The Influence of Genome Composition and Temperature on the Hatching Success and Development of the Offspring of Allotriploid Cobitis (Pisces: Cobitidae) Females
Abstract
1. Introduction
2. Results
2.1. Female Genome Structure
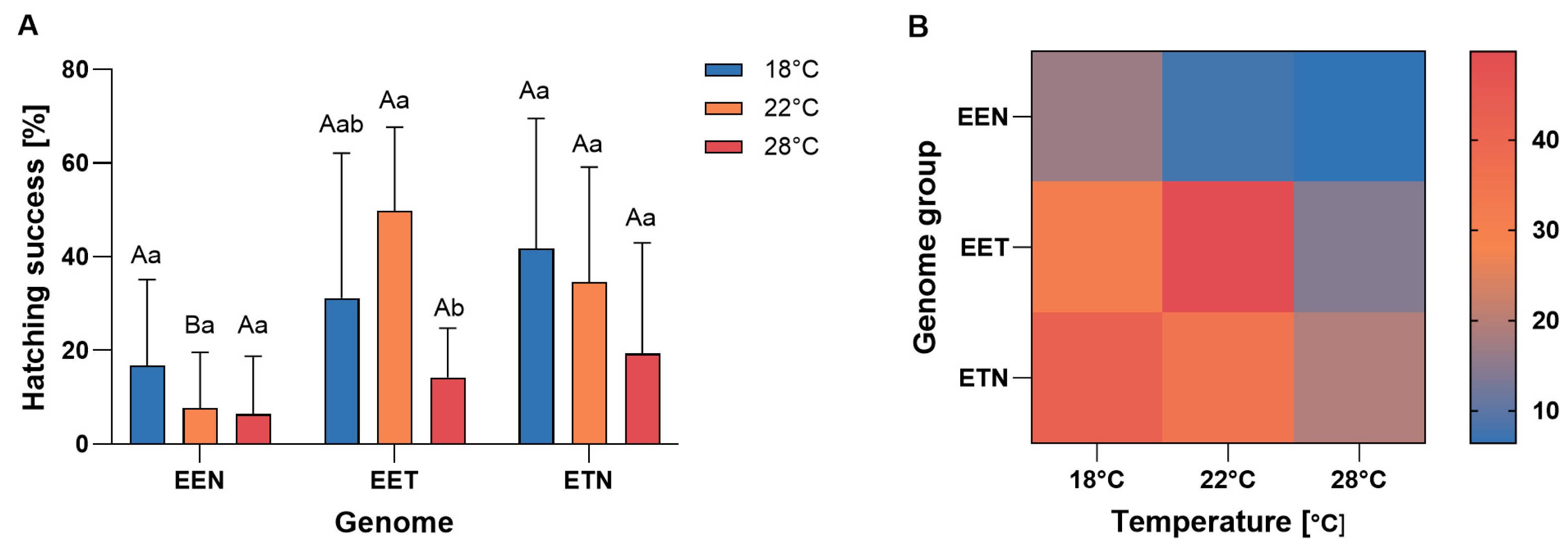
2.2. Hatching Success
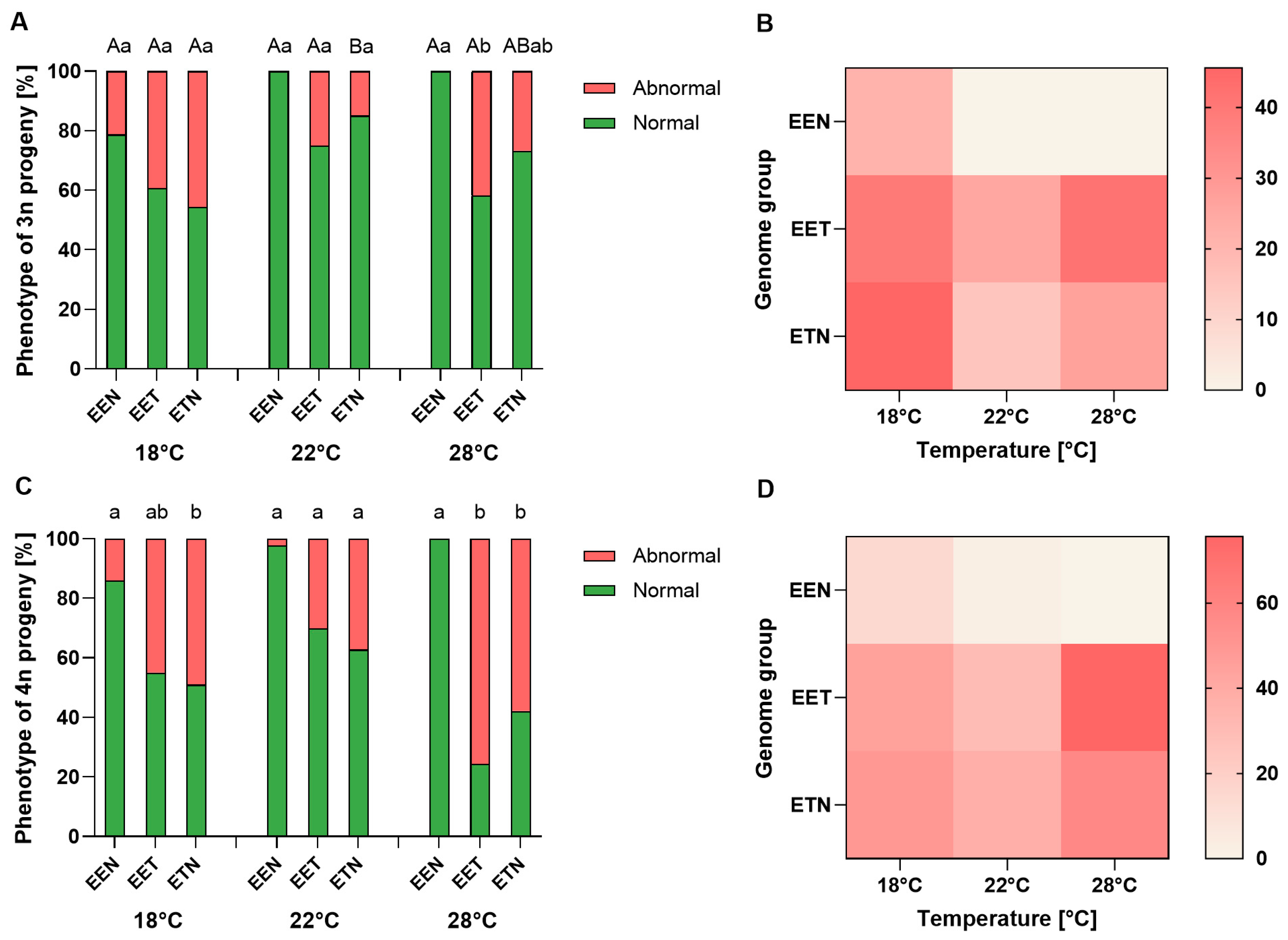
2.3. Ploidy and Phenotype of Progeny
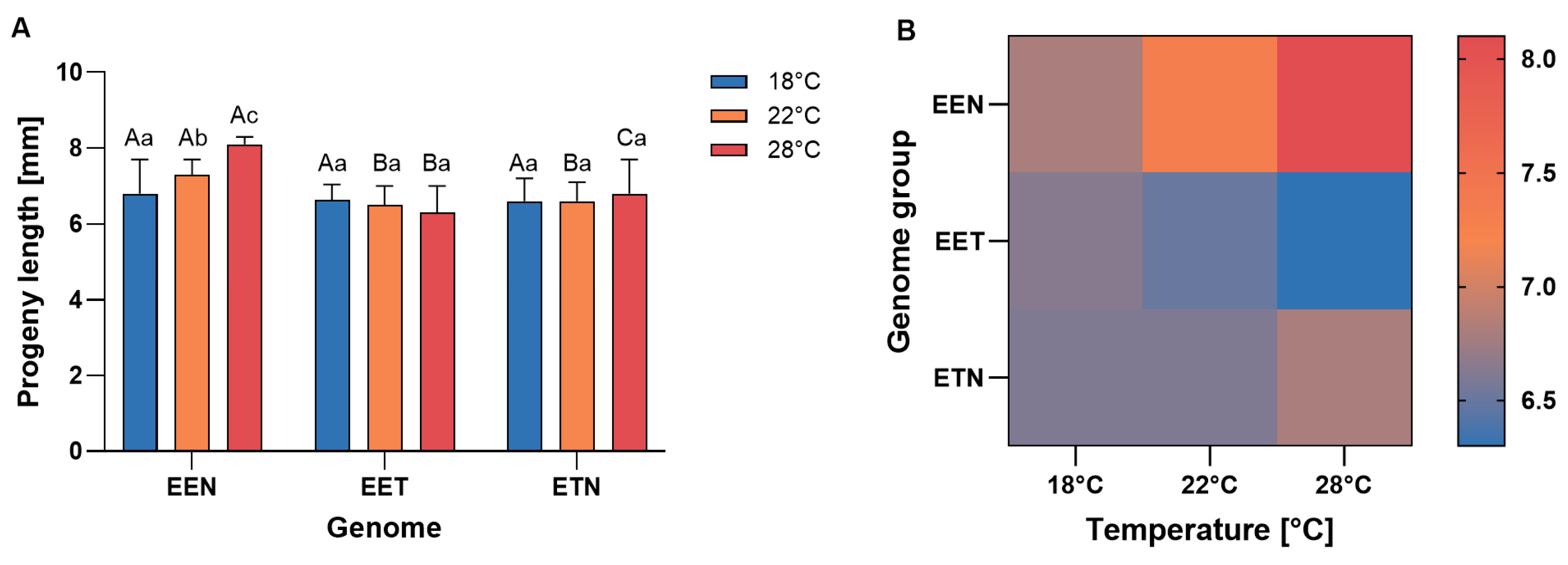
2.4. Body Length of Progeny
3. Discussion
3.1. Female Genome Structure
3.2. Hatching Success
3.3. Ploidy and Phenotype of Progeny
3.4. Body Length of Progeny
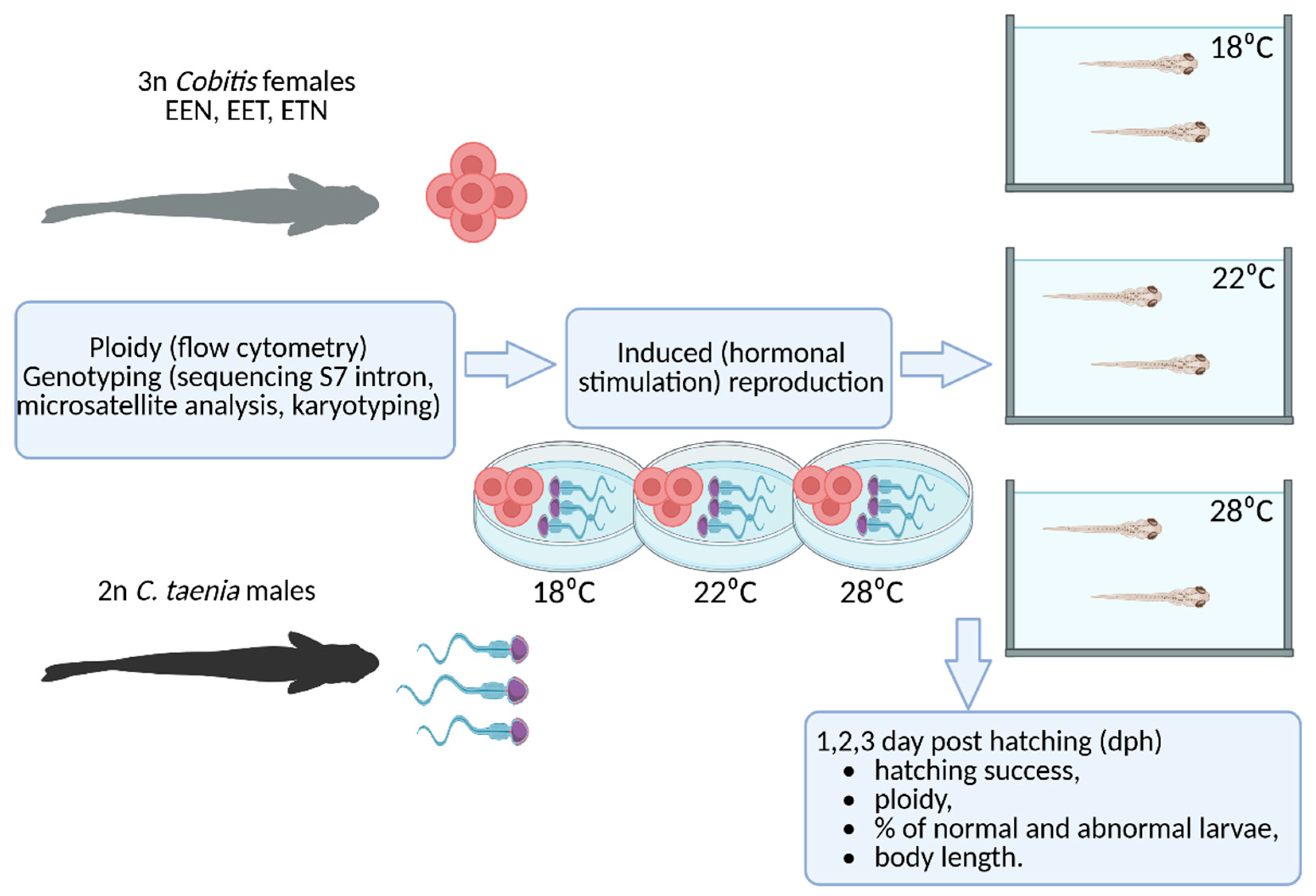
4. Materials and Methods
4.1. Fish Sampling and Genomic Identification
4.2. Crossing Experiments
4.3. Hatching Success
4.4. Ploidy Analysis of Progeny
4.5. Phenotype Analysis of Progeny
4.6. Measurement of Body Length in Progeny
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Qiang, J.; Duan, X.-J.; Zhu, H.-J.; He, J.; Tao, Y.-F.; Bao, J.-W.; Zhu, X.-W.; Xu, P. Some ‘White’ Oocytes Undergo Atresia and Fail to Mature during the Reproductive Cycle in Female Genetically Improved Farmed Tilapia (Oreochromis niloticus). Aquaculture 2021, 534, 736278. [Google Scholar] [CrossRef]
- Pino, M.E.; Braanaas, M.F.; Balseiro, P.; Kraugerud, M.; Pedrosa, C.; Imsland, A.K.D.; Handeland, S.O. Constant High Temperature Promotes Early Changes in Testis Development Associated with Sexual Maturation in Male Atlantic Salmon (Salmo salar L.) Post-Smolts. Fishes 2022, 7, 341. [Google Scholar] [CrossRef]
- Calvo-Rodríguez, L.; Ortiz-Delgado, J.B.; Cañón, L.; de Paz, P.; Fernández, I.; Riesco, M.F. Current summer heat waves impair rainbow trout (Oncorhynchus mykiss) spermatogenesis: Implications for future fish farming management practices in South Europe. Aquaculture 2025, 596, 741716. [Google Scholar] [CrossRef]
