Inhibition of Fatty Acid-Binding Protein 4 Limits High-Fat-Diet-Associated Prostate Tumorigenesis and Progression in TRAMP Mice
Abstract
1. Introduction
2. Results
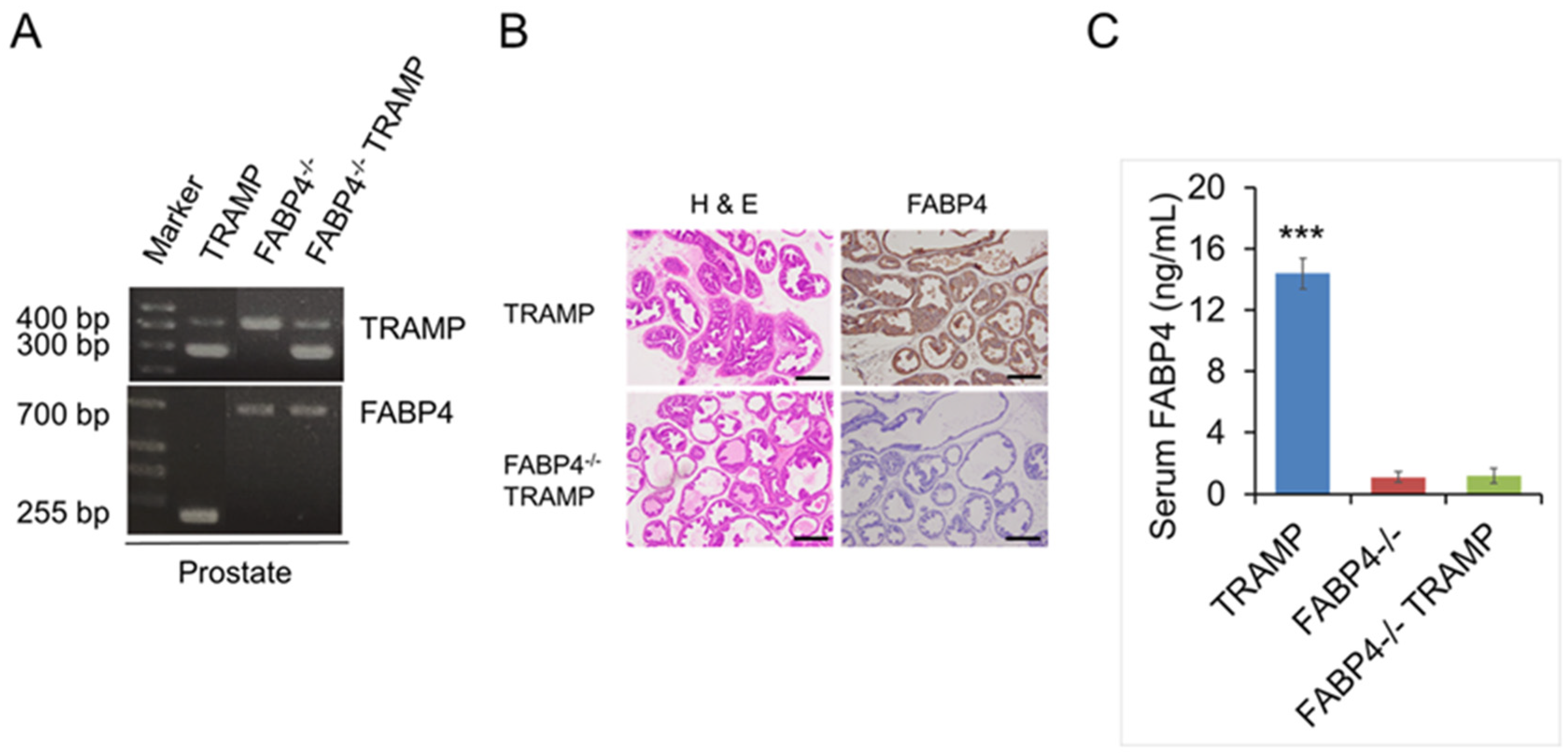
2.1. Generation of FABP4 Knockout (FABP4−/−) TRAMP Mice
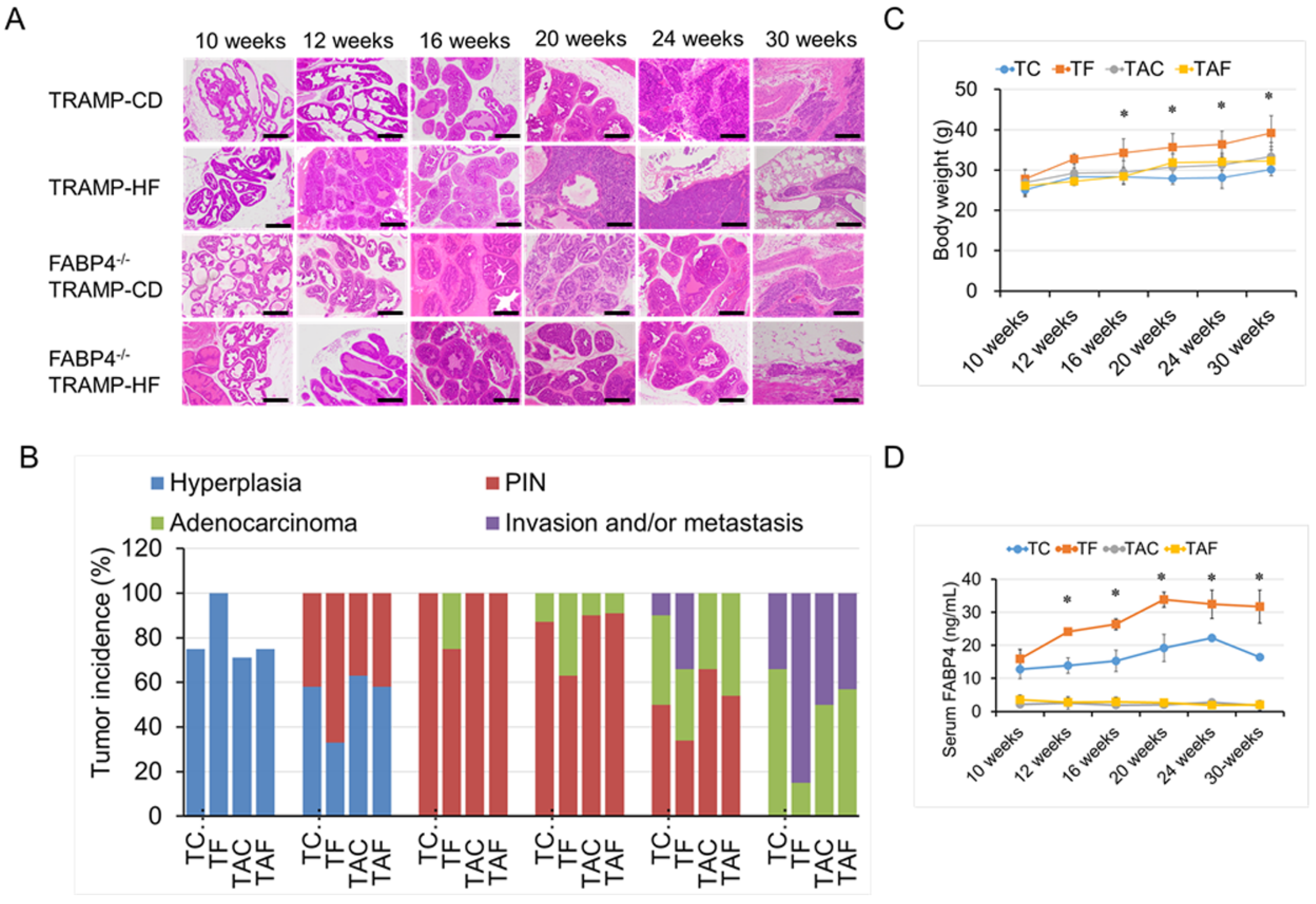
2.2. Loss of FABP4 Diminished HF-Mediated TRAMP Tumor Development and Progression
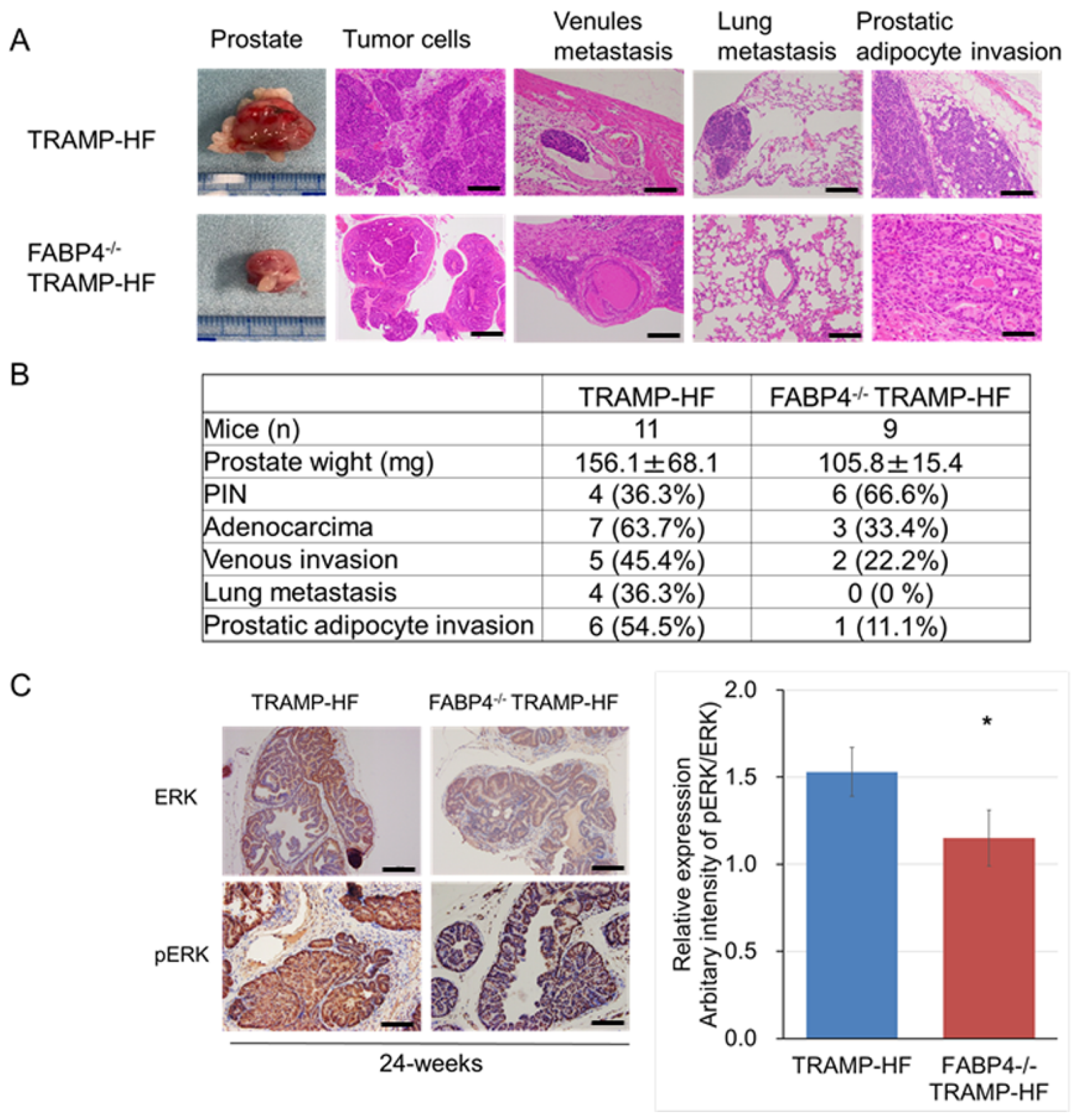
2.3. Loss of FABP4 Inhibited Prostate Tumor Progression Through Alteration of ERK Expression in TRAMP Mice
2.4. Loss of FABP4 Decreased Protumorigenic Cytokine Secretion in TRAMP Mice
2.5. Chemical Inhibition of FABP4 Delayed TRAMP Tumorigenesis and Cytokine Production
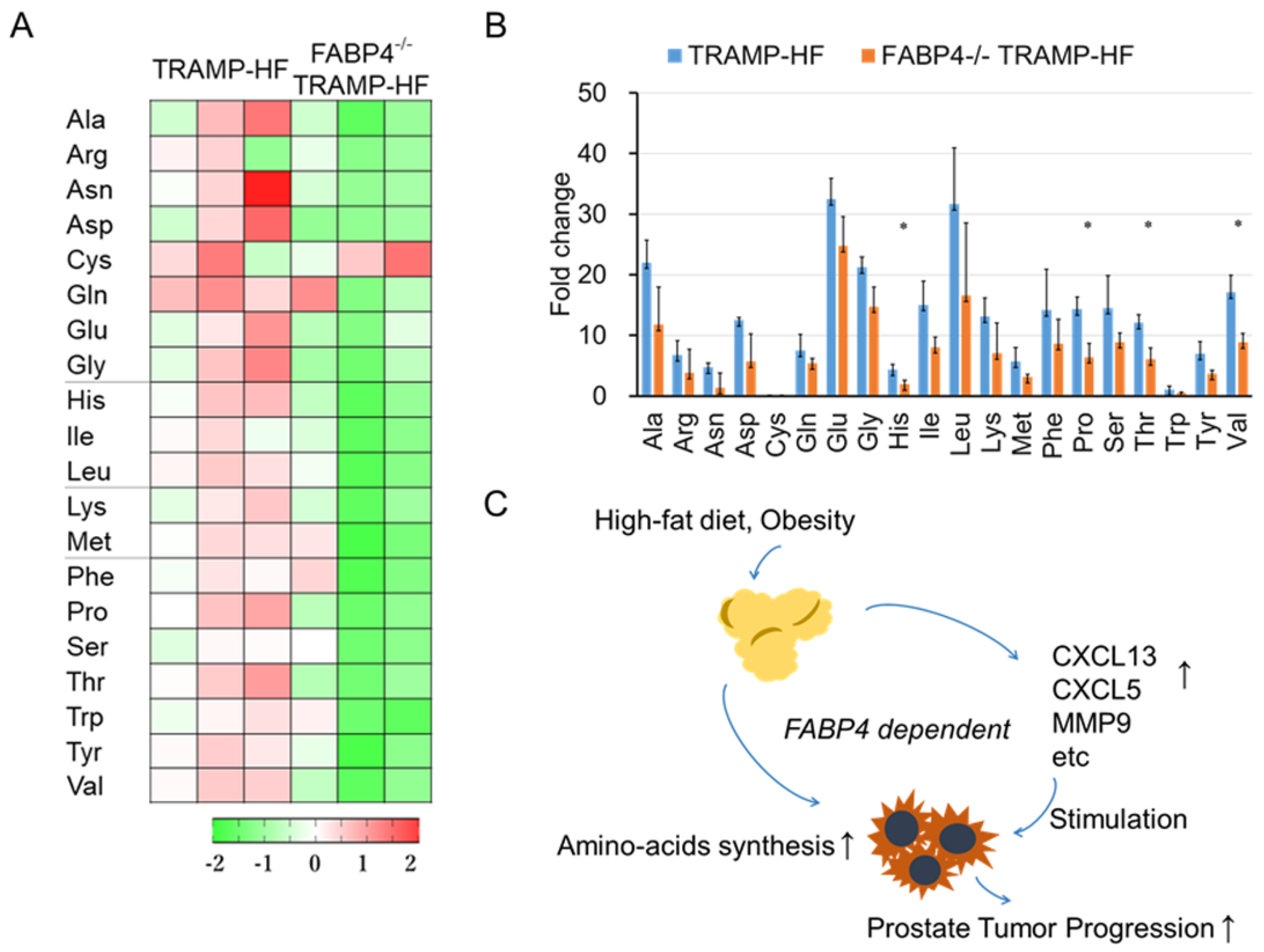
2.6. Loss of FABP4 Decreased Amino Acid Synthesis in TRAMP Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Generation of FABP4 Knockout TRAMP Mice
5.2. Animal Study
5.3. Histopathological Chemistry
5.4. Metabolome Analysis
5.5. Proteome Profiler Mouse Cytokine Array
5.6. Enzyme-Linked Immunosorbent Assay (ELISA)
5.7. Quantitative RT-PCR
5.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef]
- Ito, K. Prostate cancer in Asian men. Nat. Rev. Urol. 2014, 11, 197–212. [Google Scholar] [CrossRef]
- Wu, S.; Powers, S.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016, 529, 43–47. [Google Scholar] [CrossRef]
