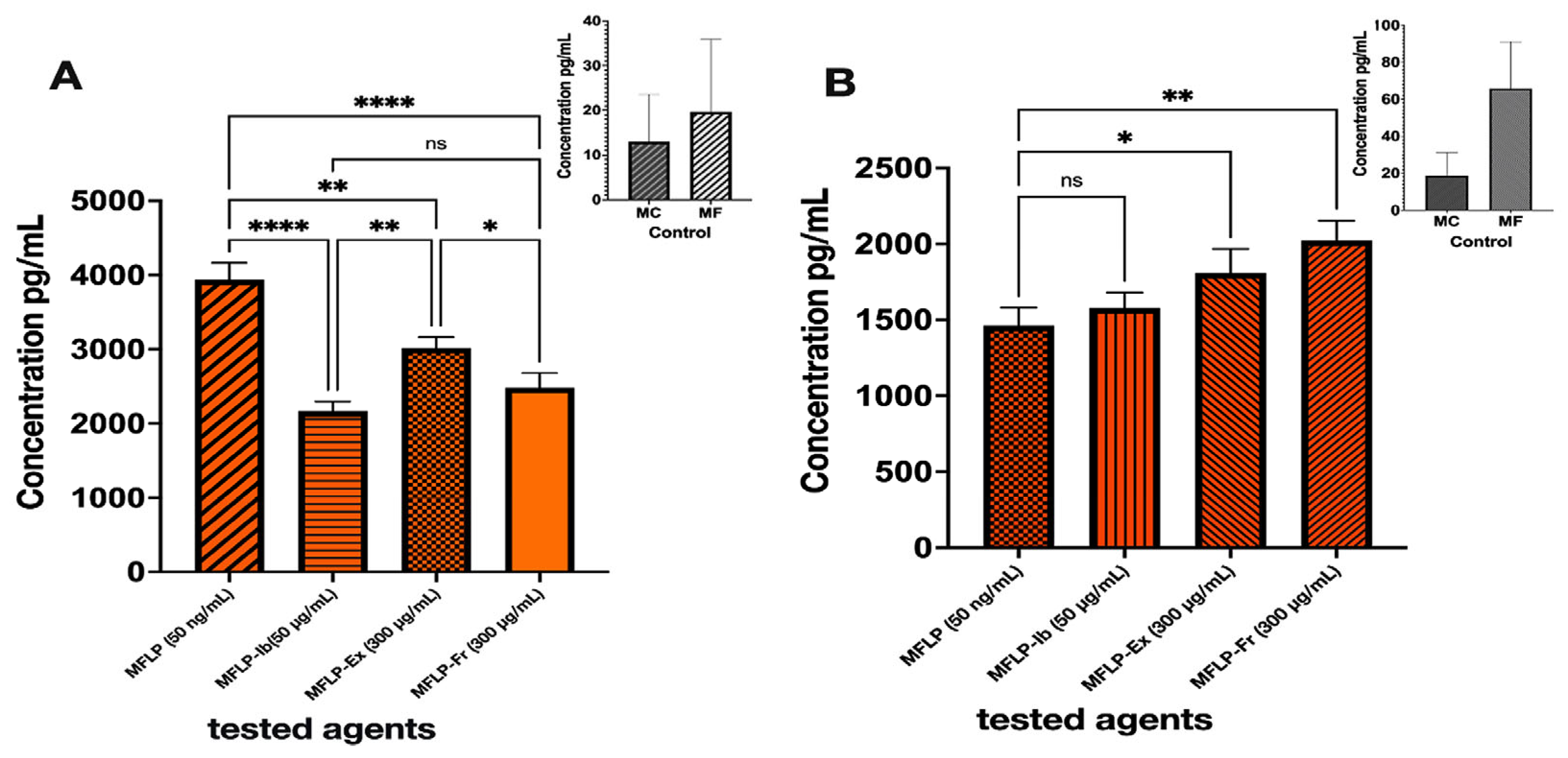
2.4.2. Anti-Inflammatory Activity
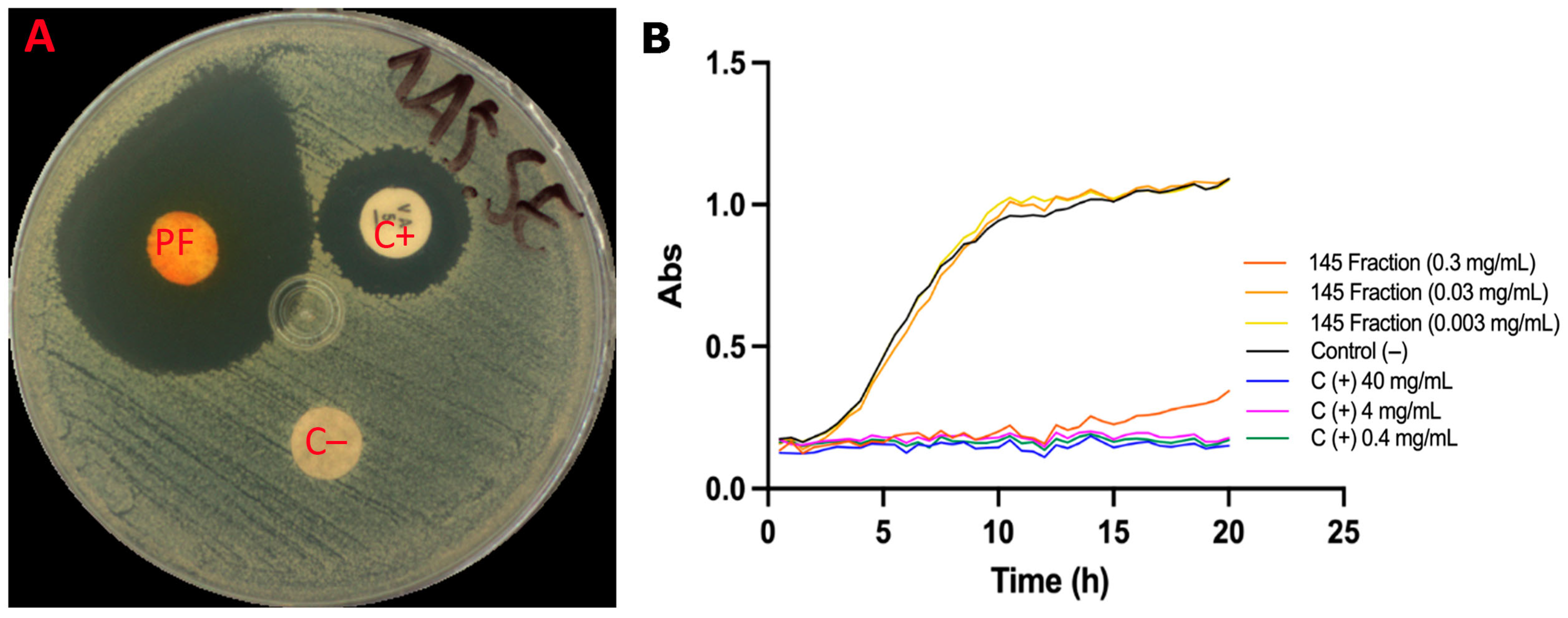
2.4.3. Anti-Acne Activity
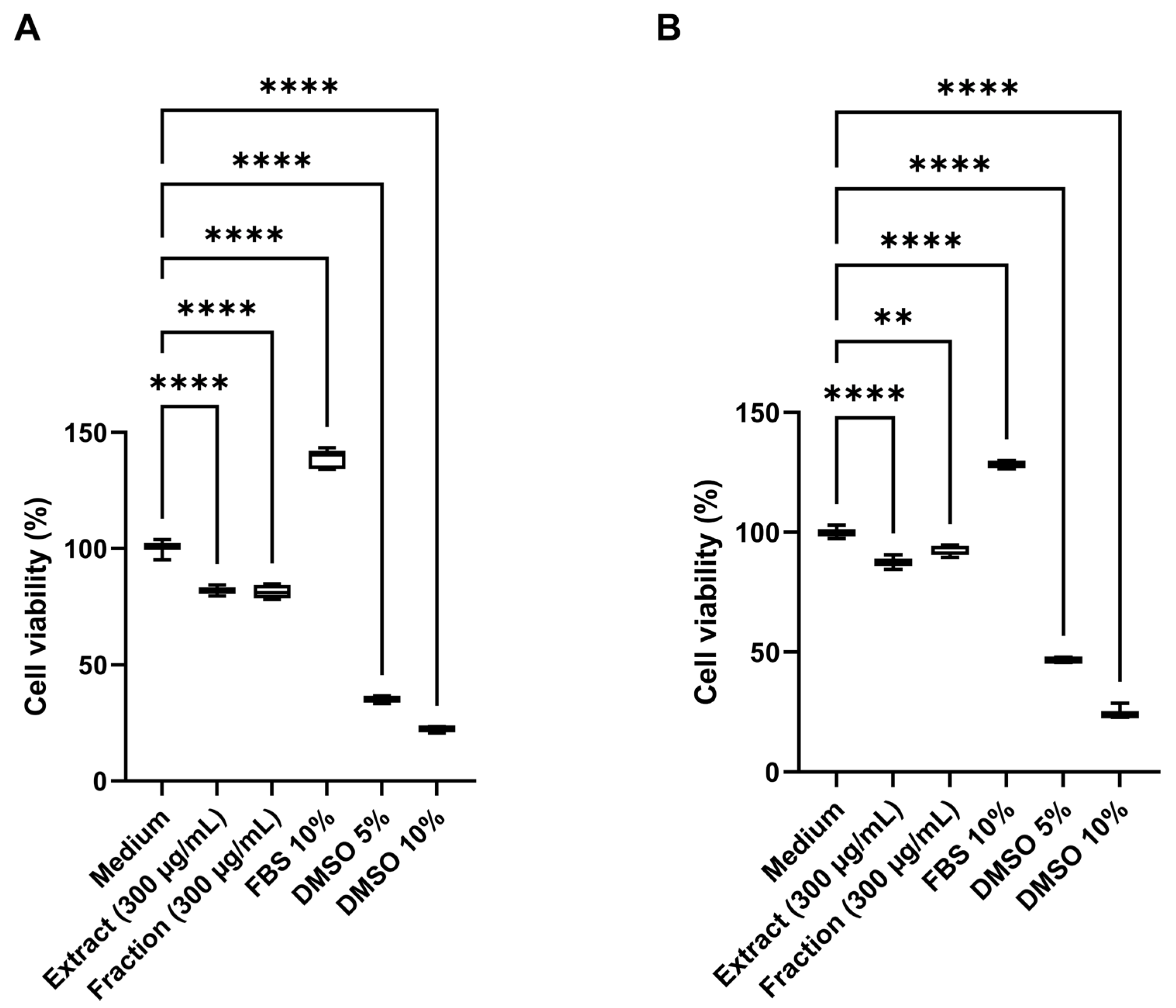
2.4.4. Cytotoxic Activity
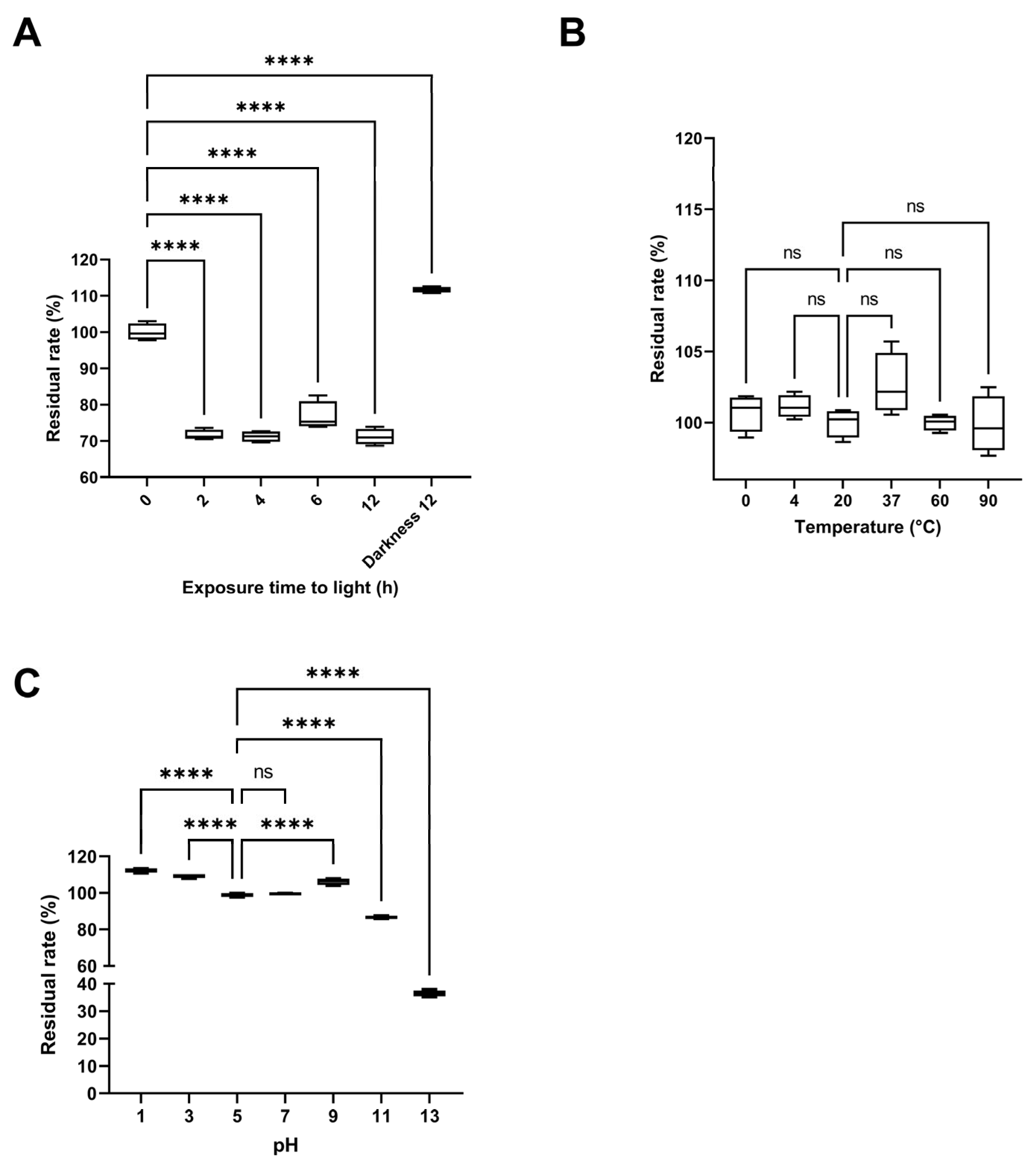
2.4.5. Stability Studies of the Pigment-Rich Fraction from S. parvulus
3. Discussion
4. Materials and Methods
4.1. Origin of Test S. parvulus Strain S145
4.1.1. Preparation of the Supernatant-Derived Extract
4.1.2. Pigment Quantification as Response Variable
4.2. Test Factors: Plackett–Burman Experimental Design
4.3. Box–Behnken Design: Bioproduction Optimization
4.4. Chemical Characterization of the Pigment-Rich Fraction from S. parvulus
4.5. 16 S rRNA Gene Sequencing
4.6. Tyrosinase Inhibition Assay
- A: Absorbance of the enzyme, substrate, and DMSO solution.
