Optimizing rhBMP-2 Therapy for Bone Regeneration: From Safety Concerns to Biomaterial-Guided Delivery Systems
Abstract
1. Introduction
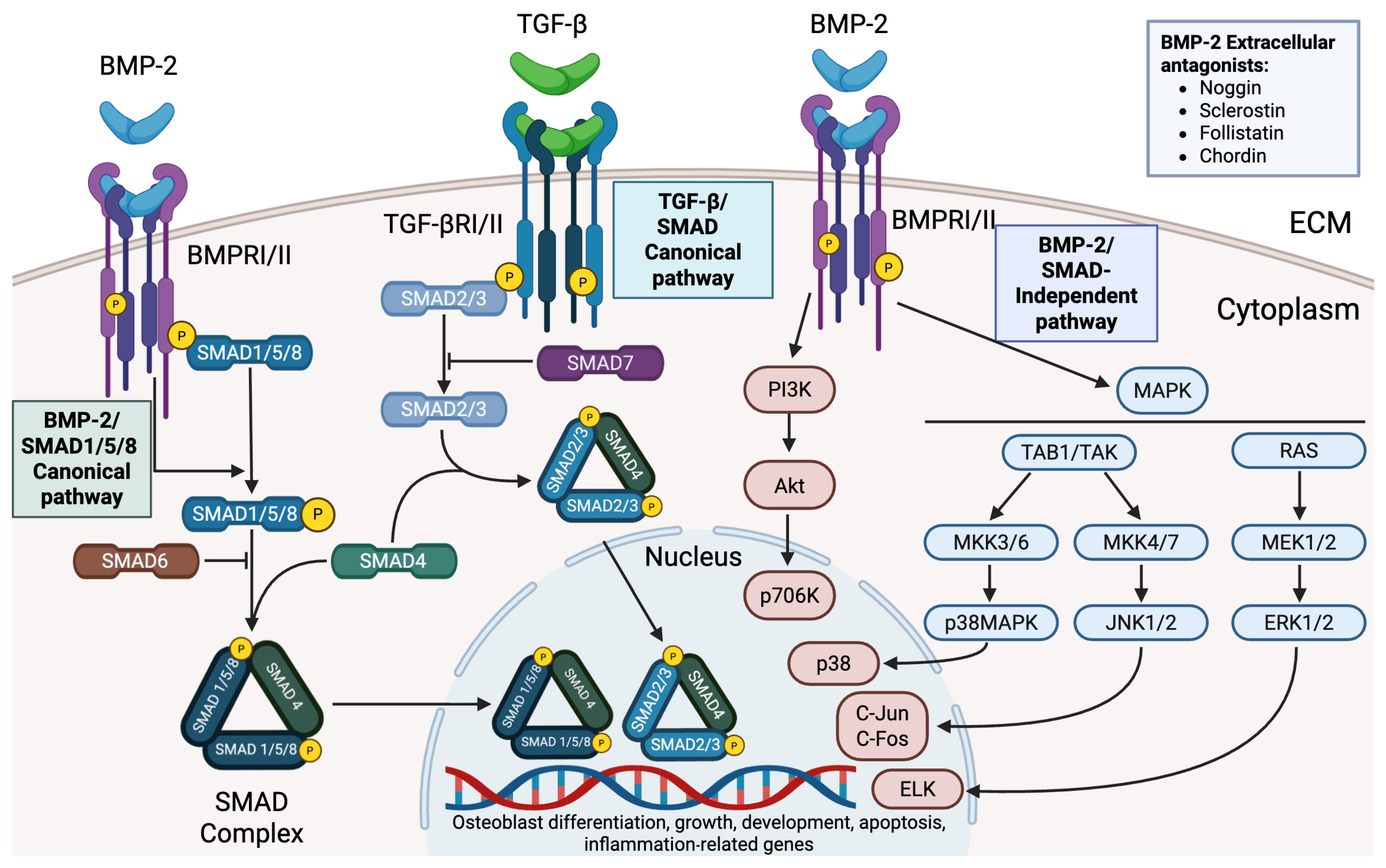
2. Biological Functions and Signaling Mechanisms of BMP-2
3. Expression Systems and Technological Approaches for the Production of rhBMP-2
Integration of rhBMP-2 into Modern Therapeutic Protocols
4. Risks and Adverse Effects of rhBMP-2 Therapy
4.1. Local Inflammation and Edema
4.2. Hyperostosis and Osteolysis
5. New Clinical Applications of rhBMP-2
Translational Barriers: Regulatory Approval, Cost, and Scalability
6. Patient-Oriented Strategies for rhBMP-2 Application
| Indication | rhBMP-2 Dose | Delivery System | Patient Factors | Key Outcomes | Citation |
|---|---|---|---|---|---|
| Lumbar spinal fusion (adult spinal deformity, 3–5 levels) | ~3.0 mg per level (≈9–15 mg total) | Hydroxyapatite (HA) carrier + rhBMP-2 (no autograft) | Adults (19–80 y) with multilevel deformity (L1–S1); osteoporosis excluded | 100% fusion at 6 and 12 mo with rhBMP-2 + HA vs. 88% with HA alone; significant pain/QoL improvement (no adverse events). | [84] |
| Lumbar interbody fusion (degenerative TLIF, 1–2 levels) | 0.5–1.0 mg per segment | rhBMP-2 (E. coli -derived) + hydroxyapatite/bone graft in interbody cage | Adults with degenerative lumbar disease undergoing 1–2 level TLIF | 100% fusion at 52 and 104 weeks; significant improvement in ODI and VAS; no rhBMP-2-related complications. | [73] |
| Maxillary sinus augmentation (for dental implants) | ~1.5 mg/mL (≈6–12 mg per sinus graft) | rhBMP-2 with HA scaffold or ACS and bone graft | Edentulous posterior maxilla with atrophic ridge (low residual bone height) | 90–100% implant survival with rhBMP-2 vs. 86–95% without; comparable graft height gain; rhBMP-2 group showed less marginal bone loss. | [85,86] |
| Alveolar ridge (socket) preservation (tooth extraction) | ~0.3 mg per socket (1.5 mg/mL, 0.2 mL) | Absorbable collagen sponge (ACS) soaked with rhBMP-2 | Adults undergoing posterior tooth extraction | Significantly less buccal bone resorption (1.8 mm less height loss vs. control) at 12 weeks; better bone fill/volume retention; no complications reported. | [87] |
| Long-bone fracture nonunion (femur, tibia, humerus) | ≤6 mg per site (avg ~ 5 mg) | HA granules + autologous cancellous bone, all mixed with rhBMP-2 | Adults with atrophic/oligotrophic or infected nonunions | 95.8% union at 6 mo, 100% at 12 mo; marked improvement in function and pain; no adverse events or antibodies. | [84] |
| Alveolar cleft reconstruction (cleft lip/palate patients) | rhBMP-2 with demineralized bone matrix (DBM) (dose varied) | DBM carrier + rhBMP-2 in cleft site | Children with unilateral/bilateral alveolar cleft | Identified “critical-size” cleft volume: graft failure rises sharply above ~885 mm3. Below that volume, rhBMP-2/DBM success ≈ 86%; above it, failure ≈ 65%. | [88] |
7. Carriers as Critical Determinants of rhBMP-2 Efficacy and Safety
7.1. Optimization of rhBMP-2 Dose and Delivery
| Procedure | Dose of rhBMP-2 (mg/level) | Study Design | Efficacy (Fusion/Timeline) | Complications | Reference |
|---|---|---|---|---|---|
| ACDF | 0.5 | RCT, >1100 patients | Faster fusion by 3 months; differences with autograft diminished by 6–12 months | Similar dysphagia rates in both groups; no serious adverse events reported | [74] |
| ACDF | ~0.5 (average dose) | Retrospective, n = 198 | Complete arthrodesis in 96% (≈15 months) | Dysphagia 11%, neck swelling 6%, pseudarthrosis 1% | [75] |
| ACDF | ≤0.7 optimal; ≥0.7: increased risk | Systematic review/ meta-analysis (29 studies; 1,539,021 patients) | Best balance of efficacy/safety at ≤0.7 mg/level | At levels ≥ 0.7 mg/L: higher risk of dysphagia, infections, and wound healing complications | [76] |
| Cervical spine (posterior approach) | >2.1 associated with a higher risk of infections | Meta-analysis (summary data) | Fusion rate 96% at mean follow-up of 15 months | Higher risk of early infections only at >2.1 mg/level; lower doses—no increased risk | [92] |
| TLIF (one-/ two-level) | 0.5–1.0 | Prospective multicenter single-arm, n = 30 | ≈98% fusion | No serious complications reported, including heterotopic ossification (HO) | [30] |
| PLF (lumbar) | Not specified (controlled doses) | RCT, n = 74 | Higher fusion quality and lower nonunion rate vs. AIBG | No serious complications reported | [72] |
| PLF (lumbar) | Various (pooled data) | Meta-analysis of 14 RCTs (789 rhBMP-2 vs. 727 ICBG) | Higher fusion rates; reduced operative time and blood loss | Fewer reoperations related to donor site morbidity | [32] |
| Deformity surgery (ASD) | Tendency to decrease: ~26.6 → 20.7 | Observational data over a decade (2008–2018) | Stable nonunion rates at lower doses | Nearly two-fold reduction in complications requiring reoperation | [57] |
7.2. Modern Carriers for Controlled and Safe Delivery of rhBMP-2
8. Molecularly Oriented Strategies for the Delivery of Osteogenic Factors
8.1. Chemically Modified RNA
8.2. Regional Gene Therapy as a Strategy for Sustained In Vivo BMP2 Expression
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| rhBMP-2 | recombinant human bone morphogenetic protein-2 |