- Narita, S.; Nara, T.; Sato, H.; Koizumi, A.; Huang, M.; Inoue, T.; Habuchi, T. Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression. J. Clin. Med. 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-Fat Diet-Induced Inflammation Accelerates Prostate Cancer Growth via IL6 Signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mo, M.; Chen, X.; Chao, D.; Zhang, Y.; Chen, X.; Wang, Y.; Zhang, N.; He, N.; Yuan, X.; et al. Targeting inhibition of prognosis-related lipid metabolism genes including CYP19A1 enhances immunotherapeutic response in colon cancer. J. Exp. Clin. Cancer Res. 2023, 42, 85. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Makowski, L.; Hotamisligil, G.S. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J. Nutr. 2004, 134, 2464S–2468S. [Google Scholar] [CrossRef]
- Furuhashi, M.; Tuncman, G.; Gorgun, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef]
- Hao, J.; Jin, R.; Yi, Y.; Jiang, X.; Yu, J.; Xu, Z.; Schnicker, N.J.; Chimenti, M.S.; Sugg, S.L.; Li, B. Development of a humanized anti-FABP4 monoclonal antibody for potential treatment of breast cancer. Breast Cancer Res. 2024, 26, 119. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Tang, Z.; Shen, Q.; Xie, H.; Zhou, X.; Li, J.; Feng, J.; Liu, H.; Wang, W.; Zhang, S.; Ni, S. Elevated expression of FABP3 and FABP4 cooperatively correlates with poor prognosis in non-small cell lung cancer (NSCLC). Oncotarget 2016, 7, 46253–46262. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Inoue, T.; Koizumi, A.; Saito, M.; Tsuruta, H.; Numakura, K.; Satoh, S.; Nanjo, H.; Sasaki, T.; et al. Fatty acid binding protein 4 enhances prostate cancer progression by upregulating matrix metalloproteinases and stromal cell cytokine production. Oncotarget 2017, 8, 111780–111794. [Google Scholar] [CrossRef]