- B: Absorbance of the substrate and DMSO solution.
- C: Absorbance of the enzyme, substrate, and extract solution.
- D: Absorbance of the substrate and extract solution.
4.7. Anti-Inflammatory Assay
4.8. Anti-Acne Activity of the Fractionated Extract
4.9. Cytotoxic Assay
4.10. Stability Assessment of the Pigment-Rich Fraction
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd-Elnaby, H.; Abo-Elala, G.; Abdel-Raouf, U.; Abd-elwahab, A.; Hamed, M. Antibacterial and Anticancer Activity of Marine Streptomyces Parvus: Optimization and Application. Biotechnol. Biotechnol. Equip. 2016, 30, 180–191. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.B.; He, H.W.; Feng, J.T.; Zhang, X.; Han, L.R. Optimization of Medium Compositions to Improve a Novel Glycoprotein Production by Streptomyces kanasenisi ZX01. AMB Express 2017, 7, 6. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; El-Ewasy, S.M. Bioproduction, Characterization, Anticancer and Antioxidant Activities of Extracellular Melanin Pigment Produced by Newly Isolated Microbial Cell Factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Wang, J.; Wang, L.G.; Liu, L.P.; Wang, C.S. Identification of an Actinomyces Producing Natural Blue Pigment and Optimization of Fermentation Conditions. Chin. J. Pharm. Biotechnol. 2014, 21, 550–553. [Google Scholar]
- Bellotti, D.; Remelli, M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021, 26, 3255. [Google Scholar] [CrossRef] [PubMed]
- Padhan, B.; Poddar, K.; Sarkar, D.; Sarkar, A. Production, Purification, and Process Optimization of Intracellular Pigment from Novel Psychrotolerant Paenibacillus sp. BPW19. Biotechnol. Reports 2021, 29, e00592. [Google Scholar] [CrossRef]
- Mansour, R. Natural Dyes and Pigments: Extraction and Applications. In Handbook of Renewable Materials for Coloration and Finishing; 2018; pp. 75–102. [Google Scholar] [CrossRef]
- Park, K.M.; Kwon, K.M.; Lee, S.H. Evaluation of the Antioxidant Activities and Tyrosinase Inhibitory Property from Mycelium Culture Extracts. Evidence-based Complement. Altern. Med. 2015, 2015, 616298. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free Radical Scavenging and Anti-Acne Activities of Mangosteen Fruit Rind Extracts Prepared by Different Extraction Methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef]
- Namjoyan, F.; Farasat, M.; Alishahi, M.; Jahangiri, A.; Mousavi, H. The Anti-Melanogenesis Activities of Some Selected Red Macroalgae from Northern Coasts of the Persian Gulf. Iran. J. Pharm. Res. 2019, 18, 383–390. [Google Scholar] [PubMed Central]
- Moreira, J.V.; Silva, S.C.M.; Cremasco, M.A. Evaluation of Carbon:Nitrogen Ratio in Semi-Defined Culture Medium to Tacrolimus Biosynthesis by Streptomyces tsukubaensis and the Effect on Bacterial Growth. Biotechnol. Rep. 2020, 26, e00440. [Google Scholar] [CrossRef]
- Krysenko, S. Current Approaches for Genetic Manipulation of Streptomyces spp.—Key Bacteria for Biotechnology and Environment. BioTech 2025, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Baserisalehi, M.; Bahador, N. Evaluation of Antimicrobial Pigment Produced by Streptomyces coeruleorubidus. Nat. Environ. Pollut. Technol. 2014, 13, 641–644. [Google Scholar]
- Al husnan, L.A.; Alkahtani, M.D.F. Molecular Identification of Streptomyces Producing Antibiotics and Their Antimicrobial Activities. Ann. Agric. Sci. 2016, 61, 251–255. [Google Scholar] [CrossRef]
- Lu, L.; Cui, H.L.; Chen, Y.N.; Yuan, S. Isolation and Identification of Streptomyces Sp. and Assay of Its Exocellular Water-Soluble Blue Pigments. Folia Microbiol. 2002, 47, 493–498. [Google Scholar] [CrossRef]
