Immunomodulatory Effects of a High-CBD Cannabis Extract: A Comparative Analysis with Conventional Therapies for Oral Lichen Planus and Graft-Versus-Host Disease
Abstract
1. Introduction
Study Hypothesis
2. Results
2.1. CAN296 and CBD:CBC (2:1) Combination Significantly Inhibited TNF-α and IFN-γ Secretion by CD8+ T Cells
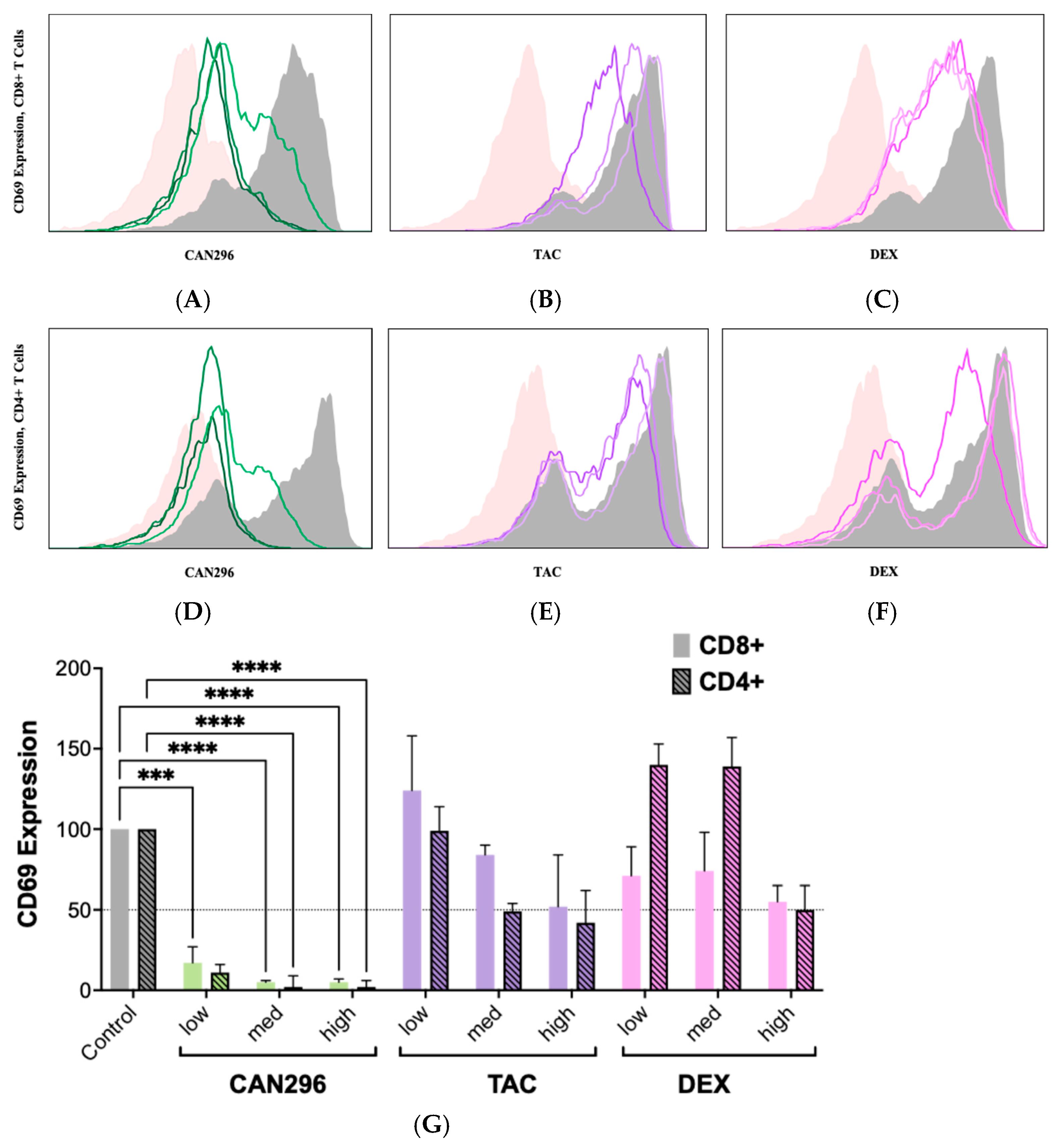
2.2. CAN296 Suppresses CD69 Expression on CD4+ and CD8+ T Cells, Outperforms Dexamethasone and Tacrolimus
2.3. CAN296 Inhibits TNF-α and IFN-γ Secretion by CD4+ and CD8+ T Cells, Outperforming Dexamethasone and Tacrolimus
2.4. CAN296 Reduces Fas-L Expression in CD8+ T Cells, Outperforming Dexamethasone and Tacrolimus
2.5. CAN296 Inhibits Granzyme B and Perforin Expression in CD8+ T Cells, Comparable to Dexamethasone and Tacrolimus
3. Discussion
4. Materials and Methods
4.1. Immunosuppressive Agents’ Preparation
4.2. Phytocannabinoid Extraction and Sample Preparation
4.3. PBMC Isolation and CD4+/CD8+ Enrichment
4.4. T Cell Activation
4.5. CD69, Granzyme B, Perforin, and Fas-L Staining
4.6. Flow Cytometry Analysis
4.7. TNF-α and IFN-γ Secretion Analysis by ELISA
4.8. Statistical Analysis
4.9. Study Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAN296 | CBD-rich cannabis extract (Type III strain) |
| CB2 | Cannabinoid receptor type 2 |
| CBC | Cannabichromene |
| CBD | Cannabidiol |
| CD4+ | Cluster of Differentiation 4 helper T cells |
| CD8+ | Cluster of Differentiation 8 cytotoxic T cells |
| DEX | Dexamethasone |
| DMSO | Dimethyl sulfoxide |
| ECS | Endocannabinoid system |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| FMO | Fluorescence-minus-one |
| GVHD | Graft-versus-host disease |
| HNSCC | Head and neck squamous cell carcinoma |
| HSCT | Hematopoietic stem cell transplantation |
| IFN-γ | Interferon gamma |
| LLLT | Low-level laser therapy |
| MFI | Median fluorescence intensity |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| OLP | Oral lichen planus |
| OPMD | Oral potentially malignant disorder |
| oGVHD | Oral graft-versus-host disease |
| PBMC | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PDT | Photodynamic therapy |
| PFA | Paraformaldehyde |
| PPAR | Peroxisome proliferator-activated receptor |
| TAC | Tacrolimus |
| T-cell | T lymphocyte |
| TNF-α | Tumor necrosis factor alpha |
| TRP | Transient receptor potential |
References
- Payeras, M.R.; Cherubini, K.; Figueiredo, M.A.; Salum, F.G. Oral lichen planus: Focus on etiopathogenesis. Arch. Oral Biol. 2013, 58, 1057–1069. [Google Scholar] [CrossRef]
- Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Zhao, Z.Z.; Zhou, X.J.; Khan, A.; Seymour, G.J.; Bigby, M. The pathogenesis of oral lichen planus. Crit. Rev. Oral Biol. Med. 2002, 13, 350–365. [Google Scholar] [CrossRef]
