6-Prenylnaringenin—Its Beneficial Biological Effects and Possible Applications
Abstract
1. Introduction
1.1. Occurrence
1.2. Pharmacokinetics of 6-PN (1)
2. Biological Activity
2.1. Antioxidant Activity
2.2. Phytoestrogen Activity
2.3. Anticancer Activity
2.4. Antimicrobial Activity
2.4.1. Antibacterial Activity
2.4.2. Antiviral Activity
2.4.3. Other Antimicrobial Activities
2.5. Impact on the Nervous System
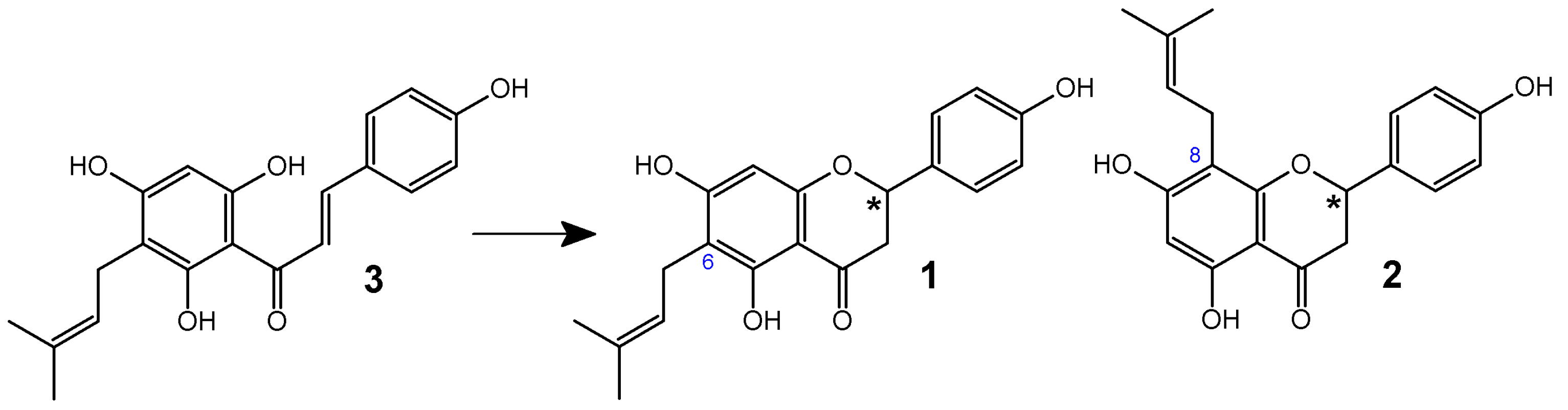
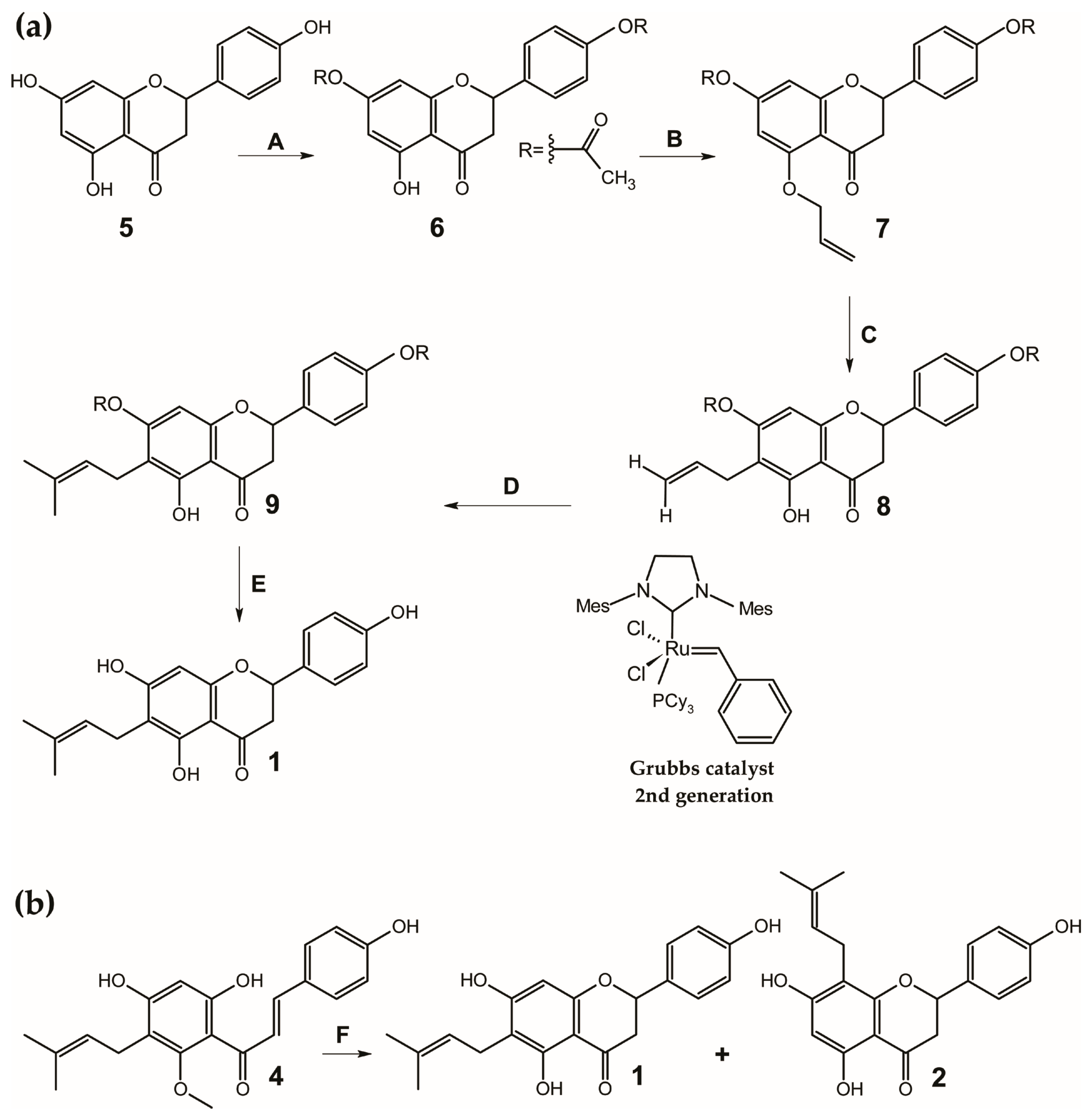
3. Synthesis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karel, Š. Cytotoxic potential of C-prenylated flavonoids. Phytochem. Rev. 2014, 13, 245–275. [Google Scholar]
- Chen, X.; Mukwaya, E.; Wong, M.-S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Dong, L.P.; Wang, S.C.; Wang, Q. The first total synthesis of sophoflavescenol, flavenochromane C, and citrusinol. Eur. J. Org. Chem. 2015, 2015, 2297–2302. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.-J. A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef]
- TGSC Information System. Available online: http://www.thegoodscentscompany.com/data/rw1859521.html (accessed on 6 August 2025).
- ChemFaces. Available online: https://www.chemfaces.com/natural/6-prenylnaringenin-CFN92017.html (accessed on 6 August 2025).
- Dhooghe, L.; Naessens, T.; Heyerick, A.; De Keukeleire, D.; Vlietinck, A.J.; Pieters, L.; Apers, S. Quantification of xanthohumol, isoxanthohumol, 8-prenylnaringenin, and 6-prenylnaringenin in hop extracts and derived capsules using secondary standards. Talanta 2010, 83, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Davies, N. Stereospecific quantitation of 6-prenylnaringenin in commercially available H. lupulus-containing natural health products and dietary supplements. Res. Pharm. Sci. 2015, 10, 182–191. [Google Scholar] [PubMed]
- Komatsu, M.; Yokoe, I.; Shirataki, Y. Studies on the constituents of Sophora species. XIII. Constituents of the aerial parts of Sophora tomentosa L. (2). Chem. Pharm. Bull. 1978, 26, 3863–3870. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. A new flavanone with antifungal activity isolated from hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar]
- McCormick, S.; Robson, K.; Bohm, B. Flavonoids from Wyethia glabra. Phytochemistry 1985, 24, 1614–1616. [Google Scholar] [CrossRef]
- McCormick, S.P.; Robson, K.A.; Maze, J.; Bohm, B.A. Flavonoids of wyethia section agnorhiza. Phytochemistry 1987, 26, 2421–2422. [Google Scholar] [CrossRef]
- Tahara, S.; Katagiri, Y.; Ingham, J.L.; Mizutani, J. Prenylated flavonoids in the roots of yellow lupin. Phytochemistry 1994, 36, 1261–1271. [Google Scholar] [CrossRef]
- Hayashi, H.; Yasuma, M.; Hiraoka, N.; Ikeshiro, Y.; Yamamoto, H.; Yeşilada, E.; Sezik, E.; Honda, G.; Tabata, M. Flavonoid variation in the leaves of Glycyrrhiza glabra. Phytochemistry 1996, 42, 701–704. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, H.-N.; Park, E.-H.; Shim, S.-H. Inhibition of human 20S proteasome by compounds from seeds of Psoralea corylifolia. Bull. Korean Chem. Soc. 2009, 30, 1867–1869. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Nikolic, D.; Burdette, J.E.; Overk, C.R.; Bolton, J.L.; van Breemen, R.B.; Fröhlich, R.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Estrogens and Congeners from Spent Hops (Humulus lupulus). J. Nat. Prod. 2004, 67, 2024–2032. [Google Scholar] [CrossRef]
