Expanding Horizons in Cholangiocarcinoma: Emerging Targets Beyond FGFR2 and IDH1
Abstract
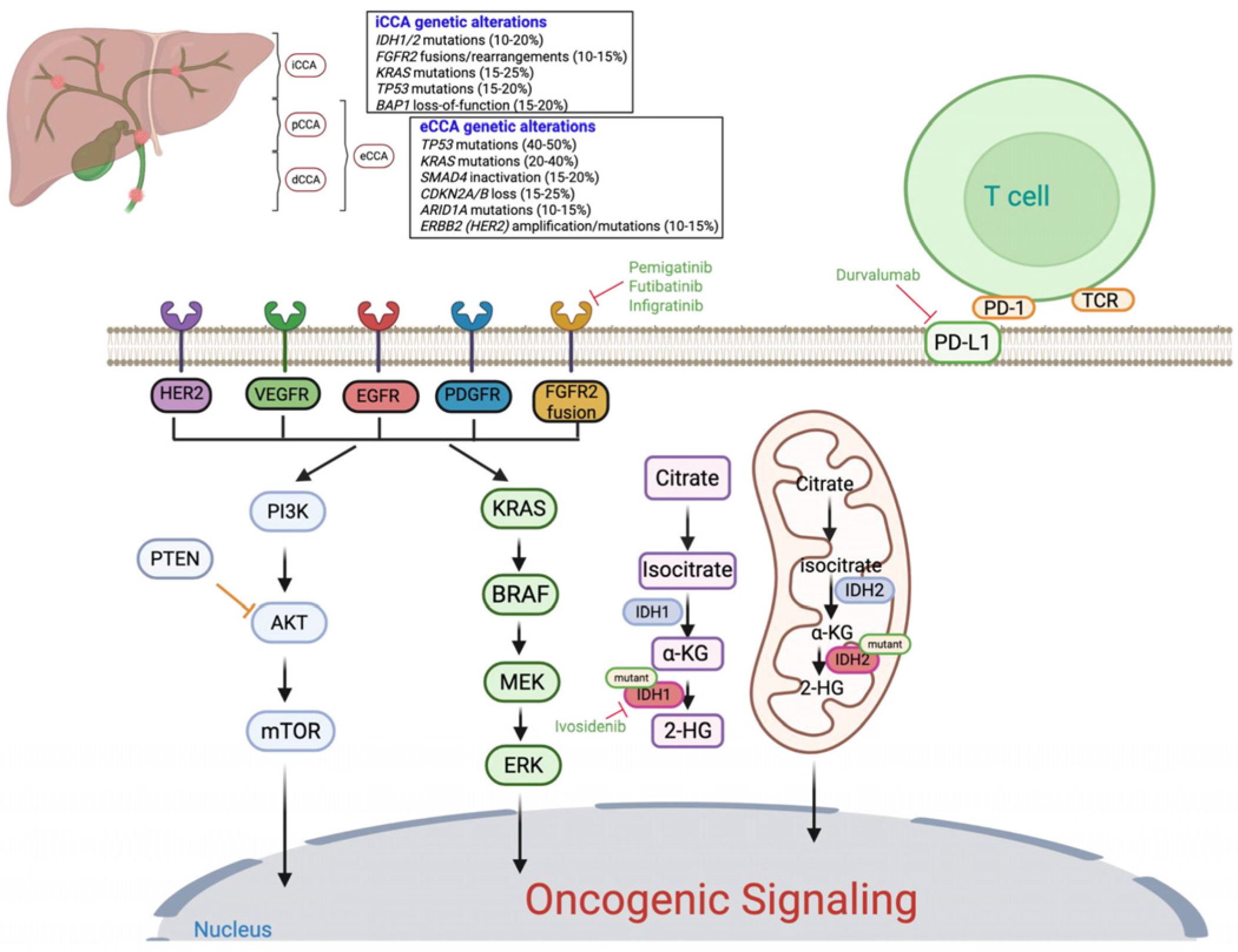
1. Introduction
2. Recent Success of Targeted Therapies in CCA: Tyrosine Kinase Inhibitors
2.1. FGFR Inhibitors
| Drug | Approval Year | Target | Patients | Efficacy Results | Most Common Grade ≥ 3 AEs | Trial No./Reference |
|---|---|---|---|---|---|---|
| Pemigatinib | 2020 | FGFR1, FGFR2, FGFR3 Little to no activity against FGFR4 | Previously treated, advanced or metastatic CCA harboring FGFR2 fusions or rearrangements | mPFS: 7.0 mo mOS: 17.5 mo | Hyperphosphatemia 14.3% Fatigue 5% Stomatitis 7% Arthralgia 6% | NCT02924376 [38] |
| Infigratinib | 2021 Withdrawn 2024 | Selective for FGFR1–3 | Previously treated, unresectable locally advanced or metastatic CCA with FGFR2 fusion/rearrangement | mPFS: 7.3 mo mOS: 12.2 mo | Hypophosphatemia 14.1% Hyperphosphatemia 12.7% Hyponatremia 11.3% Stomatitis 9.9% | NCT02150967 [39] |
| Futibatinib | 2022 | Selective for FGFR1–4 | Previously treated, unresectable locally advanced or metastatic iCCA with FGFR2 fusion/rearrangement | mPFS: 9.0 mo mOS: 21.7 mo | Hyperphosphatemia 30% Elevated AST level 7% Stomatitis 6% Fatigue 6% | NCT04093362 [40] |
| Ivosidenib | 2021 | Mutant IDH1 enzyme | Previously treated, locally advanced/metastatic CCA with an IDH1 mutation | mPFS: 2.7 months mOS: 10.3 months | Anemia 7% Increased blood bilirubin 6% Hyponatremia 6% Ascites 9% | NCT02989857 [43] |
2.2. IDH1 Inhibitors
3. Immunotherapy in CCA
4. Investigational Targeted Therapies in CCA
4.1. P53-MDM-2 Signaling
4.2. ErbB Inhibitors
4.3. VEGFR and PDGFR Inhibitors
4.4. MET Inhibitors
4.5. ALK Inhibitors
4.6. MAPK Signaling Inhibitors
4.7. PI3K/AKT/mTOR Signaling Inhibitors
4.8. JAK/STAT Pathway Inhibitors
4.9. PARP Inhibitors
5. Potential of Targeting KRAS in CCA
6. Comparative Stratification of Therapeutic Modalities
7. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCA | Cholangiocarcinoma |
| iCCA | Intrahepatic cholangiocarcinoma |
| pCCA | Perihilar cholangiocarcinoma |
| dCCA | Distal cholangiocarcinoma |
| eCCA | Extrahepatic cholangiocarcinoma |
| FGFR | Fibroblast growth factor receptor |
| IDH | Isocitrate dehydrogenase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
References
- Ilyas, S.I.; Affo, S.; Goyal, L.; Lamarca, A.; Sapisochin, G.; Yang, J.D.; Gores, G.J. Cholangiocarcinoma—Novel biological insights and therapeutic strategies. Nat. Rev. Clin. Oncol. 2023, 20, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- Sarcognato, S.; Sacchi, D.; Fassan, M.; Fabris, L.; Cadamuro, M.; Zanus, G.; Cataldo, I.; Capelli, P.; Baciorri, F.; Cacciatore, M.; et al. Cholangiocarcinoma. Pathologica 2021, 113, 158–169. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Tangkawattana, S.; Brindley, P.J. Chapter Five—Update on Pathogenesis of Opisthorchiasis and Cholangiocarcinoma. In Advances in Parasitology; Sripa, B., Brindley, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 102, pp. 97–113. [Google Scholar]
- Zheng, S.; Zhu, Y.; Zhao, Z.; Wu, Z.; Okanurak, K.; Lv, Z. Liver fluke infection and cholangiocarcinoma: A review. Parasitol. Res. 2017, 116, 11–19. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Anderson, C.D.; Wright Pinson, C.; Berlin, J.; Chari, R.S. Diagnosis and Treatment of Cholangiocarcinoma. Oncologist 2004, 9, 43–57. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Macias, R.I.R.; Andersen, J.B.; Braconi, C.; Carpino, G.; Alvaro, D.; Calvisi, D.F. Cholangiocarcinoma: State-of-the-art knowledge and challenges. Liver Int. 2019, 39, 5–6. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001, 234, 507–517. [Google Scholar] [CrossRef]
- Jung Hyun, J.; Si Young, S. Chemotherapy of Cholangiocarcinoma: Current Management and Future Directions. In Topics in the Surgery of the Biliary Tree; Hesham, A., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Zhu, A.X. Future directions in the treatment of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 355–361. [Google Scholar] [CrossRef]
- Halder, R.; Amaraneni, A.; Shroff, R.T. Cholangiocarcinoma: A review of the literature and future directions in therapy. Hepatobiliary Surg. Nutr. 2022, 11, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Kim, M.J.; Lee, J.-c.; Kim, J.; Woo, S.M.; Lee, W.J.; Lee, K.H.; Hwang, J.-H. Gemcitabine Plus Cisplatin Chemotherapy Prolongs the Survival in Advanced Hilar Cholangiocarcinoma: A Large Multicenter Study. Am. J. Clin. Oncol. 2020, 43, 422–427. [Google Scholar] [CrossRef]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014, 25, 391–398. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.T.; Kitano, M.; Lee, C.K.; Kim, J.W.; Chen, M.H.; et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): Updated overall survival from a randomised phase 3 study. Lancet Gastroenterol. Hepatol. 2024, 9, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Ramírez-Merino, N.; Aix, S.P.; Cortés-Funes, H. Chemotherapy for cholangiocarcinoma: An update. World J. Gastrointest. Oncol. 2013, 5, 171–176. [Google Scholar] [CrossRef]
