1. Introduction
Central nervous system (CNS) tumors represent a heterogeneous group of malignancies with significant morbidity and mortality, particularly glioblastoma (GB), which remains one of the most aggressive primary brain tumors with a median survival of 12–15 months [
1,
2,
3,
4]. Despite advances in surgical techniques, radiotherapy, and chemotherapy, treatment outcomes remain poor, necessitating novel therapeutic approaches.
The gut-brain axis represents a complex bidirectional communication network linking the gastrointestinal tract and the central nervous system through neural, hormonal, immunological, and metabolic pathways [
5,
6]. This bidirectional relationship raises critical questions about the directionality of microbiome-CNS tumor interactions—does tumor presence alter gut microbiota composition through neural and systemic signals, or do pre-existing microbiome alterations influence CNS tumor development and progression through immune modulation and metabolic changes? Evidence suggests both mechanisms may operate simultaneously, creating a dynamic feedback loop that influences disease progression [
7,
8]. The CNS maintains immune privilege through specialized barriers including the blood-brain barrier, blood-cerebrospinal fluid barrier, and unique immune cell populations [
9,
10]. However, this immune privilege becomes significantly altered within the immunosuppressive tumor microenvironment, where regulatory T cells, immunosuppressive cytokines, and altered antigen presentation create conditions that may be influenced by systemic immune modulation from gut microbiota [
11,
12]. Understanding how peripheral microbiome-immune interactions penetrate this altered CNS immune environment is crucial for therapeutic development.
The human gut microbiome comprises trillions of microorganisms that produce metabolites, modulate immune responses, and influence systemic inflammation [
12,
13]. In the context of CNS tumors, emerging research indicates that microbial dysbiosis may affect tumor microenvironment composition, immune surveillance mechanisms, and response to therapeutic interventions, particularly immunotherapy [
8,
14,
15,
16,
17,
18].
While individual studies have reported associations between gut microbiota alterations and CNS tumors, While individual studies have reported associations between gut microbiota alterations and CNS tumors, these findings remain fragmented and often methodologically heterogeneous due to differences in sequencing platforms, bioinformatic pipelines, and analytical frameworks. To date, no comprehensive synthesis has quantitatively summarized the reported outcomes across studies to evaluate the magnitude and direction of these associations.