- Tinkir, M.; Memiş, D.; Cheng, Y.; Xin, M.; Rodina, M.; Gela, D.; Tučková, V.; Linhart, O. Level of in Vitro Storage of the European Catfish (Silurus glanis L.) Eggs at Different Temperatures. Fish Physiol. Biochem. 2021, 47, 163–171. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.F.; Zhu, J.H.; Lu, S.Q.; Zao, Z.M.; Ma, J.L.; He, J.; Xu, P. Effects of heat stress on follicular development and atresia in Nile tilapia (Oreochromis niloticus) during one reproductive cycle and its potential regulation by autophagy and apoptosis. Aquaculture 2022, 555, 738171. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Li, G.-L.; Zhu, C.-H.; Deng, S.-P. Effects of Fish Oil on Ovarian Development in Spotted Scat (Scatophagus argus). Anim. Reprod. Sci. 2013, 141, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Li, J.; Wen, H.; Likang, L. Effects of temperature on gonadal differentiation of black rockfish (Sebastes schlegelii) and its mechanism. J. Fish. China 2019, 43, 1569–1580. [Google Scholar] [CrossRef]
- Nakatani, Y.; Shingate, P.; Ravi, V.; Pillai, N.; Prasad, A.; McLysaght, A.; Venkatesh, B. Reconstruction of proto-vertebrate, proto-cyclostome and proto-gnathostome genomes provides new insights into early vertebrate evolution. Nat. Commun. 2021, 12, 4489. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ren, Y.; Uesaka, M.; Beavan, A.; Muffato, M.; Shen, J.; Li, Y.; Sato, I.; Wan, W.; Clark, J.; et al. Hagfish genome elucidates vertebrate whole-genome duplication events and their evolutionary consequences. Nat. Ecol. Evol. 2024, 8, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Naylor, G.; Mayden, R. Deciphering Reticulate Evolution of the Largest Group of Polyploid Vertebrates, the Subfamily Cyprininae (Teleostei: Cypriniformes). Mol Phylogenet Evol. 2021, 166, 107323. [Google Scholar] [CrossRef]