- Park, J.O.; Oh, D.Y.; Hsu, C.; Chen, J.S.; Chen, L.T.; Orlando, M.; Lim, H.Y. Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review. Cancer Res. Treat. 2015, 47, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Kapacee, Z.; Breeze, M.; Bell, C.; Belcher, D.; Staiger, H.; Taylor, C.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and Other Biliary Tract Cancers. J. Clin. Med. 2020, 9, 2854. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Facchinetti, F.; DiToro, D.F.; Baiev, I.; Majeed, U.; Reyes, S.; Chen, C.; Zhang, K.; Sharman, R.; Uson Junior, P.L.S.; et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann. Oncol. 2022, 33, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Huang, T.; Jiang, T.; Wang, H. Current status and prospects of targeted therapy for cholangiocarcinoma based on molecular characteristics. Cancer Lett. 2025, 614, 217540. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Stecko, H.; Ntanasis-Stathopoulos, I.; Pawlik, T.M. Racial and Sex Differences in Genomic Profiling of Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2024, 31, 9071–9078. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Cholangiocarcinoma: Molecular Abnormalities and Cells of Origin. Technol. Cancer Res. Treat. 2023, 22, 15330338221128689. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hu, W.; Shi, X.; Liu, P.; Ma, X.; Zhao, W.; Qu, L.; Zhang, S.; Shi, W.; Liu, A.; et al. Comprehensive genomic profile of cholangiocarcinomas in China. Oncol. Lett. 2020, 19, 3101–3110. [Google Scholar] [CrossRef]
- Carotenuto, M.; Sacco, A.; Forgione, L.; Normanno, N. Genomic alterations in cholangiocarcinoma: Clinical significance and relevance to therapy. Explor. Target. Antitumor Ther. 2022, 3, 200–223. [Google Scholar] [CrossRef] [PubMed]
- Lodl, E.; Ramnaraign, B.; Sahin, I.; Wheeler, S. Updates in the use of targeted therapies for the treatment of cholangiocarcinoma. J. Oncol. Pharm. Pract. 2023, 29, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Segatto, O.; Stenzinger, A.; Saborowski, A. FGFR2 Inhibition in Cholangiocarcinoma. Annu. Rev. Med. 2023, 74, 293–306. [Google Scholar] [CrossRef]
- Salati, M.; Caputo, F.; Baldessari, C.; Carotenuto, P.; Messina, M.; Caramaschi, S.; Dominici, M.; Bonetti, L.R. The Evolving Role of FGFR2 Inhibitors in Intrahepatic Cholangiocarcinoma: From Molecular Biology to Clinical Targeting. Cancer Manag. Res. 2021, 13, 7747–7757. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Iannotti, N.O.; Gutierrez, M.; Smith, D.C.; Féliz, L.; Lihou, C.F.; Tian, C.; Silverman, I.M.; Ji, T.; Saleh, M. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann. Oncol. 2022, 33, 522–533. [Google Scholar] [CrossRef]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.M.; Melisi, D.; Al Rajabi, R.M.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. An open-label study of pemigatinib in cholangiocarcinoma: Final results from FIGHT-202. ESMO Open 2024, 9, 103488. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Subbiah, V.; Nemunaitis, J.J.; Mettu, N.B.; Papadopoulos, K.P.; Barve, M.A.; Féliz, L.; Lihou, C.F.; Tian, C.; Ji, T.; et al. Safety and efficacy of pemigatinib plus pembrolizumab combination therapy in patients (pts) with advanced malignancies: Results from FIGHT-101, an open-label phase I/II study. J. Clin. Oncol. 2020, 38, 3606. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Fassan, M.; Ruzzenente, A.; Mafficini, A.; Wood, L.D.; Corbo, V.; Melisi, D.; Malleo, G.; Vicentini, C.; Malpeli, G.; et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014, 5, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.R.; Voss, J.S.; Kerr, S.E.; Barr Fritcher, E.G.; Graham, R.P.; Zhang, L.; Highsmith, W.E.; Zhang, J.; Roberts, L.R.; Gores, G.J.; et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum. Pathol. 2012, 43, 1552–1558. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef]
- Marin, J.J.; Macias, R.I. Understanding drug resistance mechanisms in cholangiocarcinoma: Assisting the clinical development of investigational drugs. Expert. Opin. Investig. Drugs 2021, 30, 675–679. [Google Scholar] [CrossRef]
- Harding, J.J.; Lowery, M.A.; Shih, A.H.; Schvartzman, J.M.; Hou, S.; Famulare, C.; Patel, M.; Roshal, M.; Do, R.K.; Zehir, A.; et al. Isoform Switching as a Mechanism of Acquired Resistance to Mutant Isocitrate Dehydrogenase Inhibition. Cancer Discov. 2018, 8, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Rizzo, A. IDH Inhibitors and Immunotherapy for Biliary Tract Cancer: A Marriage of Convenience? Int. J. Mol. Sci. 2022, 23, 10869. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Jelinek, M.; Singh, A.; Isett, B.; Myers, E.S.; Mullett, S.J.; Eisele, Y.; Beumer, J.; Parise, R.; Urban, J.; et al. Clinical and translational study of ivosidenib plus nivolumab in advanced solid tumors harboring IDH1 mutations. medRxiv 2025. [Google Scholar] [CrossRef]
