IgG N-Glycan Profiles in Mothers and Infants Postpartum in the Context of Maternal Obesity and Gestational Diabetes
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Serum IgG N-Glycosylation Analysis of Mother-Child Pairs
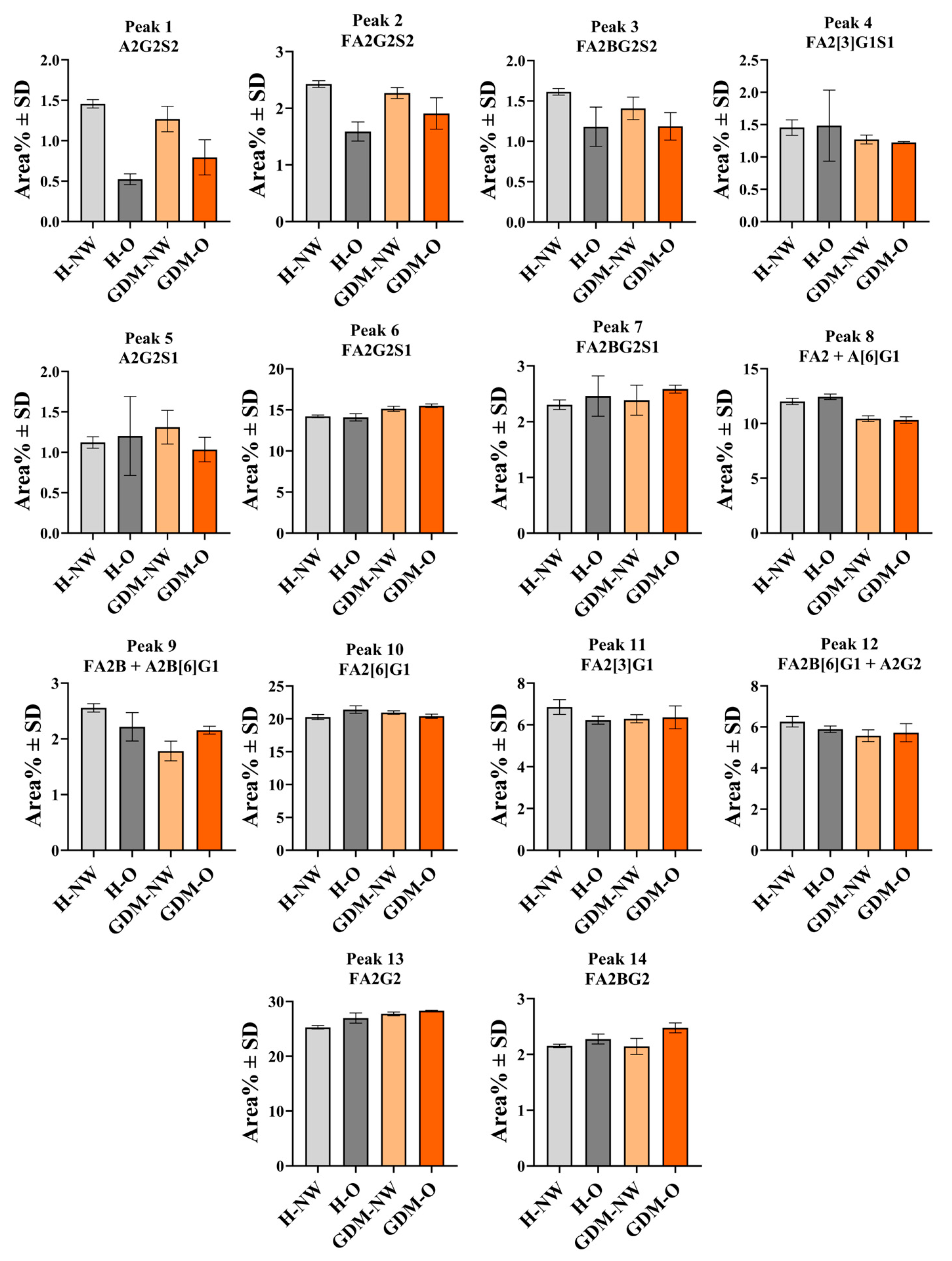
2.3. Serum IgG N-Glycan Profile in Relation to Maternal BMI and Gestational Diabetes
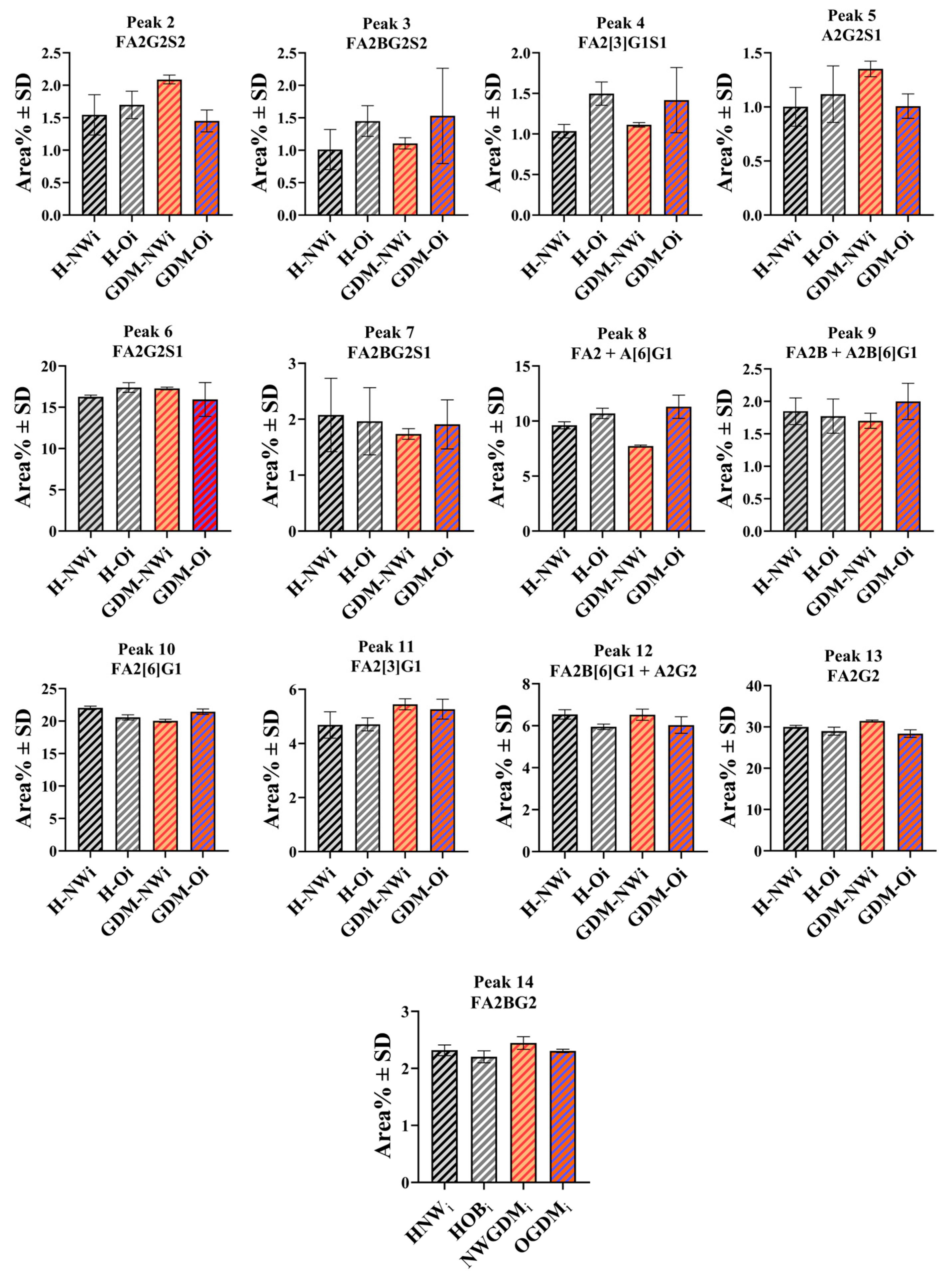
2.4. IgG N-Glycosylation of Infants Born to Normal Weight and Obese Mothers with or Without GDM
2.5. Sialo-Form to Neutral Form Ratios (SF/NF) in the IgG N-Glycosylation Patterns
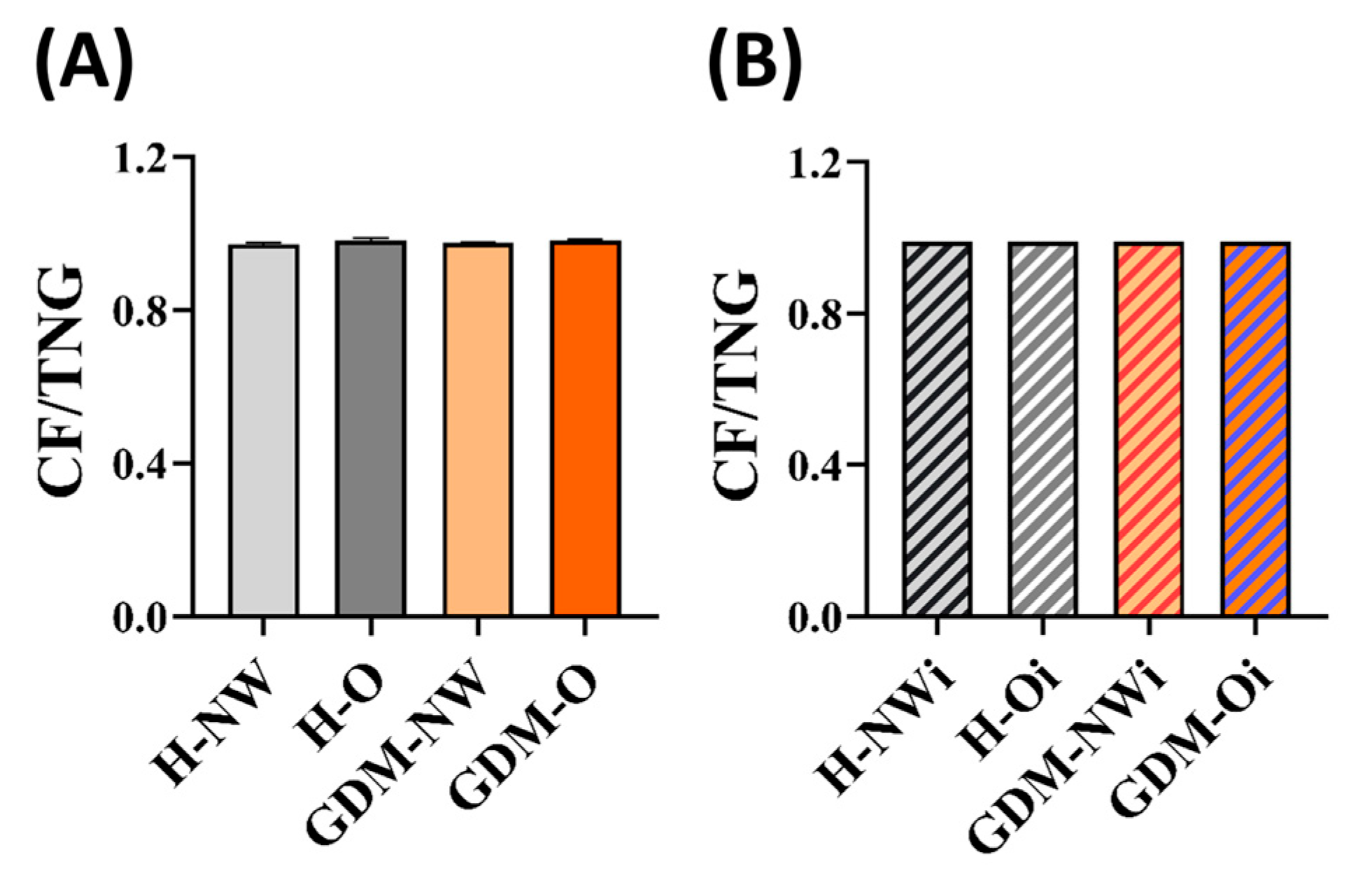
2.6. Ratio of Core Fucosylation to the Total N-Glycosylation (CF/TNG) Calculated for Maternal and Fetal Serum IgG
3. Discussion
3.1. Maternal IgG N-Glycosylation
3.2. Infant IgG N-Glycosylation
3.3. Serum IgG N-Glycosylation Properties
3.3.1. Maternal IgG
3.3.2. Maternal and Infant IgG N-Glycosylation Properties
3.4. Study Limitations
4. Materials and Methods
4.1. Samples
- (1)
- Healthy normal weight (H-NW): women with normal BMI (18.5–24.9 kg/m2) and uncomplicated pregnancy, without GDM or other complications; infants of H-NW mothers (H-NWi)