- Mable, B.; Alexandrou, M.; Taylor, M. Genome duplication in amphibians and fish: An extended synthesis. J. Zool. 2011, 284, 151–182. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. Evolutionary and Genomic Diversity of True Polyploidy in Tetrapods. Animals 2023, 13, 1033. [Google Scholar] [CrossRef]
- Majtánová, Z.; Choleva, L.; Symonová, R.; Ráb, P.; Kotusz, J.; Pekárik, L.; Janko, K. Asexual Reproduction Does Not Apparently Increase the Rate of Chromosomal Evolution: Karyotype Stability in Diploid and Triploid Clonal Hybrid Fish (Cobitis, Cypriniformes, Teleostei). PLoS ONE 2016, 11, e0146872. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, O.; Röslein, J.; Kotusz, J.; Paces, J.; Pekárik, L.; Petrtýl, M.; Halačka, K.; Štefková Kašparová, E.; Mendel, J.; Boroń, A.; et al. The Legacy of Sexual Ancestors in Phenotypic Variability, Gene Expression, and Homoeolog Regulation of Asexual Hybrids and Polyploids. Mol. Biol. Evol. 2019, 36, 1902–1920. [Google Scholar] [CrossRef]
- Mezhzherin, S.V.; Tsyba, A.O. Genomic structure and diversity of European polyploid spined loaches (Cypriniformes, Cobitidae) in a situation of genetic instability. J. Fish. Biol. 2025, 1–12. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Zou, L.; Luo, Y.; Tan, H.; Yao, J.; Zhang, M.; Liu, S. Hox Genes Reveal Variations in the Genomic DNA of Allotetraploid Hybrids Derived from Carassius auratus Red Var. (Female) × Cyprinus carpio L. (Male). BMC Genet. 2020, 21, 24. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2025. Available online: www.fishbase.org (accessed on 1 August 2025).