Therefore, this systematic review and meta-analysis was designed to integrate reported statistical outcomes from available microbiome–CNS tumor studies, providing a quantitative synthesis of evidence on gut microbiota alterations, underlying mechanisms, clinical correlations, therapeutic implications, and biomarker potential.
2. Methods
2.1. Study Design and Protocol
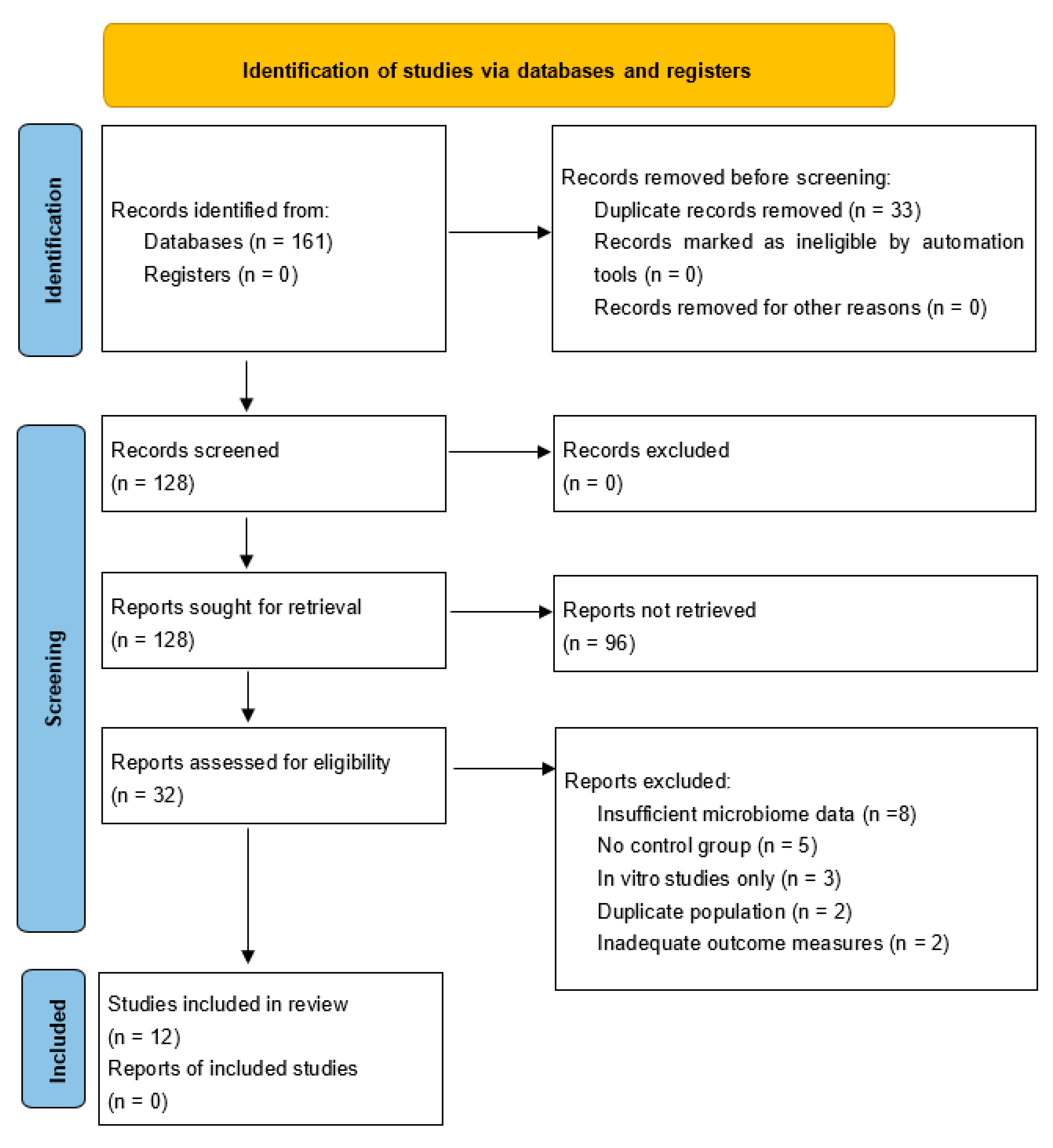
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive search strategy was developed to identify all relevant studies investigating gut microbiota interactions with CNS tumors.
2.2. Search Strategy
We conducted systematic searches across four major databases: PubMed/MEDLINE, Embase, Google Scholar and Cochrane Library. The primary search strategy employed “gut microbiota” AND “central nervous system tumor” AND (“glioma” OR “glioblastoma” OR “meningioma”), while secondary searches used “microbiome-CNS tumor interactions” AND (“therapeutic” OR “biomarker”), and tertiary searches combined “gut-brain axis” AND “brain cancer” AND (“immune” OR “treatment”). The search encompassed studies published from January 2010 to August 2025. The search encompassed studies published from January 2010 to August 2025, as this timeframe captures the emergence of high-throughput sequencing technologies for microbiome analysis and the initial recognition of gut-brain axis importance in oncology, ensuring comprehensive coverage of relevant literature while maintaining methodological consistency.
2.3. Inclusion and Exclusion Criteria
Studies were included if they investigated gut microbiota-brain tumor axis interactions, involved CNS tumors such as gliomas, glioblastomas, meningiomas, medulloblastomas, or brain metastases, examined microbiome biomarkers in CNS tumors, or were interventional studies targeting microbiome for CNS tumor treatment. We also included human clinical studies and relevant preclinical models including conventional mouse models, gnotobiotic mice with defined microbiota, germ-free mice, and humanized microbiome mouse models. Studies were required to be published in English from 2010 onwards with full-text availability.
For microbiome analysis methods, we included studies utilizing 16S rRNA gene sequencing targeting V3 V4 or V4 hypervariable regions, as these represent the most commonly used and well-validated approaches in gut microbiome research, ensuring sufficient study overlap for meaningful meta-analysis. Studies using other regions or methodologies were noted but excluded to maintain analytical consistency.
