Exploring Guanidinium Group Involvement in Hordatine Interactions with the G-Quadruplex Motif Within the c-MYC Promoter Region
Abstract
1. Introduction
2. Results and Discussion
2.1. NMR and Docking Binding Studies
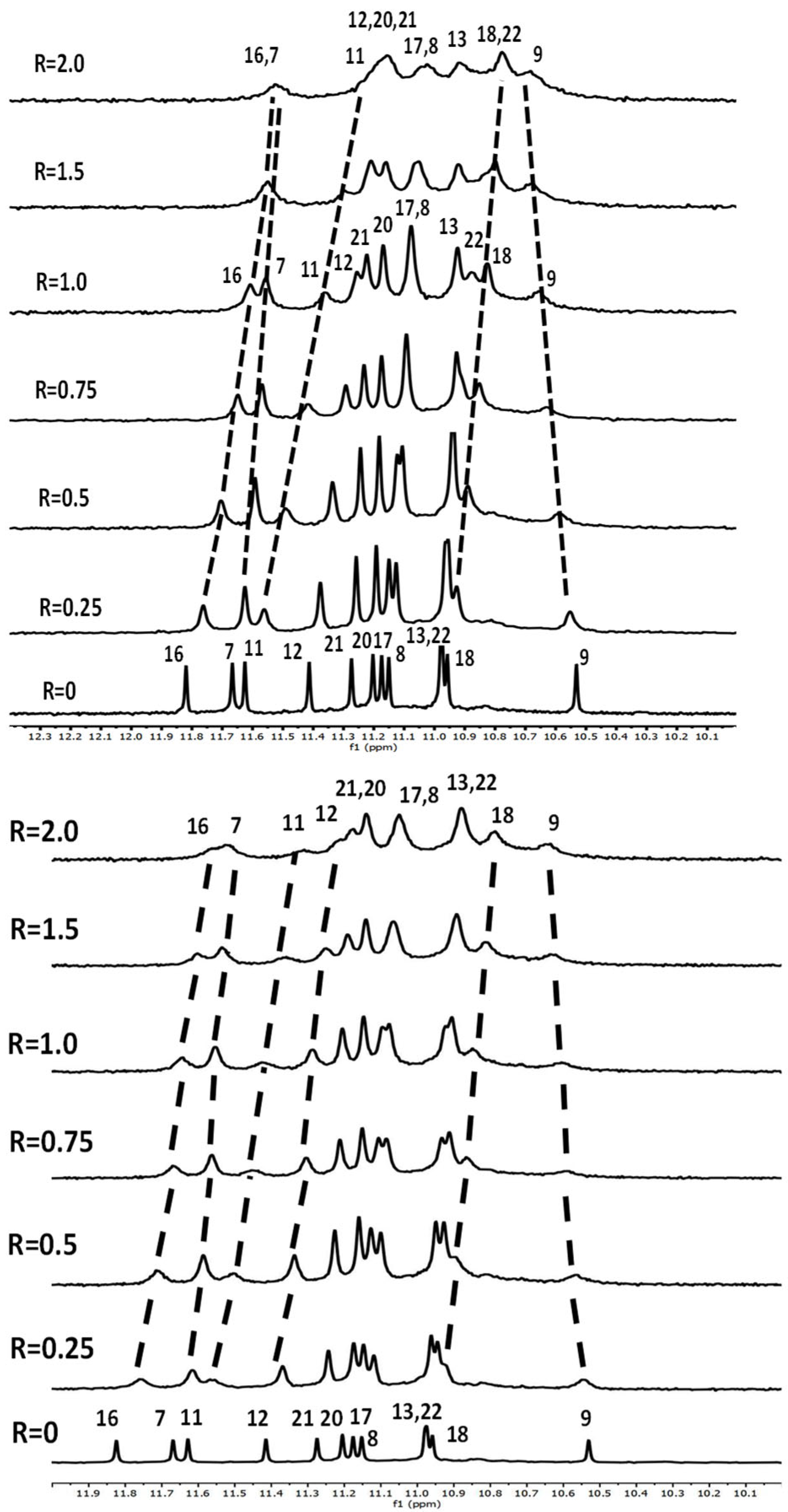
2.1.1. NMR Experiments
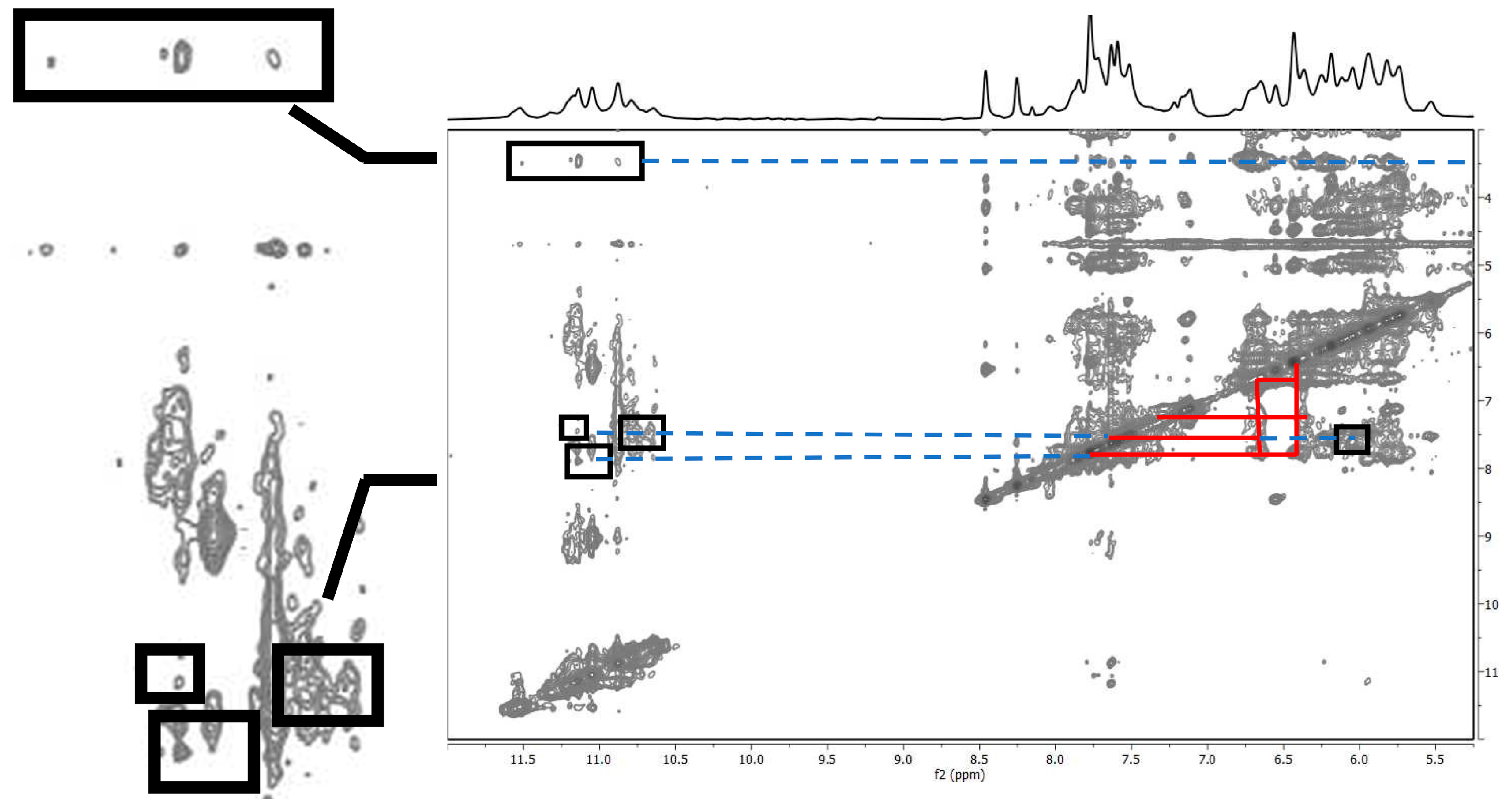
2.1.2. Interaction of Pu22T14T23 with 3
