1. Introduction
Cucurbit leaf crumple virus (CuLCrV) is a plant virus that belongs to the family Geminiviridae [
1]. It is transmitted by whiteflies and infects a wide range of cucurbit crops, including cucumber, watermelon, and squash [
2,
3]. CuLCrV infection causes severe symptoms, such as leaf crumpling, yellowing, and stunting, which can result in significant yield losses [
4,
5,
6]. CuLCrV is considered a major threat to the cucurbit industry in the United States and has been reported in many states, including Florida, California, Georgia, Texas, South Carolina, and [
4,
7,
8,
9]. Early detection and management of CuLCrV are critical for preventing the spread of the disease and minimizing its economic impact on agriculture. Traditional detection methods are available, such as PCR [
10], restriction fragment length polymorphism (RFLP) followed by PCR [
11], nucleic acid hybridization [
12], and rolling circle amplification (RCA) [
13] are effective but require expensive equipment and skilled technicians, which limit their use in field settings. ELISA is a rapid and sensitive technique that can detect viral proteins or antibodies in infected plant tissues [
14]. However, this method requires specialized equipment and reagents, and it may not be suitable for field-based diagnostics.
PCR is a molecular technique that amplifies the viral DNA or RNA sequences using specific primers, followed by detection through gel electrophoresis or hybridization. Although PCR is highly sensitive and specific, it requires expensive equipment and trained personnel, making it challenging for use in resource-limited settings. The traditional detection methods have their advantages and limitations, and the choice of the method depends on the specific needs and resources available for the detection of CuLCrV. There are several isothermal methods available for the detection of CuLCrV, including Loop-mediated isothermal amplification (LAMP), LAMP is another isothermal amplification method that uses a set of primers that recognize six distinct regions of the target DNA sequence, leading to the amplification of the target sequence. LAMP operates at a constant temperature, typically 60–65 °C, and can be completed within an hour [
15].
Recently, the development of rapid and accurate diagnostic assays based on isothermal amplification technologies, such as RPA, has provided an alternative approach for virus detection. RPA is a robust and sensitive nucleic acid amplification method that operates at a constant temperature and can be completed within 30 min. Additionally, RPA assays can be performed using simple equipment and minimal technical expertise, making it a promising tool for field-based diagnostics [
16]. More recently, a singleplex exo RPA assay has been developed for the detection of CuLCrV under field-based conditions, utilizing a battery-operated portable spectrometer [
17]. Additionally, a lab-based multiplex conventional RPA assay has been developed for three cucurbit viruses, including CuLCrV [
18]. While the exo RPA assay offers a field-based diagnostic method, it necessitates a field-operable spectrophotometer, which can be more expensive. Conversely, the lab-based RPA assay is not applicable under field-based conditions.
Furthermore, previously reported assays have all relied on instruments for virus detection, and none have been utilized for the detection of CuLCrV using the RPA-LFT (Recombinase Polymerase Amplification-Lateral Flow Test) assay. The RPA for nucleic acid amplification, followed by detection on a lateral flow test strip, enables rapid and sensitive detection of target pathogens in a user-friendly format. Rapid and reliable detection of plant viruses is essential for effective disease management, particularly in resource-limited or field settings. Traditional diagnostic methods, such as PCR and ELISA, are sensitive and specific but require sophisticated equipment and trained personnel, limiting their use outside laboratory environments. The RPA assay, when coupled with a LFT, provides a rapid, sensitive, and instrument-free method for detecting plant viruses such as CuLCrV. In this study, we introduce the use of Thermus thermophilus (Tth) endonuclease, a thermostable enzyme that enhances probe cleavage efficiency during RPA amplification, thereby improving the sensitivity and visual clarity of the RPA-LFT assay for field-based virus diagnostics. Unlike commonly used nucleases, Tth endonuclease functions efficiently at elevated temperatures and demonstrates robust activity under field-like conditions. Its ability to specifically cleave at designated sites enhances signal generation and contributes to a clearer, stronger visual output on the lateral flow strip.