- Gorouhi, F.; Davari, P.; Fazel, N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci. World J. 2014, 2014, 742826. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Scully, C.; Gil-Montoya, J.A. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2021, 50, 287–298. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. J. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Filipovich, A.H. Diagnosis and manifestations of chronic graft-versus-host disease. Best Pract. Res. Clin. Haematol. 2008, 21, 251–257. [Google Scholar] [CrossRef]
- Rizzo, J.D.; Curtis, R.E.; Socié, G.; Sobocinski, K.A.; Gilbert, E.; Landgren, O.; Travis, L.B.; Travis, W.D.; Flowers, M.E.; Friedman, D.L.; et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009, 113, 1175–1183. [Google Scholar] [CrossRef]
- Atsuta, Y.; Suzuki, R.; Yamashita, T.; Fukuda, T.; Miyamura, K.; Taniguchi, S.; Iida, H.; Uchida, T.; Ikegame, K.; Takahashi, S.; et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann. Oncol. 2014, 25, 435–441. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- El-Howati, A.; Thornhill, M.H.; Colley, H.E.; Murdoch, C. Immune mechanisms in oral lichen planus. Oral Dis. 2023, 29, 1400–1415. [Google Scholar] [CrossRef]
- Pimentel, V.N.; de Matos, L.S.; Soares, T.C.; Adam, R.; Metze, K.; Correa, M.E.; de Souza, C.A.; Cintra, M.L. Perforin and granzyme B involvement in oral lesions of lichen planus and chronic GVHD. J. Oral Pathol. Med. 2010, 39, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Solimani, F.; Hertl, M. Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef] [PubMed]
- Bachier, C.R.; Aggarwal, S.K.; Hennegan, K.; Milgroom, A.; Francis, K.; Dehipawala, S.; Rotta, M. Epidemiology and Treatment of Chronic Graft-versus-Host Disease Post-Allogeneic Hematopoietic Cell Transplantation: A US Claims Analysis. Transplant. Cell. Ther. 2021, 27, 504.e1–504.e6. [Google Scholar] [CrossRef] [PubMed]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Noceti, O.M.; Woillard, J.B.; Boumediene, A.; Esperón, P.; Taupin, J.L.; Gerona, S.; Valverde, M.; Touriño, C.; Marquet, P. Tacrolimus pharmacodynamics and pharmacogenetics along the calcineurin pathway in human lymphocytes. Clin. Chem. 2014, 60, 1336–1345. [Google Scholar] [CrossRef]
- Thongprasom, K.; Carrozzo, M.; Furness, S.; Lodi, G. Interventions for treating oral lichen planus. Cochrane Database Syst. Rev. 2011, 7, CD001168. [Google Scholar] [CrossRef]
- Lodi, G.; Carrozzo, M.; Furness, S.; Thongprasom, K. Interventions for treating oral lichen planus: A systematic review. Br. J. Dermatol. 2012, 166, 938–947. [Google Scholar] [CrossRef]