- Shirataki, Y.; Motohashi, N.; Tani, S.; Sakagami, H.; Satoh, K.; Nakashima, H.; Mahapatra, S.K.; Ganguly, K.; Dastidar, S.G.; Chakrabarty, A.N. In vitro biological activity of prenylflavanones. Anticancer Res. 2001, 21, 275–280. [Google Scholar]
- Shirataki, Y.; Motohashi, N. Flavonoids in Sophora species. In Bioactive Heterocycles VII: Flavonoids and Anthocyanins in Plants, and Latest Bioactive Heterocycles II; Springer: Berlin/Heidelberg, Germany, 2009; pp. 41–91. [Google Scholar]
- McCormick, S.; Robson, K.; Bohm, B. Flavonoids of Wyethia angustifolia and W. helenioides. Phytochemistry 1986, 25, 1723–1726. [Google Scholar] [CrossRef]
- Limper, C.; Wang, Y.; Ruhl, S.; Wang, Z.; Lou, Y.; Totzke, F.; Kubbutat, M.H.; Chovolou, Y.; Proksch, P.; Wätjen, W. Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells. J. Pharm. Pharmacol. 2013, 65, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Moir, M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Nathan, M.; Scholten, R. The complete German Commission E monographs: Therapeutic guide to herbal medicines. Ann. Intern. Med. 1999, 130, 459. [Google Scholar] [CrossRef]
- Borrás, S.; Martínez-Solís, I.; Ríos, J.L. Medicinal plants for insomnia related to anxiety: An updated review. Planta Medica 2021, 87, 738–753. [Google Scholar] [CrossRef]
- Teuber, M.; Schmalreck, A.F. Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone and humulinic acid. Arch. Microbiol. 1973, 94, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Rozalski, M.; Micota, B.; Sadowska, B.; Stochmal, A.; Jedrejek, D.; Wieckowska-Szakiel, M.; Rozalska, B. Antiadherent and antibiofilm activity of Humulus lupulus L. derived products: New pharmacological properties. BioMed Res. Int. 2013, 2013, 101089. [Google Scholar] [CrossRef] [PubMed]
- Dresel, M.; Dunkel, A.; Hofmann, T. Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer. J. Agric. Food Chem. 2015, 63, 3402–3418. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754. [Google Scholar] [CrossRef]
- Olsovska, J.; Bostikova, V.; Dusek, M.; Jandovska, V.; Bogdanova, K.; Cermak, P.; Bostik, P.; Mikyska, A.; Kolar, M. Humulus lupulus L.(hops)–a valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett 2016, 85, 19–30. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef]
- Wigg, S.; Stafford, L.D. Health warnings on alcoholic beverages: Perceptions of the health risks and intentions towards alcohol consumption. PLoS ONE 2016, 11, e0153027. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Llamas, A.; De la Cruz-Sánchez, E. Moderate beer consumption is associated with good physical and mental health status and increased social support. Nutrients 2023, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Kirin Holdings. Available online: https://www.kirinholdings.com/en/newsroom/release/2024/1219_01.html (accessed on 6 August 2025).
- Buckett, L.; Schinko, S.; Urmann, C.; Riepl, H.; Rychlik, M. Stable isotope dilution analysis of the major prenylated flavonoids found in beer, hop tea, and hops. Front. Nutr. 2020, 7, 619921. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef]