- Kelley, R.K.; Cleary, J.M.; Sahai, V.; Baretti, M.; Bridgewater, J.A.; Hua, Z.; Gliser, C.; Bian, Y.; Abou-Alfa, G.K. A phase 1/2, safety lead-in and dose expansion, open-label, multicenter trial investigating the safety, tolerability, and preliminary activity of ivosidenib in combination with nivolumab and ipilimumab in previously treated subjects with IDH1-mutated nonresectable or metastatic cholangiocarcinoma. J. Clin. Oncol. 2024, 42, TPS4197. [Google Scholar]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. S1), 75–85. [Google Scholar] [CrossRef]
- Lo, J.H.; Agarwal, R.; Goff, L.W.; Heumann, T.R. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers 2023, 15, 3312. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients with Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef]
- Delaye, M.; Assenat, E.; Dahan, L.; Blanc, J.-F.; Tougeron, D.; Metges, J.-P.; Lievre, A.; Turpin, A.; Fares, N.; Fouchardiere, C.D.L.; et al. Durvalumab (D) plus tremelimumab (T) immunotherapy in patients (Pts) with advanced biliary tract carcinoma (BTC) after failure of platinum-based chemotherapy (CTx): Interim results of the IMMUNOBIL GERCOR D18-1 PRODIGE-57 study. J. Clin. Oncol. 2022, 40, 4108. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, X.M.; Li, J.Y.; Jiang, N.; Chen, L.; Tang, L.L.; Mao, Y.P.; Li, W.F.; Zhou, G.Q.; Li, Y.Q.; et al. The immune modulation effects of gemcitabine plus cisplatin induction chemotherapy in nasopharyngeal carcinoma. Cancer Med. 2022, 11, 3437–3444. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- Italiano, A.; Miller, W.H.; Blay, J.-Y.; Gietema, J.A.; Bang, Y.-J.; Mileshkin, L.R.; Hirte, H.W.; Higgins, B.; Blotner, S.; Nichols, G.L.; et al. Phase I study of daily and weekly regimens of the orally administered MDM2 antagonist idasanutlin in patients with advanced tumors. Investig. New Drugs 2021, 39, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef]
- Fang, D.D.; Tang, Q.; Kong, Y.; Wang, Q.; Gu, J.; Fang, X.; Zou, P.; Rong, T.; Wang, J.; Yang, D.; et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 2019, 7, 327. [Google Scholar] [CrossRef]
- Takahashi, S.; Fujiwara, Y.; Nakano, K.; Shimizu, T.; Tomomatsu, J.; Koyama, T.; Ogura, M.; Tachibana, M.; Kakurai, Y.; Yamashita, T.; et al. Safety and pharmacokinetics of milademetan, a MDM2 inhibitor, in Japanese patients with solid tumors: A phase I study. Cancer Sci. 2021, 112, 2361–2370. [Google Scholar] [CrossRef]
- Dumbrava, E.E.; Stinchcombe, T.E.; Gounder, M.; Cote, G.M.; Hanna, G.J.; Sumrall, B.; Wise-Draper, T.M.; Kanaan, M.; Duffy, S.; Sumey, C.; et al. Milademetan in advanced solid tumors with MDM2 amplification and wild-type TP53: Pre-clinical and phase 2 clinical trial results. Clin. Cancer Res. 2025, 31, 4255–4264. [Google Scholar] [CrossRef]
- Cornillie, J.; Wozniak, A.; Li, H.; Gebreyohannes, Y.K.; Wellens, J.; Hompes, D.; Debiec-Rychter, M.; Sciot, R.; Schöffski, P. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin. Transl. Oncol. 2020, 22, 546–554. [Google Scholar] [CrossRef]
- Rudolph, D.; Reschke, M.; Blake, S.; Rinnenthal, J.; Wernitznig, A.; Weyer-Czernilofsky, U.; Gollner, A.; Haslinger, C.; Garin-Chesa, P.; Quant, J.; et al. Abstract 4866: BI 907828: A novel, potent MDM2 inhibitor that induces antitumor immunologic memory and acts synergistically with an anti-PD-1 antibody in syngeneic mouse models of cancer. Cancer Res. 2018, 78, 4866. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tolcher, A.; Hafez, N.; Lugowska, I.; Ramlau, R.; Macarulla, T.; Geng, J.; Li, J.; Teufel, M.; Märten, A.; et al. Efficacy and Safety of the MDM2-p53 Antagonist Brigimadlin (BI 907828) in Patients with Advanced Biliary Tract Cancer: A Case Series. Onco Targets Ther. 2024, 17, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Chen, J.S.; Hsu, C.; Chiang, N.J.; Tsai, C.S.; Tsou, H.H.; Huang, S.F.; Bai, L.Y.; Chang, I.C.; Shiah, H.S.; Ho, C.L.; et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann. Oncol. 2015, 26, 943–949. [Google Scholar] [CrossRef]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320. [Google Scholar] [CrossRef]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; Chan, J.A.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2010, 11, 48–54. [Google Scholar] [CrossRef]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2010, 102, 68–72. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.Y.; Micetich, K.C.; Kayaleh, O.R.; Lenz, H.J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef]