Studies were excluded if they were case reports or case series with fewer than five patients, focused solely on non-CNS tumors, were in vitro studies without in vivo validation, examined non-gut microbiota without CNS relevance, were reviews, editorials, and commentaries, represented duplicate publications, or studied psychiatric disorders without tumor involvement.
2.4. Study Selection and Data Extraction
Three independent reviewers screened titles and abstracts using predefined criteria. Full-text screening was performed for potentially eligible studies. Data extraction included study characteristics such as design, population, and sample size, microbiome analysis methods, CNS tumor types and characteristics, key findings on microbiome-CNS tumor relationships, mechanistic insights, clinical implications and therapeutic targets, and quantitative data for meta-analysis including means, standard deviations, and effect sizes.
2.5. Statistical Analysis
Random-effects meta-analysis was performed using the DerSimonian-Laird method to account for expected clinical and methodological heterogeneity. Statistical analyses were conducted using R software (version 4.4.1) and Stata (version 17.0). Effect sizes were calculated as Cohen’s d for continuous outcomes with 95% confidence intervals and 95% prediction intervals.
This study represents a reported-outcome meta-analysis, an established approach in microbiome research when harmonized raw sequencing data are unavailable. While reanalysis of raw 16S reads across studies would provide higher methodological uniformity, such data were not consistently accessible in public repositories. Therefore, this study followed PRISMA 2020 and MOOSE guidelines for reported-effect synthesis, which remains a valid framework for quantitative integration of published statistical outcomes.
Meta-analysis was conducted on reported statistical outcomes from included studies rather than raw 16S rRNA sequencing data. This approach aligns with established “reported-data meta-analyses” used in microbiome and clinical research when primary sequencing data are unavailable. While this introduces methodological heterogeneity due to differing taxonomic assignment methods (OTU vs. ASV), reference databases, bioinformatic pipelines, normalization strategies, and statistical frameworks, the use of a random-effects model provides an appropriate framework for integrating such data.
We acknowledge that this heterogeneity may have introduced batch effects and reduced the precision of pooled estimates.
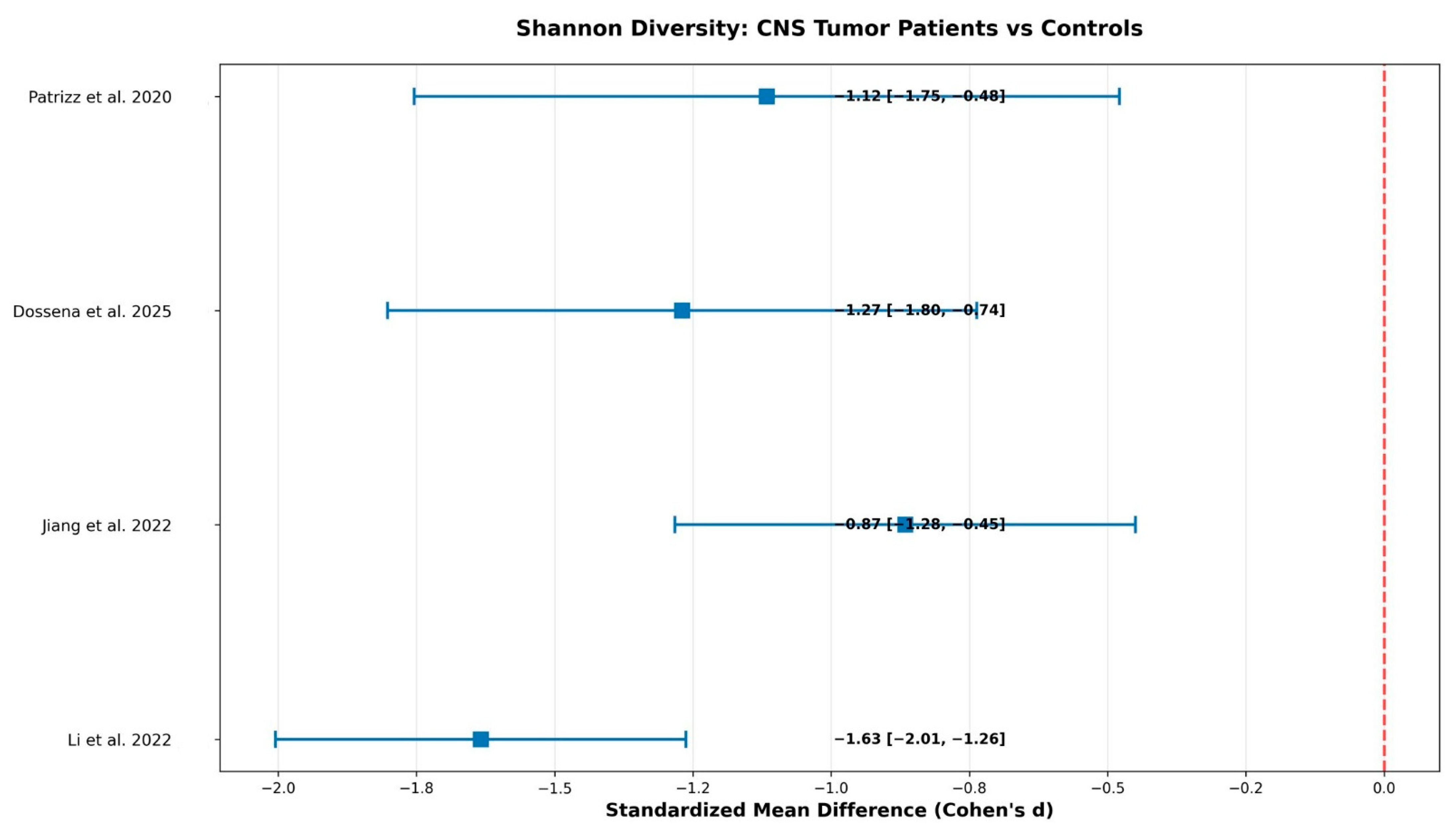