- (2)
- Healthy obese (H-O): women with obesity (BMI ≥ 30 kg/m2) and uncomplicated pregnancy, without GDM; infants of H-O mothers (H-Oi):
- (3)
- GDM with normal weight (GDM-NW): women with normal BMI and gestational diabetes mellitus; infants of GDM-NW (GDM-NWi)
- (4)
- GDM with obesity (GDM-O): women with obesity and gestational diabetes mellitus; infants of GDM-O (GDM-Oi)
4.2. Chemicals
4.3. IgG Partitioning
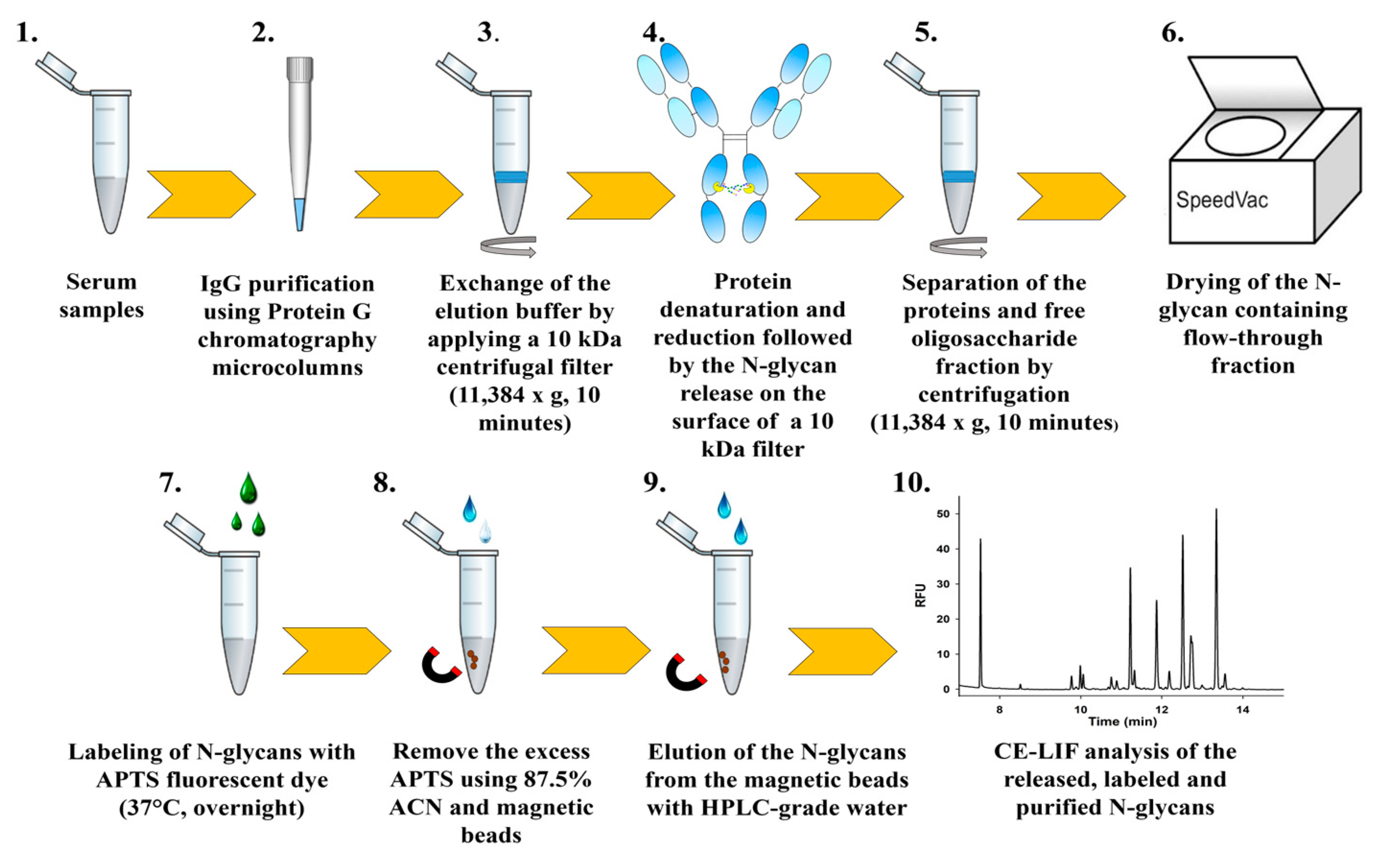
4.4. N-Glycan Release, Fluorophore Labeling and Preparation for CE-LIF Analysis
4.5. Analysis of the Labeled N-Glycans Using Capillary Electrophoresis with Laser-Induced Fluorescence Detection (CE-LIF)
4.6. Data Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | acetonitrile |
| ADCC | antibody-dependent cellular cytotoxicity |
| APTS | 8-aminopyrene-1,3,6-trisulfonic acid |
| BMI | body mass index |
| CE-LIF | Capillary electrophoresis with laser-induced fluorescent detection |
| CF/TNG ratio | ratio of core-fucosylated structures to the total N-glycosylation |