- Díez, B.H.; Torres, C.A.V.; Gaudêncio, S.P. Actinomycete-Derived Pigments: A Path Toward Sustainable Industrial Colorants. Mar. Drugs 2025, 23, 39. [Google Scholar] [CrossRef]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a Promising Biological Control Agents for Plant Pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef]
- Hemeda, N.A.; Hegazy, G.E.; Abdelgalil, S.A.; Soliman, N.A.; Abdel-Meguid, D.I.; El-Assar, S.A. Maximization of Red Pigment Production from Streptomyces sp. LS1 Structure Elucidation and Application as Antimicrobial/Antifouling against Human Pathogens and Marine Microbes. J. Genet. Eng. Biotechnol. 2022, 20, 168. [Google Scholar] [CrossRef]
- Arango, C.; Acosta-Gonzalez, A.; Parra-Giraldo, C.M.; Sánchez-Quitian, Z.A.; Kerr, R.; Díaz, L.E. Characterization of Actinobacterial Communities from Arauca River Sediments (Colombia) Reveals Antimicrobial Potential Presented in Low Abundant Isolates. Open Microbiol. J. 2018, 12, 181–194. [Google Scholar] [CrossRef]
- Abraham, J.; Chauhan, R. Profiling of Red Pigment Produced by Streptomyces sp. JAR6 and Its Bioactivity. 3 Biotech 2018, 8, 22. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current Perspective of Yellowish-Orange Pigments from Microorganisms—A Review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Fiedler, H.P.; Kulik, A.; Schüz, T.C.; Volkmann, C.; Zeeck, A. Biosynthetic Capacities of Actinomycetes. 2. Juglomycin Z, a New Naphthoquinone Antibiotic from Streptomyces tendae. J. Antibiot. 1994, 47, 1116–1122. [Google Scholar] [CrossRef]
- Ikushima, H.; Iguchi, E.; Kohsaka, M.; Aoki, H.; Imanaka, H. Streptomyces auranticolor sp. Nov., a New Anticoccidial Antibiotics Producer. J. Antibiot. 1980, 33, 1103–1106. [Google Scholar] [CrossRef]
- Shindo, K.; Kawai, H. A Novel Antibiotic, Naphthopyranomycin. J. Antibiot. 1992, 45, 584–586. [Google Scholar] [CrossRef]
- Jones, S.E.; Elliot, M.A. ‘Exploring’ the Regulation of Streptomyces Growth and Development. Curr. Opin. Microbiol. 2018, 42, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, H.; Zheng, G.; Jiang, W.; Lu, Y. Metabolic Engineering of Streptomyces coelicolor for Enhanced Prodigiosins (RED) Production. Sci. China Life Sci. 2017, 60, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Roselan, M.A.; Zakaria, N.; Faujan, N.H.; Mohammad Latif, M.A.; Mohd Faudzi, S.M.; Ab Hadi, H.; Ashari, S.E. In Vitro Cytotoxicity Assay, Mushroom Tyrosinase Inhibitory Activity and Release Analysis of Kojic Monooleate Nanodelivery System and in Silico Molecular Docking Study against 2Y9X Target Enzyme. J. Drug Deliv. Sci. Technol. 2021, 66, 102764. [Google Scholar] [CrossRef]
- Morel, S.; Sapino, S.; Peira, E.; Chirio, D.; Gallarate, M. Regulatory Requirements for Exporting Cosmetic Products to Extra-EU Countries. Cosmetics 2023, 10, 62. [Google Scholar] [CrossRef]
- Taechowisan, T.; Wanbanjob, A.; Tuntiwachwuttikul, P.; Liu, J. Anti-Inflammatory Activity of Lansais from Endophytic Streptomyces sp. SUC1 in LPS-Induced RAW 264.7 Cells. Food Agric. Immunol. 2009, 20, 67–77. [Google Scholar] [CrossRef]
- Claudel, J.-P.; Auffret, N.; Leccia, M.-T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. Evaluation of Biocompatibility and Cytotoxicity Using Keratinocyte and Fibroblast Cultures. Skin Pharmacol. Physiol. 2009, 22, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kummala, R.; Soto Véliz, D.; Fang, Z.; Xu, W.; Abitbol, T.; Xu, C.; Toivakka, M. Human Dermal Fibroblast Viability and Adhesion on Cellulose Nanomaterial Coatings: Influence of Surface Characteristics. Biomacromolecules 2020, 21, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Chavan, A.; Pawar, J.; Kakde, U.; Venkatachalam, M.; Fouillaud, M.; Dufossé, L.; Deshmukh, S.K. Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring. Fermentation 2025, 11, 395. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Kenawy, E.-R.; Ahmed, S.; El-Sapagh, S. Bioproduction and Optimization of Newly Characterized Melanin Pigment from Streptomyces djakartensis NSS-3 with Its Anticancer, Antimicrobial, and Radioprotective Properties. Microb. Cell Fact. 2024, 23, 23. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation—Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Barreto, J.V.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.; Vermelho, A.B. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef]
- Someya, N.; Nakajima, M.; Hamamoto, H.; Yamaguchi, I.; Akutsu, K. Effects of Light Conditions on Prodigiosin Stability in the Biocontrol Bacterium Serratia marcescens Strain B2. J. Gen. Plant Pathol. 2004, 70, 367–370. [Google Scholar] [CrossRef]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M.; Dufossé, L.; Joshi, N.C. Bacterial Pigments and Their Multifaceted Roles in Contemporary Biotechnology and Pharmacological Applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef]
- Ahmad, T.; Arora, P.; Nalli, Y.; Ali, A.; Riyaz-Ul-Hassan, S. Antibacterial Potential of Juglomycin A Isolated from Streptomyces achromogenes, an Endophyte of Crocus sativus Linn. J. Appl. Microbiol. 2020, 128, 1366–1377. [Google Scholar] [CrossRef]
- Lehr, N.A.; Adomas, A.; Asiegbu, F.O.; Hampp, R.; Tarkka, M.T. WS-5995 B, an Antifungal Agent Inducing Differential Gene Expression in the Conifer Pathogen Heterobasidion annosum but Not in Heterobasidion abietinum. Appl. Microbiol. Biotechnol. 2009, 85, 347–358. [Google Scholar] [CrossRef]
- Kozlovskiy, S.A.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; Sabutski, Y.E.; Polonik, S.G.; Likhatskaya, G.N.; Aminin, D.L. Anti-Inflammatory Activity of 1,4-Naphthoquinones Blocking P2 × 7 Purinergic Receptors in RAW 264.7 Macrophage Cells. Toxins. 2023, 15, 47. [Google Scholar] [CrossRef]
- Ullah, S.; Akter, J.; Kim, S.J.; Yang, J.; Park, Y.; Chun, P.; Moon, H.R. The Tyrosinase-Inhibitory Effects of 2-Phenyl-1,4-Naphthoquinone Analogs: Importance of the (E)-β-Phenyl-α,β-Unsaturated Carbonyl Scaffold of an Endomethylene Type. Med. Chem. Res. 2019, 28, 95–103. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From Oxidative Damage to Cellular and Inter-Cellular Signaling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef] [PubMed]
- Sabuakham, S.; Nasoontorn, S.; Kongtaworn, N.; Rungrotmongkol, T.; Silsirivanit, A.; Pingaew, R.; Mahalapbutr, P. Anilino-1,4-Naphthoquinones as Potent Mushroom Tyrosinase Inhibitors: In Vitro and in Silico Studies. J. Enzyme Inhib. Med. Chem. 2024, 39, 2357174. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Tovar, A.A.; Silva, L.; Sánchez-Suárez, J.; Diaz, L. Streptomyces-Derived Bioactive Pigments: Ecofriendly Source of Bioactive Compounds. Coatings 2022, 12, 1858. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Sarmiento-Tovar, A.A.; Prada-Rubio, S.J.; Gonzalez-Ronseria, J.; Coy-Barrera, E.; Diaz, L. Exploration of the Bioactivity of Pigmented Extracts from Streptomyces Strains Isolated Along the Banks of the Guaviare and Arauca Rivers (Colombia). Fermentation 2024, 10, 529. [Google Scholar] [CrossRef]
- Omae, I. General Aspects of Tin-Free Antifouling Paints. Chem. Rev. 2003, 103, 3431–3448. [Google Scholar] [CrossRef]
- Selvameenal, L.; Radhakrishnan, M.; Balagurunathan, R. Antibiotic Pigment from Desert Soil Actinomycetes; Biological Activity, Purification and Chemical Screening. Indian J. Pharm. Sci. 2009, 71, 499–504. [Google Scholar] [CrossRef]