- Tan, N.S.; Shaw, N.S.; Vinckenbosch, N.; Liu, P.; Yasmin, R.; Desvergne, B.; Wahli, W.; Noy, N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell Biol. 2002, 22, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.W.; Jiang, M.; Murphy, T.A.; Yi, Y.; Konvinse, K.C.; Franco, O.E.; Wang, Y.; Young, J.D.; Hayward, S.W. PPARgamma isoforms differentially regulate metabolic networks to mediate mouse prostatic epithelial differentiation. Cell Death Dis. 2012, 3, e361. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Pan, M.; Zorbas, C.; Sugaya, M.; Ishiguro, K.; Kato, M.; Nishida, M.; Zhang, H.F.; Candeias, M.M.; Okamoto, A.; Ishikawa, T.; et al. Glutamine deficiency in solid tumor cells confers resistance to ribosomal RNA synthesis inhibitors. Nat. Commun. 2022, 13, 3706. [Google Scholar] [CrossRef]
- Nomura, M.; Ohuchi, M.; Sakamoto, Y.; Kudo, K.; Yaku, K.; Soga, T.; Sugiura, Y.; Morita, M.; Hayashi, K.; Miyahara, S.; et al. Niacin restriction with NAMPT-inhibition is synthetic lethal to neuroendocrine carcinoma. Nat. Commun. 2023, 14, 8095. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsu, S.C.; Chung, T.Y.; Chu, C.Y.; Wang, H.J.; Hsiao, P.W.; Yeh, S.D.; Ann, D.K.; Yen, Y.; Kung, H.J. Arginine is an epigenetic regulator targeting TEAD4 to modulate OXPHOS in prostate cancer cells. Nat. Commun. 2021, 12, 2398. [Google Scholar] [CrossRef]