| cmRNA | chemically modified messenger RNA |
| BMP-2 | bone morphogenetic protein 2 |
| MAPK | mitogen-activated protein kinase |
| PDGF | platelet-derived growth factor |
| FDA | U.S. Food and Drug Administration |
| CHO | Chinese hamster ovary |
| HEK293 | human embryonic kidney 293 |
| RTT | repeated transient transfection |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| RCTs | randomized clinical trials |
| HA | hydroxyapatite |
| BCP | biphasic calcium phosphate |
| β-TCP | β-tricalcium phosphate |
| ICBG | iliac crest bone graft |
| ACDF | anterior cervical discectomy and fusion |
| PLF | posterolateral lumbar fusion |
| TLIF | transforaminal lumbar interbody fusion |
| PE-PLIF | posterior extreme lateral interbody fusion |
| ODI | Oswestry Disability Index |
| NRS | Numeric Rating Scale |
| ASD | adult sagittal imbalance |
| LLIF | lateral lumbar interbody fusion |
| DBM | demineralized bone matrix |
| MDS | myelodysplastic syndrome |
| PLGA | poly(lactic-co-glycolic acid) |
| nHA | nanohydroxyapatite |
| PLLA | poly-l-lactic acid |
| PLA | poly(lactic acid) |
| PEG | polyethylene glycol |
| MSCs | mesenchymal stem cells |
| mRNA | messenger RNA |
| GelMA | gelatin methacrylate |
| BMSCs | bone marrow mesenchymal stem cells |
| UCB-MSCs | umbilical cord blood-derived mesenchymal stem cells |
| LV-BMP-2 | lentivirus encoding BMP-2 |
| VL-PSL | visible light projection stereolithography |
| SCID | severe combined immunodeficiency |
| AAVs | adeno-associated viruses |
| GAS | gene-activated scaffold |
| PEI | polyethylenimine |
| PDGF-B | platelet-derived growth factor subunit B |
References
- Sun, S.-H.; Tsai, W.-W.; Shiu, S.-I.; Chen, C.-H. Induced Membrane Technique for Large Bone Defects: A Systematic Review and Individual Participant Data Meta-Analysis. Medicine 2022, 101, e29292. [Google Scholar] [CrossRef]
- Hoveidaei, A.H.; Ghaseminejad-Raeini, A.; Esmaeili, S.; Sharafi, A.; Ghaderi, A.; Pirahesh, K.; Azarboo, A.; Nwankwo, B.O.; Conway, J.D. Effectiveness of Synthetic versus Autologous Bone Grafts in Foot and Ankle Surgery: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2024, 25, 539. [Google Scholar] [CrossRef] [PubMed]
- Manocha, A.; Suresh, D.K.; Malik, R. Bone Morphogenetic Proteins: A Potential Stimulant in Bone Regeneration; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2012; ISBN 9783659130694. [Google Scholar]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 Activity, Although Dispensable for Bone Formation, Is Required for the Initiation of Fracture Healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Castranio, T.; Mishina, Y. Bmp2 Is Required for Cephalic Neural Tube Closure in the Mouse. Dev. Dyn. 2009, 238, 110–122. [Google Scholar] [CrossRef]
- Heng, S.; Paule, S.; Hardman, B.; Li, Y.; Singh, H.; Rainczuk, A.; Stephens, A.N.; Nie, G. Posttranslational Activation of Bone Morphogenetic Protein 2 Is Mediated by Proprotein Convertase 6 during Decidualization for Pregnancy Establishment. Endocrinology 2010, 151, 3909–3917. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Koosha, E.; Eames, B.F. Two Modulators of Skeletal Development: BMPs and Proteoglycans. J. Dev. Biol. 2022, 10, 15. [Google Scholar] [CrossRef]
- Kim, N.-H.; Jung, S.-K.; Lee, J.; Chang, P.-S.; Kang, S.-H. Modulation of Osteogenic Differentiation by Escherichia Coli-Derived Recombinant Bone Morphogenetic Protein-2. AMB Express 2022, 12, 106. [Google Scholar] [CrossRef]
- Shah, I.; Chopra, M.; Ton, A.; Hah, R.J.; Wang, J.C.; Alluri, R.K. Osteobiologics in Degenerative Spine Disease: Current Clinical Applications of Recombinant Human Bone Morphogenetic Proteins—A Narrative Review. AME Med. J. 2024, 9, 5. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; De Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Hiepen, C.; Benn, A.; Denkis, A.; Lukonin, I.; Weise, C.; Boergermann, J.H.; Knaus, P. BMP2-Induced Chemotaxis Requires PI3K P55γ/P110α-Dependent Phosphatidylinositol (3,4,5)-Triphosphate Production and LL5β Recruitment at the Cytocortex. BMC Biol. 2014, 12, 43. [Google Scholar] [CrossRef]
- Goto, K.; Kamiya, Y.; Imamura, T.; Miyazono, K.; Miyazawa, K. Selective Inhibitory Effects of Smad6 on Bone Morphogenetic Protein Type I Receptors. J. Biol. Chem. 2007, 282, 20603–20611. [Google Scholar] [CrossRef]
- Novak, S.; Madunic, J.; Shum, L.; Vucetic, M.; Wang, X.; Tanigawa, H.; Ghosh, M.; Sanjay, A.; Kalajzic, I. PDGF Inhibits BMP2-Induced Bone Healing. npj Regen. Med. 2023, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone Morphogenetic Protein 2 Stimulates Endochondral Ossification by Regulating Periosteal Cell Fate during Bone Repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Von Benecke, J.P.; Tarsitano, E.; Zimmermann, L.-M.A.; Shakesheff, K.M.; Walsh, W.R.; Bae, H.W. A Narrative Review on Recombinant Human Bone Morphogenetic Protein 2: Where Are We Now? Cureus 2024, 16, e67785. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). InFUSE Bone Graft/LT-CAGE Lumbar Tapered Fusion Devices—P000058 2002.8. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf/P000058c.pdf (accessed on 25 August 2025).