- Lee, S.; Shroff, R.T.; Makawita, S.; Xiao, L.; Danner De Armas, A.; Bhosale, P.; Reddy, K.; Shalaby, A.; Raghav, K.; Pant, S.; et al. Phase II Study of Ramucirumab in Advanced Biliary Tract Cancer Previously Treated by Gemcitabine-Based Chemotherapy. Clin. Cancer Res. 2022, 28, 2229–2236. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2018, 109, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Yarchoan, M.; O’Connor, A.; Gallagher, D.; Zahurak, M.L.; Rosner, G.; Ohaji, C.; Sartorius-Mergenthaler, S.; Subbiah, V.; Zinner, R.; et al. The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. Br. J. Cancer 2017, 116, 1402–1407, Erratum in Br. J. Cancer 2018, 118, e2. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef]
- Buzzoni, R.; Pusceddu, S.; Bajetta, E.; De Braud, F.; Platania, M.; Iannacone, C.; Cantore, M.; Mambrini, A.; Bertolini, A.; Alabiso, O.; et al. Activity and safety of RAD001 (everolimus) in patients affected by biliary tract cancer progressing after prior chemotherapy: A phase II ITMO study. Ann. Oncol. 2014, 25, 1597–1603. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Goff, L.W.; Crysler, O.V.; Enzler, T.; Zalupski, M. Phase 2 multicenter trial of rucaparib and nivolumab as maintenance therapy following first-line platinum-based chemotherapy in patients with advanced biliary tract cancer (BTC): BilT-02. J. Clin. Oncol. 2023, 41, 4113. [Google Scholar] [CrossRef]
- Sadagopan, N.; Wang, H.; Yin, C.; Weinberg, B.A.; Noel, M.S.; Mukherji, R.; Geng, X.; Marshall, J.L.; He, A.R. A phase II single-arm study of combination pembrolizumab and olaparib in the treatment of patients with advanced biliary tract cancer. Npj Precis. Oncol. 2025, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Lamarca, A.; Choi, H.J.; Vogel, A.; Pishvaian, M.J.; Goyal, L.; Ueno, M.; Märten, A.; Teufel, M.; Geng, L.; et al. Brightline-2: A phase IIa/IIb trial of brigimadlin (BI 907828) in advanced biliary tract cancer, pancreatic ductal adenocarcinoma or other solid tumors. Future Oncol. 2024, 20, 1069–1077. [Google Scholar] [CrossRef]
- Sirica, A.E. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2008, 14, 7033–7058. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Tovar, V.; Moeini, A.; Llovet, J.M. Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for molecular therapies. Oncogene 2013, 32, 4861–4870. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2008, 98, 418–425. [Google Scholar] [CrossRef]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2016, 122, 574–581. [Google Scholar] [CrossRef]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Villalona Calero, M.; Bekaii-Saab, T. HER2/neu may not be an interesting target in biliary cancers: Results of an early phase II study with lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Arrivas Bajardi, E.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. 2015, 26, 542–547. [Google Scholar] [CrossRef]
- Boonjaraspinyo, S.; Wu, Z.; Boonmars, T.; Kaewkes, S.; Loilome, W.; Sithithaworn, P.; Nagano, I.; Takahashi, Y.; Yongvanit, P.; Bhudhisawasdi, V. Overexpression of PDGFA and its receptor during carcinogenesis of Opisthorchis viverrini-associated cholangiocarcinoma. Parasitol. Int. 2012, 61, 145–150. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.J.; Ben-Josef, E.; Lenz, H.J.; Gold, P.J.; Hamilton, R.D.; Govindarajan, R.; Eng, C.; Blanke, C.D. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig. New Drugs 2012, 30, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Ojima, H.; Iwasaki, M.; Shimizu, H.; Kokubu, A.; Hiraoka, N.; Kosuge, T.; Yoshikawa, D.; Kono, T.; Furukawa, H.; et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br. J. Cancer 2011, 105, 131–138. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.-Y.; Orlova, R.; Van Cutsem, E.; Adeva, J.; Chen, L.-T.; Obermannova, R.; et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: A randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1468–1482, Correction in Lancet Oncol. 2021, 22, e472. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Saleh, M.; Bendell, J.; Infante, J.R.; Jones, S.; Kurkjian, C.D.; Moore, K.M.; Kazakin, J.; Abbadessa, G.; Wang, Y.; et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann. Oncol. 2014, 25, 1416–1421. [Google Scholar] [CrossRef]
- Paik Paul, K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot Alexis, B.; Garassino Marina, C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients with MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-Term Follow-Up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 1260–1266, Correction in JAMA Oncol. 2023, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Administration USFD. FDA Approves Tepotinib for Metastatic Non-Small Cell Lung Cancer. Food Drug Adm. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tepotinib-metastatic-non-small-cell-lung-cancer (accessed on 23 October 2025).