- Atta, H.M. Biochemical Studies on Antibiotic Production from Streptomyces Sp.: Taxonomy, Fermentation, Isolation and Biological Properties. J. Saudi Chem. Soc. 2015, 19, 12–22. [Google Scholar] [CrossRef]
- Manikkam, R.; Venugopal, G.; Ramasamy, B.; Kumar, V. Effect of Critical Medium Components and Culture Conditions on Antitubercular Pigment Production from Novel Streptomyces sp D25 Isolated from Thar Desert, Rajasthan. J. Appl. Pharm. Sci. 2015, 5, 15–19. [Google Scholar] [CrossRef]
- Joseph, F.-J.R.S.; Iniyan, A.M.; Vincent, S.G.P. HR-LC-MS Based Analysis of Two Antibacterial Metabolites from a Marine Sponge Symbiont Streptomyces pharmamarensis ICN40. Microb. Pathog. 2017, 111, 450–457. [Google Scholar] [CrossRef]
- Feng, Y.; Qaseem, A.; Moumbock, A.F.A.; Pan, S.; Kirchner, P.A.; Simoben, C.V.; Malange, Y.I.; Babiaka, S.B.; Gao, M.; Günther, S. StreptomeDB 4.0: A Comprehensive Database of Streptomycetes Natural Products Enriched with Protein Interactions and Interactive Spectral Visualization. Nucleic Acids Res. 2025, 53, D724–D729. [Google Scholar] [CrossRef] [PubMed]
- Azmin, N.R.A.M.; Sarmin, N.I.M.; Khan, H.B.S.G.; Zainal, M. Identification of Endophytic Actinomycetes Isolated From Hopea ferrea and Its Antibacterial Activity Against Cariogenic Bacterium. Malaysian Appl. Biol. 2024, 53, 37–46. [Google Scholar] [CrossRef]
- Nurul Zarith, M.Z.; Nor Asmara Tasrip, N.A.T.; Mohd Nasir, M.D.; Cheah Yoke Kqueen, C.Y.K.; Zainul Amiruddin Zakaria, Z.A.Z.; Rukman Awang Hamat, R.A.H.; Mariana Nor Shamsudin, M.N.S. Characterization and Antimicrobial Activities of Two Streptomyces Isolates from Soil in the Periphery of Universiti Putra Malaysia. Trop. Biomed. 2011, 28, 651–660. [Google Scholar] [PubMed]
- Zhang, R.; Chen, J.; Mao, X.; Qi, P.; Zhang, X. Anti-Inflammatory and Anti-Aging Evaluation of Pigment–Protein Complex Extracted from Chlorella pyrenoidosa. Mar. Drugs 2019, 17, 586. [Google Scholar] [CrossRef]
- Asha Shalini, A.; Syed Ali, M.; Anuradha, V.; Yogananth, N.; Bhuvana, P. GCMS Analysis and In vitro Antibacterial and Anti-Inflammatory Study on Methanolic Extract of Thalassiosira weissflogii. Biocatal. Agric. Biotechnol. 2019, 19, 101148. [Google Scholar] [CrossRef]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on Secondary Metabolite Profiling, Anti-Inflammatory Potential, in Vitro Photoprotective and Skin-Aging Related Enzyme Inhibitory Activities of Malaxis acuminata, a Threatened Orchid of Nutraceutical Importance. J. Photochem. Photobiol. B Biol. 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, Anti-Inflammatory and Antiarthritic Potential of Moringa oleifera Lam: An Ethnomedicinal Plant of Moringaceae Family. South Afr. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Muddathir, A.M.; Mitsunaga, T. Evaluation of Anti-Acne Activity of Selected Sudanese Medicinal Plants. J. Wood Sci. 2013, 59, 73–79. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhan, J.X.; Su, K.M.; Zhang, Y.X. A Kind of Potential Food Additive Produced by Streptomyces coelicolor: Characteristics of Blue Pigment and Identification of a Novel Compound, Lambda-Actinorhodin. Food Chem. 2006, 95, 186–192. [Google Scholar] [CrossRef]
- Zhu, Y.; Shang, X.; Yang, L.; Zheng, S.; Liu, K.; Li, X. Purification, Identification and Properties of a New Blue Pigment Produced from Streptomyces sp. A1013Y. Food Chem. 2020, 308, 125600. [Google Scholar] [CrossRef]

| Source | DF | Adjust SC | Adjust MC | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 6 | 10,314.6 | 1719.1 | 24.12 | 0.002 |