| DC-SIGN | dendritic cell-specific ICAM-3 grabbing non-integrin |
| DTT | dithiothreitol |
| Fc | fragment crystallizable |
| GDM | gestational diabetes mellitus |
| GDM-O | GDM with obesity |
| H-NW | Healthy Normal Weight |
| H-NWi | infant of healthy normal weight mothers |
| H-O | Healthy Obese |
| H-Oi | infant of healthy obese mothers |
| IgG | immunoglobulin G |
| NCHO | N-linked carbohydrate separation gel buffer |
| GDM-NW | GDM with normal weight |
| GDM-NWi | Infant of GDM with normal weight mothers |
| GDM-Oi | Infant of GDM with obesity mothers |
| OGTT | Oral glucose tolerance test |
| PNGase F | Peptide N-Glycosidase F |
| RFU | relative fluorescence unit |
| SDS | sodium dodecyl sulfate |
| SF/NF ratio | ratio of the sialo form to neutral structures |
| WHO | World Health Organization |
References
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S183–S192. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period; National Institute for Health and Care Excellence: London, UK, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555331/ (accessed on 25 September 2025).
- Schafer-Graf, U.M.; Gembruch, U.; Kainer, F.; Groten, T.; Hummel, S.; Hosli, I.; Grieshop, M.; Kaltheuner, M.; Buhrer, C.; Kautzky-Willer, A.; et al. Gestational Diabetes Mellitus (GDM)—Diagnosis, Treatment and Follow-Up. Guideline of the DDG and DGGG (S3 Level, AWMF Registry Number 057/008, February 2018). Geburtshilfe Frauenheilkd. 2018, 78, 1219–1231. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- de Wit, L.; Bos, D.M.; van Rossum, A.P.; van Rijn, B.B.; Boers, K.E. Repeated oral glucose tolerance tests in women at risk for gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 242, 79–85. [Google Scholar] [CrossRef]
- Maor-Sagie, E.; Hallak, M.; Toledano, Y.; Gabbay-Benziv, R. Oral Glucose Tolerance Test Performed after 28 Gestational Weeks and Risk for Future Diabetes-A 5-Year Cohort Study. J. Clin. Med. 2023, 12, 6072. [Google Scholar] [CrossRef]
- Miailhe, G.; Kayem, G.; Girard, G.; Legardeur, H.; Mandelbrot, L. Selective rather than universal screening for gestational diabetes mellitus? Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 95–100. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 September 2025).