- Rahnemai-Azar, A.A.; Weisbrod, A.B.; Dillhoff, M.; Schmidt, C.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Current management and emerging therapies. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Takeyasu, Y.; Okuma, H.S.; Kojima, Y.; Nishikawa, T.; Tanioka, M.; Sudo, K.; Shimoi, T.; Noguchi, E.; Arakawa, A.; Mori, T.; et al. Impact of ALK Inhibitors in Patients with ALK-Rearranged Nonlung Solid Tumors. JCO Precis. Oncol. 2021, 5, 756–766. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Palomba, G.; Doneddu, V.; Cossu, A.; Paliogiannis, P.; Manca, A.; Casula, M.; Colombino, M.; Lanzillo, A.; Defraia, E.; Pazzola, A.; et al. Prognostic impact of KRAS, NRAS, BRAF, and PIK3CA mutations in primary colorectal carcinomas: A population-based study. J. Transl. Med. 2016, 14, 292. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Walter, D.; Hartmann, S.; Waidmann, O. Update on cholangiocarcinoma: Potential impact of genomic studies on clinical management. Z. Gastroenterol. 2017, 55, 575–581. [Google Scholar] [CrossRef]
- Subbiah, V.; Puzanov, I.; Blay, J.Y.; Chau, I.; Lockhart, A.C.; Raje, N.S.; Wolf, J.; Baselga, J.; Meric-Bernstam, F.; Roszik, J.; et al. Pan-Cancer Efficacy of Vemurafenib in BRAF (V600)-Mutant Non-Melanoma Cancers. Cancer Discov. 2020, 10, 657–663. [Google Scholar] [CrossRef]
- Silkin, S.V.; Startsev, S.S.; Krasnova, M.E.; Raskin, G.A.; Mitiushkina, N.V.; Iyevleva, A.G.; Sokolenko, A.P.; Imyanitov, E.N. Complete Clinical Response of BRAF-Mutated Cholangiocarcinoma to Vemurafenib, Panitumumab, and Irinotecan. J. Gastrointest. Cancer 2016, 47, 502–505. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; McEntee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153, Erratum in BMC Cancer 2016, 16, 369. [Google Scholar]
- Doherty, M.K.; Tam, V.C.; McNamara, M.G.; Jang, R.; Hedley, D.; Chen, E.; Dhani, N.; Tang, P.; Sim, H.W.; O’Kane, G.M.; et al. Randomised, Phase II study of selumetinib, an oral inhibitor of MEK, in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer. Br. J. Cancer 2022, 127, 1473–1478. [Google Scholar] [CrossRef]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef]
- Heumann, T.R.; Yarchoan, M.; Murray, J.; Lu, J.; Li, D.; Kunk, P.R.; Iyer, R.V.; Dayyani, F.; Fonkoua, L.A.K.; Kalyan, A.; et al. A randomized phase 2 study of combination atezolizumab and varlilumab (CDX-1127) with or without addition of cobimetinib in previously treated unresectable biliary tract cancer. J. Clin. Oncol. 2024, 42, 4017. [Google Scholar] [CrossRef]
- Schmitz, K.J.; Lang, H.; Wohlschlaeger, J.; Sotiropoulos, G.C.; Reis, H.; Schmid, K.W.; Baba, H.A. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2007, 13, 6470–6477. [Google Scholar] [CrossRef]
- Javle, M.M.; Yu, J.; Khoury, T.; Chadha, K.S.; Iyer, R.V.; Foster, J.; Kuvshinoff, B.W.; Gibbs, J.F.; Geradts, J.; Black, J.D.; et al. Akt expression may predict favorable prognosis in cholangiocarcinoma. J. Gastroenterol. Hepatol. 2006, 21, 1744–1751. [Google Scholar] [CrossRef]
- Lau, D.K.; Tay, R.Y.; Yeung, Y.H.; Chionh, F.; Mooi, J.; Murone, C.; Skrinos, E.; Price, T.J.; Mariadason, J.M.; Tebbutt, N.C. Phase II study of everolimus (RAD001) monotherapy as first-line treatment in advanced biliary tract cancer with biomarker exploration: The RADiChol Study. Br. J. Cancer 2018, 118, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Alberts, S.R.; Peña, C.; Genvresse, I.; Ajavon-Hartmann, A.; Xia, C.; Kelly, A.; Grilley-Olson, J.E. Phase I dose-escalation study of copanlisib in combination with gemcitabine or cisplatin plus gemcitabine in patients with advanced cancer. Br. J. Cancer 2018, 118, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Li, J.; Wei, L.; Doyle, A.; Marshall, J.L.; Schaaf, L.J.; Phelps, M.A.; Villalona-Calero, M.A.; Bekaii-Saab, T. Results of an abbreviated phase-II study with the Akt. Inhibitor MK-2206 in Patients with Advanced Biliary Cancer. Sci. Rep. 2015, 5, 12122. [Google Scholar] [CrossRef]
- Yang, R.; Song, Y.; Shakoor, K.; Yi, W.; Peng, C.; Liu, S. Insights into the role of STAT3 in intrahepatic cholangiocarcinoma (Review). Mol. Med. Rep. 2022, 25, 171. [Google Scholar] [CrossRef]