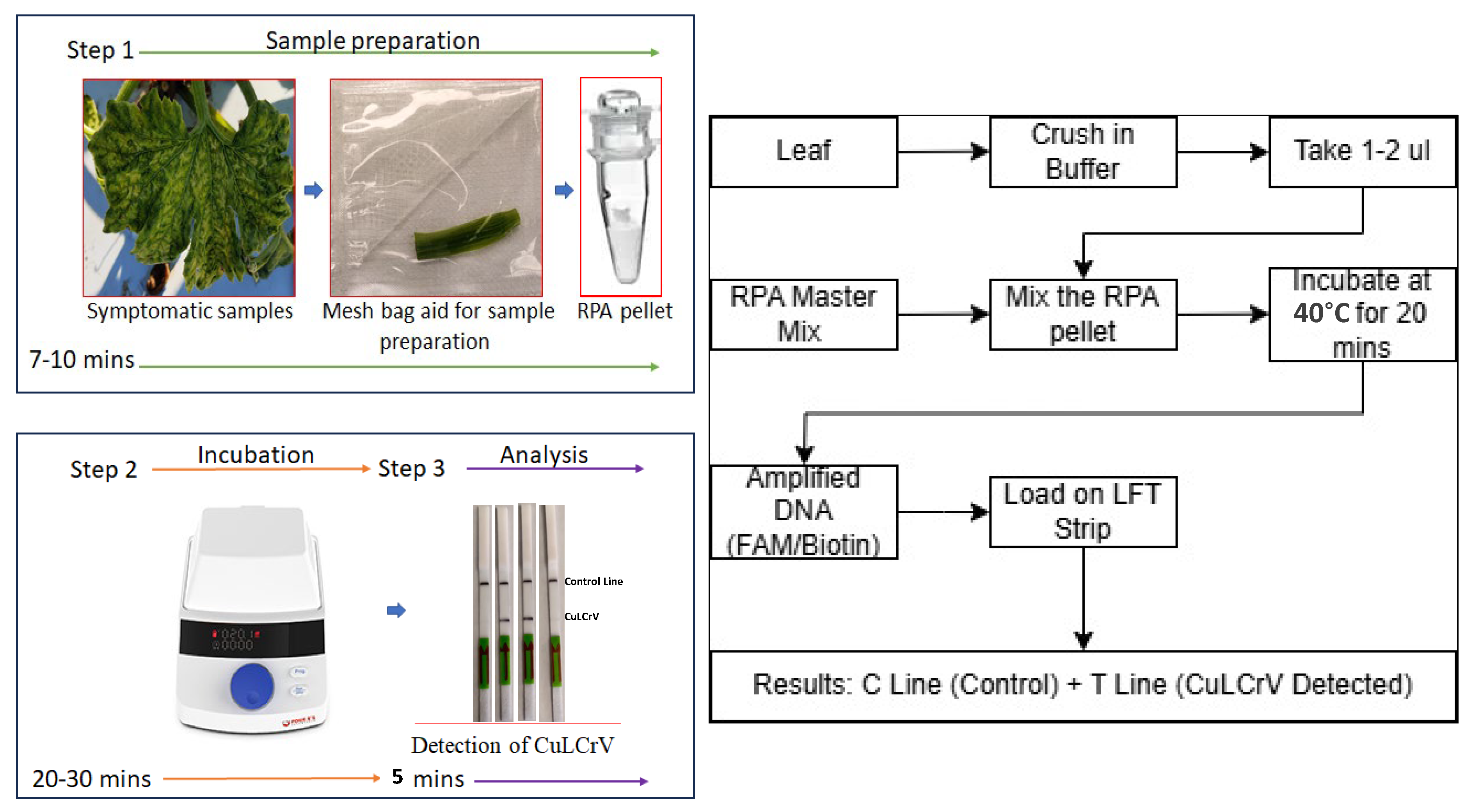
In this study, we developed a new RPA-based lateral flow test (LFT) assay for the detection of CuLCrV using the Tth endonuclease. This LFT assay is a user-friendly, rapid, and cost-effective diagnostic tool that combines the amplification and detection of the target nucleic acid in a single reaction. The LFT assay employs gold nanoparticle-conjugated probes that bind to the amplified product and produce a visible signal on the test strip. This signal can be easily read by the naked eye, eliminating the need for specialized equipment or trained personnel. The objective of this study was to evaluate the sensitivity and specificity of the RPA-based LFT assay for the detection of CuLCrV, and to compare its performance with that of traditional detection methods. The results of this study further demonstrate the potential of RPA-based LFT assays as a rapid and reliable diagnostic tool for the detection of CuLCrV in field setting, which can aid in the effective management and control of this destructive plant virus. The figure and flowchart’s schematic representations are explained in the field-based RPA-LFT assay development of CuLCrV detection (
Figure 1).
3. Discussion
Cucurbit leaf crumple virus (CuLCrV) poses a significant threat to cucurbit production across the United States due to its rapid spread, broad host range, and association with severe yield losses [
21,
22]. Current diagnostic techniques such as PCR, qPCR, ELISA, and nucleic acid hybridization provide reliable virus detection but are often constrained by their dependence on expensive equipment, skilled personnel, and laboratory infrastructure [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. This limits their deployment in field conditions where timely detection is essential for managing disease outbreaks. Although LAMP assays have improved the feasibility of field diagnostics [
15], their complexity in primer design and operation temperature still restricts widespread application.
To address these limitations, we developed and validated a RPA-LFT assay that offers a rapid, sensitive, and equipment-independent method for detecting CuLCrV in both plant and vector samples. RPA is an isothermal amplification method that operates at low temperatures (~40 °C) and can be completed in less than 30 min, making it well suited for field-based diagnostics [
23,
24]. The lateral flow detection system allows for immediate visual interpretation of results without the need for instrumentation, addressing the current gap in cost-effective, field-deployable assays for CuLCrV [
16].