- Garrison, K.; Donell, S.; Ryder, J.; Shemilt, I.; Mugford, M.; Harvey, I.; Song, F. Clinical Effectiveness and Cost-Effectiveness of Bone Morphogenetic Proteins in the Non-Healing of Fractures and Spinal Fusion: A Systematic Review. Health Technol. Assess. 2007, 11, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; On, S.W.; Song, S.I. Bone Regenerative Effect of Recombinant Human Bone Morphogenetic Protein-2 after Cyst Enucleation. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 22. [Google Scholar] [CrossRef]
- Seo, J.I.; Lim, J.H.; Jo, W.M.; Lee, J.K.; Song, S.I. Effects of rhBMP-2 with Various Carriers on Maxillofacial Bone Regeneration through Computed Tomography Evaluation. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 40. [Google Scholar] [CrossRef]
- Quaas, B.; Burmeister, L.; Li, Z.; Nimtz, M.; Hoffmann, A.; Rinas, U. Properties of Dimeric, Disulfide-Linked rhBMP-2 Recovered from E. Coli Derived Inclusion Bodies by Mild Extraction or Chaotropic Solubilization and Subsequent Refolding. Process Biochem. 2018, 67, 80–87. [Google Scholar] [CrossRef]
- Jérôme, V.; Thoring, L.; Salzig, D.; Kubick, S.; Freitag, R. Comparison of Cell-based versus Cell-free Mammalian Systems for the Production of a Recombinant Human Bone Morphogenic Growth Factor. Eng. Life Sci. 2017, 17, 1097–1107. [Google Scholar] [CrossRef]
- Riedl, S.A.B.; Jérôme, V.; Freitag, R. Repeated Transient Transfection: An Alternative for the Recombinant Production of Difficult-to-Express Proteins Like BMP2. Processes 2022, 10, 1064. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Wu, P.-Y.; Chang, C.-H.; Huang, L.-F. High-Yield BMP2 Expression in Rice Cells via CRISPR and Endogenous αAmy3 Promoter. Appl. Microbiol. Biotechnol. 2024, 108, 206. [Google Scholar] [CrossRef]
- Heinks, T.; Hettwer, A.; Hiepen, C.; Weise, C.; Gorka, M.; Knaus, P.; Mueller, T.D.; Loidl-Stahlhofen, A. Optimized Expression and Purification of a Soluble BMP2 Variant Based on In-Silico Design. Protein Expr. Purif. 2021, 186, 105918. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Wu, Y.; Su, X. Soluble Expression and Purification of High-Bioactivity Recombinant Human Bone Morphogenetic Protein-2 by Codon Optimisation in Escherichia coli. Protein Eng. Des. Sel. 2019, 32, 153–157. [Google Scholar] [CrossRef]
- Kang, M.; Lee, S.; Seo, J.; Lee, E.; Ahn, D.; Shin, J.; Paik, Y.-K.; Jo, D. Cell-Permeable Bone Morphogenetic Protein 2 Facilitates Bone Regeneration by Promoting Osteogenesis. Mater. Today Bio 2024, 25, 100983. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Hyun, S.-J.; Lee, C.H.; Youn, J.H.; Ryu, M.Y.; Kim, K.-J. Safety and Efficacy of Recombinant Human Bone Morphogenetic Protein-2 in Multilevel Posterolateral Lumbar Fusion in a Prospective, Randomized, Controlled Trial. Neurospine 2022, 19, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.P.; Francis, D.L.; Thirumoorthi, H.; Rahul; Rathi, M.; Singh, H.; Karmakar, S.; Pradhan, S. Effectiveness of Recombinant Human Bone Morphogenetic Protein-2 in Socket Preservation: A Randomized Controlled Clinical and Sequential Human Histological Trial (BMP-2 TRIAL). Clin. Exp. Dent. Res. 2025, 11, e70134. [Google Scholar] [CrossRef]
- Wei, L.; Sun, Y.; Yu, D.; Pieterse, H.; Wismeijer, D.; Liu, Y.; Wu, Y. The Clinical Efficacy and Safety of ErhBMP-2/BioCaP/β-TCP as a Novel Bone Substitute Using the Tooth-Extraction-Socket-Healing Model: A Proof-of-Concept Randomized Controlled Trial. J. Clin. Periodontol. 2025, 52, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, B.; Chen, L.; Wang, X.; Abdelrahim, M.E.A.; Wei, C. Bone Morphogenetic Protein-2 against Iliac Crest Bone Graft for the Posterolateral Fusion of the Lumbar Spine: A Meta-analysis. Int. J. Clin. Pract. 2021, 75, e13911. [Google Scholar] [CrossRef]
- Ratko, T.A.; Belinson, S.E.; Samson, D.J.; Bonnell, C.; Ziegler, K.M.; Aronson, N. Bone Morphogenetic Protein: The State of the Evidence of On-Label and Off-Label Use; AHRQ Technology Assessments; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2010. [Google Scholar]
- Tateiwa, D.; Kaito, T. Advances in Bone Regeneration with Growth Factors for Spinal Fusion: A Literature Review. N. Am. Spine Soc. J. 2023, 13, 100193. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.; Tan, E.T.; Sneag, D.B.; Lebl, D.R.; Chazen, J.L. Postoperative Lumbar Fusion Bone Morphogenic Protein–Related Epidural Cyst Formation. Am. J. Neuroradiol. 2023, 44, 351–355. [Google Scholar] [CrossRef]
- Mazur-Hart, D.J.; Yamamoto, E.A.; Yoo, J.; Orina, J.N. Bone Morphogenetic Protein and Cancer in Spinal Fusion: A Propensity Score–Matched Analysis. J. Neurosurg. Spine 2023, 39, 722–728. [Google Scholar] [CrossRef]
- Simmonds, M.C.; Brown, J.V.E.; Heirs, M.K.; Higgins, J.P.T.; Mannion, R.J.; Rodgers, M.A.; Stewart, L.A. Safety and Effectiveness of Recombinant Human Bone Morphogenetic Protein-2 for Spinal Fusion: A Meta-Analysis of Individual-Participant Data. Ann. Intern. Med. 2013, 158, 877–889. [Google Scholar] [CrossRef]
- Wu, G.; Huang, F.; Chen, Y.; Zhuang, Y.; Huang, Y.; Xie, Y. High Levels of BMP2 Promote Liver Cancer Growth via the Activation of Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 194. [Google Scholar] [CrossRef]
- Huang, P.; Chen, A.; He, W.; Li, Z.; Zhang, G.; Liu, Z.; Liu, G.; Liu, X.; He, S.; Xiao, G.; et al. BMP-2 Induces EMT and Breast Cancer Stemness through Rb and CD44. Cell Death Discov. 2017, 3, 17039. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, Y.; Ruan, J.; Wang, F.; He, Y.; Yang, L.; Yu, T.; Du, F.; Zhang, N.; Cao, X. The Effects of BMP2 and the Mechanisms Involved in the Invasion and Angiogenesis of IDH1 Mutant Glioma Cells. J. Neurooncol. 2024, 170, 161–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Qiao, M.; Zhang, B.-Q.; Wang, N.; Zhang, Z.; Liao, Z.; Zeng, L.; Deng, Y.; Deng, F.; et al. Bone Morphogenetic Protein 2 Inhibits the Proliferation and Growth of Human Colorectal Cancer Cells. Oncol. Rep. 2014, 32, 1013–1020. [Google Scholar] [CrossRef]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A Critical Review of Recombinant Human Bone Morphogenetic Protein-2 Trials in Spinal Surgery: Emerging Safety Concerns and Lessons Learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. Development of a Novel Compression-Resistant Carrier for Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) and Preliminary Clinical Results. In Bone Morphogenetic Proteins: From Local to Systemic Therapeutics; Vukicevic, S., Sampath, K.T., Eds.; Progress in Inflammation Research; Birkhäuser Basel: Basel, Switzerland, 2008. [Google Scholar]
- Wang, Y.; Zhang, G.; Wang, H.; Tan, Y.; Wang, X. Preinduction with Bone Morphogenetic Protein-2 Enhances Cardiomyogenic Differentiation of c-Kit+ Mesenchymal Stem Cells and Repair of Infarcted Myocardium. Int. J. Cardiol. 2018, 265, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, J.; Wang, H.; Tan, Y. Delivery of Stem Cells and BMP-2 With Functionalized Self-Assembling Peptide Enhances Regeneration of Infarcted Myocardium. Stem Cell Rev. Rep. 2024, 20, 1540–1554. [Google Scholar] [CrossRef]
- Pulkkinen, H.H.; Kivistö-Rahnasto, A.; Korpela, H.; Heikkilä, M.; Järveläinen, N.; Siimes, S.; Kilpeläinen, L.; Laham-Karam, N.; Ylä-Herttuala, S.; Laakkonen, J.P. BMP2 Gene Transfer Induces Pericardial Effusion and Inflammatory Response in the Ischemic Porcine Myocardium. Front. Cardiovasc. Med. 2023, 10, 1279613. [Google Scholar] [CrossRef]
- McClellan, J.W.; Mulconrey, D.S.; Forbes, R.J.; Fullmer, N. Vertebral Bone Resorption After Transforaminal Lumbar Interbody Fusion with Bone Morphogenetic Protein (rhBMP-2). J. Spinal Disord. Tech. 2006, 19, 483–486. [Google Scholar] [CrossRef]
- Shields, L.B.E.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse Effects Associated with High-Dose Recombinant Human Bone Morphogenetic Protein-2 Use in Anterior Cervical Spine Fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Public Health Notification: Life-Threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion. 1 July 2008. Available online: https://www.patientsafety.va.gov/docs/alerts/AL09-13MedtronicInfuse.pdf (accessed on 26 August 2025).