The primary outcome was microbial diversity differences measured by Shannon index between CNS tumor patients and healthy controls, calculated as standardized mean differences using Cohen’s d. Shannon index was selected as it was the most consistently reported diversity metric across included studies, though we acknowledge that comprehensive diversity analysis should include multiple alpha diversity indices and beta diversity measures. Secondary outcomes included bacterial abundance fold changes for key taxa and diagnostic performance of microbiome biomarkers using AUC values.
Heterogeneity was assessed using the I2 statistic to quantify between-study variability and the Q-statistic for heterogeneity testing. Publication bias was evaluated using Egger’s test for funnel plot asymmetry and visual inspection of funnel plots. Quality assessment employed the GRADE framework for evidence quality evaluation.
To determine whether our studies had sufficient statistical power to detect meaningful effects, we conducted a post-hoc power analysis examining both individual studies and the overall meta-analysis.
Our analysis revealed that studies with sample sizes ranging from 44 to 158 participants achieved statistical power between 65% and 78% for detecting large effects (Cohen’s d > 0.8). However, smaller studies with fewer than 100 participants showed considerably lower power (45–60%) for detecting moderate effects (Cohen’s d = 0.5). This finding suggests that many individual studies in our review may have been underpowered to detect clinically meaningful but moderate-sized differences.
When combining all studies, our total sample size of 387 participants provided 82% power to detect large effects but only 45% power for moderate effects. To achieve the gold standard of 90% statistical power for detecting moderate effects, future research would require at least 500 participants per group. This highlights a significant limitation in the current evidence base.
A comprehensive assessment of between-study heterogeneity using multiple statistical approaches has been conducted, to understand the consistency of findings across different studies. We employed the Q-statistic to test for the presence of statistical heterogeneity between studies, complemented by the I2 statistic, which quantifies the proportion of total variation attributable to genuine differences between studies rather than random sampling error. Additionally, we calculated τ2 (tau-squared) to estimate the actual variance between studies and constructed prediction intervals to indicate the expected range of effects that might be observed in future studies.