Traditional RPA-LFT assays often utilize endonuclease IV (Nfo) for probe cleavage. However, this study explored the use of Tth endonuclease, a thermostable enzyme known for its robust activity at elevated temperatures [
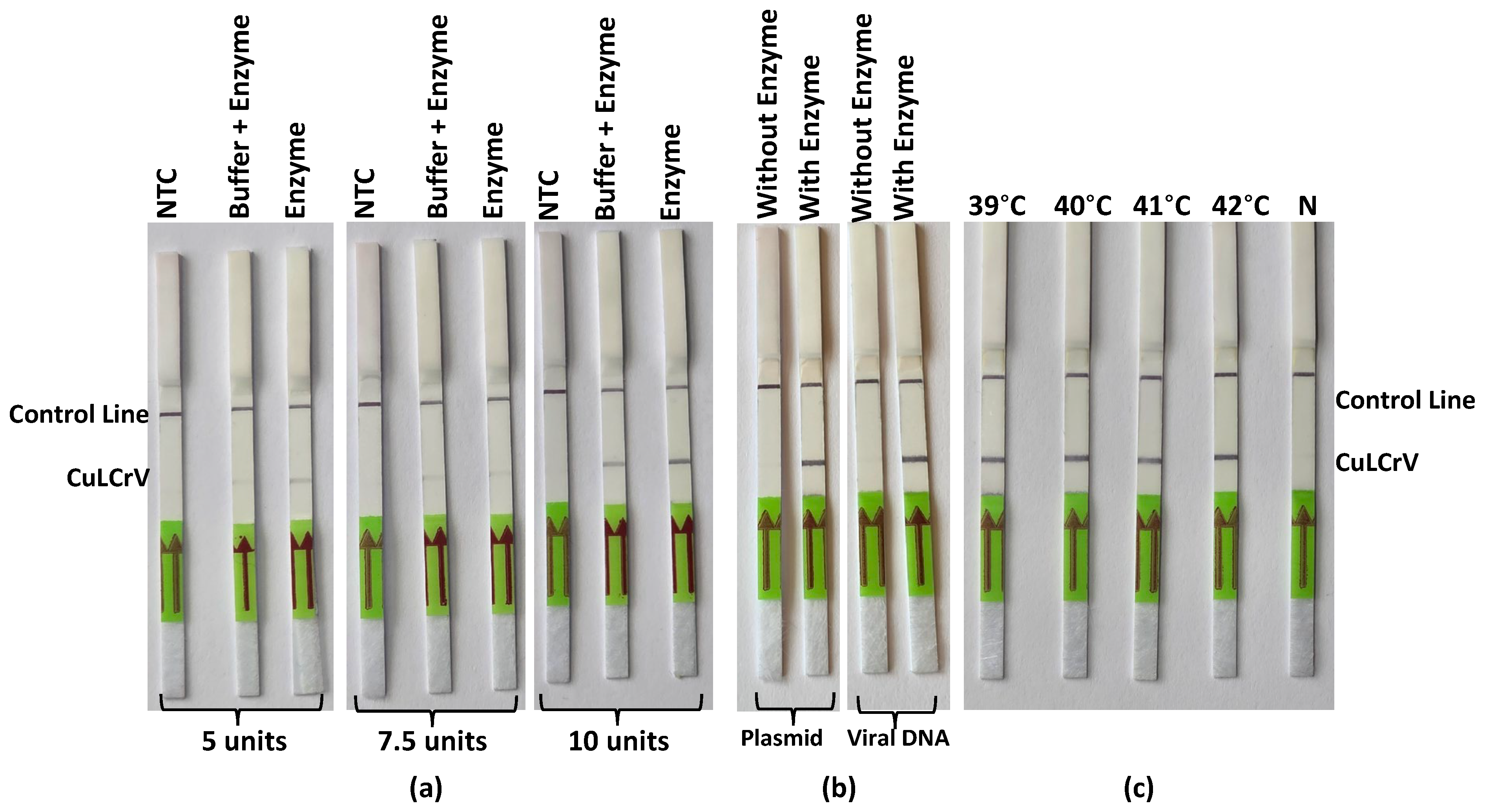
25]. By optimizing the concentration of Tth endonuclease to 10 units per reaction, the assay achieved clearer and more reproducible test and control lines on the lateral flow strips. This enhancement is attributed to Tth endonuclease’s efficient cleavage of the probe, facilitating better signal development. Unlike previous RPA-LFT assays reported for plant viruses, this study is the first to incorporate the thermostable Tth endonuclease as an alternative to Nfo for probe cleavage, enabling improved signal consistency and enhanced performance under field conditions. The thermostability of Tth endonuclease allows the RPA-LFT assay to operate effectively at 40 °C, a temperature conducive to field conditions. This attribute is particularly beneficial for on-site diagnostics, where precise temperature control may be challenging. The successful application of Tth endonuclease in this context underscores its potential utility in other isothermal amplification assays requiring robust enzyme activity under variable environmental conditions.
A key distinction between the present assay and conventional RPA systems lies in the use of Tth endonuclease instead of the commonly employed Endonuclease IV (Nfo). The Tth enzyme exhibits higher thermostability and remains catalytically active across a wider temperature range (35–65 °C), which is advantageous for field conditions where precise temperature control is difficult [
24]. In contrast, Nfo is less thermostable and performs optimally below 42 °C, limiting its robustness in warmer environments [
16]. Although both enzymes efficiently cleave double-stranded probe intermediates during RPA, our findings demonstrate that Tth-based RPA-LFT maintains comparable sensitivity and reliability to Nfo-based assays while offering greater operational flexibility and cost efficiency for on-site plant virus detection.