- Bolca, S.; Possemiers, S.; Maervoet, V.; Huybrechts, I.; Heyerick, A.; Vervarcke, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; De Henauw, S. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: A dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br. J. Nutr. 2007, 98, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Wyns, C.; Possemiers, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; Verstraete, W.; Heyerick, A. Cosupplementation of isoflavones, prenylflavonoids, and lignans alters human exposure to phytoestrogen-derived 17β-estradiol equivalents. J. Nutr. 2009, 139, 2293–2300. [Google Scholar] [CrossRef]
- Schaefer, O.; Bohlmann, R.; Schleuning, W.-D.; Schulze-Forster, K.; Hümpel, M. Development of a radioimmunoassay for the quantitative determination of 8-prenylnaringenin in biological matrices. J. Agric. Food Chem. 2005, 53, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nikolić, D.; Chen, S.; Alvarenga, R.R.; Pauli, G.; van Breemen, R. Structure Determination of Isoxanthohumol and 8-Prenylnaringenin Glucuronides Formed by Human Liver Microsomes. Planta Medica 2013, 79, PR4. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S. The oral bioavailability of 8-prenylnaringenin from hops (Humulus Lupulus L.) in healthy women and men is significantly higher than that of its positional isomer 6-prenylnaringenin in a randomized crossover trial. Mol. Nutr. Food Res. 2018, 62, 1700838. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Nešpor, J.; Hanko, V.; Karabín, M.; Jelínek, L.; Dostálek, P. Prenylated flavonoids as valuable biologically active compounds from hops. Kvas. Prum. 2017, 63, 164–172. [Google Scholar] [CrossRef]
- Santos, C.M.; Silva, A.M. The antioxidant activity of prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef]
- Schmandke, H. Prenylflavonoids in hops and beer-biochemical and biological activities. Ernährungs-Umschau 2006, 53, 225–229, 210. [Google Scholar]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-W.; Wang, Q.-L.; Luo, M.; Zhu, M.-D.; Liang, H.-M.; Li, W.-J.; Cai, H.; Zhou, Z.-B.; Wang, H.; Tong, S.-Q. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch. Pharmacal Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłonski, J.; Huszcza, E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug Metab. 2013, 14, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Matsubara, C.; Hanada, A.; Omoe, Y.; Ogata, T.; Isegawa, Y. Effect of Structural Differences in Naringenin, Prenylated Naringenin, and Their Derivatives on the Anti-Influenza Virus Activity and Cellular Uptake of Their Flavanones. Pharmaceuticals 2022, 15, 1480. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.-L.; Catteau, J.-P.; Pommery, J.; Wallet, J.-C.; Gaydou, E.M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Ambrož, M.; Lněničková, K.; Matoušková, P. Antiproliferative effects of hop-derived prenylflavonoids and their influence on the efficacy of oxaliplatine, 5-fluorouracil and irinotecan in human colorectalC cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef]