- Janko, K.; Flajšhans, M.; Choleva, L.; Bohlen, J.; Šlechtová, V.; Rábová, M.; Lajbner, Z.; Šlechta, V.; Ivanova, P.; Dobrovolov, I.; et al. Diversity of European Spined Loaches (Genus Cobitis L.): An Update of the Geographic Distribution of the Cobitis taenia Hybrid Complex with a Description of New Molecular Tools for Species and Hybrid Determination. J. Fish Biol. 2007, 71, 387–408. [Google Scholar] [CrossRef]
- Boroń, A.; Grabowska, A.; Jablonska, O.; Kirtiklis, L.; Duda, S.; Juchno, D. Chromosomal rDNA Distribution Patterns in Clonal Cobitis Triploid Hybrids (Teleostei, Cobitidae): Insights into Parental Genomic Contributions. Genes 2025, 16, 68. [Google Scholar] [CrossRef]
- Janko, K.; Kotusz, J.; De Gelas, K.; Šlechtová, V.; Opoldusová, Z.; Drozd, P.; Choleva, L.; Popiołek, M.; Baláž, M. Dynamic Formation of Asexual Diploid and Polyploid Lineages: Multilocus Analysis of Cobitis Reveals the Mechanisms Maintaining the Diversity of Clones. PLoS ONE 2012, 7, e45384. [Google Scholar] [CrossRef] [PubMed]
- Juchno, D.; Pecio, A.; Boroń, A.; Leska, A.; Jablonska, O.; Cejko, B.I.; Kowalski, R.K.; Judycka, S.; Przybylski, M. Evidence of the Sterility of Allotetraploid Cobitis Loaches (Teleostei, Cobitidae) Using Testes Ultrastructure. J. Exp. Zool. Pt. A 2017, 327, 66–74. [Google Scholar] [CrossRef]
- Mezhzherin, S.V.; Tsyba, A.A.; Kryvokhyzha, D. Cryptic expansion of hybrid polyploid spined loaches Cobitis in the rivers of Eastern Europe. Hydrobiologia 2022, 849, 1689–1700. [Google Scholar] [CrossRef]
- Choleva, L.; Musilova, Z.; Kohoutova-Sediva, A.; Paces, J.; Rab, P.; Janko, K. Distinguishing between Incomplete Lineage Sorting and Genomic Introgressions: Complete Fixation of Allospecific Mitochondrial DNA in a Sexually Reproducing Fish (Cobitis; Teleostei), despite Clonal Reproduction of Hybrids. PLoS ONE 2014, 9, e80641. [Google Scholar] [CrossRef] [PubMed]
- Juchno, D.; Boroń, A.; Gołaszewski, J. Comparative Morphology and Histology of the Ovaries of the Spined Loach Cobitis taenia L. and Natural Allopolyploids of Cobitis (Cobitidae). J. Fish Biol. 2007, 70, 1392–1411. [Google Scholar] [CrossRef]
- Dedukh, D.; Majtánová, Z.; Marta, A.; Pšenička, M.; Kotusz, J.; Klíma, J.; Juchno, D.; Boron, A.; Janko, K. Parthenogenesis as a Solution to Hybrid Sterility: The Mechanistic Basis of Meiotic Distortions in Clonal and Sterile Hybrids. Genetics 2020, 215, 975–987. [Google Scholar] [CrossRef]
- Marta, A.; Tichopád, T.; Bartoš, O.; Klíma, J.; Shah, M.A.; Bohlen, V.Š.; Bohlen, J.; Halačka, K.; Choleva, L.; Stöck, M.; et al. Genetic and Karyotype Divergence between Parents Affect Clonality and Sterility in Hybrids. eLife 2023, 12, RP88366. [Google Scholar] [CrossRef]
- Dedukh, D.; Marta, A.; Janko, K. Challenges and Costs of Asexuality: Variation in Premeiotic Genome Duplication in Gynogenetic Hybrids from Cobitis taenia Complex. Int. J. Mol. Sci. 2021, 22, 12117. [Google Scholar] [CrossRef]
- Juchno, D.; Jabłońska, O.; Boroń, A.; Kujawa, R.; Leska, A.; Grabowska, A.; Nynca, A.; Świgońska, S.; Król, M.; Spóz, A.; et al. Ploidy-Dependent Survival of Progeny Arising from Crosses between Natural Allotriploid Cobitis Females and Diploid C. taenia Males (Pisces, Cobitidae). Genetica 2014, 142, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, S.; Hu, J.; He, G.; Liu, Y.; Chen, X.; Lei, T.; Li, Q.; Yang, L.; Li, W.; et al. Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell Tissue Organ Cult. 2020, 140, 315–325. [Google Scholar] [CrossRef]
- Glennon, K.L.; Niemann, H.J.; Archibald, S. Fire-mediated effects on polyploid biology. Trends Ecol. Evol. 2024, 39, 424–426. [Google Scholar] [CrossRef]
- Hermaniuk, A.; Rybacki, M.; Taylor, J.R.E. Low Temperature and Polyploidy Result in Larger Cell and Body Size in an Ectothermic Vertebrate. Physiol. Biochem. Zool. 2016, 89, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Maciak, S.; Janko, K.; Kotusz, J.; Choleva, L.; Boroń, A.; Juchno, D.; Kujawa, R.; Kozłowski, J.; Konarzewski, M. Standard metabolic rate (SMR) is inversely related to erythrocyte and genome size in allopolyploid fish of the Cobitis taenia hybrid complex. Funct. Ecol. 2011, 25, 1072–1078. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Yuan, X.; Jiang, Y. Stage-specific Influence of Temperature on the Growth Rate of Japanese Spanish Mackerel (Scomberomorus niphonius) in Early Life. J. Fish Biol. 2022, 100, 498–506. [Google Scholar] [CrossRef]