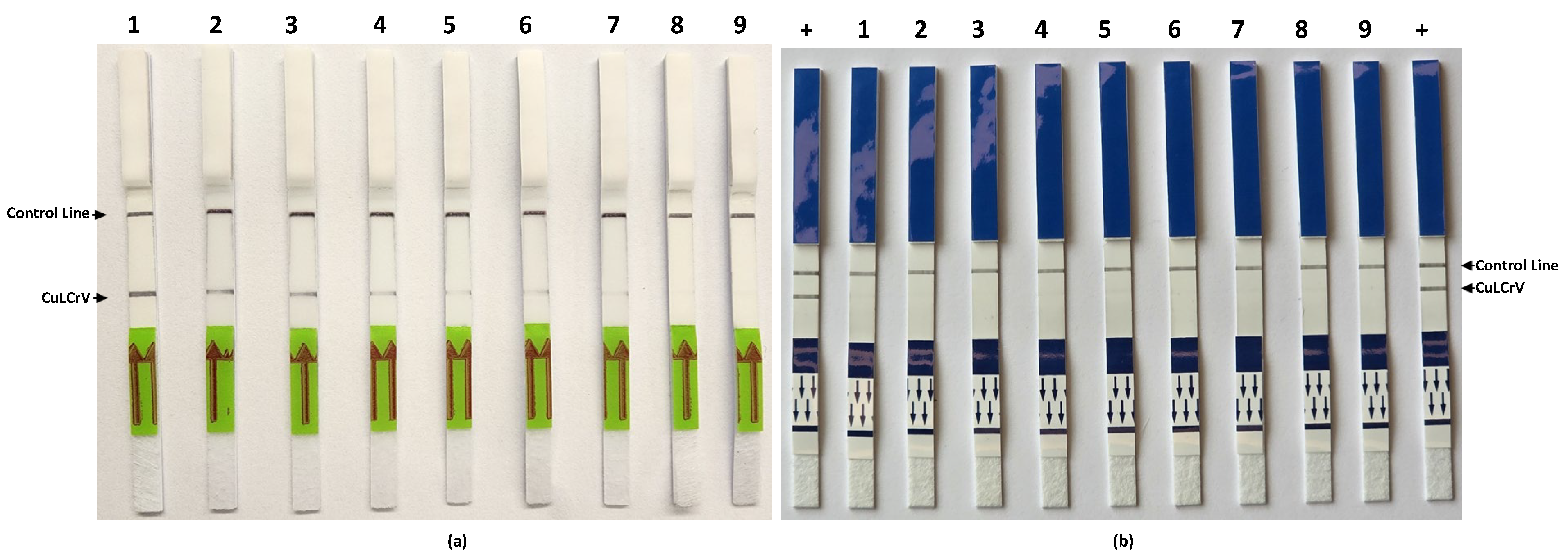
Our assay demonstrated high analytical sensitivity, detecting CuLCrV down to 10 copies of plasmid DNA, and exceptional specificity, showing no cross-reactivity with other cucurbit-infecting viruses including CCYV, CYSDV, SqVYV, PRSV-W, WMV, ZYMV, ToLCNDV, WaLCV1, and 2. These findings confirm the robustness of the primer-probe set designed specifically to target a conserved region in the CuLCrV genome and validated through in silico and empirical approaches. We further optimized the assay by testing new endonuclease enzyme and reaction conditions. The use of Tth endonuclease provided stronger, more consistent signal development than Nfo, suggesting that enzyme selection can significantly enhance assay performance. Additionally, 40 °C was found to be the optimal temperature for consistent amplification and detection, confirming earlier studies on RPA assay optimization [