- Galluzzo, P.; Marino, M. Nutritional flavonoids impact on nuclear and extranuclear estrogen receptor activities. Genes Nutr. 2006, 1, 161–176. [Google Scholar] [CrossRef]
- Kiyama, R. Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef] [PubMed]
- Nastainczyk, W. Untersuchung über die Östrogene Wirkung des Hopfens und des Bieres. Ph.D. Thesis, Universitat des Saarlandes, Saarbrucken, Germany, 1972. [Google Scholar]
- Hänsel, R.; Schulz, J. Desmethylxanthohumol: Isolierung aus hopfen und cyclisierung zu flavanonen. Arch. Pharm. 1988, 321, 37–40. [Google Scholar] [CrossRef]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.; Kalita, J.; Pocock, V.; Van De Kauter, V.; Stevens, J.; Deinzer, M.; Rong, H.; De Keukeleire, D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. [Google Scholar] [CrossRef]
- Milligan, S.; Kalita, J.; Pocock, V.; Heyerick, A.; De Cooman, L.; Rong, H.; De Keukeleire, D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reprod. Camb. 2002, 123, 235–242. [Google Scholar] [CrossRef]
- Rong, H.; Boterberg, T.; Maubach, J.; Stove, C.; Depypere, H.; Van Slambrouck, S.; Serreyn, R.; De Keukeleire, D.; Mareel, M.; Bracke, M. 8-Prenylnaringenin, the phytoestrogen in hops and beer, upregulates the function of the E-cadherin/catenin complex in human mammary carcinoma cells. Eur. J. Cell Biol. 2001, 80, 580–585. [Google Scholar] [CrossRef]
- Coldham, N.G.; Sauer, M.J. Identification, quantitation and biological activity of phytoestrogens in a dietary supplement for breast enhancement. Food Chem. Toxicol. 2001, 39, 1211–1224. [Google Scholar] [CrossRef]
- Zierau, O.; Gester, S.; Schwab, P.; Metz, P.; Kolba, S.; Wulf, M.; Vollmer, G. Estrogenic activity of the phytoestrogens naringenin, 6-(1, 1-dimethylallyl) naringenin and 8-prenylnaringenin. Planta Medica 2002, 68, 449–451. [Google Scholar] [CrossRef]
- Berek, J.S.; Berek, D.L.; Hengst, T.C. Berek & Novak’s Gynecology, 15th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Davis, S.R.; Dinatale, I.; Rivera-Woll, L.; Davison, S. Postmenopausal hormone therapy: From monkey glands to transdermal patches. J. Endocrinol. 2005, 185, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.S.; Nakajima, S.T. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet. Gynecol. Clin. 2015, 42, 163–179. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Kawa, K.; Eckl, V.; Morton, C.; Stredney, R. Herbal supplement sales in US increased 8.5% in 2017, topping $8 billion. HerbalGram 2018, 119, 62–71. [Google Scholar]
- Holmberg, L.; Anderson, H. HABITS (hormonal replacement therapy after breast cancer—Is it safe?), a randomised comparison: Trial stopped. Lancet 2004, 363, 453–455. [Google Scholar] [CrossRef]
- D’Alonzo, M.; Bounous, V.E.; Villa, M.; Biglia, N. Current evidence of the oncological benefit-risk profile of hormone replacement therapy. Medicina 2019, 55, 573. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.E.; Johnsen, S.A.; Monroe, D.G.; Spelsberg, T.C.; Westendorf, J.J. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005, 96, 387–399. [Google Scholar] [CrossRef]
- Global cancer burden growing, amidst mounting need for services. Saudi Med. J. 2024, 45, 326–327.