- Woo, E.J. Adverse Events After Recombinant Human BMP2 in Nonspinal Orthopaedic Procedures. Clin. Orthop. 2013, 471, 1707–1711. [Google Scholar] [CrossRef]
- Pan, H.C.; Lee, S.; Ting, K.; Shen, J.; Wang, C.; Nguyen, A.; Berthiaume, E.A.; Zara, J.N.; Turner, A.S.; Seim, H.B.; et al. Cyst-Like Osteolytic Formations in Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Augmented Sheep Spinal Fusion. Am. J. Pathol. 2017, 187, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Byvaltsev, V.A.; Kalinin, A.A.; Hernandez, P.A.; Shepelev, V.V.; Pestryakov, Y.Y.; Aliyev, M.A.; Giers, M.B. Molecular and Genetic Mechanisms of Spinal Stenosis Formation: Systematic Review. Int. J. Mol. Sci. 2022, 23, 13479. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.; Glassman, S.D.; Howard, J.M.; Djurasovic, M.; Witten, J.L.; Carreon, L.Y. Perioperative Complications with rhBMP-2 in Transforaminal Lumbar Interbody Fusion. Eur. Spine J. 2011, 20, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Nandyala, S.V.; Marquez-Lara, A.; Cha, T.D.; Khan, S.N.; Fineberg, S.J.; Pelton, M.A. Clinical Sequelae after rhBMP-2 Use in a Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine J. 2013, 13, 1118–1125. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- De Stefano, F.A.; Elarjani, T.; Burks, J.D.; Burks, S.S.; Levi, A.D. Dose Adjustment Associated Complications of Bone Morphogenetic Protein: A Longitudinal Assessment. World Neurosurg. 2021, 156, e64–e71. [Google Scholar] [CrossRef]
- Bannwarth, M.; Smith, J.S.; Bess, S.; Klineberg, E.O.; Ames, C.P.; Mundis, G.M.; Kim, H.J.; Lafage, R.; Gupta, M.C.; Burton, D.C.; et al. Use of rhBMP-2 for Adult Spinal Deformity Surgery: Patterns of Usage and Changes over the Past Decade. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Onafowokan, O.O.; Uzosike, A.C.; Sharma, A.; Galetta, M.; Lorentz, N.; Montgomery, S.; Fisher, M.R.; Yung, A.; Tahmasebpour, P.; Seo, L.; et al. Treatment of Adult Spine Deformity: A Retrospective Comparison of Bone Morphogenic Protein and Bone Marrow Aspirate with Bone Allograft. Acta Neurochir. 2024, 166, 448. [Google Scholar] [CrossRef]
- Lin, S.; Maekawa, H.; Moeinzadeh, S.; Lui, E.; Alizadeh, H.V.; Li, J.; Kim, S.; Poland, M.; Gadomski, B.C.; Easley, J.T.; et al. An Osteoinductive and Biodegradable Intramedullary Implant Accelerates Bone Healing and Mitigates Complications of Bone Transport in Male Rats. Nat. Commun. 2023, 14, 4455. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Wu, Y.; Shi, J.; Chen, R. Application of RhBMP-2 in Percutaneous Endoscopic Posterior Lumbar Interbody Fusion. BMC Surg. 2024, 24, 376, Erratum in BMC Surg. 2025, 25, 16. [Google Scholar] [CrossRef]
- Hani, U.; Farber, S.H.; Pfortmiller, D.; Kim, P.K.; Bohl, M.A.; Holland, C.M.; McGirt, M.J. Comparison of Transforaminal and Posterior Lumbar Interbody Fusion Outcomes in Patients Receiving a Novel Allograft versus rhBMP-2: A Radiographic and Patient-Reported Outcomes Analysis. J. Neurosurg. Spine 2024, 41, 236–245. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Inductos to Be Suspended in the EU; EMA/683814/2015. 20 November 2015. Available online: https://www.ema.europa.eu/en/documents/referral/inductos-article-20-procedure-inductos-be-suspended-eu_en.pdf (accessed on 3 October 2025).
- Epstein, N. Pros, Cons, and Costs of INFUSE in Spinal Surgery. Surg. Neurol. Int. 2011, 2, 10. [Google Scholar] [CrossRef]
- Carreon, L.Y.; Glassman, S.D.; Djurasovic, M.; Campbell, M.J.; Puno, R.M.; Johnson, J.R.; Dimar, J.R. RhBMP-2 Versus Iliac Crest Bone Graft for Lumbar Spine Fusion in Patients Over 60 Years of Age: A Cost-Utility Study. Spine 2009, 34, 238–243. [Google Scholar] [CrossRef]
- Nunna, R.S.; Gruber, M.D.; Karuparti, S.; Taylor, Z.; Genovese, S.; Jumah, F.; Godolias, P.; Tataryn, Z.; Hollern, D.; Oskouian, R.; et al. Cost-Effectiveness Analyses of Bone Morphogenetic Protein 2 (rhBMP-2) in Spinal Fusion: A Systematic Review. Glob. Spine J. 2025, 15, 2514–2535. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Bohnert, J.S.; O’Brien, M.; Alnuaimi, S.; Carnwath, T.P.; Dunivan, Q.; Coffin, D.W.; Smith, T.L. Keratin Scaffold Formulation Impacts rhBMP-2 Biodistribution and Bone Regeneration in a Rat Femur Defect Model. Tissue Eng. Regen. Med. 2025. [Google Scholar] [CrossRef]
- Kitahara, T.; Tateiwa, D.; Hirai, H.; Ikuta, M.; Furuichi, T.; Bun, M.; Ukon, Y.; Kanie, Y.; Furuya, M.; Fujimori, T.; et al. rhBMP-2-Loaded Hydroxyapatite/Beta-Tricalcium Phosphate Microsphere/Hydrogel Composite Promotes Bone Regeneration in a Novel Rat Femoral Nonunion Model. Front. Bioeng. Biotechnol. 2024, 12, 1461260. [Google Scholar] [CrossRef] [PubMed]