26].
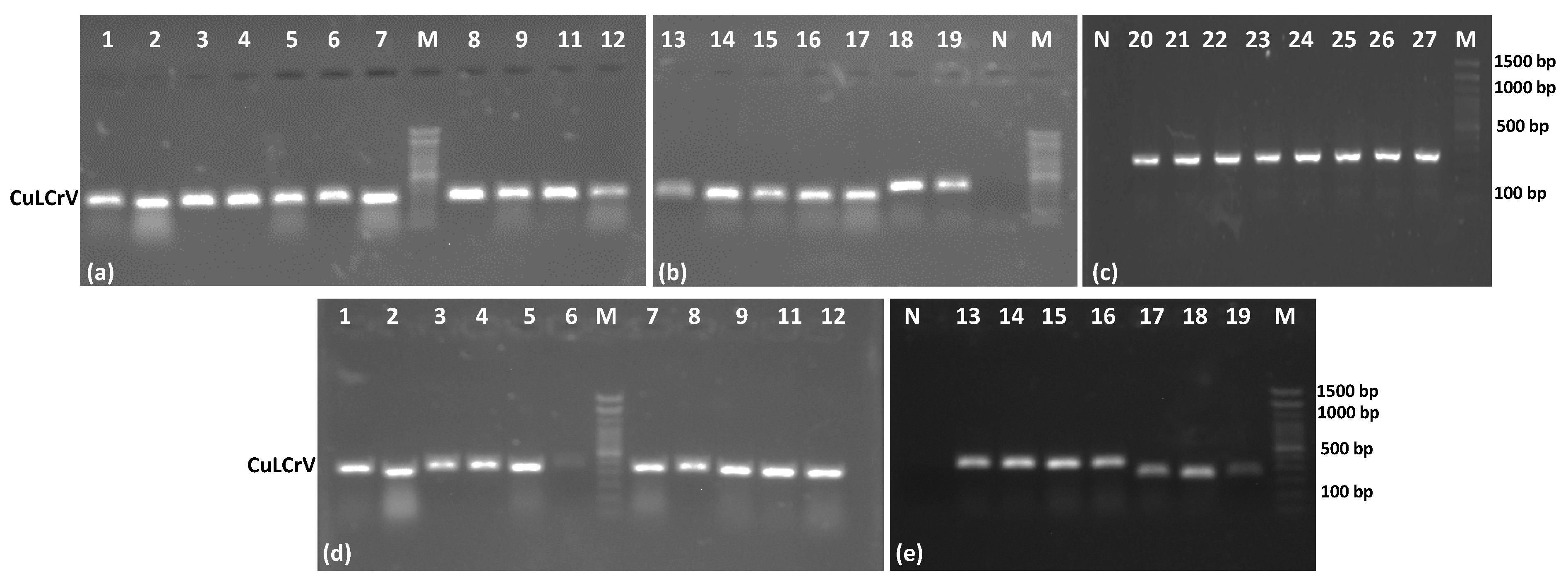
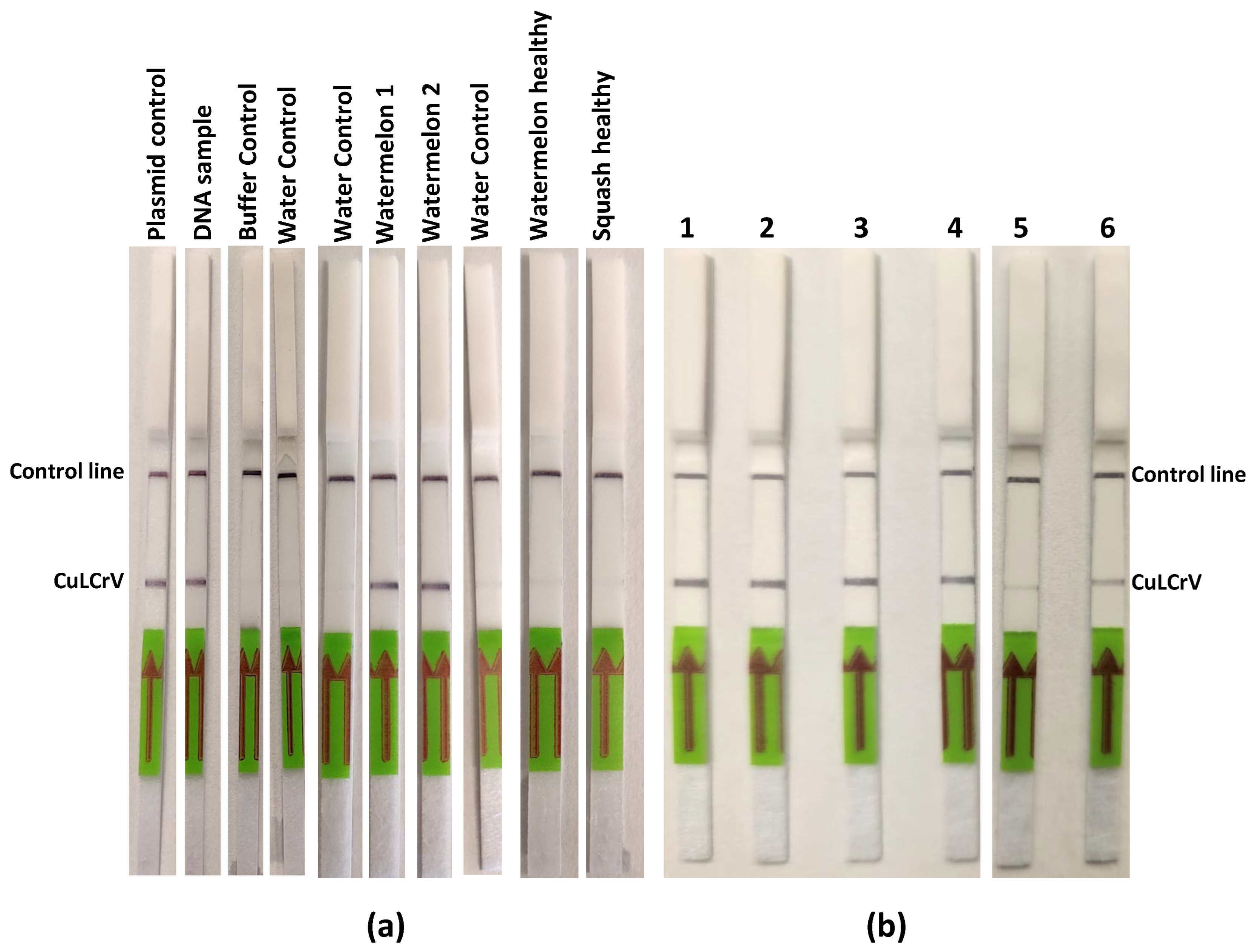
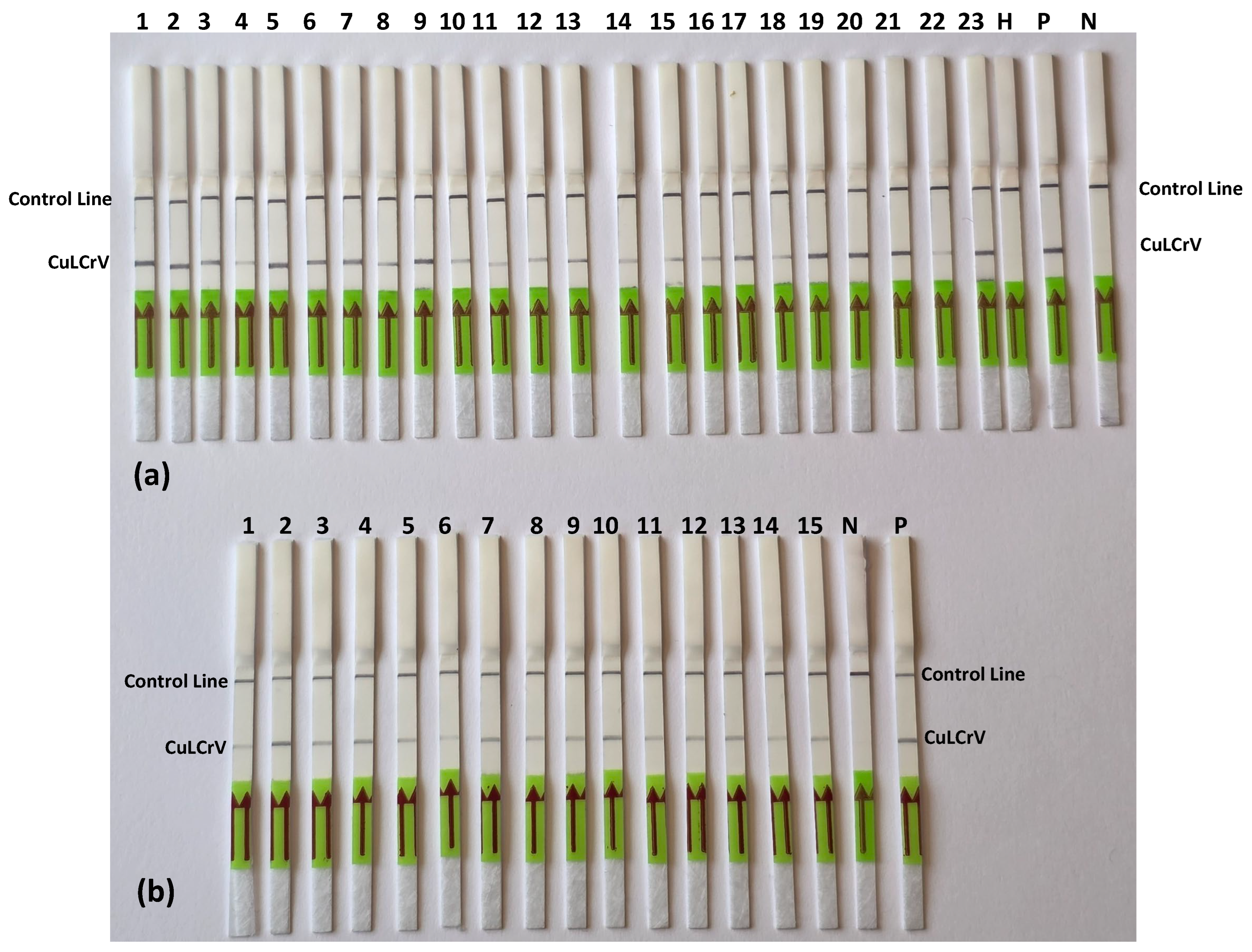
In field sample validation, the RPA-LFT assay successfully detected CuLCrV in all 27 symptomatic cucurbit samples (squash, watermelon, and pumpkin), as well as in 15 vector (whitefly) samples. These results were entirely consistent with conventional PCR, qPCR, and standard RPA assays, confirming the diagnostic accuracy of the developed LFT method. Notably, the assay also worked effectively using crude whitefly extracts, eliminating the need for nucleic acid extraction and further streamlining field testing. This adaptability offers a significant advantage for pest surveillance and rapid intervention in infected fields [
15].
Importantly, this study is the first report of a Tth-based RPA-LFT assay for CuLCrV detection, and one of the few to demonstrate successful integration of enzyme optimization and field sample testing across plant and insect hosts. By providing an instrument-free, sensitive, and user-friendly diagnostic tool, the RPA-LFT assay represents a practical solution for CuLCrV monitoring in low-resource and remote agricultural settings. The potential of this platform extends beyond CuLCrV. Given the modularity of RPA and the flexibility of lateral flow detection, similar diagnostic formats could be developed for other whitefly-transmitted viruses and emerging plant pathogens. Future directions may include the integration of this assay with smartphone-based readers or lyophilized reagent formats to further enhance portability, shelf life, and accessibility. The recent development of gold nanoparticle-based lateral flow tests (AuNPs-LFTs), an avenue for rapid, high-throughput field diagnostics [
27].