- Kahan, B.D. Cyclosporine: A revolution in transplantation. Transplant. Proc. 1999, 31, 14s–15s. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Goa, K.L.; Gillis, J.C. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 1997, 54, 925–975. [Google Scholar] [CrossRef] [PubMed]
- Digiovanna, J.J.; Mauro, T.; Milstone, L.M.; Schmuth, M.; Toro, J.R. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol. Ther. 2013, 26, 26–38. [Google Scholar] [CrossRef]
- Collins, M.D.; Mao, G.E. Teratology of retinoids. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 399–430. [Google Scholar] [CrossRef]
- Kvaal, S.I.; Angell-Petersen, E.; Warloe, T. Photodynamic treatment of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 62–70. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Pour, N.M. Photodynamic treatment of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 257. [Google Scholar] [CrossRef]
- Akram, Z.; Abduljabbar, T.; Vohra, F.; Javed, F. Efficacy of low-level laser therapy compared to steroid therapy in the treatment of oral lichen planus: A systematic review. J. Oral Pathol. Med. 2018, 47, 11–17. [Google Scholar] [CrossRef]
- Devi, S.; Zimmermann-Klemd, A.M.; Fiebich, B.L.; Heinrich, M.; Gründemann, C.; Steinberger, P.; Kowarschik, S.; Huber, R. Immunosuppressive activity of non-psychoactive Cannabis sativa L. extract on the function of human T lymphocytes. Int. Immunopharmacol. 2022, 103, 108448. [Google Scholar] [CrossRef]
- Aswad, M.; Hamza, H.; Pechkovsky, A.; Zikrach, A.; Popov, T.; Zohar, Y.; Shahar, E.; Louria-Hayon, I. High-CBD Extract (CBD-X) Downregulates Cytokine Storm Systemically and Locally in Inflamed Lungs. Front. Immunol. 2022, 13, 875546. [Google Scholar] [CrossRef]
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory Potential of Cannabidiol in Multiple Sclerosis: A Systematic Review. J. Neuroimmune Pharmacol. 2021, 16, 251–269. [Google Scholar] [CrossRef]
- Blal, K.; Besser, E.; Procaccia, S.; Schwob, O.; Lerenthal, Y.; Abu Tair, J.; Meiri, D.; Benny, O. The Effect of Cannabis Plant Extracts on Head and Neck Squamous Cell Carcinoma and the Quest for Cannabis-Based Personalized Therapy. Cancers 2023, 15, 497, Erratum in Cancers 2023, 15, 2481. [Google Scholar] [CrossRef]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Besser, E.; Gelfand, A.; Procaccia, S.; Berman, P.; Meiri, D. Cannabinoid combination targets NOTCH1-mutated T-cell acute lymphoblastic leukemia through the integrated stress response pathway. eLife 2024, 12, RP90854. [Google Scholar] [CrossRef]