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Gaillard, R.; Felix, J.F.; Duijts, L.; Jaddoe, V.W. Childhood consequences of maternal obesity and excessive weight gain during pregnancy. Acta Obstet. Gynecol. Scand. 2014, 93, 1085–1089. [Google Scholar] [CrossRef]
- Neri, C.; Edlow, A.G. Effects of Maternal Obesity on Fetal Programming: Molecular Approaches. Cold Spring Harb. Perspect. Med. 2015, 6, a026591. [Google Scholar] [CrossRef]
- Aebersold, R.; Agar, J.N.; Amster, I.J.; Baker, M.S.; Bertozzi, C.R.; Boja, E.S.; Costello, C.E.; Cravatt, B.F.; Fenselau, C.; Garcia, B.A.; et al. How many human proteoforms are there? Nat. Chem. Biol. 2018, 14, 206–214. [Google Scholar] [CrossRef]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell. Mol. Immunol. 2023, 20, 1101–1113. [Google Scholar] [CrossRef]
- Colley, K.J.; Varki, A.; Haltiwanger, R.S.; Kinoshita, T. Cellular Organization of Glycosylation. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579926/ (accessed on 25 September 2025).
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target Ther. 2024, 9, 194. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kizuka, Y. Glycans and cancer: Role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11–51. [Google Scholar] [CrossRef]
- Packer, N.H.; von der Lieth, C.W.; Aoki-Kinoshita, K.F.; Lebrilla, C.B.; Paulson, J.C.; Raman, R.; Rudd, P.; Sasisekharan, R.; Taniguchi, N.; York, W.S. Frontiers in glycomics: Bioinformatics and biomarkers in disease. An NIH white paper prepared from discussions by the focus groups at a workshop on the NIH campus, Bethesda MD (11–13 September 2006). Proteomics 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Kam, R.K.T.; Poon, T.C.W. The Potentials of Glycomics in Biomarker Discovery. Clin. Proteom. 2008, 4, 67–79. [Google Scholar] [CrossRef]
- Anthony, R.M.; Kobayashi, T.; Wermeling, F.; Ravetch, J.V. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 2011, 475, 110–113. [Google Scholar] [CrossRef]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef]
- Biermann, M.H.; Griffante, G.; Podolska, M.J.; Boeltz, S.; Sturmer, J.; Munoz, L.E.; Bilyy, R.; Herrmann, M. Sweet but dangerous—The role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus 2016, 25, 934–942. [Google Scholar] [CrossRef]
- Scallon, B.J.; Tam, S.H.; McCarthy, S.G.; Cai, A.N.; Raju, T.S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 2007, 44, 1524–1534. [Google Scholar] [CrossRef]
- Quast, I.; Keller, C.W.; Maurer, M.A.; Giddens, J.P.; Tackenberg, B.; Wang, L.X.; Munz, C.; Nimmerjahn, F.; Dalakas, M.C.; Lunemann, J.D. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Investig. 2015, 125, 4160–4170. [Google Scholar] [CrossRef]
- Abes, R.; Teillaud, J.L. Impact of Glycosylation on Effector Functions of Therapeutic IgG. Pharmaceuticals 2010, 3, 146–157. [Google Scholar] [CrossRef]
- Lemmers, R.F.H.; Vilaj, M.; Urda, D.; Agakov, F.; Simurina, M.; Klaric, L.; Rudan, I.; Campbell, H.; Hayward, C.; Wilson, J.F.; et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2240–2249. [Google Scholar] [CrossRef]
- Tanigaki, K.; Sacharidou, A.; Peng, J.; Chambliss, K.L.; Yuhanna, I.S.; Ghosh, D.; Ahmed, M.; Szalai, A.J.; Vongpatanasin, W.; Mattrey, R.F.; et al. Hyposialylated IgG activates endothelial IgG receptor FcgammaRIIB to promote obesity-induced insulin resistance. J. Clin. Investig. 2018, 128, 309–322. [Google Scholar] [CrossRef]
- Stambuk, T.; Kifer, D.; Smircic-Duvnjak, L.; Vucic Lovrencic, M.; Gornik, O. Associations between plasma protein, IgG and IgA N-glycosylation and metabolic health markers in pregnancy and gestational diabetes. PLoS ONE 2023, 18, e0284838. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Pucic Bakovic, M.; Kristic, J.; Novokmet, M.; Huffman, J.E.; Vitart, V.; Hayward, C.; Rudan, I.; Wilson, J.F.; Campbell, H.; et al. The association between galactosylation of immunoglobulin G and body mass index. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 20–25. [Google Scholar] [CrossRef]
- Liu, D.; Li, Q.; Dong, J.; Li, D.; Xu, X.; Xing, W.; Zhang, X.; Cao, W.; Hou, H.; Wang, H.; et al. The Association Between Normal BMI with Central Adiposity And Proinflammatory Potential Immunoglobulin G N-Glycosylation. Diabetes Metab. Syndr. Obes. 2019, 12, 2373–2385. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile And Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 25 September 2025).