- Bohlen, J. Reproduction of Spined Loach, Cobitis taenia, (Cypriniformes; Cobitidae) under Laboratory Conditions. J. Appl. Ichthyol. 1999, 15, 49–53. [Google Scholar] [CrossRef]
- Niu, J.; Huss, M.; Vasemägi, A.; Gårdmark, A. Decades of Warming Alters Maturation and Reproductive Investment in Fish. Ecosphere 2023, 14, e4381. [Google Scholar] [CrossRef]
- Fraz, S.; Laframboise, L.; Manzon, R.; Somers, C.M.; Wilson, J.Y. Embryonic and Larval Development of Yellow Perch (Perca flavescens) and Its Sensitivity to Incubation Temperature. J. Fish Biol. 2024, 105, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Sardi, A.E.; Bégout, M.L.; Lalles, A.L.; Cousin, X.; Budzinski, H. Temperature and feeding frequency impact the survival, growth, and metamorphosis success of Solea solea larvae. PLoS ONE 2023, 18, e0281193. [Google Scholar] [CrossRef]
- Howard, C.; Taylor, J.F.; Migaud, H.; Gutierrez, A.P.; Bekaert, M. Comparison of Diploid and Triploid Atlantic Salmon (Salmo salar) Physiological Embryonic Development. Animals 2023, 13, 3352. [Google Scholar] [CrossRef]
- Juchno, D.; Boroń, A.; Szlachciak, J.; Kujawa, R. Early Development and Post Embryonic Skeletal Morphology of the Progeny of Spined Loach Cobitis taenia L. (Teleostei, Cobitidae) and its Naturally Occurring Allotriploids. Folia Biol. 2016, 64, 153–162. [Google Scholar] [CrossRef]
- Kočí, J.; Röslein, J.; Pačes, J.; Kotusz, J.; Halačka, K.; Koščo, J.; Fedorčák, J.; Iakovenko, N.; Janko, K. No evidence for accumulation of deleterious mutations and fitness degradation in clonal fish hybrids: Abandoning sex without regrets. Mol. Ecol. 2020, 29, 3038–3055. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, J.; Zeng, M.; Xu, K.; Tao, M.; Zhang, C.; Duan, W.; Liu, W.; Luo, K.; Liu, Y.; et al. Genomic Variation in the Hybrids of White Crucian Carp and Red Crucian Carp: Evidence from Ribosomal DNA. Sci. China Life Sci. 2015, 58, 590–601. [Google Scholar] [CrossRef]
- Káldy, J.; Mozsár, A.; Fazekas, G.; Farkas, M.; Fazekas, D.L.; Fazekas, G.L.; Goda, K.; Gyöngy, Z.; Kovács, B.; Semmens, K.; et al. Hybridization of Russian Sturgeon (Acipenser gueldenstaedtii, Brandt and Ratzeberg, 1833) and American Paddlefish (Polyodon spathula, Walbaum 1792) and Evaluation of Their Progeny. Genes 2020, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, K.; Nomoto, K.; Machida, Y.; Ichimura, M.; Koizumi, I. No reduction of hatching rates among F1hybrids of naturally hybridizing three Far Eastern daces, genus Tribolodon (Cypriniformes, Cyprinidae). Ichthyol. Res. 2017, 65, 165–167. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, J.; Xu, W.; He, S.; Long, M.; Liao, Q.; Liu, J.; Peng, L.; Liu, W.; Xiao, Y. Characteristics of hatching enzymes and egg envelope in cross progenies from crucian carp (Carassius auratus var.) and zebrafish (Barchydanio rerio var.). Reprod. Breed. 2021, 1, 81–88. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Sun, Z.; Zhao, Y.; Du, W.; Cui, J.; Hou, J.; Wang, Y. Induction of gyno-tetraploidy in Japanese flounder Paralichthys olivaceus. J. Oceanol. Limnol. 2019, 38, 288–293. [Google Scholar] [CrossRef]
- Ren, L.; Tang, C.; Li, W.; Cui, J.; Tan, X.; Xiong, Y.; Chen, J.; Wang, J.; Xiao, J.; Zhou, Y.; et al. Determination of dosage compensation and comparison of gene expression in a triploid hybrid fish. BMC Genom. 2017, 18, 38. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Tan, H.; Luo, L.; Cui, J.; Hu, J.; Wang, S.; Liu, Q.; Hu, F.; Tang, C.; et al. Asymmetric expression patterns reveal a strong maternal effect and dosage compensation in polyploid hybrid fish. BMC Genom. 2018, 19, 517. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, X.; Li, J.; Yan, X.; Gao, X.; Cui, J.; Tang, C.; Liu, S. Diverse transcriptional patterns of homoeologous recombinant transcripts in triploid fish (Cyprinidae). Sci. China Life Sci. 2021, 64, 1491–1501. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Xu, W.; Wang, K.; Wu, B.; Xu, M.; Chen, Y.; Miao, L.; Wang, Z.; Li, Z.; et al. Comparative genome anatomy reveals evolutionary insights into a unique amphitriploid fish. Nat Ecol Evol. 2022, 6, 1354–1366. [Google Scholar] [CrossRef]
- Luo, J.; Chai, J.; Wen, Y.; Tao, M.; Lin, G.; Liu, X.; Ren, L.; Chen, Z.; Wu, S.; Li, S.; et al. From asymmetrical to balanced genomic diversification during rediploidization: Subgenomic evolution in allotetraploid fish. Sci. Adv. 2020, 6, eaaz7677. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liao, Z.; Brock, J.; Du, K.; Li, G.; Chen, Z.; Wang, Y.; Gao, Z.; Agarwal, G.; Wei, K.; et al. Maternal dominance contributes to subgenome differentiation in allopolyploid fishes. Nat. Commun. 2023, 14, 8357. [Google Scholar] [CrossRef]