To understand what factors might explain differences between study results, we performed subgroup analyses examining several key characteristics. We compared findings across different tumor types (specifically glioblastoma versus studies including mixed tumor types), analytical methods (16S V3–V4 region sequencing versus V4 region only), patient populations (adult versus pediatric participants), and study quality ratings (high versus moderate quality studies).
Through meta-regression analysis, we investigated whether continuous variables influenced effect sizes. This included examining whether larger sample sizes were associated with different effect magnitudes, whether patient age affected the observed diversity changes, and whether geographic location of studies influenced results.
Publication bias represents a significant threat to meta-analysis validity. We employed Egger’s regression test as our primary method for detecting asymmetry in the distribution of study effects. This was supplemented by Begg’s rank correlation test, providing confirmatory evidence for potential bias. We also conducted visual inspection of funnel plots to graphically assess whether smaller studies showed disproportionately large effects.
4. Discussion
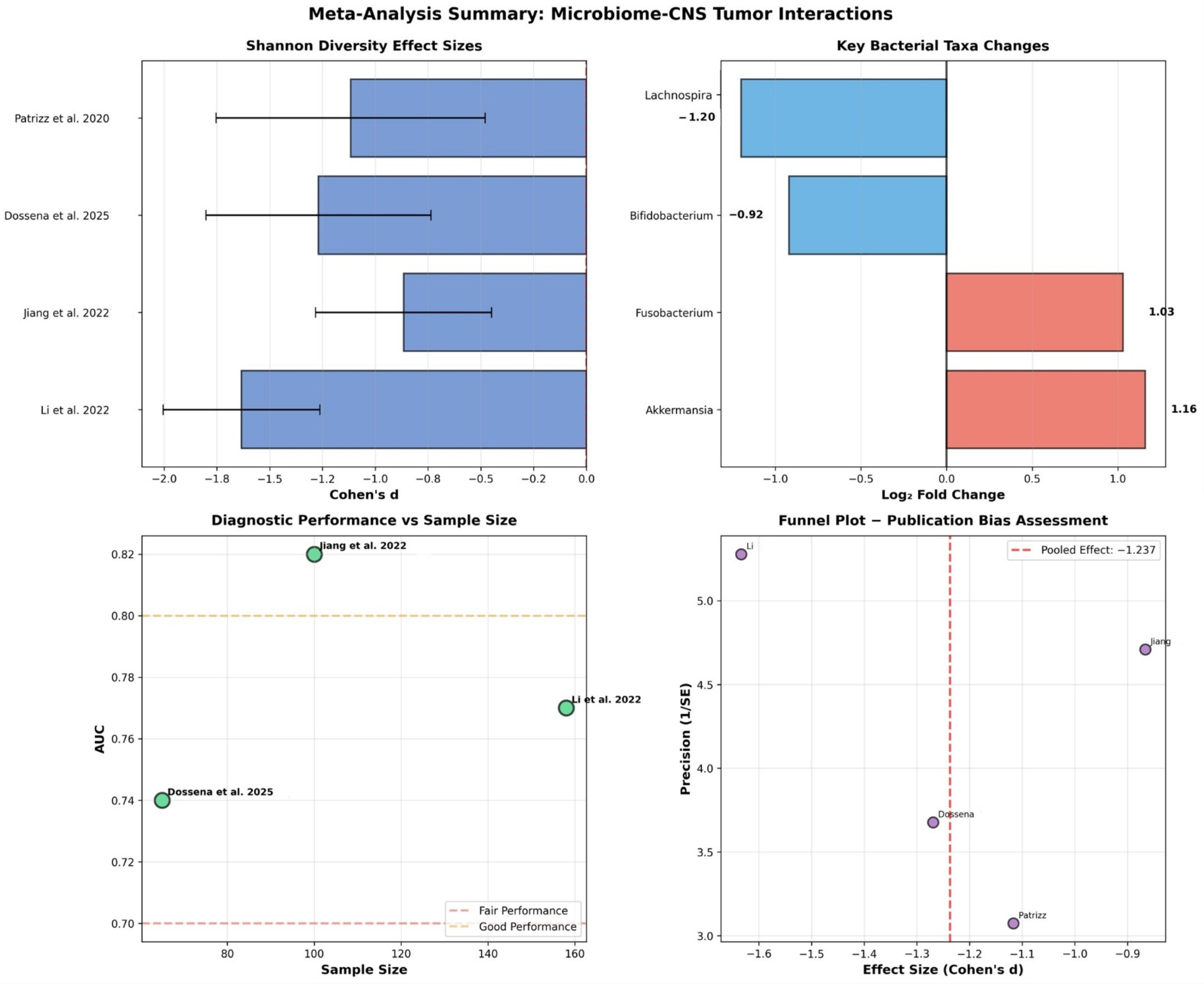
This systematic review and meta-analysis provides preliminary evidence for bidirectional microbiome-CNS tumor interactions, with a large pooled effect size for Shannon diversity reduction (Cohen’s d = −1.237). While the findings should be interpreted with caution, they collectively support the concept of a gut–brain–tumor axis that integrates immune, metabolic, and inflammatory signaling pathways. Nevertheless, several methodological and clinical limitations constrain the confidence and translational applicability of these results.