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s Pharmacology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Le Marchand, L. Cancer preventive effects of flavonoids-a review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.P.; Daglia, M.; Atanasov, A.G. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Molčanová, L.; Janošíková, D.; Dall’ Acqua, S.; Šmejkal, K. C-prenylated flavonoids with potential cytotoxic activity against solid tumor cell lines. Phytochem. Rev. 2019, 18, 1051–1100. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated flavonoids with selective toxicity against human cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef]
- Tan, K.W.; Cooney, J.; Jensen, D.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Hop-derived prenylflavonoids are substrates and inhibitors of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Mol. Nutr. Food Res. 2014, 58, 2099–2110. [Google Scholar] [CrossRef]
- Delmulle, L.; Bellahcène, A.; Dhooge, W.; Comhaire, F.; Roelens, F.; Huvaere, K.; Heyerick, A.; Castronovo, V.; De Keukeleire, D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine 2006, 13, 732–734. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly cancer selective antiproliferative activity of natural prenylated flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Busch, C.; Noor, S.; Leischner, C.; Burkard, M.; Lauer, U.M.; Venturelli, S. Anti-proliferative activity of hop-derived prenylflavonoids against human cancer cell lines. Wien. Med. Wochenschr. (1946) 2015, 165, 258–261. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Mahapatra, S.K.; Ganguly, K.; Chakrabarty, A.N.; Shirataki, Y.; Motohashi, N. Antimicrobial activity of prenylflavanones. In Vivo 2001, 15, 519–523. [Google Scholar]
- Osorio, M.; Carvajal, M.; Vergara, A.; Butassi, E.; Zacchino, S.; Mascayano, C.; Montoya, M.; Mejías, S.; Martín, M.C.-S.; Vásquez-Martínez, Y. Prenylated flavonoids with potential antimicrobial activity: Synthesis, biological activity, and in silico study. Int. J. Mol. Sci. 2021, 22, 5472. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Niessner, H.; Sinnberg, T.; Berger, A.; Burkard, M.; Urmann, C.; Donaubauer, K.; Böcker, A.; Leischner, C.; Riepl, H. 6-and 8-Prenylnaringenin, novel natural histone deacetylase inhibitors found in hops, exert antitumor activity on melanoma cells. Cell. Physiol. Biochem. 2018, 51, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Delmulle, L.; Vanden Berghe, T.; De Keukeleire, D.; Vandenabeele, P. Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase-independent form of cell death. Phytother. Res. 2008, 22, 197–203. [Google Scholar] [CrossRef]
- Miranda, C.L.; Aponso, G.L.M.; Stevens, J.F.; Deinzer, M.L.; Buhler, D.R. Prenylated chalcones and flavanones as inducers of quinone reductase in mouse Hepa 1c1c7 cells. Cancer Lett. 2000, 149, 21–29. [Google Scholar] [CrossRef]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.-N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. Toxicol. 2016, 29, 1142–1150. [Google Scholar] [CrossRef]
- Stubert, J.; Gerber, B. Isoflavones–mechanism of action and impact on breast cancer risk. Breast Care 2009, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Diel, P.; Thomae, R.B.; Caldarelli, A.; Zierau, O.; Kolba, S.; Schmidt, S.; Schwab, P.; Metz, P.; Vollmer, G. Regulation of gene expression by 8-prenylnaringenin in uterus and liver of Wistar rats. Planta Medica 2004, 70, 39–44. [Google Scholar] [CrossRef]
- Zierau, O.; Kretzschmar, G.; Möller, F.; Weigt, C.; Vollmer, G. Time dependency of uterine effects of naringenin type phytoestrogens in vivo. Mol. Cell. Endocrinol. 2008, 294, 92–99. [Google Scholar] [CrossRef]
- Piersen, C.E. Phytoestrogens in botanical dietary supplements: Implications for cancer. Integr. Cancer Ther. 2003, 2, 120–138. [Google Scholar] [CrossRef]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. In Silico Pharmacol. 2015, 3, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, M.V.; Gastiazoro, M.P.; Kretzschmar, G.; Wober, J.; Vollmer, G.; Varayoud, J.; Durando, M.; Zierau, O. AHR agonistic effects of 6-PN contribute to potential beneficial effects of Hops extract. Mol. Cell. Endocrinol. 2022, 543, 111540. [Google Scholar] [CrossRef] [PubMed]
- Hitzman, R.T.; Dunlap, T.L.; Howell, C.E.; Chen, S.-N.; Vollmer, G.n.; Pauli, G.F.; Bolton, J.L.; Dietz, B.M. 6-Prenylnaringenin from hops disrupts ERα-mediated downregulation of CYP1A1 to facilitate estrogen detoxification. Chem. Res. Toxicol. 2020, 33, 2793–2803. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial properties of spent hops extracts, flavonoids isolated therefrom, and their derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Tani, N.; Nagayama, M.; Yamaguchi, R. Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2006, 43, 243–248. [Google Scholar] [CrossRef]
- das Chagas Almeida, A.; Azevedo Rodrigues, L.; dos Santos Paulino, G.; Pereira Aguilar, A.; Andrade Almeida, A.; Olavo Ferreira, S.; Brandão, G.C.; Viana Leite, J.P.; de Oliveira Barros Ribon, A. Prenylated flavonoid-enriched fraction from Maclura tinctoria shows biological activity against Staphylococcus aureus and protects Galleria mellonella larvae from bacterial infection. BMC Complement. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Zuo, G.-Y.; Yang, C.-X.; Han, J.; Li, Y.-Q.; Wang, G.-C. Synergism of prenylflavonoids from Morus alba root bark against clinical MRSA isolates. Phytomedicine 2018, 39, 93–99. [Google Scholar] [CrossRef]
- Sohn, H.-Y.; Son, K.; Kwon, C.-S.; Kwon, G.-S.; Kang, S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017–2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- One Health Trust. Available online: https://onehealthtrust.org/wp-content/uploads/2015/09/the-state-of-the-worlds-antibiotics-_2015.pdf (accessed on 7 August 2025).