- Berglund, E.; Maaskola, J.; Schultz, N.; Friedrich, S.; Marklund, M.; Bergenstrahle, J.; Tarish, F.; Tanoglidi, A.; Vickovic, S.; Larsson, L.; et al. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat. Commun. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Narita, S.; Koizumi, A.; Nara, T.; Numakura, K.; Satoh, S.; Nanjo, H.; Habuchi, T. Macrophage inhibitory cytokine-1 induced by a high-fat diet promotes prostate cancer progression by stimulating tumor-promoting cytokine production from tumor stromal cells. Cancer Commun. 2021, 41, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Guerard, A.; Mazerolles, C.; Le Gonidec, S.; Toulet, A.; Nieto, L.; Zaidi, F.; Majed, B.; Garandeau, D.; Socrier, Y.; et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016, 7, 10230. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.R.; Barrios, R.J.; Kattan, M.W.; Nahm, H.S.; Finegold, M.J.; Greenberg, N.M. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997, 57, 4687–4691. [Google Scholar]
- Yu, L.; Wei, W.; Lv, J.; Lu, Y.; Wang, Z.; Cai, C. FABP4-mediated lipid metabolism promotes TNBC progression and breast cancer stem cell activity. Cancer Lett. 2024, 604, 217271. [Google Scholar] [CrossRef]
- Eason, K.; Sadanandam, A. Molecular or Metabolic Reprograming: What Triggers Tumor Subtypes? Cancer Res. 2016, 76, 5195–5200. [Google Scholar] [CrossRef]
- Vander Heiden, M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef]