- Laurie, A.L.; Chen, Y.; Chou, R.; Fu, R. Meta-Analysis of the Impact of Patient Characteristics on Estimates of Effectiveness and Harms of Recombinant Human Bone Morphogenetic Protein-2 in Lumbar Spinal Fusion. Spine 2016, 41, E1115–E1123. [Google Scholar] [CrossRef]
- Overley, S.C.; Mcanany, S.J.; Anwar, M.A.; Merrill, R.K.; Lovy, A.; Guzman, J.Z.; Zhadanov, S.; Doshi, A.; Rothenberg, E.; Vaishnav, A.; et al. Predictive Factors and Rates of Fusion in Minimally Invasive Transforaminal Lumbar Interbody Fusion Utilizing rhBMP-2 or Mesenchymal Stem Cells. Int. J. Spine Surg. 2019, 13, 46–52. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, Y.; Shin, J.-W.; Ha, J.-W.; Choi, H.-M.; Kim, H.-S.; Moon, S.-H.; Suk, K.-S.; Park, S.-Y.; Lee, B.-H.; et al. Accelerated Fusion Dynamics by Recombinant Human Bone Morphogenetic Protein-2 Following Transforaminal Lumbar Interbody Fusion, Particularly in Osteoporotic Conditions. Spine J. 2024, 24, 2078–2085. [Google Scholar] [CrossRef]
- Singh, V.; Oppermann, M.; Evaniew, N.; Soroceanu, A.; Nicholls, F.; Jacobs, W.B.; Thomas, K.; Swamy, G. Lateral Lumbar Interbody Fusion with rhBMP-2 Can Achieve High Fusion Rates in Adult Spine Deformity Surgeries. Glob. Spine J. 2024, 14, 244–256. [Google Scholar] [CrossRef]
- Cho, M.; You, K.-H.; Yeom, J.S.; Kim, H.; Lee, K.B.; Cho, J.H.; Yang, J.J.; Lee, J.H. Mid-Term Efficacy and Safety of Escherichia Coli-Derived rhBMP-2/Hydroxyapatite Carrier in Lumbar Posterolateral Fusion: A Randomized, Multicenter Study. Eur. Spine J. 2023, 32, 353–360. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Cho, J.H.; Lee, J.B.; Kim, J.H. Evaluation of the Fusion Rate and Safety of Escherichia coli-Derived rhBMP-2 in Transforaminal Lumbar Interbody Fusion for Patients with Degenerative Lumbar Disease: A Prospective, Multicenter, Single-Arm Trial. J. Clin. Med. 2024, 13, 1733. [Google Scholar] [CrossRef]
- Chen, T.; Wu, X.; Li, X.; Shi, J.; Zhu, Y.; Zhou, H.; Zhuang, Y.; Sun, H.; Jiang, W.; Liu, Y. A Randomized Controlled Trial Evaluating Efficacy and Complications of Low-Dose rhBMP-2 for Anterior Cervical Diskectomy and Fusion. Neurosurgery 2025, 97, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, S.K.; Priddy, B.H.; Mobasser, J.-P.; Potts, E.A. Safety and Efficacy of Low-Dose rhBMP-2 Use for Anterior Cervical Fusion. Neurosurg. Focus 2021, 50, E2. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-D.; Jiang, W.-M.; Yang, H.-L.; Shi, J.-H. Exploratory Meta-Analysis on Dose-Related Efficacy and Complications of rhBMP-2 in Anterior Cervical Discectomy and Fusion: 1,539,021 Cases from 2003 to 2017 Studies. J. Orthop. Transl. 2020, 24, 166–174. [Google Scholar] [CrossRef]
- Miguez, P.A.; De Paiva Gonçalves, V.; Musskopf, M.L.; Rivera-Concepcion, A.; McGaughey, S.; Yu, C.; Lee, D.J.; Tuin, S.A.; Ali, A. Mitigation of BMP-Induced Inflammation in Craniofacial Bone Regeneration and Improvement of Bone Parameters by Dietary Hesperidin. Sci. Rep. 2024, 14, 2602. [Google Scholar] [CrossRef]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg.–Am. Vol. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Boyne, P.J.; Lilly, L.C.; Marx, R.E.; Moy, P.K.; Nevins, M.; Spagnoli, D.B.; Triplett, R.G. De Novo Bone Induction by Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Maxillary Sinus Floor Augmentation. J. Oral Maxillofac. Surg. 2005, 63, 1693–1707. [Google Scholar] [CrossRef]
- Paul, J.C.; Lonner, B.S.; Vira, S.; Kaye, I.D.; Errico, T.J. Reoperation Rates After Long Posterior Spinal Fusion: Use of Recombinant Bone Morphogenetic Protein in Idiopathic and Non-Idiopathic Scoliosis. Spine Deform. 2016, 4, 304–309. [Google Scholar] [CrossRef]
- Azad, V.; Breitbart, E.; Al-Zube, L.; Yeh, S.; O’Connor, J.P.; Lin, S.S. rhBMP-2 Enhances the Bone Healing Response in a Diabetic Rat Segmental Defect Model. J. Orthop. Trauma 2009, 23, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Tombyln, S.; Wasnick, D.C.; Hughes, M.R.; Ellenburg, M.D.; Callahan, M.F.; Smith, T.L.; Van Dyke, M.E.; Burnett, L.R.; Saul, J.M. Reduction of Ectopic Bone Growth in Critically-Sized Rat Mandible Defects by Delivery of rhBMP-2 from Kerateine Biomaterials. Biomaterials 2014, 35, 3220–3228. [Google Scholar] [CrossRef] [PubMed]