- Nicodème, J.; Paulin, E.N.; Zingg, M.; Uçkay, I.; Malacarne, S.; Suva, D. Pied diabétique infecté. Rev. Médicale Suisse 2015, 11, 1238–1241. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T. Phenolic compounds from Humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef]
- Omar, R.M.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Ebel, R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma brucei. Phytochem. Anal. PCA 2016, 27, 107–115. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Han, Q.; Chen, J.; Lv, Y. Xanthohumol enhances antiviral effect of interferon α-2b against bovine viral diarrhea virus, a surrogate of hepatitis C virus. Phytomedicine 2010, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Buckwold, V.E.; Wilson, R.J.H.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.A.; Buckheit, R.W.; Wei, J.; Wenzel-Mathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Z.-H.; Liu, J.-K.; Zheng, Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Bouback, T.A.; Aljohani, A.M.; Albeshri, A.; Al-Talhi, H.; Moatasim, Y.; GabAllah, M.; Badierah, R.; Albiheyri, R.; Al-Sarraj, F.; Ali, M.A. Antiviral activity of Humulus lupulus (HOP) aqueous extract against MERS-CoV and SARS-CoV-2: In-vitro and in-silico study. Biotechnol. Biotechnol. Equip. 2023, 37, 167–179. [Google Scholar] [CrossRef]
- Herzog, A.-M.; Göbel, K.; Marongiu, L.; Ruetalo, N.; Alonso, M.C.; Leischner, C.; Busch, C.; Burkard, M.; Lauer, U.M.; Geurink, P.P. Compounds derived from Humulus lupulus inhibit SARS-CoV-2 papain-like protease and virus replication. Phytomedicine 2024, 123, 155176. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human african trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Urmann, C.; Oberbauer, E.; Couillard-Després, S.; Aigner, L.; Riepl, H. Neurodifferentiating potential of 8-prenylnaringenin and related compounds in neural precursor cells and correlation with estrogen-like activity. Planta Medica 2015, 81, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Goetz, T.; Arslan, A.; Wisden, W.; Wulff, P. GABAA receptors: Structure and function in the basal ganglia. Prog. Brain Res. 2007, 160, 21–41. [Google Scholar]
- Hänsel, R.; Wohlfart, R.; Coper, H. Versuche, sedativ-hypnotische Wirkstoffe im Hopfen nachzuweisen, II/Narcotic Action of 2-Methyl-3-butene-2-ol Contained in the Exhalation of Hops. Z. Für Naturforschung C 1980, 35, 1096–1097. [Google Scholar] [CrossRef]
- Bagatin, M.C.; Tozatti, C.S.S.; Abiko, L.A.; dos Santos Yamazaki, D.A.; Silva, P.R.A.; Perego, L.M.; Audi, E.A.; Seixas, F.A.V.; Basso, E.A.; de Freitas Gauze, G. Molecular docking and panicolytic effect of 8-Prenylnaringenin in the elevated T-Maze. Chem. Pharm. Bull. 2014, 62, 1231–1237. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Eerola, K.; Soini, S.L.; Uusi-Oukari, M. Humulone modulation of GABAA receptors and its role in hops sleep-promoting activity. Front. Neurosci. 2020, 14, 594708. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Logrén, N.; Somborac, T.; Kortesniemi, M.; Soini, S.L.; Yang, B.; Salo-Ahen, O.M.; Laaksonen, O.; Uusi-Oukari, M. Hops compounds modulatory effects and 6-prenylnaringenin dual mode of action on GABAA receptors. Eur. J. Pharmacol. 2020, 873, 172962. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Soini, S.L.; Stompor, M.; Uusi-Oukari, M. Positive allosteric modulation of native and recombinant GABAA receptors by hops prenylflavonoids. Eur. J. Pharmacol. 2019, 852, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Fujita, T.; Deguchi, T.; Yamaoka, S.; Tomochika, K.; Tsubota, M.; Ono, S.; Horaguchi, Y.; Ichii, M.; Ichikawa, M. Blockade of T-type calcium channels by 6-prenylnaringenin, a hop component, alleviates neuropathic and visceral pain in mice. Neuropharmacology 2018, 138, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Du Nguyen, H.; Okada, T.; Kitamura, S.; Yamaoka, S.; Horaguchi, Y.; Kasanami, Y.; Sekiguchi, F.; Tsubota, M.; Yoshida, S.; Nishikawa, H. Design and synthesis of novel anti-hyperalgesic agents based on 6-prenylnaringenin as the T-type calcium channel blockers. Bioorganic Med. Chem. 2018, 26, 4410–4427. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Okada, T.; Sekiguchi, F.; Tsubota, M.; Nishikawa, H.; Kawabata, A.; Toyooka, N. Prenylflavanones as novel T-type calcium channel blockers useful for pain therapy. Nat. Prod. Commun. 2019, 14, 1934578X19873441. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, G.D. Prenylated Flavonoids: Pharmacology and Biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, R.; Sarpal, P. Synthesis of (±) lupinifoun, di-O-methyl xanthohumol and isoxanthohumol and related compounds. Tetrahedron 1978, 34, 3563–3567. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, R.C.; Sarpal, P.D. SYNTHESIS OF RACEMIC 8-C-PRENYL-6″,6″-DIMETHYLPYRANO(2″, 3″:7,6)NARINGENIN. Chem. Lett. 1978, 7, 995–998. [Google Scholar] [CrossRef]
- Nagar, A.; Gujral, V.K.; Gupta, S.R. Synthesis of lupinifolin. Tetrahedron Lett. 1978, 19, 2031–2034. [Google Scholar] [CrossRef]
- Bu, X.; Zhao, L.; Li, Y. A facile synthesis of 6-C-prenylflavanones. Synthesis 1997, 1997, 1246–1248. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D. Asymmetric O- and C-Alkylation of Phenols. J. Am. Chem. Soc. 1998, 120, 815–816. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Sanders, D.P.; Grubbs, R.H. Synthesis of symmetrical trisubstituted olefins by cross metathesis. Org. Lett. 2002, 4, 1939–1942. [Google Scholar] [CrossRef]
- Tischer, S.; Metz, P. Selective C-6 Prenylation of Flavonoids via Europium(III)-Catalyzed Claisen Rearrangement and Cross-Metathesis. Adv. Synth. Catal. 2007, 349, 147–151. [Google Scholar] [CrossRef]
- Urmann, C.; Riepl, H. Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments. Molecules 2020, 25, 4007. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Liu, Y.; Wu, Y.; Zhao, L.; Pei, J. Biochemical characterization of a novel prenyltransferase from Streptomyces sp. NT11 and development of a recombinant strain for the production of 6-prenylnaringenin. J. Agric. Food Chem. 2021, 69, 14231–14240. [Google Scholar] [CrossRef] [PubMed]

| Source | Content in Dry Mass [%] |
|---|---|
| Sophora sp. | detected [9,17,18] |
| Wyethia sp. | 0.051 [19] |
| Lupinus luteus | detected [13] |
| Glycyrrhiza glabra | 0.48 [14] |
| Psoralea corylifolia | 0.068 [20] |
| Humulus lupulus | 0.004 [16] |
| Hop extract | 0.170 [7] |
| Type of Beverages | 6-PN (1) (C.V., n = 2) [μg/L] | 6-PN (1) [μg/L] (±SD) | Total Prenylflavonoids [μg/L] |
|---|---|---|---|
| Lager/pilsner | 31–38 (1.0–4.0) | 14.2 (0.58) | 460–750 [32]/693 [38] |
| American Porter | 560 (2.2) | 2900 [32] | |
| Dunkel (dark beer) | 355 (3.1) | 2375 [38] | |
| American Hefeweizen | 11 (2.1) | 330 [32] | |
| Strong ale | 200 (0.3) | 4000 [32] | |
| India Pale Ale (IPA) | 146 (2.4) | 368 (15.9) | 2446 [38] |
| Double IPA | 421 (9.7) | 2331 [38] | |
| Imported stout | 170 (0.7) | 2680 [32] | |
| Coffe stout | 351 (11.2) | 2445 [38] | |
| Helles (pale lager) | 46.7 (0.4) | 1260 [38] | |
| Imported lager | 1 (28) | 14.2 (0.1) | 40 [32]/693 [38] |
| Imported pilsner | 22–55 (2.0–7.7) | 22.8 (0.2) | 680 [32]/1051 [38] |
| Bock | 156 (3.0) | 1879 [38] | |
| Doppelbock beer A | 203 (2.4) | 1346 [38] | |
| Doppelbock beer B | 63.6 (0.6) | 1614 [38] | |
| Wheat bock beer | 91.2 (3.2) | 771 [38] | |
| Festbeer (festival beer) | 51.8 (0.90) | 1234 [38] | |
| Hopped beer A | 278 (5.6) | 2042 [38] | |
| Hopped beer B | 120 (6.7) | 1993 [38] | |
| IPA alcohol free | 104 (3.2) | 1332 [38] | |
| Non-alcohol beer | 7 (8.2) | 120 [32] | |
| Herb tea | 4 (5.4) | 14.2 (0.58) | 20 [32] |
| Product | Recommended Dose 1 | Country of Manufacture |
|---|---|---|
| Nature’s Own™ MenoSleep | 2 tablets | Australia |
| AOR™ Advanced Series Estro Detox™ | 2 capsules | Canada |
| Life Extension® Natural Female Support | 1 capsule | United States |
| BioCeuticals® MenoPlus 8-PN™ | 1 tablet | Australia |
| Garden of Life® Oceans 3™ Healthy Hormones® | 3 softgels | United States |