- Hasan, M.; Sultana Mely, S.; Faruk, A.; Nayeem Hossain, M. Climate Change Effects on Hatching Success, Embryonic Development and Larvae Survival of Freshwater Fish: A Critical Review. Int. J. Ecotoxicol. Ecobiol. 2023, 16, 24. [Google Scholar] [CrossRef]
- Ashaf-Ud-Doulah, M.; Islam, S.M.M.; Zahangir, M.M.; Islam, M.S.; Brown, C.; Shahjahan, M. Increased water temperature interrupts embryonic and larval development of Indian major carp rohu Labeo rohita. Aquacult Int. 2021, 29, 711–722. [Google Scholar] [CrossRef]
- Schmitz, A.M.; Sepulveda Villet, O.J. Short term temperature fluctuations affect embryonic and larval development of yellow perch (Perca flavescens). J. Aquac. Mar. Biol. 2021, 10, 168–176. [Google Scholar] [CrossRef]
- Pepin, P.; Orr, D.C.; Anderson, J.T. Time to Hatch and Larval Size in Relation to Temperature and Egg Size in Atlantic Cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 1997, 54, 2–10. [Google Scholar] [CrossRef]
- El-Gamal, A.E.-H.E. Effect of Temperature on Hatching and Larval Development and Mucin Secretion in Common Carp, Cyprinus carpio (Linnaeus, 1758). Glob. Vet. 2009, 3, 80–90. [Google Scholar]
- Dionísio, G.; Campos, C.; Valente, L.M.P.; Conceição, L.E.C.; Cancela, M.L.; Gavaia, P.J. Effect of Egg Incubation Temperature on the Occurrence of Skeletal Deformities in Solea senegalensis: Effect of Incubation Temperature on Skeletal Deformities of Sole. J. Appl. Ichthyol. 2012, 28, 471–476. [Google Scholar] [CrossRef]
- De Souza, A.M.; da Silva Junior, F.C.; Dantas, É.D.; Galvão-Pereira, M.C.; de Medeiros, S.R.B.; Luchiari, A.C. Temperature effects on development and lifelong behavior in zebrafish. Sci. Total Environ. 2025, 973, 179172. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Nguyen, T.; Singchat, W.; Punthum, T.; Kraichak, E.; Panochit, P.; Maneeaphai, W.; Phuonnim, A.; Sengtrakool, S.; Sriphairoj, K.; et al. Impact of higher temperatures on yolk sac absorption and early development in hybrid catfish between Clarias gariepinus and C. macrocephalus. J. World Aquac. Soc. 2025, 56, e13119. [Google Scholar] [CrossRef]
- Myers, J.; Chatakondi, N.; Dunham, R.; Butts, I. Genetic architecture of early life history traits for channel catfish, Ictalurus punctatus ♀ × blue catfish, I. furcatus ♂ hybrid production. Aquaculture 2020, 514, 734436. [Google Scholar] [CrossRef]
- Huang, J.; Chen, G.; Wang, Z.; Zhang, J. Use of response surface methodology to study the combined effects of temperature and salinity on hatching and deformity of the hybrid grouper, Epinephelus fuscoguttatus (♀)× Epinephelus polyphekadion (♂). Aquac. Res. 2018, 49, 1997–2005. [Google Scholar] [CrossRef]
- Lee, J.; Han, K.; Choi, W.; Yu, T.; Kim, H.; Lee, S. Effects of Water Temperature and Salinity on the Growth and Survival of Hybrid Pufferfish Larvae (Takifugu obscurus ♀ × T. rubripes ♂). Aquac. Res. 2024, 2024, 8511980. [Google Scholar] [CrossRef]
- Bohlen, J. Spawning habitat in the spined loach, Cobitis taenia (Cypriniformes: Cobitidae). Ichthyol Res. 2003, 50, 0098–0101. [Google Scholar] [CrossRef]
- Bohlen, J.; Ritterbusch, D. Which factors affect sex ratio of spined loach (genus Cobitis) in Lake Müggelsee? Environ. Biol. Fishes 2000, 59, 347–352. [Google Scholar] [CrossRef]
- Aristarkhova, E.; Fedoniuk, T.; Romanchuk, L.; Latushynskyi, S.; Kot, I. Features of the surface water oxygen regime in the Ukrainian Polesie Region. J. Water Land Dev. 2023, 49, 104–110. [Google Scholar] [CrossRef]
- Fraser, G.; Bestgen, K.; Winkelman, D.; Thompson, K. Temperature—Not Flow—Predicts Native Fish Reproduction with Implications for Climate Change. Trans. Am. Fish. Soc. 2019, 148, 509–527. [Google Scholar] [CrossRef]
- Koenigbauer, S.; Cubbage, M.; Warren, L.; Tellier, J.; Selz, O.; Sass, G.; Höök, T. Fish reproductive phenology shifts with increasing temperature and year. Biol. Lett. 2025, 21, 20240240. [Google Scholar] [CrossRef]
- Pankhurst, N.; Munday, P. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Servili, A.; Canário, A.; Mouchel, O.; Muñoz-Cueto, J. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef]
- Lema, S.; Luckenbach, J.; Yamamoto, Y.; Housh, M. Fish reproduction in a warming world: Vulnerable points in hormone regulation from sex determination to spawning. Philos. Trans. R. Soc. B 2024, 379, 20220516. [Google Scholar] [CrossRef]