- Willett, N.J.; Li, M.-T.A.; Uhrig, B.A.; Boerckel, J.D.; Huebsch, N.; Lundgren, T.S.; Warren, G.L.; Guldberg, R.E. Attenuated Human Bone Morphogenetic Protein-2–Mediated Bone Regeneration in a Rat Model of Composite Bone and Muscle Injury. Tissue Eng. Part C Methods 2013, 19, 316–325. [Google Scholar] [CrossRef]
- Choi, W.; Kim, B.-S.; Cho, W.-T.; Lim, E.J.; Choi, J.S.; Ryu, Y.K.; Cho, J.-W.; Sakong, S.; Oh, J.-K. Efficacy and Safety of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Combined with Autologous Bone for the Treatment of Long Bone Nonunion: A Report of a Prospective Case Series. Injury 2024, 55, 111711. [Google Scholar] [CrossRef]
- Lee, J.-H.; Seo, H.; Cho, Y.-C.; Sung, I.-Y.; Son, J.-H. Addition of Recombinant Human Bone Morphogenic Protein-2 to the Graft Materials Improves the Clinical Outcomes of Implants Placed in Grafted Maxillary Sinus. J. Dent. Sci. 2024, 19, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Moon, J.E.; Lee, E.-H.; Yang, H.J.; Hwang, S.J. Clinical and Radiographic Outcomes of Dental Implant after Maxillary Sinus Floor Augmentation with rhBMP-2/Hydroxyapatite Compared to Deproteinized Bovine Bone. PLoS ONE 2022, 17, e0273399. [Google Scholar] [CrossRef]
- Thammanichanon, P.; Ouyyamwongs, W.; Mai-ngam, K.; Rittipakorn, P. Clinical Outcomes of rhBMP-2-Loaded Collagen Sponge for Alveolar Ridge Preservation: A Pilot Study. Eur. J. Dent. 2025. [Google Scholar] [CrossRef]
- Roohani, I.; Youn, S.; Alfeerawi, S.; Shakoori, P.; Trotter, C.; Choi, D.G.; Fahradyan, A.; Urata, M.M.; Magee, W.P.; Hammoudeh, J.A. Failure Rates Based on Alveolar Cleft Volume: Analysis of the Critical-Size Defect for Alveolar Bone Grafting. Plast. Reconstr. Surg. 2025, 155, 377e–386e. [Google Scholar] [CrossRef]
- Seeherman, H.; Wozney, J.M. Delivery of Bone Morphogenetic Proteins for Orthopedic Tissue Regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [Google Scholar] [CrossRef]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Cohen, D.J.; Jacobs, T.W.; Wilson, D.S.; Mancini, M.C.; Van Duyn, C.; Schwartz, Z.; Boyan, B.D. Rapidly Polymerizing Click Hydrogel Provides Localized Delivery of rhBMP2 to Promote Bone Formation. Pharmacol. Res. Perspect. 2025, 13, e70119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential Dual-Drug Delivery of BMP-2 and Alendronate from Hydroxyapatite-Collagen Scaffolds for Enhanced Bone Regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Xiao, Y. Sequential Drug Delivery of Vancomycin and rhBMP-2 via Pore-Closed PLGA Microparticles Embedded Photo-Crosslinked Chitosan Hydrogel for Enhanced Osteointegration. Int. J. Biol. Macromol. 2021, 182, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kim, B.; Yeo, D.; Lim, E.J.; Sakong, S.; Lim, J.; Park, S.; Jeong, Y.; Jung, T.; Choi, H.; et al. 3D-printed, Bioactive Ceramic Scaffold with rhBMP-2 in Treating Critical Femoral Bone Defects in Rabbits Using the Induced Membrane Technique. J. Orthop. Res. 2021, 39, 2671–2680. [Google Scholar] [CrossRef]
- Tateiwa, D.; Nakagawa, S.; Tsukazaki, H.; Okada, R.; Kodama, J.; Kushioka, J.; Bal, Z.; Ukon, Y.; Hirai, H.; Kaito, T. A Novel BMP-2–Loaded Hydroxyapatite/Beta-Tricalcium Phosphate Microsphere/Hydrogel Composite for Bone Regeneration. Sci. Rep. 2021, 11, 16924. [Google Scholar] [CrossRef]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental Study of rhBMP-2 Chitosan Nano-Sustained Release Carrier-Loaded PLGA/nHA Scaffolds to Construct Mandibular Tissue-Engineered Bone. Arch. Oral Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef]
- Hong, Y.R.; Kim, T.-H.; Park, K.-H.; Kang, J.; Lee, K.; Park, E.K.; Kwon, T.-G.; Lim, J.O.; Oh, C.-W. rhBMP-2-Conjugated Three-Dimensional-Printed Poly(l-Lactide) Scaffold Is an Effective Bone Substitute. Tissue Eng. Regen. Med. 2023, 20, 69–81. [Google Scholar] [CrossRef]
- Xia, P.; Wang, S.; Qi, Z.; Zhang, W.; Sun, Y. BMP-2-Releasing Gelatin Microspheres/PLGA Scaffolds for Bone Repairment of X-Ray-Radiated Rabbit Radius Defects. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, H.; Mi, Y.; Ma, P.; Fan, D. Chitosan and Hyaluronic Acid Based Injectable Dual Network Hydrogels—Mediating Antimicrobial and Inflammatory Modulation to Promote Healing of Infected Bone Defects. Int. J. Biol. Macromol. 2024, 274, 133124. [Google Scholar] [CrossRef]
- Viveros-Moreno, N.G.; Garcia-Lorenzana, M.; Peña-Mercado, E.; García-Sanmartín, J.; Narro-Íñiguez, J.; Salazar-García, M.; Huerta-Yepez, S.; Sanchez-Gomez, C.; Martínez, A.; Beltran-Vargas, N.E. In Vivo Biocompatibility Testing of Nanoparticle-Functionalized Alginate–Chitosan Scaffolds for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2023, 11, 1295626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, L.; Lin, M.; Cao, S.; Feng, Y.; Sun, S. RhBMP-2-Loaded PLGA/Titanium Nanotube Delivery System Synergistically Enhances Osseointegration. ACS Omega 2021, 6, 16364–16372. [Google Scholar] [CrossRef]