4. Materials and Methods
4.1. Sample Collection and Nucleic Acid Extraction
Samples of infected plants displaying symptoms of CuLCrV were collected from watermelon, pumpkins, and squash plants at the NFREC research fields in Quincy, FL, USA. Representative samples with visible signs such as crumpled leaves, yellowing, and stunted growth were carefully chosen. To prevent any contamination between samples, aseptic techniques and disposable gloves were employed. Each sample was assigned a unique identifier to ensure traceability. To minimize the degradation of viral nucleic acids, the collected plant samples were promptly transported to the laboratory and stored in a cool, dark location. In cases where immediate processing was not possible, the samples were stored at −80 °C until further analysis. The Quick-DNA Plant Mini Kit from Qiagen (Redwood City, CA, USA) was utilized to extract total nucleic acids from the infected plant samples, following the manufacturer’s instructions. The extracted nucleic acids were evaluated for quality, integrity, concentration, and purity using a Nano Spectrometer from Thermo Scientific (Waltham, MA, USA). The presence of CuLCrV was confirmed by conducting a conventional PCR with specific primers targeting the CP region, as described by Jailani et al. (2021) [
19]. Gel electrophoresis was employed to visualize the amplified CP region and confirm the presence of the target product. The extracted nucleic acids were stored at −80 °C for further validation and the RPA-LFT assay.
4.2. Crude Extract Preparation from Plant Tissue Samples
Fresh leaf tissue (approximately 100 mg, equivalent to a small leaf disc or a 1 cm2 section) was placed in a disposable extraction bag containing 1000 µL of GBE 1 extraction buffer. The tissue was manually ground using the integrated roller of the extraction bag until a uniform homogenate was obtained. The crude extract was then allowed to settle for 1–2 min at room temperature. A 1–2 µL aliquot of the clear supernatant was immediately used as the template for the RPA reaction without further purification. This simple, rapid extraction approach minimized processing time and was suitable for field-based molecular detection assays.
4.3. Nucleic Acid Extraction from Whiteflies
For the extraction of whitefly nucleic acids, ethanol-preserved adult whiteflies (
Bemisia tabaci) were first washed twice with sterile double-distilled water to remove external contaminants. Excess water was carefully removed, and the insects were air-dried on sterile filter paper. The dried whiteflies were then homogenized in sterile 2 mL microcentrifuge tubes using an autoclaved pestle. A minor modification was introduced to the standard extraction protocol—30 µL of extraction buffer was used in the initial step instead of the recommended 450 µL to accommodate the small tissue volume. Both single and pooled whitefly samples were homogenized in 30 µL of the modified extraction buffer supplied with the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Total nucleic acids were then extracted following the same procedure used for plant tissue samples, as described by Jailani et al. (2024) [
20].
4.4. RPA Primer and RPA-LFT Probe Design for CuLCrV Detection
In order to design primers and probe for CuLCrV, a specific region (coat protein) within the CuLCrV genome was identified. This region needed to exhibit high conservation and be appropriate for primer design. It was essential for the selected target region to be unique to CuLCrV and possess minimal sequence similarity to other viruses that infect cucurbits or cucurbit genomes. Bioedit software 7.7 was utilized to design primers that specifically bind to the chosen target region, ensuring a high level of specificity for CuLCrV detection. The primers and probe were designed following the TwistDx manufacturer’s protocol for the RPA assay, adhering to the recommended length of 30–35 base pairs for primers and 46–54 base pairs for probe and achieving a melting temperature (Tm) suitable for RPA conditions. To validate the RPA primers and RPA-LFT probe, in silico analysis was conducted against known CuLCrV sequences and other closely related viruses to assess their specificity. Additionally, an analysis of the primer and probe sequences were performed against the NCBI GenBank database to avoid any potential cross-reactivity. The RPA primers were synthesized by Integrated DNA Technology (Skokie, IL, USA) as per manufacture protocol. The RPA-LFt probe was synthesized by LGC Biosearch Technologies (Petaluma, CA, USA) (
Table 3).
4.5. Conventional RPA Reaction
The RPA reaction was conducted to prepare a 50-µL reaction volume, following the guidelines provided in the RPA-basic kit (TwistDx, Cambridge, UK). The reaction mixture consisted of 29.5 µL of rehydration buffer, 2.1 µL of forward primer, 2.1 µL of reverse primer (both at a concentration of 10 µM), a single lyophilized enzyme pellet, 1 µL of DNA template (plant 10–50 ng and whitefly 2–10 ng), and 12.8 µL of nuclease-free water. The reaction was initiated by adding 2.5 µL of 280 mM magnesium acetate. After an incubation period of 40 min at 40 °C, the amplicons were purified using the Qiagen PCR purification Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol, and subsequently subjected to electrophoresis on 2.0% agarose gels.
4.6. Optimization of Endonuclease Enzyme Performance for RPA-LFT
To determine the optimal concentration of endonuclease enzyme for the RPA-LFT assay, different units (5, 7.5, and 10 units) of Thermus thermophilus (Tth) (Cat. No. M0294S, NEB, Ipswich, MA, USA) endonuclease were tested in the reaction mixture. Each reaction was prepared with TwistAmp® Basic kit (TwistDx, Maidenhead, UK) components, probe (10 μM) and amplification results were evaluated based on test line intensity and consistency on lateral flow strips (HybriDetect-Universal Lateral Flow Assay Kit, Milenia Biotec GmbH, Giessen, Germany). In parallel, the effect of buffer addition with the enzyme was assessed by preparing reactions with enzyme alone and enzyme plus buffer to determine any influence on signal development.