- Mitra, A.; Abdel-Gawad, F.; Bassem, S.; Barua, P.; Assisi, L.; Parisi, C.; Temraz, T.; Vangone, R.; Kajbaf, K.; Kumar, V.; et al. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture. Water 2023, 15, 725. [Google Scholar] [CrossRef]
- Choleva, L.; Janko, K.; De Gelas, K.; Bohlen, J.; Šlechtová, V.; Rábová, M.; Ráb, P. Synthesis of clonality and polyploidy in vertebrate animals by hybridization between two sexual species. Evolution 2012, 66, 2191–2203. [Google Scholar] [CrossRef]
- Luo, K.; Xiao, J.; Liu, S.; Wang, J.; He, W.; Hu, J.; Qin, Q.; Zhang, C.; Tao, M.; Liu, Y. Massive Production of All-Female Diploids and Triploids in the Crucian Carp. Int. J. Biol. Sci. 2011, 7, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Y.; Gui, J.-F. Genetic Evidence for Gonochoristic Reproduction in Gynogenetic Silver Crucian Carp (Carassius auratus gibelio Bloch) as Revealed by RAPD Assays. J. Mol. Evol. 2000, 51, 498–506. [Google Scholar] [CrossRef]
- Arai, K.; Fujimoto, T. Genomic Constitution and Atypical Reproduction in Polyploid and Unisexual Lineages of the Misgurnus Loach, a Teleost Fish. Cytogenet. Genome Res. 2013, 140, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Arai, K. Meiosis and gametogenesis in hybrid, polyploid, and clonal fishes: Case studies in the dojo loach Misgurnus anguillicaudatus. Fish. Sci. 2023, 89, 537–570. [Google Scholar] [CrossRef]
- Janko, K.; Bartoš, O.; Kočí, J.; Roslein, J.; Janková Drdová, E.; Kotusz, J.; Eisner, J.; Mokrejš, M.; Štefková-Kašparová, E. Genome Fractionation and Loss of Heterozygosity in Hybrids and Polyploids: Mechanisms, Consequences for Selection, and Link to Gene Function. Mol. Biol. Evol. 2021, 38, 5255–5274. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, A.R.; Blanchard, J.L.; Wotherspoon, S.; Stuart-Smith, R.D.; Edgar, G.J.; Barrett, N.; Audzijonyte, A. Mean Reef Fish Body Size Decreases towards Warmer Waters. Ecol. Lett. 2024, 27, e14375. [Google Scholar] [CrossRef]
- Lindmark, M.; Audzijonyte, A.; Blanchard, J.L.; Gårdmark, A. Temperature Impacts on Fish Physiology and Resource Abundance Lead to Faster Growth but Smaller Fish Sizes and Yields under Warming. Glob. Chang. Biol. 2022, 28, 6239–6253. [Google Scholar] [CrossRef]
- Park, J.W.; Yoo, H.; Jung, H.; Park, H.J.; Bae, K.; Kang, C.K.; Lee, C. Effects of water temperature changes on the early life stages (egg and larvae) of walleye Pollock (Gadus chalcogrammus)—Laboratory experiments and field applications. J. Exp. Mar. Biol. Ecol. 2024, 571, 151980. [Google Scholar] [CrossRef]
- Miller, N.A.; Stillman, J.H. Physiological Optima and Critical Limits. Nat. Educ. Knowl. 2012, 3, 1. [Google Scholar]
- Qiang, J.; Zhong, C.; Bao, J.; Liang, M.; Liang, C.; Li, H.; He, J.; Xu, P. The effects of temperature and dissolved oxygen on the growth, survival and oxidative capacity of newly hatched hybrid yellow catfish larvae (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂). J. Therm. Biol. 2019, 86, 102436. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, M.; Kašpar, V.; Tichopád, T.; Rodina, M.; Flajšhans, M. How do suboptimal temperatures affect polyploid sterlet Acipenser ruthenus during early development? J. Fish Biol. 2022, 101, 77–91. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Ohlberger, J. Climate Warming and Ectotherm Body Size—From Individual Physiology to Community Ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Spies, I.; Canino, M.; Dorn, M.; Jimenez-Hidalgo, I.; Hauser, L. Growth Patterns of Larval Walleye Pollock Gadus chalcogrammus from Core and Peripheral Habitat Differ in Response to Temperature. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2022, 199, 105083. [Google Scholar] [CrossRef]
- Réalis-Doyelle, E.; Pasquet, A.; Fontaine, P.; Teletchea, F. Effects of Temperature on the Survival and Development of the Early Life Stages of Northern Pike (Esox lucius). Knowl. Manag. Aquat. Ecosyst. 2022, 423, 10. [Google Scholar] [CrossRef]
- Jablonska, O.; Duda, S.; Gajowniczek, S.; Nitkiewicz, A.; Fopp-Bayat, D. Toll-like Receptor Type 2 and 13 Gene Expression and Immune Cell Profiles in Diploid and Triploid Sterlets (Acipenser ruthenus): Insights into Immune Competence in Polyploid Fish. Int. J. Mol. Sci. 2025, 26, 3986. [Google Scholar] [CrossRef]
- De Gelas, K.; Janko, K.; Volckaert, F.A.M.; De Charleroy, D.; Van Houdt, J.K.J. Development of Nine Polymorphic Microsatellite Loci in the Spined Loach, Cobitis taenia, and Cross-species Amplification in the Related Species C. elongatoides, C. taurica and C. tanaitica. Mol. Ecol. Resour. 2008, 8, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for Centromeric Position on Chromosomes. Hereditas 2009, 52, 201–220. [Google Scholar] [CrossRef]