- Xu, H.; Luo, H.; Chen, J.; Chen, G.; Yu, X.; Ye, Z. BMP-2 Releasing Mineral-Coated Microparticle-Integrated Hydrogel System for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1217335. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Tian, P.; Zhao, J.; Xin, H.; Ma, X.; Yuan, X. The Sequential and Systemic Administration of BMP-2 and SDF-1α Nanocapsules for Promoting Osteoporotic Fracture Healing. Biomimetics 2023, 8, 369. [Google Scholar] [CrossRef]
- Del Castillo-Santaella, T.; Ortega-Oller, I.; Padial-Molina, M.; O’Valle, F.; Galindo-Moreno, P.; Jódar-Reyes, A.B.; Peula-García, J.M. Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration. Pharmaceutics 2019, 11, 388. [Google Scholar] [CrossRef]
- Chien, K.R.; Zangi, L.; Lui, K.O. Synthetic Chemically Modified mRNA (modRNA): Toward a New Technology Platform for Cardiovascular Biology and Medicine. Cold Spring Harb. Perspect. Med. 2015, 5, a014035. [Google Scholar] [CrossRef]
- De La Vega, R.E.; Van Griensven, M.; Zhang, W.; Coenen, M.J.; Nagelli, C.V.; Panos, J.A.; Peniche Silva, C.J.; Geiger, J.; Plank, C.; Evans, C.H.; et al. Efficient Healing of Large Osseous Segmental Defects Using Optimized Chemically Modified Messenger RNA Encoding BMP-2. Sci. Adv. 2022, 8, eabl6242. [Google Scholar] [CrossRef] [PubMed]
- Jriyasetapong, K.; Lawtrakulngam, N.; Vivatbutsiri, P.; Namano, S.; Wattanasirmkit, K.; Angkhanawiriyarak, S.; Suwanwela, J. mRNA Encoding Bone Morphogenetic Protein-2 Facilitated Peri-Implant Bone Formation of Titanium Implants Placed in Rat Femurs. Sci. Rep. 2025, 15, 17852. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Gan, X.; Wei, M.; Wang, C.; Yang, G.; Zhao, Y.; Zhu, Z.; Wang, Z. Hydrogel Armed with Bmp2 mRNA-Enriched Exosomes Enhances Bone Regeneration. J. Nanobiotechnol. 2023, 21, 119. [Google Scholar] [CrossRef]
- Surisaeng, T.; Wisitrasameewong, W.; Champaiboon, C.; Sa-Ard-Iam, N.; Chanamuangkon, T.; Thongnuek, P.; Tam, Y.K.; Muramatsu, H.; Weissman, D.; Pardi, N.; et al. BMP-2 mRNA-Transfected BMSCs Promote Superior Calvarial Bone Regeneration. Sci. Rep. 2025, 15, 15022. [Google Scholar] [CrossRef]
- Bougioukli, S.; Saitta, B.; Sugiyama, O.; Tang, A.H.; Elphingstone, J.; Evseenko, D.; Lieberman, J.R. Lentiviral Gene Therapy for Bone Repair Using Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells. Hum. Gene Ther. 2019, 30, 906–917. [Google Scholar] [CrossRef]
- Sarkar, A.; Gallo, M.C.; Bell, J.A.; Mayfield, C.K.; Ball, J.R.; Ayad, M.; Lechtholz-Zey, E.; Chang, S.W.; Sugiyama, O.; Evseenko, D.; et al. Ex Vivo Regional Gene Therapy Compared to Recombinant BMP-2 for the Treatment of Critical-Size Bone Defects: An In Vivo Single-Cell RNA-Sequencing Study. Bioengineering 2025, 12, 29. [Google Scholar] [CrossRef]
- Ihn, H.; Kang, H.; Iglesias, B.; Sugiyama, O.; Tang, A.; Hollis, R.; Bougioukli, S.; Skorka, T.; Park, S.; Longjohn, D.; et al. Regional Gene Therapy with Transduced Human Cells: The Influence of “Cell Dose” on Bone Repair. Tissue Eng. Part A 2021, 27, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in Vivo Bone Formation by BMP-2 Engineered Human Mesenchymal Stem Cells Encapsulated in a Projection Stereolithographically Fabricated Hydrogel Scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef]
- Alaee, F.; Sugiyama, O.; Virk, M.S.; Tang, H.; Drissi, H.; Lichtler, A.C.; Lieberman, J.R. Suicide Gene Approach Using a Dual-Expression Lentiviral Vector to Enhance the Safety of Ex Vivo Gene Therapy for Bone Repair. Gene Ther. 2014, 21, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. How Safe Is a Popular Gene Therapy Vector? Science 2020, 367, 131. [Google Scholar] [CrossRef]
- Warnock, J.N.; Daigre, C.; Al-Rubeai, M. Introduction to Viral Vectors. In Viral Vectors for Gene Therapy; Merten, O.-W., Al-Rubeai, M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 737, pp. 1–25. ISBN 978-1-61779-094-2. [Google Scholar]
- Kay, M.A. State-of-the-Art Gene-Based Therapies: The Road Ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Sieker, J.T.; Kunz, M.; Weißenberger, M.; Gilbert, F.; Frey, S.; Rudert, M.; Steinert, A.F. Direct Bone Morphogenetic Protein 2 and Indian Hedgehog Gene Transfer for Articular Cartilage Repair Using Bone Marrow Coagulates. Osteoarthr. Cartil. 2015, 23, 433–442. [Google Scholar] [CrossRef]