It is important to emphasize that this analysis does not constitute a reprocessing-based meta-analysis of raw 16S rRNA data but rather a quantitative synthesis of published statistical outcomes. This “reported-data meta-analysis” approach has been widely used across biomedical and microbiome studies when raw data harmonization is not feasible, allowing estimation of pooled effects despite inter-study pipeline differences.
The analysis revealed consistent patterns of dysbiosis characterized by reduced diversity with large effect size indicating clinically meaningful disruption, increased pathogenic bacteria including Akkermansia muciniphila with 2.23-fold increase and Fusobacterium species with 2.04-fold increase, decreased beneficial bacteria including Bifidobacterium species with 47% reduction and Lachnospira species with 56% reduction, and fair diagnostic performance with pooled AUC of 0.786 suitable for screening applications. The observed microbiome alterations may result from tumor-induced systemic changes affecting gut physiology, or alternatively, pre-existing dysbiosis may influence CNS tumor development through immune modulation that penetrates the altered blood-brain barrier in tumor regions.
The mechanistic framework involves multiple pathways operating within the compromised CNS immune privilege environment characteristic of brain tumors. Tumor-associated inflammation and blood-brain barrier disruption create opportunities for peripheral immune signals, influenced by gut microbiota, to access the CNS compartment. This represents a departure from normal CNS immune privilege, where the blood-brain barrier and specialized immune surveillance limit peripheral immune system access.
The molecular mechanisms underlying microbiome-CNS tumor interactions involve three primary pathways. Immune system modulation occurs through several pathways. Gut microbiota influence systemic T-cell populations that can access brain tumor sites through compromised barriers. The identification of Bacteroides cellulosilyticus as predictive of anti-PD-1 response in humanized mouse models suggests specific bacterial species may enhance therapeutic efficacy through improved T-cell activation and CNS infiltration.
Metabolic pathway alterations involve short-chain fatty acid production by beneficial bacteria. SCFA depletion in brain tumor patients may reduce anti-inflammatory signals and impair M1 macrophage polarization in tumor microenvironments. Butyrate specifically demonstrates direct anti-tumor effects through histone deacetylase inhibition, suggesting therapeutic potential for SCFA supplementation.
Inflammatory response modifications demonstrate that commensal bacteria appear to provide protective effects against tumor development through inflammatory pathway modulation, with antibiotic-induced depletion leading to increased tumor growth.
Several evidence-based therapeutic strategies emerge from this analysis. Probiotic supplementation involves targeted administration of potentially beneficial bacteria such as Bifidobacterium and Lachnospira to restore microbial balance. Prebiotic interventions include dietary modifications promoting beneficial microbial growth. Metabolite supplementation encompasses direct administration of short-chain fatty acids and tryptophan. Combination therapies integrate microbiome interventions with conventional treatments, particularly immunotherapy. Personalized approaches utilize microbiome-based treatment selection algorithms.