| Lineal | 6 | 10,314.6 | 1719.1 | 24.12 | 0.002 |
| Temp | 1 | 1587.0 | 1587.00 | 22.27 | 0.005 |
| pH | 1 | 2760.3 | 2760.33 | 38.74 | 0.002 |
| Ti | 1 | 1382.5 | 1382.45 | 19.4 | 0.007 |
| CN | 1 | 62.20 | 62.2 | 0.87 | 0.393 |
| CC | 1 | 1077.7 | 1077.69 | 15.12 | 0.012 |
| rpm | 1 | 3444.92 | 3444.92 | 48.34 | 0.001 |
| Error | 4 | 71.26 | 71.26 | ||
| Total | 11 | ||||
| S | R2 | R2 (Adjust) | R2 (Pred) | ||
| 8.44148 | 96.66% | 92.65% | 80.77% | ||
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 3.117 × 105 | 1 | 77,920.09 | 22.66 | <0.0001 * |
| A—Temperature | 1.367 × 105 | 1 | 1.367 × 105 | 39.76 | <0.0001 * |
| B—rpm | 27,109.21 | 1 | 27,109.21 | 7.88 | 0.0185 * |
| AB | 27,888.84 | 1 | 27,888.84 | 8.11 | 0.0173 * |
| B2 | 78,613.75 | 1 | 78,613.75 | 22.87 | 0.0007 * |
| Residual | 34,381.57 | 10 | 3438.16 | ||
| Lack of Fit | 17,814.71 | 8 | 2226.84 | 0.2688 | 0.9279 |
| Pure Error | 16,566.86 | 2 | 8283.43 | ||
| Cor Total | 3.461 × 105 | 14 | |||
| St Dev. | 58.64 | R2 | 0.9006 | ||
| Mean | 191.81 | fitted R2 | 0.8609 | ||
| C.V % | 30.57 | Predicted R2 | 0.7914 | ||
| PRESS | 72,193.05 | Adeq precision | 14.4596 |
| Run | T (°C) | pH | Ti (days) | C. N (%) | C. C (%) | rpm | Pigment (µg/mL) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | −1 | 1 | −1 | −1 | −1 | 85.33 | |
| 2 | −1 | 1 | 1 | −1 | 1 | −1 | 13.33 | |
| 3 | 1 | 1 | −1 | 1 | −1 | −1 | 43.40 | |
| 4 | 1 | −1 | 1 | −1 | 1 | 1 | 7.40 | |
| 5 | −1 | 1 | 1 | 1 | 1 | −1 | 62.00 | |
| 6 | 1 | −1 | 1 | 1 | −1 | 1 | 36.07 | |
| 7 | 1 | 1 | −1 | −1 | 1 | 1 | 12.00 | |
| 8 | 1 | −1 | −1 | 1 | 1 | −1 | 6.73 | |
| 9 | −1 | −1 | −1 | 1 | 1 | 1 | 60.07 | |
| 10 | −1 | −1 | 1 | 1 | −1 | 1 | 70.73 | |
| 11 | −1 | 1 | −1 | −1 | −1 | 1 | 78.73 | |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | 0.07 | |
| Levels | 1 | 35 | 8 | 7 | 0.2 | 0.2 | 200 | |
| −1 | 25 | 6 | 3 | 0.1 | 0.1 | 100 |
| Run | T (°C) | rpm | Ti (days) | Pigment (µg/mL) | |
|---|---|---|---|---|---|
| 1 | −1 | 0 | 1 | 445.00 | |
| 2 | −1 | 1 | 0 | 142.00 | |
| 3 | 1 | −1 | 0 | 42.73 | |
| 4 | 1 | 0 | −1 | 76.40 | |
| 5 | 0 | 0 | 0 | 210.40 | |
| 6 | −1 | −1 | 0 | 440.06 | |
| 7 | 0 | −1 | −1 | 247.40 | |
| 8 | 1 | 0 | 1 | 205.40 | |
| 9 | 0 | 0 | 0 | 304.10 | |
| 10 | 0 | 1 | 1 | 8.70 | |
| 11 | 1 | 1 | 0 | 15.40 | |
| 12 | 0 | −1 | 1 | 284.40 | |
| 13 | 0 | 1 | −1 | 7.40 | |
| 14 | 0 | 0 | 0 | 392.40 | |
| 15 | −1 | 0 | −1 | 55.40 | |
| Levels | 1 | 40 | 150 | 9 | |
| 0 | 35 | 100 | 7 | ||
| −1 | 30 | 50 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Arias, L.D.; Díaz, L.; Coy-Barrera, E. Bioproduction Optimization, Characterization, and Bioactivity of Extracellular Pigment Produced by Streptomyces parvulus. Int. J. Mol. Sci. 2025, 26, 10762. https://doi.org/10.3390/ijms262110762
Silva-Arias LD, Díaz L, Coy-Barrera E. Bioproduction Optimization, Characterization, and Bioactivity of Extracellular Pigment Produced by Streptomyces parvulus. International Journal of Molecular Sciences. 2025; 26(21):10762. https://doi.org/10.3390/ijms262110762
Chicago/Turabian StyleSilva-Arias, Laura Daniela, Luis Díaz, and Ericsson Coy-Barrera. 2025. "Bioproduction Optimization, Characterization, and Bioactivity of Extracellular Pigment Produced by Streptomyces parvulus" International Journal of Molecular Sciences 26, no. 21: 10762. https://doi.org/10.3390/ijms262110762
APA StyleSilva-Arias, L. D., Díaz, L., & Coy-Barrera, E. (2025). Bioproduction Optimization, Characterization, and Bioactivity of Extracellular Pigment Produced by Streptomyces parvulus. International Journal of Molecular Sciences, 26(21), 10762. https://doi.org/10.3390/ijms262110762