2.1.3. Interaction of Pu22T14T23 with 4
2.2. Docking Studies
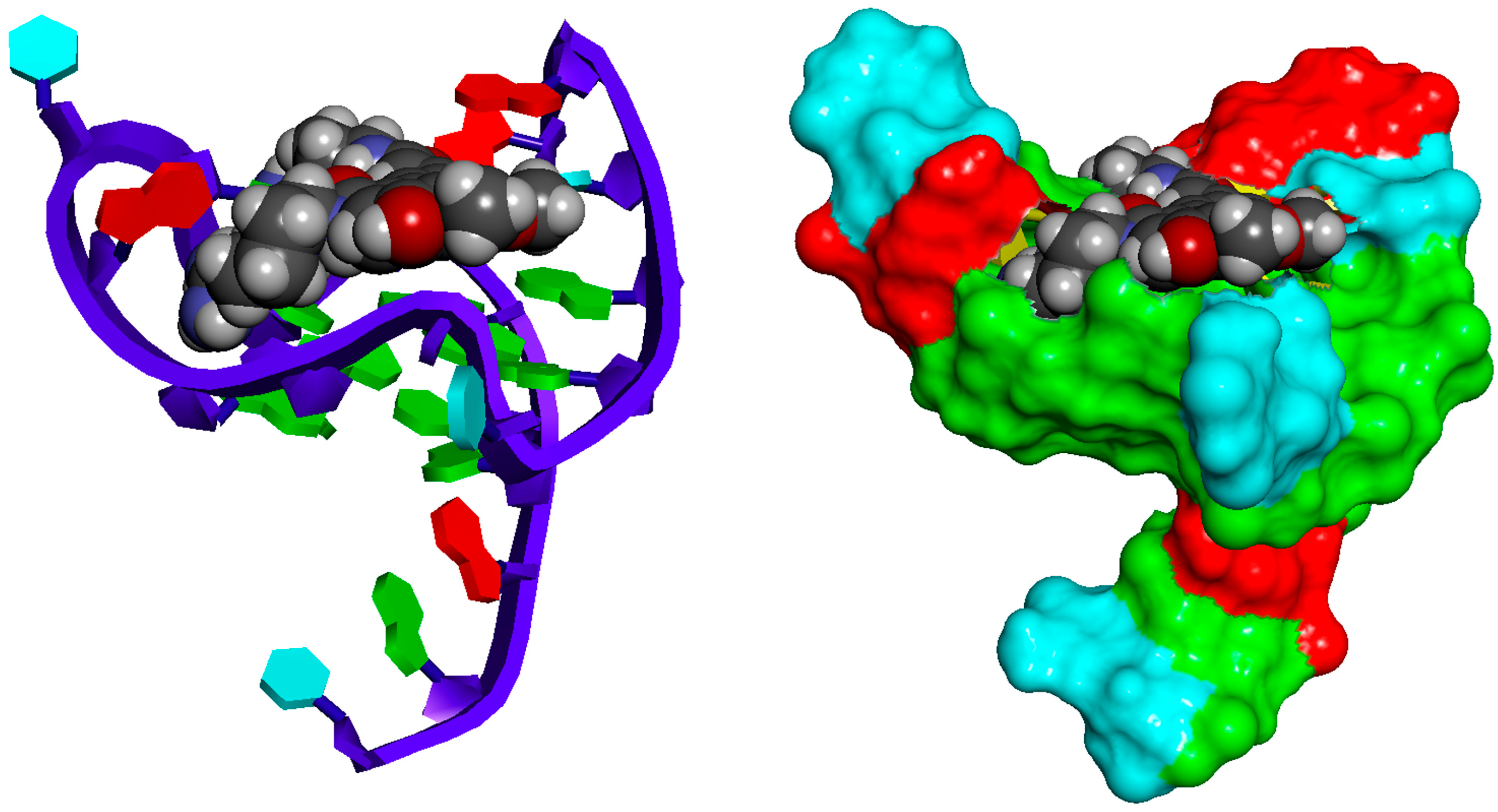
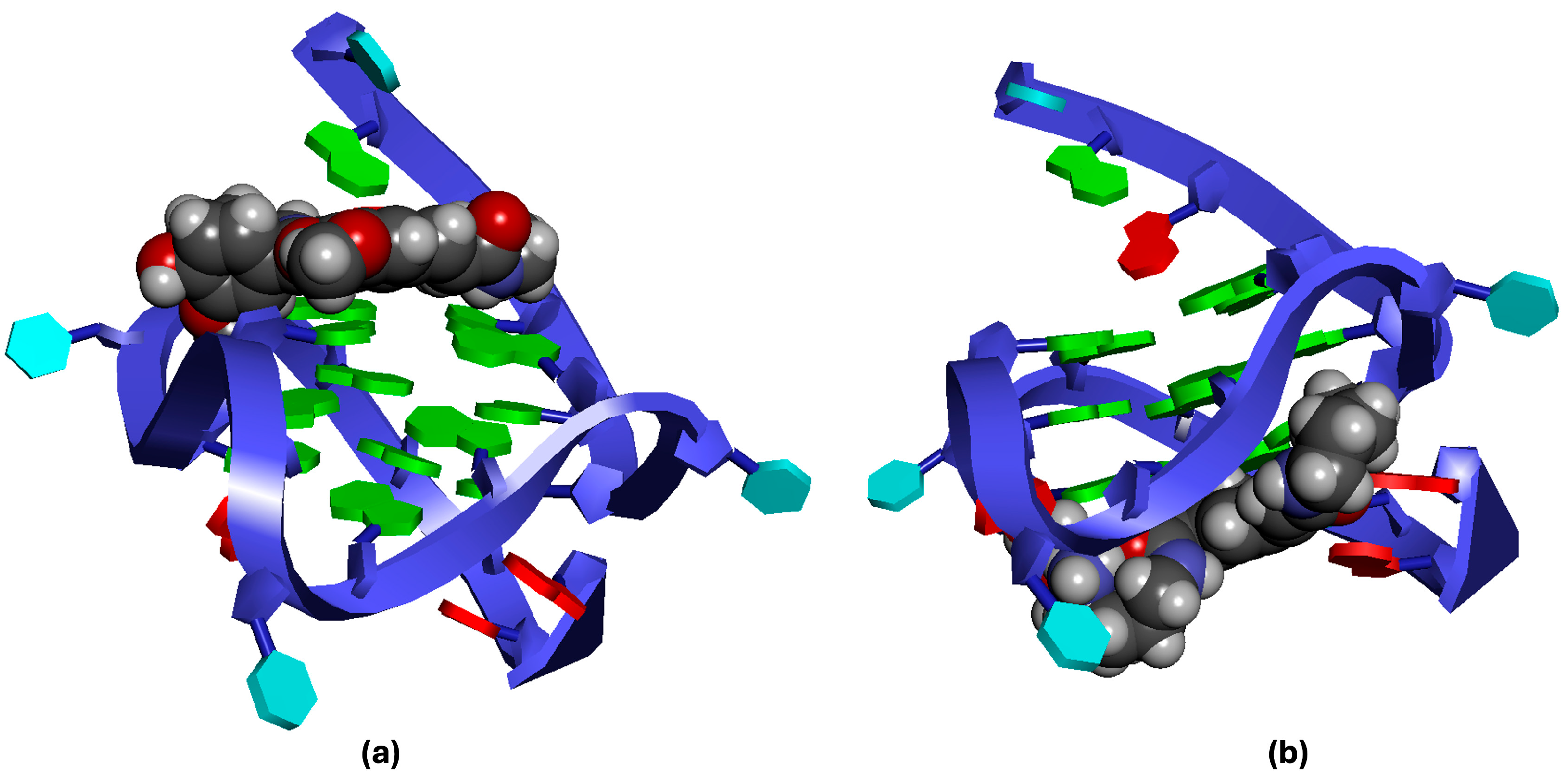
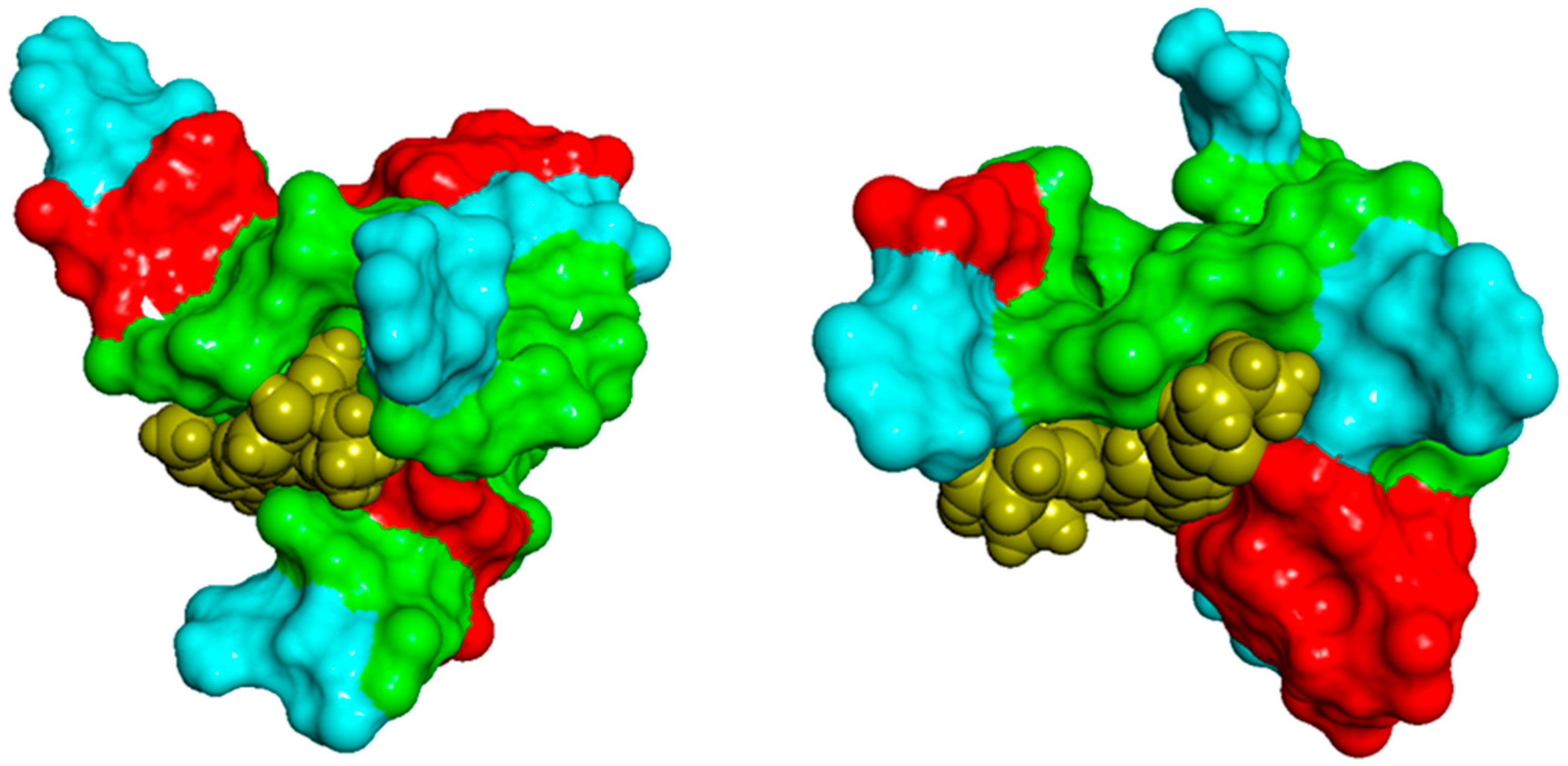
2.2.1. Docking Analysis of the Interaction Between Pu22T14T23 and 3
2.2.2. Docking Analysis of the Interaction Between Pu22T14T23 and 4
2.3. Cytotoxicity Evaluation
3. Materials and Methods