- Jarnicki, A.; Putoczki, T.; Ernst, M. Stat3: Linking inflammation to epithelial cancer—More than a “gut” feeling? Cell Div. 2010, 5, 14. [Google Scholar] [CrossRef]
- Isomoto, H.; Mott, J.L.; Kobayashi, S.; Werneburg, N.W.; Bronk, S.F.; Haan, S.; Gores, G.J. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology 2007, 132, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Li, L.; Hou, G.J.; Yan, X.Z.; Xu, Q.G.; Chen, L.; Zhang, B.H.; Shen, F. STAT3 overexpression promotes metastasis in intrahepatic cholangiocarcinoma and correlates negatively with surgical outcome. Oncotarget 2017, 8, 7710–7721. [Google Scholar] [PubMed]
- Li, X.; Jiang, W.; Dong, S.; Li, W.; Zhu, W.; Zhou, W. STAT3 Inhibitors: A Novel Insight for Anticancer Therapy of Pancreatic Cancer. Biomolecules 2022, 12, 1450. [Google Scholar] [CrossRef]
- Hong, D.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; Zhou, T.; Schmidt, J.; Jo, M.; et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl. Med. 2015, 7, 314ra185. [Google Scholar] [CrossRef]
- Han, A.; Purwin, T.J.; Aplin, A.E. Roles of the BAP1 Tumor Suppressor in Cell Metabolism. Cancer Res. 2021, 81, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Carloni, R.; Ricci, A.D.; Di Federico, A.; Guven, D.C.; Yalcin, S.; Brandi, G. Molecular Profile and Prognostic Value of BAP1 Mutations in Intrahepatic Cholangiocarcinoma: A Genomic Database Analysis. J. Pers. Med. 2022, 12, 1247. [Google Scholar] [CrossRef]
- Sabbatino, F.; Liguori, L.; Malapelle, U.; Schiavi, F.; Tortora, V.; Conti, V.; Filippelli, A.; Tortora, G.; Ferrone, C.R.; Pepe, S. Case Report: BAP1 Mutation and RAD21 Amplification as Predictive Biomarkers to PARP Inhibitor in Metastatic Intrahepatic Cholangiocarcinoma. Front. Oncol. 2020, 10, 567289. [Google Scholar] [CrossRef]
- Mohan, A.; Quingalahua, E.; Gunchick, V.; Paul, S.; Kumar-Sinha, C.; Crysler, O.; Zalupski, M.M.; Sahai, V. PARP inhibitor therapy in patients with IDH1 mutated cholangiocarcinoma. Oncologist 2024, 29, 725–730. [Google Scholar] [CrossRef]
- Tsang, E.S.; O’Kane, G.M.; Knox, J.J.; Chen, E.X. A phase II study of olaparib and durvalumab in patients with IDH-mutated cholangiocarcinoma. J. Clin. Oncol. 2023, 41, 4099. [Google Scholar] [CrossRef]
- Zhou, T.; Mahn, R.; Möhring, C.; Sadeghlar, F.; Meyer, C.; Toma, M.; Kreppel, B.; Essler, M.; Glowka, T.; Matthaei, H.; et al. Case Report: Sustained complete remission on combination therapy with olaparib and pembrolizumab in BRCA2-mutated and PD-L1-positive metastatic cholangiocarcinoma after platinum derivate. Front. Oncol. 2022, 12, 933943. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Riener, M.O.; Bawohl, M.; Clavien, P.A.; Jochum, W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosomes Cancer 2008, 47, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Ohnishi, H.; Ohtsuka, K.; Matsushima, S.; Ohkura, Y.; Furuse, J.; Watanabe, T.; Mori, T.; Sugiyama, M. KRAS Mutation as a Potential Prognostic Biomarker of Biliary Tract Cancers. Jpn. Clin. Med. 2016, 7, 33–39. [Google Scholar] [CrossRef]
- The Lancet Oncology. Undruggable KRAS-Time Rebrand? Lancet Oncol. 2021, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.R.; Garassino, M.C.; Nadal, E.; Öhrling, K.; Scheffler, M.; Mazières, J. On target: Rational approaches to KRAS inhibition for treatment of non-small cell lung carcinoma. Lung Cancer 2021, 160, 152–165. [Google Scholar] [CrossRef]
- Uprety, D.; Adjei, A.A. KRAS: From undruggable to a druggable Cancer Target. Cancer Treat. Rev. 2020, 89, 102070. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Yaeger, R.; Spira, A.I.; Pelster, M.S.; Sabari, J.K.; Hafez, N.; Barve, M.; Velastegui, K.; Yan, X.; Shetty, A.; et al. Adagrasib in Advanced Solid Tumors Harboring a KRAS(G12C) Mutation. J. Clin. Oncol. 2023, 41, 4097–4106. [Google Scholar] [CrossRef]
- Bouattour, M.; Valle, J.W.; Vogel, A.; Kim, J.W.; Kitano, M.; Chen, J.-S.; Zaucha, R.; Qin, S.; Evesque, L.; Zhen, D.B.; et al. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J. Clin. Oncol. 2023, 41, 531. [Google Scholar] [CrossRef]
| Treatment | Phase | Line | Patients | Efficacy Results | Most Common Grade ≥ 3 AEs | Trial No./Reference |
|---|---|---|---|---|---|---|