- Harvey, D.J.; Merry, A.H.; Royle, L.; Campbell, M.P.; Rudd, P.M. Symbol nomenclature for representing glycan structures: Extension to cover different carbohydrate types. Proteomics 2011, 11, 4291–4295. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Anthony, R.M.; Ravetch, J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. USA 2007, 104, 8433–8437. [Google Scholar] [CrossRef]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Simister, N.E.; Mostov, K.E. An Fc receptor structurally related to MHC class I antigens. Nature 1989, 337, 184–187. [Google Scholar] [CrossRef]
- Farkas, A.; Suranyi, A.; Kolcsar, B.; Gyurkovits, Z.; Kozinszky, Z.; Vari, S.G.; Guttman, A. Potential Glycobiomarkers in Maternal Obesity and Gestational Diabetes During Human Pregnancy. J. Clin. Med. 2025, 14, 1626. [Google Scholar] [CrossRef]
- Bondt, A.; Rombouts, Y.; Selman, M.H.; Hensbergen, P.J.; Reiding, K.R.; Hazes, J.M.; Dolhain, R.J.; Wuhrer, M. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteom. 2014, 13, 3029–3039. [Google Scholar] [CrossRef]
- Simunic-Briski, N.; Dukaric, V.; Ocic, M.; Madzar, T.; Vinicki, M.; Frkatovic-Hodzic, A.; Knjaz, D.; Lauc, G. Regular moderate physical exercise decreases Glycan Age index of biological age and reduces inflammatory potential of Immunoglobulin G. Glycoconj. J. 2024, 41, 67–76, Erratum in Glycoconj. J. 2024, 41, 77–78. [Google Scholar] [CrossRef]
- Darmawan, D.; Raychaudhuri, S.; Lakshminrusimha, S.; Dimitriades, V.R. Hypogammaglobulinemia in neonates: Illustrative cases and review of the literature. J. Perinatol. 2024, 44, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Arkwright, P.D.; Rudd, P.; Scragg, I.G.; Edge, C.J.; Wormald, M.R.; Rademacher, T.W. Short communication: Selective placental transport of maternal IgG to the fetus. Placenta 1995, 16, 749–756. [Google Scholar] [CrossRef]
- Kibe, T.; Fujimoto, S.; Ishida, C.; Togari, H.; Wada, Y.; Okada, S.; Nakagawa, H.; Tsukamoto, Y.; Takahashi, N. Glycosylation and Placental Transport of Immunoglobulin G. J. Clin. Biochem. Nutr. 1996, 21, 57–63. [Google Scholar] [CrossRef]
- Einarsdottir, H.K.; Selman, M.H.; Kapur, R.; Scherjon, S.; Koeleman, C.A.; Deelder, A.M.; van der Schoot, C.E.; Vidarsson, G.; Wuhrer, M. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 2013, 30, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.C.; Bondt, A.; Reiding, K.R.; Scherjon, S.A.; Vidarsson, G.; Wuhrer, M. MALDI-TOF-MS reveals differential N-linked plasma- and IgG-glycosylation profiles between mothers and their newborns. Sci. Rep. 2016, 6, 34001. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, J.; Pabst, M.; Altmann, F. Analytical and Functional Aspects of Antibody Sialylation. J. Clin. Immunol. 2010, 30, 15–19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/WHO-NMH-MND-13.2 (accessed on 25 September 2025).