| Biological Activity | Cell Line/Enzyme | Concentration: IC50 A (μM) or CC50 B (μg/mL) | Reference |
|---|---|---|---|
| Antiproliferative Activity | PC-3 | 18.4 A ± 1.2 | [86] |
| DU 145 | 29.1 A ± 1.1 | ||
| T-47D | 16.01 A ± 3.74 | [87] | |
| MCF7 | 43.25 A ± 4.37 | ||
| MDA-MB-231 | 62.64 A ± 19.54 | ||
| A2780 | 44.16 A ± 14.71 | ||
| A2780cis | 81.73 A ± 17.68 | ||
| HT-29 | 64.61 A ± 17.07 | ||
| UO.31, PC-3 | - | [88] | |
| Inhibition of EGFR, MEK, ERK kinase | H4IIE | 33 ± 5 μM | [20] |
| Hct116 | 34 ± 4 μM | ||
| C6 | >50 μM | ||
| Cytotoxic activity | HSC-2 | 22 B ± 0.065 | [89] |
| HSG | 32 B ± 0.094 | ||
| HGF | 35 B ± 0.103 | ||
| MDA-MB-231 | 53.94 B ± 9.66 | [90] | |
| B16-F10 | 49.14 B ± 3.38 | ||
| MEF | 48.45 B ± 3.44 | ||
| Inhibition of cellular histone deacetylases (HDACs) | SK-MEL-28 | 100 µM | [91] |
| BLM | 100 µM | ||
| Induced caspase-independent form of cell death | DU 145 | >200 µM | [92] |
| PC-3 | <200 µM | ||
| Induction of quinone reductase (QR) activity | Hepa-1c1c7 | - | [93] |
| Inhibition of metabolic activation of procarcinogens | P450 CYP1A | 0.09 A | [94] |
| P450 CYP1A1 | 0.63 ± 0.08 | ||
| P450 CYP1B1 | 0.21 ± 0.02 |
| Biological Activity | Organism/Virus | Concentration: MIC50 A, CC50 B, EC50 C, IC50 D [μg/mL] | Reference |
|---|---|---|---|
| Anti-G-positive bacteria activity | Bacillus subtilis VB1 | >100 A | [89] |
| Staphylococcus aureus 8531 | 50 A | ||
| Staphylococcus aureus 8530 | 50 A | ||
| Staphylococcus typhimurium 4 | >10 A | ||
| Staphylococcus typhimurium 57 | >100 A | ||
| Staphylococcus typhimurium 59 | >100 A | ||
| Staphylococcus aureus 6571 | 25 A | ||
| Staphylococcus aureus MRSA 97-7 | 5 A | [90] | |
| Staphylococcus aureus MRSA 622-4 | 25 A | ||
| Staphylococcus aureus ATCC 6538 | 10 A | ||
| Anti-G-negative bacteria activity | Klebsiella spp. 14 | >100 A | [89] |
| Providencia spp. 1 | >100 A | ||
| Shigella dysenteriae 1 | 50 A | ||
| Shigella sonnei 2 | >100 A | ||
| Vibrio cholerae 865 | 25 A | ||
| Escherichia coli R832 | 25 A | ||
| Escherichia coli ROW | >100 A | ||
| Helicobacter pylori | >100 A | ||
| Escherichia coli | >200 A | [10] | |
| Antifungal activity | Aspergillus flavus ATCC9170 | >250 A | [90] |
| Aspergillus fumigatus ATCC26934 | >250 A | ||
| Aspergillus niger ATCC9029 | >250 A | ||
| Candida albicans ATCC10231 | >250 A | ||
| Candida albicans | >200 A | [10] | |
| Cryptococcus neoformans ATCC32264 | 125 A | [90] | |
| Cryptococcus neoformans clinical isolates | 250 A | ||
| Microsporum gypseum CCC115 | 62.5 A | ||
| Trichophyton mentagrophytes ATCC9972 | 62.5 A | ||
| Trichophyton rubrum CCC113 | 62.5 A | ||
| Trichophyton mentagrophytes | 3.13 A | [10] | |
| Trichophyton rubrum | 3.13 A | ||
| Fusarium oxysporum | >200 A | ||
| Mucor rouxianous | 50 A | ||
| Antiviral activity | Anti-HIV activity | 125 B | [89] |
| >200 C | |||
| <1 (SI (CC50/EC50)) | |||
| Anti-Influenza | 38 ± 4.7 D | [53] | |
| SARS-CoV-2 | 7.3 D | [90] | |
| Antiparasitic activity | Trypanosoma brucei | 11.4 ± 0.34 A | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tronina, T.; Łój, D.; Popłoński, J.; Bartmańska, A. 6-Prenylnaringenin—Its Beneficial Biological Effects and Possible Applications. Int. J. Mol. Sci. 2025, 26, 10662. https://doi.org/10.3390/ijms262110662
Tronina T, Łój D, Popłoński J, Bartmańska A. 6-Prenylnaringenin—Its Beneficial Biological Effects and Possible Applications. International Journal of Molecular Sciences. 2025; 26(21):10662. https://doi.org/10.3390/ijms262110662
Chicago/Turabian StyleTronina, Tomasz, Daniel Łój, Jarosław Popłoński, and Agnieszka Bartmańska. 2025. "6-Prenylnaringenin—Its Beneficial Biological Effects and Possible Applications" International Journal of Molecular Sciences 26, no. 21: 10662. https://doi.org/10.3390/ijms262110662
APA StyleTronina, T., Łój, D., Popłoński, J., & Bartmańska, A. (2025). 6-Prenylnaringenin—Its Beneficial Biological Effects and Possible Applications. International Journal of Molecular Sciences, 26(21), 10662. https://doi.org/10.3390/ijms262110662