| Pembrolizumab | II | 2nd | Adults with advanced/metastatic solid tumors (including CCA), MSI-H/dMMR tumors, prior therapy failure | ORR: 5.8% mPFS: 2.0 mo mOS: 7.4 mo | Common all grade AEs: Fatigue 14.4%, rash 11.5%, pruritus 8.7% | NCT02628067 [54] |
| Nivolumab | II | 2nd | Adults with advanced/metastatic CCA, refractory to ≥1 prior therapy | ORR: 11% mPFS: 3.7 mo mOS: 14.2 mo | Hyponatremia 6% Increased alkaline phosphatase 4% | NCT02829918 [55] |
| Ipilimumab/Nivolumab | II | 2nd | Adults with advanced/metastatic CCA, refractory to ≥1 prior therapy | ORR: 23.1% mPFS: 2.9 mo mOS: 5.7 mo | Immune-related (any grade) 49% Immune-related (≥3) 15% | NCT02923934 [56] |
| Durvalumab/Tremelimumab (interim) | II | 2nd | Adults with advanced/metastatic CCA, who failed prior platinum-based chemotherapy | ORR: 9.7% mPFS: 2.5 mo mOS: 8.0 mo | Common any grade AE: Fatigue, pruritus, increased liver enzymes | NCT03704480 [57] |
| GemCis + nivolumab Nivolumab + Ipilimumab | II | 1st | Adults with advanced/unresectable CCA | ORR: 22.9 vs. 3.0% mPFS: 6.6 vs. 3.9 mo mOS: 10.6 vs. 8.2 mo | Arm A: Neutropenia 34%, anemia 23%, fatigue 8.6%; Arm B: Elevated transaminases 9.1% | NCT03101566 [58] |
| GemCis, then GemCis + durvalumab + tremelimumab GemCis + durvalumab GemCis + durvalumab + tremelimumab | II | 1st | Adults with previously untreated advanced CCA | ORR: 50 vs. 72.3 vs. 70.2% mPFS: 6.6 vs. 11.8 vs. 12.3 mo mOS: 15 vs. 20.2 vs. 18.7 mo | Across all patients: Neutropenia 53% Anemia 40% Thrombocytopenia 19% | NCT03046862 [59] |
| GemCis + durvalumab GemCis | III | 1st | Adults with previously untreated advanced (unresectable or metastatic) CCA, or recurrent disease ≥6 months post-surgery/adjuvant therapy | ORR: 26.7 vs. 18.7% mPFS: 7.2 vs. 5.7 mo mOS: 12.9 vs. 11.3 mo | Neutropenia 21 vs. 25% Anemia 19 vs. 19% Thrombocytopenia 10 vs. 11% Fatigue 6 vs. 5% | NCT03875235 [22] |
| GemCis + pembrolizumab GemCis | III | 1st | Adults with previously untreated, advanced/metastatic/unresectable CCA | ORR: 28.7 vs. 28.5% mPFS: 6.5 vs. 5.6 mo mOS: 12.7 vs. 10.9 mo | Neutropenia 21 vs. 25% Anemia 19 vs. 19% Thrombocytopenia 10 vs. 11% Fatigue 6 vs. 5% | NCT04003636 [23] |
| Treatment | Phase | Line | Patients | Efficacy Results | Most Common Grade ≥ 3 AEs | Trial No./Reference |
|---|---|---|---|---|---|---|
| Brigimadlin (MDM2–p53 antagonist) Brigimadlin + Ezabenlimab | II | 2nd | Advanced or metastatic, MDM2-amplified and p53-wt solid tumors including CCA | ORR: 33 vs. 67% | Neutropenia 26 vs. 25% Thrombocytopenia 24 vs. 22% Anemia 15 vs. 14% | NCT03449381 NCT03964233 [69] |
| GEMOX GEMOX + Erlotinib (EGFR inhibitor) | III | 1st | Treatment-naïve patients with unresectable locally advanced or metastatic CCA | ORR: 15.8 vs. 29.6% mPFS: 4.2 vs. 5.8 mo mOS: 9.5 vs. 9.5 mo | Neutropenia 6 vs. 4% Thrombocytopenia 4 vs. 4% Peripheral neuropathy 3% (GEMOX) Diarrhea 7% (GEMOX + Erlotinib) | NCT01149122 [70] |
| GEMOX + Cetuximab (EGFR inhibitor) | II | 1st | Treatment-naïve, unresectable advanced or metastatic CCA | ORR: 23% | Skin rash 13.3% Peripheral neuropathy 13.3% Thrombocytopenia 10% | NCT01216345 [71] |
| GEMOX GEMOX + Cetuximab | II | 1st | Treatment-naïve patients with unresectable locally advanced or metastatic CCA | ORR: 15 vs. 27% mPFS: 4.1 vs. 6.7 mo mOS: No difference | Neutropenia 6.7 vs. 11.3% Skin rash 0 vs. 6.5% Allergic/infusion reaction 0 vs. 4.8% | NCT01267344 [72] |
| Neratinib (HER2 inhibitor) | II | 2nd | Advanced/metastatic CCA, HER2-mutant, refractory | ORR: 16% mPFS: 2.8 mo mOS: 5.4 mo | Diarrhea | NCT01953926 [73] |
| GEMOX + Bevacizumab | II | 1st | Advanced, measurable CCA ≤1 prior chemo | mPFS: 7.0 mo mOS: 12.7 mo | Neutropenia 20% Sepsis 9.4% Thrombocytopenia 8.6% | NCT00361231 [74] |
| Sorafenib (VEGFR, PDGFR, Raf inhibitor) | II | 2nd | Advanced CCA who progressed after prior chemotherapy | ORR: 2% mPFS: 2.3 mo mOS: 4.4 mo | Skin rash 15% Gastrointestinal toxicity 15% Hand-foot skin reaction 11% | [75] |
| Sorafenib + Erlotinib | II | 1st or 2nd | Unresectable or metastatic disease and could have received 0–1 prior systemic chemotherapy | mPFS: 2 mo mOS: 6 mo | Hypertension Elevated liver enzymes Diarrhea | [76] |