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes. Res. 1998, 6, 51s–209s. [Google Scholar]
- Váradi, C.; Lew, C.; Guttman, A. Rapid magnetic bead based sample preparation for automated and high throughput N-glycan analysis of therapeutic antibodies. Anal. Chem. 2014, 86, 5682–5687. [Google Scholar] [CrossRef]
- Varadi, C.; Mittermayr, S.; Szekrenyes, A.; Kadas, J.; Takacs, L.; Kurucz, I.; Guttman, A. Analysis of haptoglobin N-glycome alterations in inflammatory and malignant lung diseases by capillary electrophoresis. Electrophoresis 2013, 34, 2287–2294. [Google Scholar] [CrossRef]
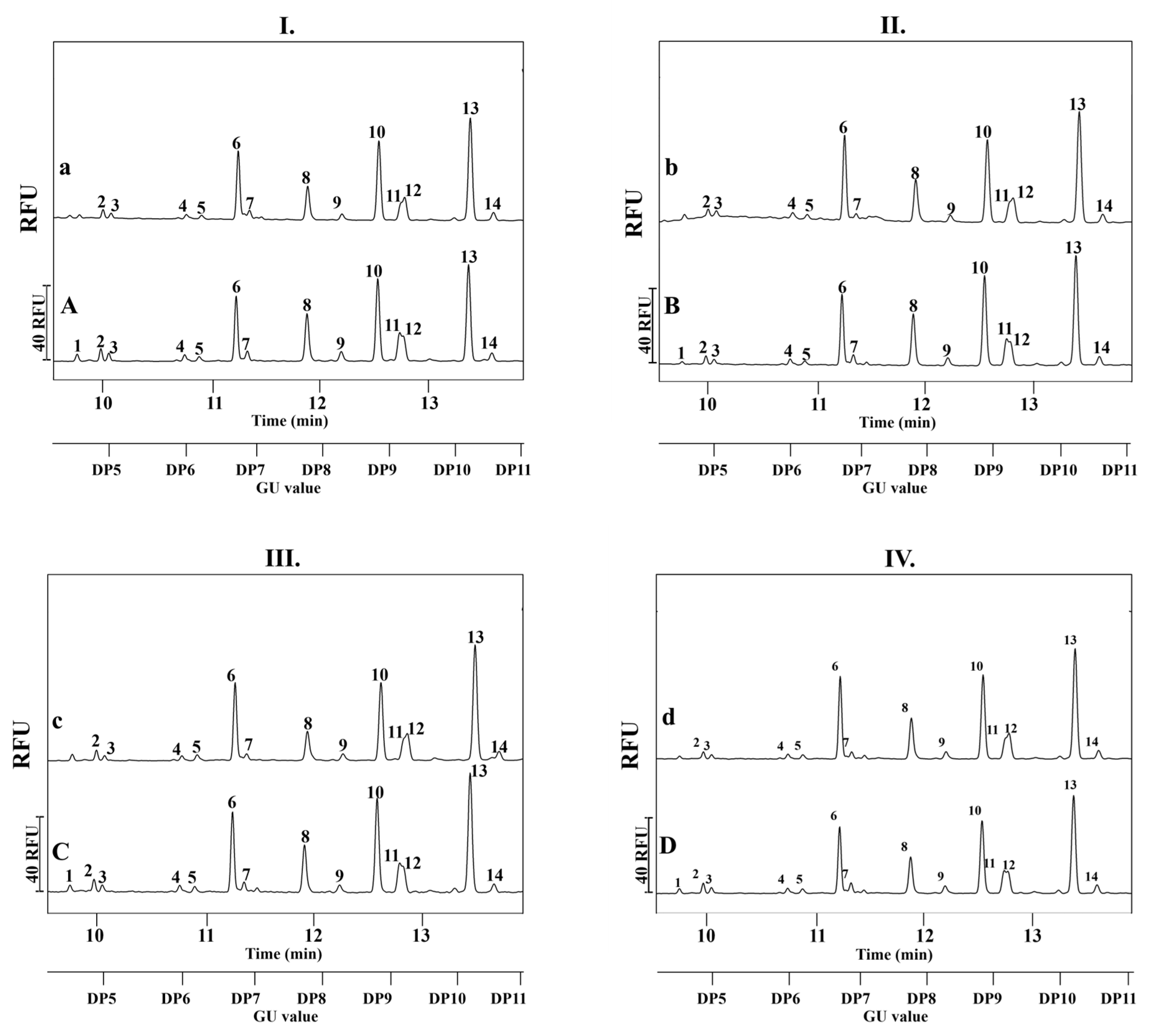

| Group | Age of Mothers (Mean Year ± SD) | Maternal BMI (kg/m2) (Mean ± SD) | Gestational Age (Weeks ± SD) | Delivery Mode in % (Natural/Cesarean-Section) |
|---|---|---|---|---|
| Healthy Normal Weight (H-NW, n = 15). | 26.47 ± 4.98 ns | 22.75 ± 1.21 | 38.94 ± 1.91 | 80%/20% |
| Healthy Obese (H-O, n = 15) | 26.20 ± 5.27 ns | 31.36 ± 1.43 a,**** | 37.96 ± 1.41 b,** | 86.66%/13.34% |
| GDM + Normal Weight (GDM-NW, n = 15) | 27.87 ± 5.19 ns | 23.07 ± 1.83 | 38.59 ± 0.88 | 53.34%/46.66% |
| GDM + Obese (GDM-O, n = 15) | 30.73 ± 7.21 ns | 32.49 ± 2.15 a,**** | 39.96 ± 1.66 a,** | 73.34%/26.66% |
| Peak No. | N-Glycan | GU-Value | Sample | Groups | Peak No. | N-Glycan | GU-Value | Sample | Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-NW | H-O | GDM-NW | GDM-O | H-NW | H-O | GDM-NW | GDM-O | ||||||||
| Mean Area % ± SD | Mean Area % ± SD | ||||||||||||||
| 1 | A2G2S2 | 4.62 ± 0.01 | Maternal | 1.46 ± 0.05 | 0.52 ± 0.07 | 1.27 ± 0.16 | 0.79 ± 0.22 | 8 | FA2; A[6]G1 | 7.77 ± 0.03 | Maternal | 12.02 ± 0.29 | 12.45 ± 0.25 | 10.44 ± 0.27 | 10.32 ± 0.30 |
| Infants | N/A | N/A | N/A | N/A | Infants | 9.62 ± 0.32 | 10.69 ± 0.47 | 7.73 ± 0.09 | 11.30 ± 1.05 | ||||||
| 2 | FA2G2S2 | 4.90 ± 0.01 | Maternal | 2.43 ± 0.06 | 1.59 ± 0.17 | 2.27 ± 0.10 | 1.91 ± 0.28 | 9 | FA2B; A2B[6]G1 | 8.29 ± 0.03 | Maternal | 2.56 ± 0.07 | 2.22 ± 0.26 | 1.78 ± 0.18 | 2.16 ± 0.07 |
| Infants | 1.55 ± 0.31 | 1.70 ± 0.21 | 2.09 ± 0.07 | 1.45 ± 0.17 | Infants | 1.85 ± 0.20 | 1.77 ± 0.26 | 1.70 ± 0.12 | 2.00 ± 0.28 | ||||||
| 3 | FA2BG2S2 | 5.00 ± 0.01 | Maternal | 1.61 ± 0.04 | 1.18 ± 0.24 | 1.41 ± 0.14 | 1.19 ± 0.17 | 10 | FA2[6]G1 | 8.85 ± 0.03 | Maternal | 20.27 ± 0.39 | 21.41 ± 0.57 | 20.96 ± 0.25 | 20.41 ± 0.32 |
| Infants | 1.01 ± 0.31 | 1.45 ± 0.24 | 1.11 ± 0.09 | 1.53 ± 0.73 | Infants | 22.03 ± 0.28 | 20.57 ± 0.39 | 20.02 ± 0.25 | 21.44 ± 0.41 | ||||||
| 4 | FA2[3]G1S1 | 5.98 ± 0.02 | Maternal | 1.45 ± 0.12 | 1.48 ± 0.55 | 1.27 ± 0.07 | 1.22 ± 0.01 | 11 | FA2[3]G1 | 9.18 ± 0.03 | Maternal | 6.86 ± 0.35 | 6.23 ± 0.19 | 6.30 ± 0.19 | 6.36 ± 0.55 |
| Infants | 1.04 ± 0.08 | 1.50 ± 0.14 | 1.11 ± 0.03 | 1.42 ± 0.40 | Infants | 4.69 ± 0.49 | 4.71 ± 0.24 | 5.45 ± 0.20 | 5.27 ± 0.37 | ||||||