4.7. Validation of Endonuclease Activity with Different Template Types
To confirm the functionality of Tth endonuclease in the RPA-LFT assay, reactions were prepared using both CuLCrV-infected plant DNA and plasmid DNA as templates. Parallel reactions were performed with and without the addition of endonuclease to evaluate its necessity in signal generation. Lateral flow test strips were used to detect amplification products, and visual signal presence or absence was recorded to assess assay specificity and enzyme activity.
4.8. Temperature Optimization for Endonuclease-Based RPA-LFT Assay
To identify the optimal incubation temperature for the endonuclease-based RPA-LFT assay, reactions were incubated at different temperatures ranging from 39 °C to 42 °C with a battery-powered mini heat block. All reactions included the full set of RPA-LFT reagents and 10 units of Tth endonuclease. After amplification, lateral flow strips were used to visualize the results, and the clarity and intensity of test lines were compared across the tested temperatures to establish optimal conditions for sensitive detection of CuLCrV.
4.9. RPA-LFT Assay Development
Designing primers and probes for the RPA-LFT assay involves identifying conserved regions in the target pathogen, such as CuLCrV, and designing specific primers and probes according to the Twist DX, UK protocol. The designed primers and probes are then validated for strength and specificity using bioinformatics software such as BioEdit 7.7. The RPA reaction for the LFT assay was conducted under the same reaction conditions as mentioned previously. Additionally, for the RPA reaction in the LFT assay, Tth (1 μL/reaction) (NEB, Ipswich, MA, USA), were added as alternatives to the Nfo enzyme performances. The enzyme units were optimized at different levels, and we utilized the final standardized units in the above RPA-LFT assay. In the RPA assay, the probe and primer concentrations were adjusted slightly according to the manufacturer’s protocol used for the RPA-LFT assay with the TwistDX Nfo kit.
4.10. Lateral Flow Test (LFT) Visualization
Following the completion of the CuLCrV RPA assay, the amplified products were analyzed using Milenia HybriDetect lateral flow test (LFT) strips for the detection of biotin- and FITC-labeled analytes. According to the manufacturer’s protocol, a 5 μL volume of the CuLCrV RPA amplification product was directly pipetted onto the sample application area of the Milenia® HybriDetect LFT strip. Subsequently, the test strips were immersed in a tube containing 100 μL of HybriDetect Assay Buffer and incubated for 5–15 min to observe the results.
4.11. Sensitivity and Specificity Evaluation
To evaluate the sensitivity of the RT-RPA assay and compare it with the conventional RPA assay, we utilized tenfold serial dilutions (100 to 106) of an artificial plasmid control (APC) as the template for this sensitivity assessment. Subsequently, these APCs were subjected to analysis using conventional PCR. For assessing the specificity of the CuLCrV RPA-LFT assay, we investigated potential cross-reactivity with common cucurbit-infecting viruses, including cucurbit yellow stunting disorder virus (CYSDV), cucurbit chlorotic yellows virus (CCYV), squash vein yellowing virus (SqVYV), papaya ringspot virus-watermelon strain (PRSV-W), watermelon mosaic virus-2 (WMV-2), and watermelon crinkle leaf-associated viruses 1 & 2 (WCLaV1). The primers and probe designed specifically for CuLCrV in this study were utilized for this assessment to ensure the assay’s specificity. PCRD FLEX duplex LFT strips (Cytiva, Amersham, UK) were used for specificity assays due to supply delays, while Milenia HybriDetect single-plex strips were used for other tests. The appearance of test and control lines indicated positive and valid reactions, respectively.
5. Conclusions
This study reports, for the first time, the successful development and validation of a Tth endonuclease-based RPA-LFT assay for the rapid detection of CuLCrV in both plant tissues and insect vectors. The novel incorporation of Tth endonuclease into the RPA-LFT platform significantly enhanced visual signal clarity and detection consistency compared to traditional enzyme systems such as endonuclease IV (Nfo), offering a substantial improvement in the assay’s diagnostic performance. The RPA-LFT assay exhibited excellent analytical sensitivity, detecting as few as 10 copies of the target DNA, and demonstrated complete specificity, with no cross-reactivity observed with closely related cucurbit-infecting viruses. Importantly, the assay produced reliable results using both purified DNA and crude extracts, underscoring its adaptability for field-based diagnostics without the need for complex nucleic acid extraction protocols or sophisticated equipment.
The portability, rapid turnaround time (results within 10–15 min), and user-friendly nature of this assay make it especially suitable for on-site deployment in cucurbit-growing regions where early detection and rapid response are critical for managing CuLCrV outbreaks. By enabling timely and accurate virus identification at the point of need, this assay has the potential to greatly enhance integrated pest and disease management strategies, support quarantine and surveillance programs, and ultimately mitigate the economic losses associated with CuLCrV infections. Overall, the Tth endonuclease–optimized RPA-LFT assay represents a significant advancement in isothermal plant virus diagnostics. Its robust performance and field-applicability make it a promising diagnostic platform not only for CuLCrV, but also for adaptation to other agriculturally important plant pathogens. Future integration with lyophilized reagents and lateral flow devices may further streamline its use in resource-limited and remote agricultural settings.


_Kim.png)