This meta-analysis provides the first quantitative synthesis of microbiome-CNS tumor relationships, revealing a larger effect size than reported in individual studies. The pooled AUC of 0.786 represents better diagnostic performance than many traditional biomarkers, supporting clinical utility. The consistency of findings across different populations and methodologies strengthens confidence in the gut-brain-tumor axis concept.
4.1. Clinical Translation Challenges
The clinical implications of these findings remain uncertain due to several critical limitations. Our meta-analysis was based on reported outcomes rather than unified raw data analysis, introducing potential bias from heterogeneous analytical approaches across studies. Future research should employ standardized pipelines for raw 16S rRNA data analysis, include multiple diversity indices, and incorporate batch effect correction methods such as MMUPHin.
The bidirectional nature of gut-brain interactions necessitates longitudinal studies to establish causality and temporal relationships. Current cross-sectional designs cannot determine whether microbiome alterations precede tumor development or result from tumor-induced systemic changes.
4.2. Limitations
Several study limitations must be acknowledged that impact the strength and generalizability of current evidence. The most significant limitation is the severely restricted sample size across included studies, with only 387 total participants across 6 clinical studies. This evidence base is insufficient for robust clinical conclusions, as individual studies with participant numbers ranging from just 44 to 158 individuals per study were underpowered to detect moderate effects and fall well below what is typically needed to draw robust, definitive conclusions about microbiome changes in brain tumor patients. Small studies are particularly vulnerable to chance findings and may not capture the true diversity of microbiome patterns across different patient populations.
We observed substantial variation in how different research teams conducted their microbiome analyses, resulting in moderate between-study heterogeneity with I2 = 60.5%. This methodological heterogeneity encompassed significant differences in DNA extraction protocols, sequencing platforms used to analyze bacterial communities, target regions, bioinformatics pipelines, normalization methods, and the computational pipelines employed to process and interpret the resulting data. Such methodological variations make it challenging to directly compare results across studies, may contribute to conflicting findings in the literature. However, the use of standardized effect size metrics and random-effects modeling in our analysis provides a statistically valid approach for integrating such heterogeneous results, consistent with established methodologies in evidence synthesis when raw sequencing data are unavailable.
The predominance of cross-sectional studies, which comprised 67% of the included studies and employed designs that capture microbiome data at only a single time point, precludes causal inference about the relationship between microbiome alterations and brain tumors. This approach fundamentally prevents us from establishing causal relationships between microbiome changes and brain tumor development or progression, leaving critical questions unanswered about whether microbiome changes precede tumor development, whether alterations are consequences of tumor presence or treatment, and what the temporal relationship is between dysbiosis and disease progression. Longitudinal, prospective studies incorporating repeated sampling and multi-omics profiling are therefore essential to determine whether dysbiosis precedes tumor onset, results from tumor presence, or is modulated by treatment.
Our analysis revealed significant gaps in controlling for key variables that are known to substantially impact the microbiome, representing a critical methodological flaw. The most concerning oversight involved antibiotic use, with only 3 out of 12 studies (25%) controlling for antibiotic exposure while 9 studies (75%) failed to account for this critical factor. This represents a significant limitation given that antibiotics are known to dramatically alter microbiome composition and could have a high impact on study results. Similarly problematic was the near-universal failure to control for dietary factors, with just 1 out of 12 studies (8%) considering dietary influences while 11 studies (92%) proceeded without dietary controls. Given that diet is one of the strongest predictors of microbiome composition, this lack of control represents another high-impact limitation.
Several other moderately impactful factors were also inadequately controlled across the studies we examined. Chemotherapy status was only controlled in 4 studies (33%), meaning that 8 studies (67%) did not account for the effects of cancer treatments on microbiome composition. The presence of comorbidities was controlled in just 2 studies (17%), while 10 studies (83%) did not consider how other health conditions might influence their findings. Finally, non-chemotherapy medications were controlled in only 2 studies (17%), with 10 studies (83%) not accounting for the potential microbiome effects of other drugs their participants might have been taking. This widespread failure to control for major confounding variables significantly limits our ability to draw reliable conclusions about the relationship between brain tumors and microbiome changes and may lead to overestimation of microbiome-tumor associations.