- Azuma, K.; Xiang, H.; Tagami, T.; Kasajima, R.; Kato, Y.; Karakawa, S.; Kikuchi, S.; Imaizumi, A.; Matsuo, N.; Ishii, H.; et al. Clinical significance of plasma-free amino acids and tryptophan metabolites in patients with non-small cell lung cancer receiving PD-1 inhibitor: A pilot cohort study for developing a prognostic multivariate model. J. Immunother. Cancer 2022, 10, e004420. [Google Scholar] [CrossRef]
- Katagiri, R.; Goto, A.; Nakagawa, T.; Nishiumi, S.; Kobayashi, T.; Hidaka, A.; Budhathoki, S.; Yamaji, T.; Sawada, N.; Shimazu, T.; et al. Increased Levels of Branched-Chain Amino Acid Associated With Increased Risk of Pancreatic Cancer in a Prospective Case-Control Study of a Large Cohort. Gastroenterology 2018, 155, 1474–1482.e1. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Okada, M.; Yang, L.; Takano, T.; Tabata, S.; Soga, T.; Ho, D.M.; Chung, J.; Minami, Y.; Yoo, S.K. Methionine restriction breaks obligatory coupling of cell proliferation and death by an oncogene Src in Drosophila. Elife 2021, 10, e59809. [Google Scholar] [CrossRef]
- Hirayama, A.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; Ochiai, A.; Tomita, M.; et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009, 69, 4918–4925. [Google Scholar] [CrossRef]
- Sato, H.; Narita, S.; Ishida, M.; Takahashi, Y.; Mingguo, H.; Kashima, S.; Yamamoto, R.; Koizumi, A.; Nara, T.; Numakura, K.; et al. Specific Gut Microbial Environment in Lard Diet-Induced Prostate Cancer Development and Progression. Int. J. Mol. Sci. 2022, 23, 2214. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Narita, S.; Sato, H.; Sekine, Y.; Kobayashi, M.; Kashima, S.; Yamamoto, R.; Koizumi, A.; Nara, T.; Numakura, K.; et al. Inhibition of Fatty Acid-Binding Protein 4 Limits High-Fat-Diet-Associated Prostate Tumorigenesis and Progression in TRAMP Mice. Int. J. Mol. Sci. 2025, 26, 10621. https://doi.org/10.3390/ijms262110621
Huang M, Narita S, Sato H, Sekine Y, Kobayashi M, Kashima S, Yamamoto R, Koizumi A, Nara T, Numakura K, et al. Inhibition of Fatty Acid-Binding Protein 4 Limits High-Fat-Diet-Associated Prostate Tumorigenesis and Progression in TRAMP Mice. International Journal of Molecular Sciences. 2025; 26(21):10621. https://doi.org/10.3390/ijms262110621
Chicago/Turabian StyleHuang, Mingguo, Shintaro Narita, Hiromi Sato, Yuya Sekine, Mizuki Kobayashi, Soki Kashima, Ryohei Yamamoto, Atsushi Koizumi, Taketoshi Nara, Kazuyuki Numakura, and et al. 2025. "Inhibition of Fatty Acid-Binding Protein 4 Limits High-Fat-Diet-Associated Prostate Tumorigenesis and Progression in TRAMP Mice" International Journal of Molecular Sciences 26, no. 21: 10621. https://doi.org/10.3390/ijms262110621
APA StyleHuang, M., Narita, S., Sato, H., Sekine, Y., Kobayashi, M., Kashima, S., Yamamoto, R., Koizumi, A., Nara, T., Numakura, K., Saito, M., Nanjo, H., Ikezoe, T., & Habuchi, T. (2025). Inhibition of Fatty Acid-Binding Protein 4 Limits High-Fat-Diet-Associated Prostate Tumorigenesis and Progression in TRAMP Mice. International Journal of Molecular Sciences, 26(21), 10621. https://doi.org/10.3390/ijms262110621