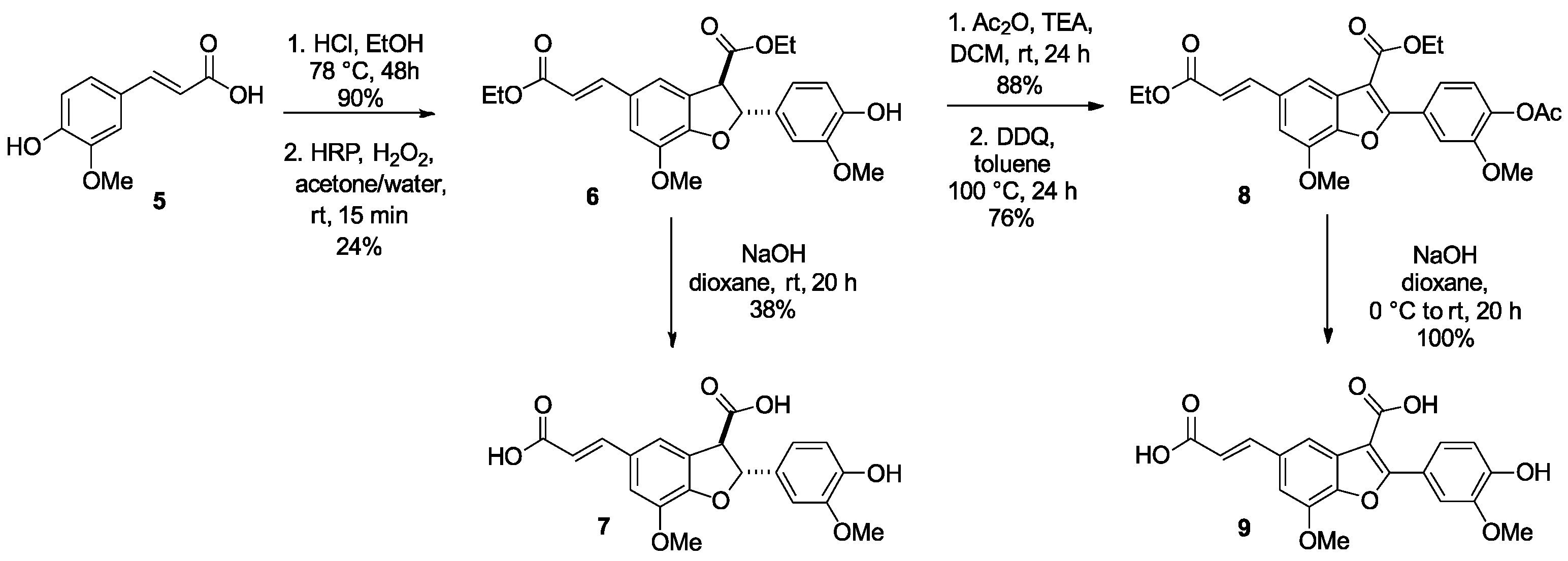
3.1. Chemistry
3.1.1. Synthetic Procedures
Synthesis of Ethyl (2R,3R)-5-((E)-3-Ethoxy-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxylate (6) and (2R,3R)-5-((E)-2-Carboxyvinyl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxylic acid (7)
Synthesis of (E)-5-(2-Carboxyvinyl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxylic acid (9)