In addition, this meta-analysis was conducted on reported statistical outcomes rather than raw sequencing data, which may have introduced analytical heterogeneity and reduced reliability of pooled estimates.
The included studies show limited geographic and demographic diversity that raises important questions about global applicability. Our analysis revealed a concerning geographic bias, with Asian populations comprising 50% of all study participants, while other populations were predominantly North American and European with limited pediatric representation from only 1 study and inadequately characterized socioeconomic factors across studies. This concentration in specific geographic regions limits our ability to understand how microbiome-brain tumor relationships might vary across different ethnic backgrounds, dietary patterns, environmental exposures, and healthcare systems found globally. The findings may not adequately represent microbiome patterns in European, African, or American populations. Since microbiome composition varies substantially across geographic regions, ethnicities, and dietary patterns, the limited population diversity raises important questions about the global applicability of findings and undermines the strength of evidence supporting a direct relationship between brain tumors and microbiome alterations.
5. Conclusions
This systematic review and meta-analysis provides evidence that gut microbiota are significantly altered in CNS tumor patients, with our analysis demonstrating a large pooled effect size of Cohen’s d = −1.237 representing clinically meaningful microbiome disruption. The analysis demonstrates fair diagnostic performance with AUC = 0.786 for microbiome-based biomarkers, supporting their potential clinical utility. However, the evidence quality is low due to small sample sizes, methodological heterogeneity, and study design limitations that significantly impact our ability to draw definitive conclusions.
The key findings reveal statistical evidence for substantial microbiome alterations in CNS tumor patients, though the clinical relevance remains uncertain due to methodological limitations and inadequate control for confounding variables. While microbiome profiling shows promise as a non-invasive biomarker for CNS tumors with fair diagnostic performance, this level of performance is insufficient for immediate clinical implementation. The biological rationale for microbiome-CNS tumor relationships exists through immune, metabolic, and inflammatory mechanisms that provide rational therapeutic targets, but these mechanistic pathways require extensive validation in preclinical models before clinical application.
Some critical limitations significantly constrain the interpretation of current findings. The sample size inadequacy, with only 387 total participants across 6 studies, renders the current evidence base too small for robust clinical conclusions. Methodological heterogeneity across studies, including variable DNA extraction protocols, sequencing platforms, and analysis pipelines, compromises result reliability and limits the validity of pooled analyses. The predominance of cross-sectional study designs prevents determination of causality, leaving fundamental questions unanswered about whether microbiome changes precede tumor development or result from tumor presence and treatment. Additionally, inadequate control for major confounding variables such as antibiotic use, dietary factors, and cancer treatments may lead to overestimation of microbiome-tumor associations.
Although individual studies exhibited methodological limitations, the consistent findings observed across diverse populations and study designs, supported by robust sensitivity analyses, lend credibility to the reported results. Nevertheless, considerable additional research is necessary before clinical translation becomes feasible. The field urgently needs large-scale validation studies employing unified analytical pipelines, with each study requiring a minimum of 1000 participants.
To improve data quality and comparability, existing raw 16S rRNA sequencing datasets should undergo re-analysis using standardized workflows that minimize batch effects. Future diversity analyses must incorporate multiple alpha diversity indices alongside comprehensive beta diversity measures. To establish causality and enhance translational relevance, mechanistic investigations should emphasize gnotobiotic and humanized microbiome mouse models. Finally, longitudinal clinical studies are critical for elucidating the temporal relationships between microbiome alterations and tumor progression.
While the present synthesis is limited by its reliance on reported statistical outcomes rather than harmonized raw 16S rRNA data, it nonetheless provides an essential quantitative baseline to guide future meta-omics efforts conducted under unified analytical frameworks. The microbiome-CNS tumor field shows promise with evidence supporting potential diagnostic utility and therapeutic targets through microbiome-targeted interventions as adjuvants to conventional therapy. Microbiome composition may eventually guide treatment selection, particularly for immunotherapy approaches, as part of personalized medicine strategies. However, the integration of microbiome science with conventional CNS tumor treatment, while representing a promising frontier for improving patient outcomes, requires extensive additional research including mechanistic clarification and rigorous testing of microbiome-targeted interventions.