| Ramucirumab (VEGFR2 inhibitor) | II | 2nd or later | Advanced, unresectable CCA; prior gemcitabine-based treatment | ORR: 1.7% mPFS: 3.2 mo mOS: 9.5 mo | Hypertension Proteinuria Pulmonary embolism | [77] |
| Regorafenib (VEGFR2 inhibitor) | II | 2nd or later | Advanced or metastatic CCA, progression after first-line chemotherapy. | ORR: 10.7% mPFS: 3.6 mo mOS: 7.3 mo | Hypophosphatemia 40% Hyperbilirubinemia 26% Hypertension 23% | NCT02053376 [78] |
| Selumetinib (MEK1/2 inhibitor) | II | 2nd or later | Metastatic CCA | ORR: 12%; SD: 44% mPFS: 3.7 mo mOS: 9.8 mo | Diarrhea Nausea Fatigue | [79] |
| Trametinib (MEK1/2 inhibitor) | II | 2nd | Advanced or metastatic CCA | SD: 65%; PD: 35% mPFS: 10.6 wks 1-year OS: 20% | Rash, Diarrhea Fatigue Elevated liver enzymes | NCT01943864 [80] |
| Pazopanib (VEGFR inhibitor) + Trametinib | II | 2nd or later | Advanced, unresectable CCA | ORR: 5% mPFS: 3.6 mo mOS: 6.4 mo | Thrombocytopenia 24% Rash 12% Elevated liver enzymes 12% | [81] |
| Dabrafenib (targets BRAFV600E mutation) + Trametinib | II | 2nd or later | BRAFV600E-mutated, unresectable, metastatic, locally advanced, or recurrent CCA | ORR: 47% mPFS: 7.2 mo mOS: 11.3 mo | Increase γ-GGT 12% Decreased WBC 7% Hypertension 7% | NCT02034110 [82] |
| Atezolizumab (anti-PD-L1) + Cobimetinib (MEK inhibitor) Atezolizumab | II | 2nd or later | Unresectable CCA, 1–2 prior systemic therapies | ORR: 3% both arms mPFS: 3.65 vs. 1.87 mo | Arm A: Rash 16%, thrombocytopenia 11%, elevated AST/ALT 8%; Arm B: Fatigue 5%, hypertension 5%, elevated AST/ALT 5% | NCT03201458 [83] |
| Everolimus monotherapy (mTOR inhibitor) | II | 2nd | Advanced CCA who progressed after 1st-line chemotherapy | DCR: 44.7% (at 12 wks) mPFS: 3.2 mo mOS: 7.7 mo | Grade ≥ 3 AE not reported All grade AEs: Fatigue 43.6%, Thrombocytopenia 35.6%, Pyrexia 30.8% | EudraCT 2008-007152-94 [84] |
| Rucaparib (PARP inhibitor) + Nivolumab | II | 2nd or later | Advanced/metastatic CCA, post ≥4–6 mo platinum-based therapy without progression | PR: 6.4%, SD: 71% mPFS: 4.6 mo mOS: 15.9 mo | Fatigue 29% Anemia 22.6% Neutropenia 19.3% | NCT03991832 [85] |
| Olaparib (PARP inhibitor) + Pembrolizumab | II | 2nd or later | Advanced or metastatic CCA who have progressed after prior systemic therapy | ORR: 15.4% mPFS: 5.45 mo mOS: 7.2 mo | Anemia 35.7% Decreased neutrophil count 7.1% Diarrhea 7.1% | NCT04306367 [86] |
| Treatment | Phase | Line | Patients | Efficacy Results | Most Common Grade ≥ 3 AEs | Trial No./Reference |
|---|---|---|---|---|---|---|
| Adagrasib (KRASG12C inhibitor) monotherapy | II | 2nd or later | Advanced solid tumors including CCA harboring a KRASG12C mutation who progressed after prior therapies | ORR: 50.0% mPFS: 11.3 mo mOS: 15.1 mo | Grade 3 AEs overall: 25.4% Common grade 3 events: Fatigue 6.3%, QT prolongation 6.3% | [142] |
| LY4066434 (pan-KRAS inhibitor) monotherapy | I | 2nd or later | KRAS mutant advanced or metastatic solid tumors, including CCA | Not yet reported | Not yet reported | NCT06607185 |
| MRTX 1133 (KRASG12D inhibitor) monotherapy | I/II | 2nd or later | KRASG12D mutant advanced or metastatic solid tumors, including CCA | Not yet reported | Not yet reported | NCT05737706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darman, L.; Kaurich, Q.; Hassan, M.S.; von Holzen, U.; Awasthi, N. Expanding Horizons in Cholangiocarcinoma: Emerging Targets Beyond FGFR2 and IDH1. Int. J. Mol. Sci. 2025, 26, 10755. https://doi.org/10.3390/ijms262110755
Darman L, Kaurich Q, Hassan MS, von Holzen U, Awasthi N. Expanding Horizons in Cholangiocarcinoma: Emerging Targets Beyond FGFR2 and IDH1. International Journal of Molecular Sciences. 2025; 26(21):10755. https://doi.org/10.3390/ijms262110755
Chicago/Turabian StyleDarman, Lily, Quinn Kaurich, Md Sazzad Hassan, Urs von Holzen, and Niranjan Awasthi. 2025. "Expanding Horizons in Cholangiocarcinoma: Emerging Targets Beyond FGFR2 and IDH1" International Journal of Molecular Sciences 26, no. 21: 10755. https://doi.org/10.3390/ijms262110755
APA StyleDarman, L., Kaurich, Q., Hassan, M. S., von Holzen, U., & Awasthi, N. (2025). Expanding Horizons in Cholangiocarcinoma: Emerging Targets Beyond FGFR2 and IDH1. International Journal of Molecular Sciences, 26(21), 10755. https://doi.org/10.3390/ijms262110755