- Sun, K.; Lin, H.; Tang, Y.; Xiang, S.; Xue, J.; Yin, W.; Tan, J.; Peng, H.; Alexander, P.G.; Tuan, R.S.; et al. Injectable BMP-2 Gene-Activated Scaffold for the Repair of Cranial Bone Defect in Mice. Stem Cells Transl. Med. 2020, 9, 1631–1642. [Google Scholar] [CrossRef]
- Khvorostina, M.; Mironov, A.; Nedorubova, I.; Bukharova, T.; Vasilyev, A.; Goldshtein, D.; Komlev, V.; Popov, V. Osteogenesis Enhancement with 3D Printed Gene-Activated Sodium Alginate Scaffolds. Gels 2023, 9, 315. [Google Scholar] [CrossRef]
- Walsh, D.P.; Raftery, R.M.; Murphy, R.; Chen, G.; Heise, A.; O’Brien, F.J.; Cryan, S.-A. Gene Activated Scaffolds Incorporating Star-Shaped Polypeptide-pDNA Nanomedicines Accelerate Bone Tissue Regeneration in Vivo. Biomater. Sci. 2021, 9, 4984–4999. [Google Scholar] [CrossRef] [PubMed]
- Meglei, A.Y.; Nedorubova, I.A.; Basina, V.P.; Chernomyrdina, V.O.; Nedorubov, A.A.; Kuznetsova, V.S.; Vasilyev, A.V.; Kutsev, S.I.; Goldshtein, D.V.; Bukharova, T.B. Collagen–Platelet-Rich Plasma Mixed Hydrogels as a pBMP2 Delivery System for Bone Defect Regeneration. Biomedicines 2024, 12, 2461. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Tigli Aydın, R.S.; Godzik, K.P.; Acri, T.M.; Heo, D.N.; Rizk, E.; Wee, H.; Lewis, G.S.; Salem, A.K.; Ozbolat, I.T. Controlled Co-Delivery of pPDGF-B and pBMP-2 from Intraoperatively Bioprinted Bone Constructs Improves the Repair of Calvarial Defects in Rats. Biomaterials 2022, 281, 121333. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Nedorubova, I.A.; Chernomyrdina, V.O.; Meglei, A.Y.; Basina, V.P.; Mironov, A.V.; Kuznetsova, V.S.; Sinelnikova, V.A.; Mironova, O.A.; Trifanova, E.M.; et al. Antisolvent 3D Printing of Gene-Activated Scaffolds for Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 13300. [Google Scholar] [CrossRef]
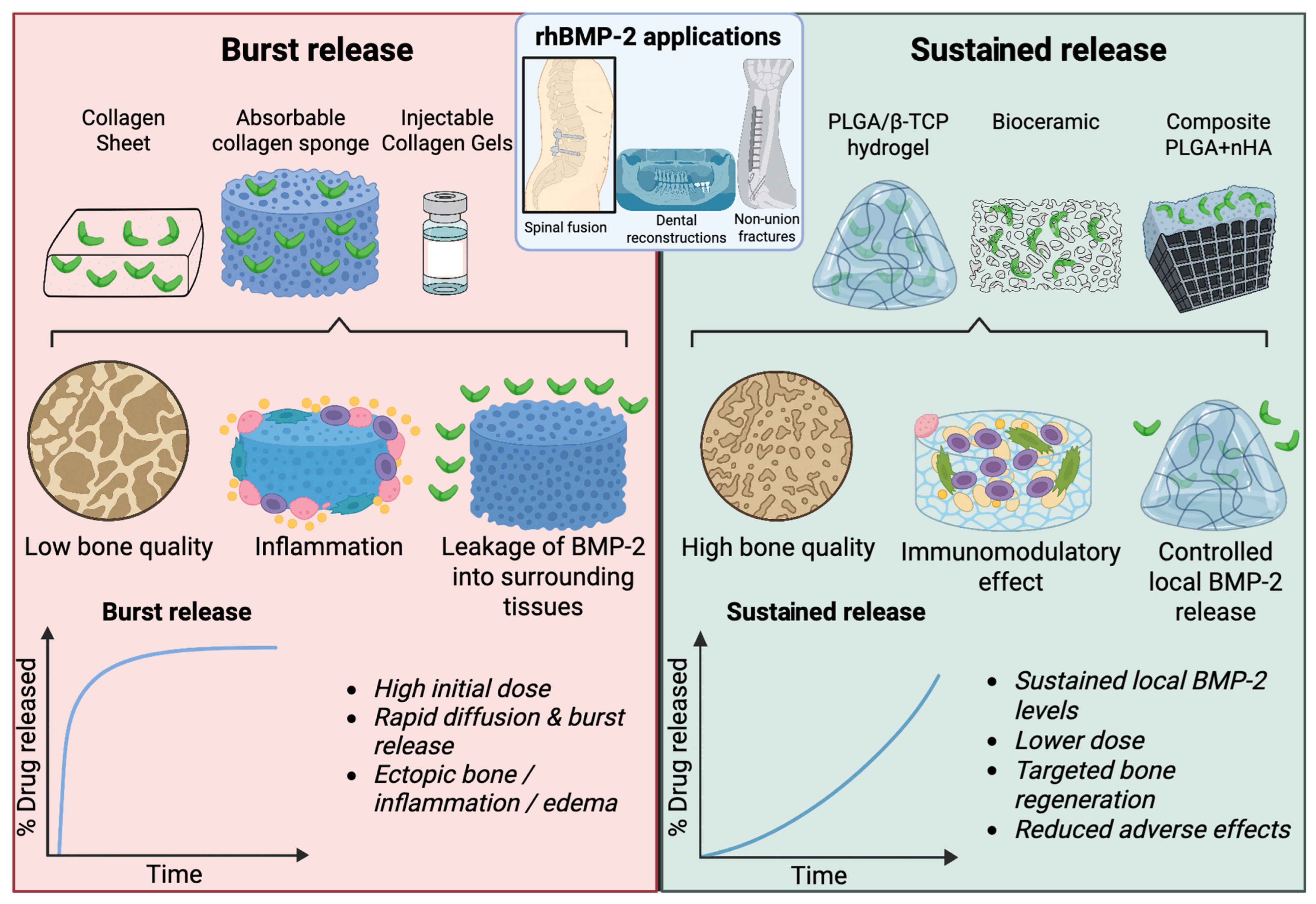
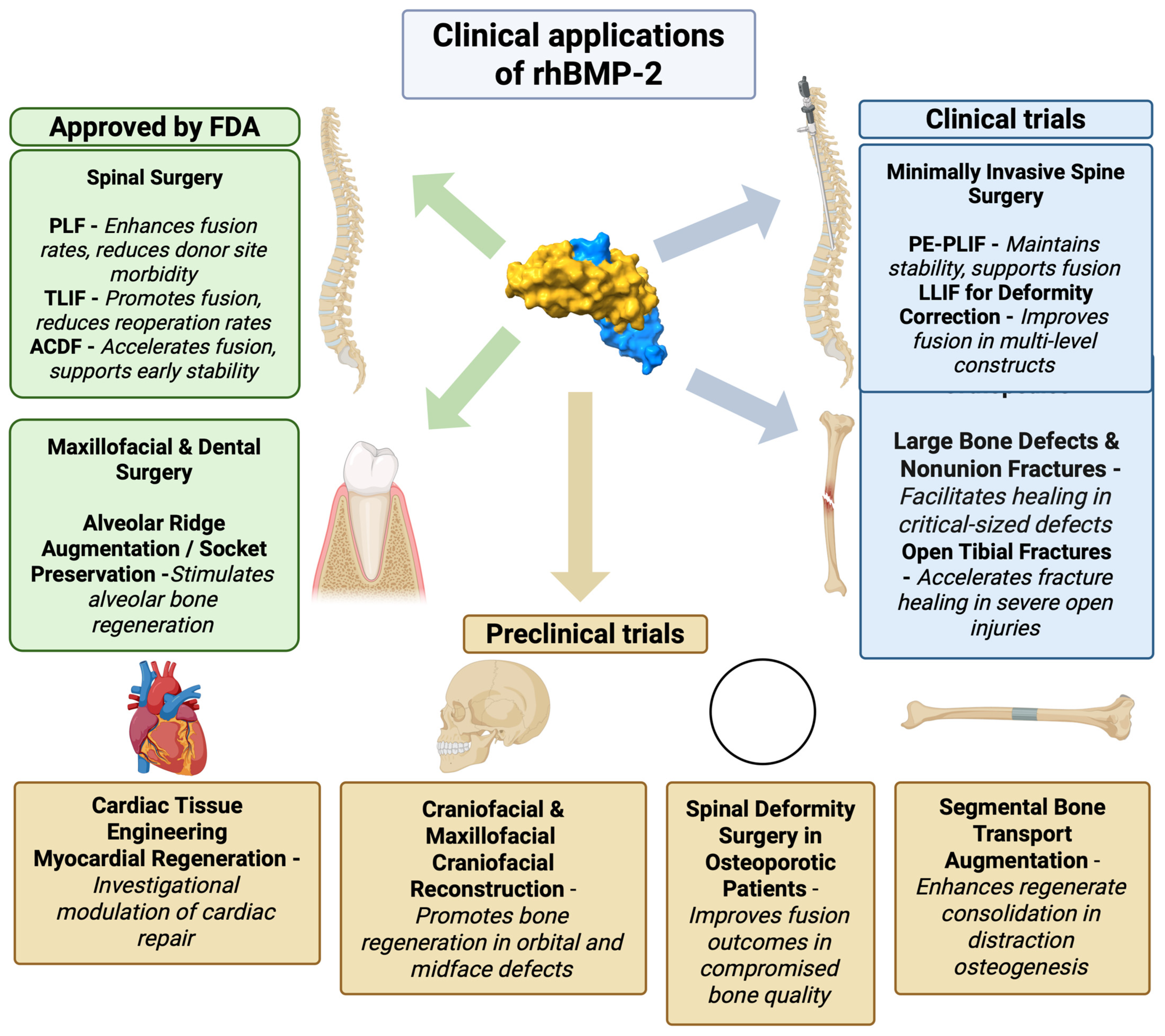
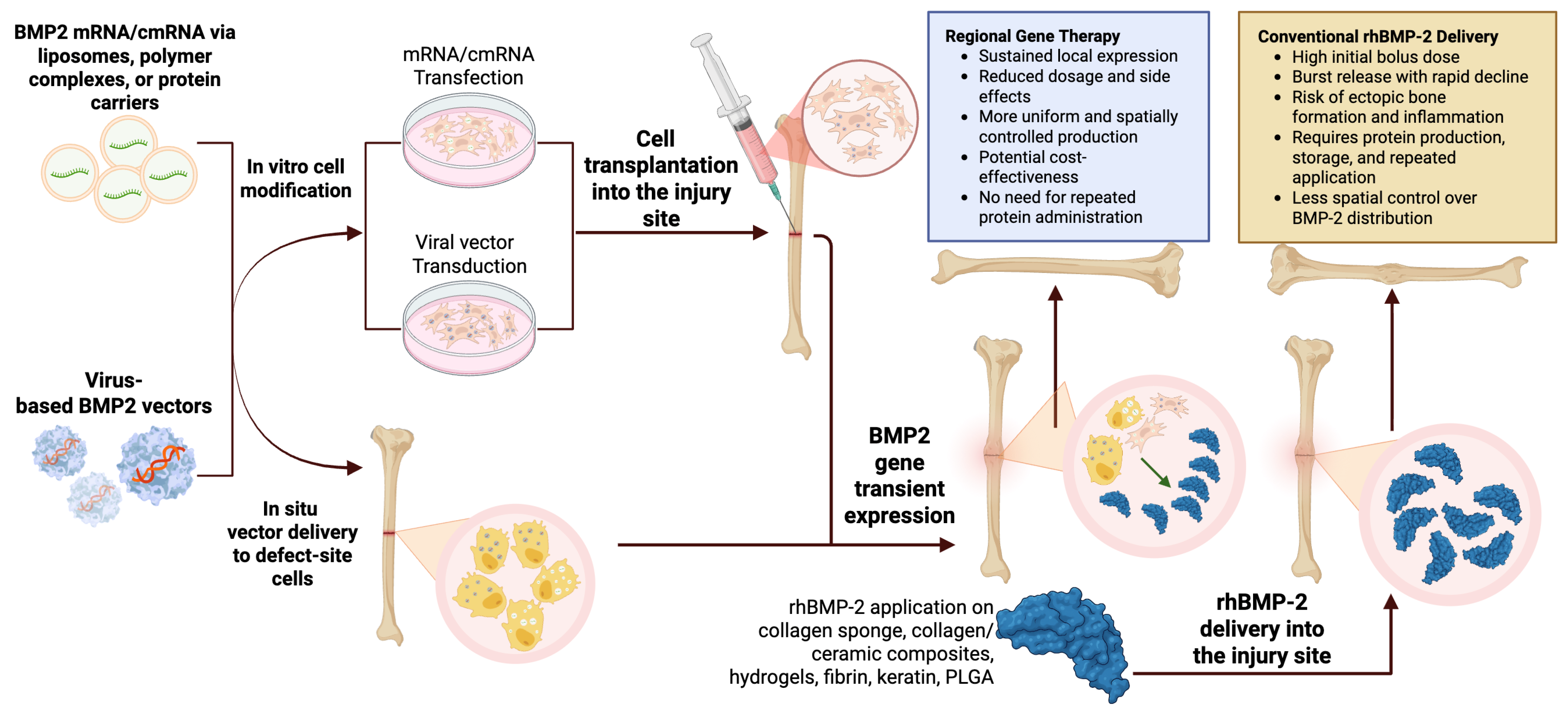
| Carrier Type | Duration of rhBMP-2 Release | Efficacy | Risk of Complications | Experimental Model | Description |
|---|---|---|---|---|---|
| Collagen Sponge | Short (1–3 days, burst release) | High osteoinduction, but unstable bone structure | High (inflammation, ectopic ossification, osteolysis) | In vivo, Clinical | Classical carrier; requires high doses (mg), which increases the incidence of side effects |
| Hydrogel | Intermediate (7–14 days) | Moderately high osteoinduction, good integration | Medium | In vivo | Can be modified for dual release (e.g., rhBMP-2 + antibiotic) |
| Bioceramic Scaffold (HA, β-TCP) | Intermediate–long (14–30 days) | High mineralization and mechanical strength | Medium–low | In vivo, Clinical | Provides good osteoconduction and stability; HA granules enhance fusion quality |
| Composite Scaffold (polymer + ceramic) | Long (up to 21–30 days) | High mineralization, uniform bone formation | Low | In vivo | Allows reduction of rhBMP-2 dose by 5–10 times compared with collagen |
| 3D-Printed Scaffold | Long, controlled (14–28 days) | High osteoinduction and architectural adaptation | Low | In vivo | Enables patient-specific design tailored to the defect |
| Nanoparticles/ Nanofibers | Very long (up to 60–75 days) | High osteoinduction at low doses | Low | In vivo | Minimal burst release, targeted delivery, and excellent biocompatibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysheva, M.; Ruchko, E.; Eremeev, A. Optimizing rhBMP-2 Therapy for Bone Regeneration: From Safety Concerns to Biomaterial-Guided Delivery Systems. Int. J. Mol. Sci. 2025, 26, 10723. https://doi.org/10.3390/ijms262110723
Chernysheva M, Ruchko E, Eremeev A. Optimizing rhBMP-2 Therapy for Bone Regeneration: From Safety Concerns to Biomaterial-Guided Delivery Systems. International Journal of Molecular Sciences. 2025; 26(21):10723. https://doi.org/10.3390/ijms262110723
Chicago/Turabian StyleChernysheva, Maria, Evgenii Ruchko, and Artem Eremeev. 2025. "Optimizing rhBMP-2 Therapy for Bone Regeneration: From Safety Concerns to Biomaterial-Guided Delivery Systems" International Journal of Molecular Sciences 26, no. 21: 10723. https://doi.org/10.3390/ijms262110723
APA StyleChernysheva, M., Ruchko, E., & Eremeev, A. (2025). Optimizing rhBMP-2 Therapy for Bone Regeneration: From Safety Concerns to Biomaterial-Guided Delivery Systems. International Journal of Molecular Sciences, 26(21), 10723. https://doi.org/10.3390/ijms262110723