| 5 | A2G2S1 | 6.19 ± 0.02 | Maternal | 1.12 ± 0.07 | 1.20 ± 0.49 | 1.31 ± 0.21 | 1.03 ± 0.15 | 12 | FA2B[6]G; A2G2 | 9.23 ± 0.03 | Maternal | 6.26 ± 0.26 | 5.89 ± 0.16 | 5.57 ± 0.29 | 5.72 ± 0.44 |
| Infants | 1.00 ± 0.18 | 1.12 ± 0.26 | 1.35 ± 0.07 | 1.01 ± 0.11 | Infants | 6.54 ± 0.22 | 5.95 ± 0.13 | 6.52 ± 0.27 | 6.03 ± 0.40 | ||||||
| 6 | FA2G2S1 | 6.71 ± 0.02 | Maternal | 14.20 ± 0.16 | 14.10 ± 0.45 | 15.14 ± 0.31 | 15.51 ± 0.21 | 13 | FA2G2 | 10.22 ± 0.04 | Maternal | 25.30 ± 0.32 | 26.99 ± 0.92 | 27.75 ± 0.34 | 28.32 ± 0.09 |
| Infants | 16.28 ± 0.19 | 17.39 ± 0.58 | 17.28 ± 0.17 | 15.94 ± 2.04 | Infants | 30.02 ± 0.37 | 28.98 ± 0.96 | 31.45 ± 0.23 | 28.40 ± 0.91 | ||||||
| 7 | FA2BG2S1 | 6.87 ± 0.02 | Maternal | 2.30 ± 0.09 | 2.46 ± 0.36 | 2.39 ± 0.27 | 2.59 ± 0.07 | 14 | FA2BG2 | 10.57 ± 0.04 | Maternal | 2.15 ± 0.03 | 2.28 ± 0.09 | 2.15 ± 0.14 | 2.48 ± 0.09 |
| Infants | 2.08 ± 0.66 | 1.96 ± 0.60 | 1.74 ± 0.09 | 1.91 ± 0.44 | Infants | 2.32 ± 0.09 | 2.20 ± 0.11 | 2.45 ± 0.11 | 2.31 ± 0.03 | ||||||
| SF/NF ratio | Maternal | 0.33 ± 0.01 | 0.29 ± 0.02 | 0.33 ± 0.02 | 0.32 ± 0.02 | CF/TNG ratio | Maternal | 0.97 ± 0.00 | 0.98 ± 0.00 | 0.97 ± 0.00 | 0.98 ± 0.00 | ||||
| Infants | 0.30 ± 0.03 | 0.34 ± 0.02 | 0.33 ± 0.00 | 0.30 ± 0.01 | Infants | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | ||||||
| Classification | BMI (kg/m2) | Obesity Class |
|---|---|---|
| Underweight | <18.5 | |
| Normal | 18.5–24.9 | |
| Overweight | 25.0–29.9 | |
| Obesity | 30.0–34.9 | I |
| 35.0–39.9 | II | |
| Extreme Obesity | ≥40 | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, A.; Matsyura, O.; Besh, L.; Guttman, A.; Vari, S.G. IgG N-Glycan Profiles in Mothers and Infants Postpartum in the Context of Maternal Obesity and Gestational Diabetes. Int. J. Mol. Sci. 2025, 26, 10641. https://doi.org/10.3390/ijms262110641
Farkas A, Matsyura O, Besh L, Guttman A, Vari SG. IgG N-Glycan Profiles in Mothers and Infants Postpartum in the Context of Maternal Obesity and Gestational Diabetes. International Journal of Molecular Sciences. 2025; 26(21):10641. https://doi.org/10.3390/ijms262110641
Chicago/Turabian StyleFarkas, Anna, Oksana Matsyura, Lesya Besh, Andras Guttman, and Sandor G. Vari. 2025. "IgG N-Glycan Profiles in Mothers and Infants Postpartum in the Context of Maternal Obesity and Gestational Diabetes" International Journal of Molecular Sciences 26, no. 21: 10641. https://doi.org/10.3390/ijms262110641
APA StyleFarkas, A., Matsyura, O., Besh, L., Guttman, A., & Vari, S. G. (2025). IgG N-Glycan Profiles in Mothers and Infants Postpartum in the Context of Maternal Obesity and Gestational Diabetes. International Journal of Molecular Sciences, 26(21), 10641. https://doi.org/10.3390/ijms262110641