- Aziz, A.I.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur. J. Pharmacol. 2019, 843, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.; Haas, C.; d’Argouges, S.; Fisch, T.; Kufer, P.; Brischwein, K.; Prang, N.; Bargou, R.; Suzich, J.; Baeuerle, P.A.; et al. The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol. Immunother. 2007, 56, 1551–1563. [Google Scholar] [CrossRef]
- Wyrobnik, I.; Steinberg, M.; Gelfand, A.; Rosenblum, R.; Eid Mutlak, Y.; Sulimani, L.; Procaccia, S.; Ofran, Y.; Novak-Kotzer, H.; Meiri, D. Decreased melanoma CSF-1 secretion by Cannabigerol treatment reprograms regulatory myeloid cells and reduces tumor progression. Oncoimmunology 2023, 12, 2219164. [Google Scholar] [CrossRef]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef]
- Morgan, D.J.; Davis, D.M. Distinct Effects of Dexamethasone on Human Natural Killer Cell Responses Dependent on Cytokines. Front. Immunol. 2017, 8, 432. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091–4106. [Google Scholar] [CrossRef]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef]
- Qi, X.; Lin, W.; Wu, Y.; Li, Q.; Zhou, X.; Li, H.; Xiao, Q.; Wang, Y.; Shao, B.; Yuan, Q. CBD Promotes Oral Ulcer Healing via Inhibiting CMPK2-Mediated Inflammasome. J. Dent. Res. 2022, 101, 206–215. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blal, K.; Rosenblum, R.; Novak-Kotzer, H.; Procaccia, S.; Abu Tair, J.; Casap, N.; Meiri, D.; Benny, O. Immunomodulatory Effects of a High-CBD Cannabis Extract: A Comparative Analysis with Conventional Therapies for Oral Lichen Planus and Graft-Versus-Host Disease. Int. J. Mol. Sci. 2025, 26, 10711. https://doi.org/10.3390/ijms262110711
Blal K, Rosenblum R, Novak-Kotzer H, Procaccia S, Abu Tair J, Casap N, Meiri D, Benny O. Immunomodulatory Effects of a High-CBD Cannabis Extract: A Comparative Analysis with Conventional Therapies for Oral Lichen Planus and Graft-Versus-Host Disease. International Journal of Molecular Sciences. 2025; 26(21):10711. https://doi.org/10.3390/ijms262110711
Chicago/Turabian StyleBlal, Kifah, Ronen Rosenblum, Hila Novak-Kotzer, Shiri Procaccia, Jawad Abu Tair, Nardy Casap, David Meiri, and Ofra Benny. 2025. "Immunomodulatory Effects of a High-CBD Cannabis Extract: A Comparative Analysis with Conventional Therapies for Oral Lichen Planus and Graft-Versus-Host Disease" International Journal of Molecular Sciences 26, no. 21: 10711. https://doi.org/10.3390/ijms262110711
APA StyleBlal, K., Rosenblum, R., Novak-Kotzer, H., Procaccia, S., Abu Tair, J., Casap, N., Meiri, D., & Benny, O. (2025). Immunomodulatory Effects of a High-CBD Cannabis Extract: A Comparative Analysis with Conventional Therapies for Oral Lichen Planus and Graft-Versus-Host Disease. International Journal of Molecular Sciences, 26(21), 10711. https://doi.org/10.3390/ijms262110711