- Jezierska, B.; Lugowska, K.; Witeska, M.; Sarnowski, P. Malformations of newly hatched common carp larvae. Electron. J. Pol. Agric. Univ. 2000, 3, 1. [Google Scholar]
- Alix, M.; Zarski, D.; Chardard, D.; Fontaine, P.; Schaerlinger, B. Deformities in Newly Hatched Embryos of Eurasian Perch Populations Originating from Two Different Rearing Systems. J. Zool. 2017, 302, 126–137. [Google Scholar] [CrossRef]


| Family | Number of Eggs | Number of Hatched Larvae | Hatching Success (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 18 °C | 22 °C | 28 °C | 18 °C | 22 °C | 28 °C | 18 °C | 22 °C | 28 °C | |
| Triploid Cobitis females of EEN genome composition | |||||||||
| F3M3 | 259 | 351 | 350 | 87 | 100 | 99 | 33.6 | 28.5 | 28.3 |
| F35M35 | 243 | 224 | 186 | 96 | 4 | 0 | 39.5 | 1.8 | 0.0 |
| F37M36 | 139 | 145 | 139 | 10 | 10 | 0 | 7.2 | 6.9 | 0.0 |
| F39M37 | 187 | 175 | 237 | 7 | 2 | 0 | 3.7 | 1.1 | 0.0 |
| F47M47 | 343 | 304 | 234 | 0 | 0 | 9 | 0.0 | 0.0 | 3.9 |
| Mean | 16.8 | 7.7 | 6.4 | ||||||
| Triploid Cobitis females of EET genome composition | |||||||||
| F67M55 | 268 | 388 | 447 | 63 | 188 | 8 | 23.5 | 48.5 | 1.8 |
| F69M57 | 233 | 251 | 271 | 38 | 63 | 20 | 16.3 | 25.1 | 7.4 |
| F70M58 | 213 | 322 | 212 | 10 | 133 | 31 | 4.7 | 41.3 | 14.6 |
| F71M59 | 127 | 140 | 128 | 34 | 92 | 23 | 26.8 | 65.7 | 18.0 |
| F72M60 | 168 | 199 | 208 | 142 | 136 | 61 | 84.5 | 68.3 | 29.3 |
| Mean | 31.2 | 49.8 | 14.2 | ||||||
| Triploid Cobitis females of ETN genome composition | |||||||||
| F5M5 | 167 | 123 | 128 | 77 | 39 | 0 | 46.1 | 31.7 | 0.0 |
| F10M10 | 213 | 190 | 216 | 84 | 146 | 0 | 39.4 | 76.8 | 0.0 |
| F11M11 | 192 | 272 | 314 | 55 | 4 | 130 | 28.7 | 1.5 | 41.4 |
| F12M12 | 289 | 363 | 334 | 0 | 43 | 0 | 0.0 | 11.9 | 0.0 |
| F46M46 | 169 | 186 | 197 | 26 | 35 | 23 | 15.4 | 18.8 | 11.7 |
| F48M48 | 141 | 124 | 120 | 35 | 57 | 31 | 24.8 | 46.0 | 25.8 |
| F57M52 | 199 | 253 | 219 | 137 | 51 | 3 | 68.8 | 20.2 | 1.4 |
| F73M61 | 85 | 124 | 157 | 57 | 34 | 58 | 67.1 | 27.4 | 36.9 |
| F75M63 | 106 | 94 | 92 | 37 | 38 | 6 | 34.9 | 40.4 | 6.5 |
| F78M66 | 163 | 125 | 135 | 151 | 89 | 94 | 92.6 | 71.2 | 69.6 |
| Mean | 41.8 | 34.6 | 19.3 | ||||||
| Ploidy | Range | Mean | ±SE | ||
|---|---|---|---|---|---|
| EEN | 3n | 0.0–52 | 37.6 | 6.0 | |
| 4n | 20.0–55.6 | 41.6 | 4.5 | ||
| Female | EET | 3n | 8.3–75.0 | 52.6 | 4.3 |
| genome | 4n | 25.0–91.7 | 43.3 | 4.6 | |
| ETN | 3n | 4.8–73.1 | 41.4 | 4.4 | |
| 4n | 10.0–80.0 | 40.5 | 4.0 | ||
| 18 °C | 3n | 0.0–72.2 | 45.5 | 4.8 | |
| 4n | 16.7–80.0 | 39.2 | 4.4 | ||
| Temperature | 22 °C | 3n | 8.3–70.6 | 42.8 | 4.1 |
| 4n | 10.0–91.7 | 43.0 | 5.0 | ||
| 28 °C | 3n | 0.0–75.0 | 40.1 | 7.7 | |
| 4n | 17.1–100.0 | 48.3 | 6.7 |
| Temperature | Female Genome | Number of Larvae |
|---|---|---|
| 18 °C | EEN | 51 |
| EET | 45 | |
| ETN | 143 | |
| Total | 239 | |
| 22 °C | EEN | 38 |
| EET | 53 | |
| ETN | 124 | |
| Total | 215 | |
| 28 °C | EEN | 23 |
| EET | 62 | |
| ETN | 97 | |
| Total | 182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda, S.; Jablonska, O.; Boroń, A.; Kujawa, R.; Janko, K.; Juchno, D. The Influence of Genome Composition and Temperature on the Hatching Success and Development of the Offspring of Allotriploid Cobitis (Pisces: Cobitidae) Females. Int. J. Mol. Sci. 2025, 26, 10669. https://doi.org/10.3390/ijms262110669
Duda S, Jablonska O, Boroń A, Kujawa R, Janko K, Juchno D. The Influence of Genome Composition and Temperature on the Hatching Success and Development of the Offspring of Allotriploid Cobitis (Pisces: Cobitidae) Females. International Journal of Molecular Sciences. 2025; 26(21):10669. https://doi.org/10.3390/ijms262110669
Chicago/Turabian StyleDuda, Sara, Olga Jablonska, Alicja Boroń, Roman Kujawa, Karel Janko, and Dorota Juchno. 2025. "The Influence of Genome Composition and Temperature on the Hatching Success and Development of the Offspring of Allotriploid Cobitis (Pisces: Cobitidae) Females" International Journal of Molecular Sciences 26, no. 21: 10669. https://doi.org/10.3390/ijms262110669
APA StyleDuda, S., Jablonska, O., Boroń, A., Kujawa, R., Janko, K., & Juchno, D. (2025). The Influence of Genome Composition and Temperature on the Hatching Success and Development of the Offspring of Allotriploid Cobitis (Pisces: Cobitidae) Females. International Journal of Molecular Sciences, 26(21), 10669. https://doi.org/10.3390/ijms262110669