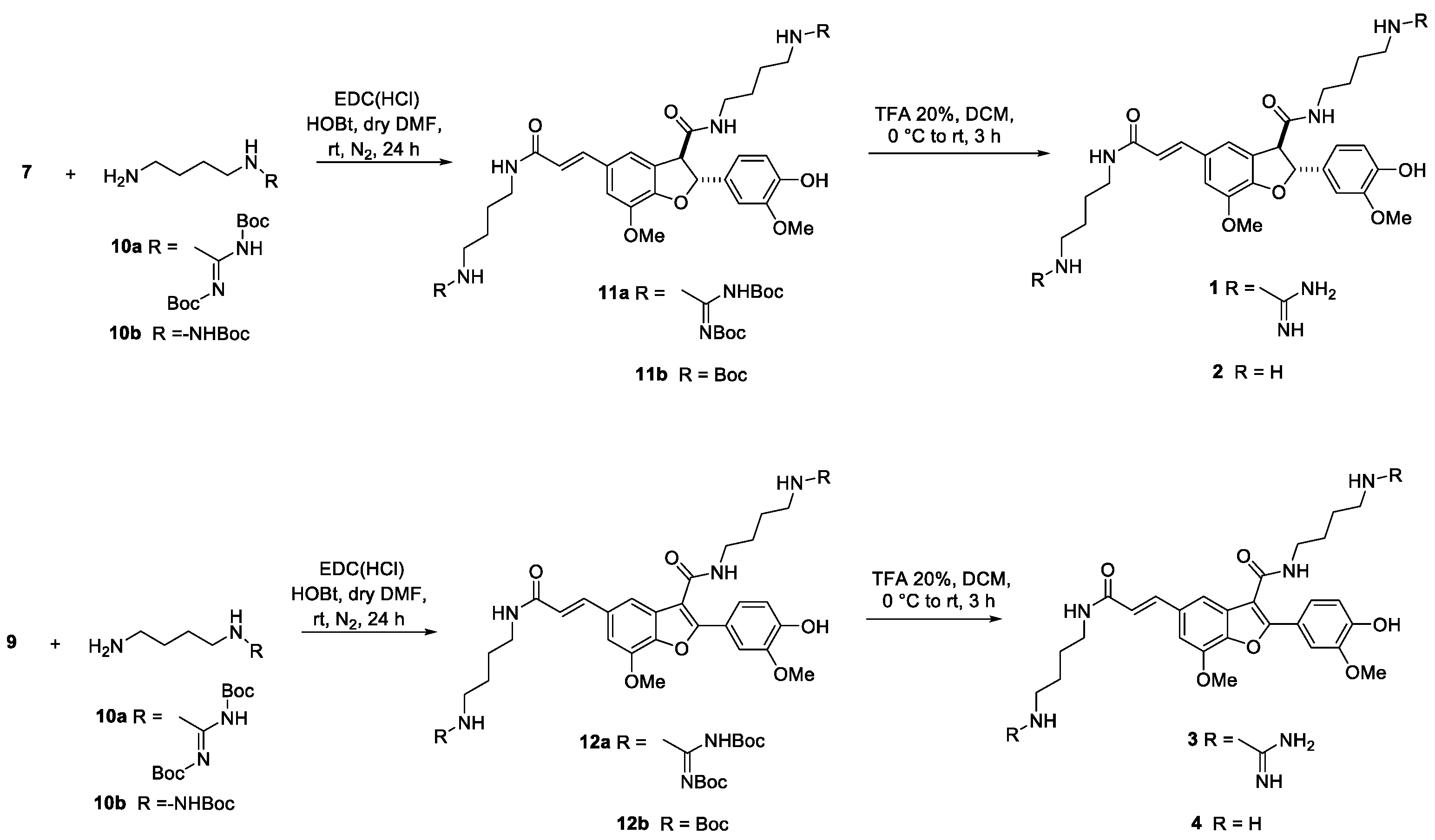
General Procedure for the Synthesis of Compounds (2R,3R)-N-(4-Guanidinobutyl)-5-((E)-3-((4-guanidinobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxamide (1) and (E)-N-(4-Guanidinobutyl)-5-(3-((4-guanidinobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxamide (3)
Tetra-Boc-Protected (2R,3R)-N-(4-Guanidinobutyl)-5-((E)-3-((4-guanidinobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxamide) (11a)
Tetra-Boc-Protected (E)-N-(4-Guanidinobutyl)-5-(3-((4-guanidinobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxamide (12a)
(2R,3R)-N-(4-Guanidinobutyl)-5-((E)-3-((4-guanidinobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxamide (rac-Hordatine C, 1)
(E)-N-(4-Guanidinobutyl)-5-(3-((4-guanidinobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxamide (3)
General Procedure for the Synthesis of (2R,3R)-N-(4-Aminobutyl)-5-((E)-3-((4-aminobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxamide (2) and (E)-N-(4-Aminobutyl)-5-(3-((4-aminobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxamide (4)
Bis-Boc-Protected (2R,3R)-N-(4-Aminobutyl)-5-((E)-3-((4-aminobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxamide (11b)
Bis-Boc-Protected (E)-N-(4-Aminobutyl)-5-(3-((4-aminobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxamide (12b)
(2R,3R)-N-(4-Aminobutyl)-5-((E)-3-((4-aminobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxy-2,3-dihydrobenzofuran-3-carboxamide (2)
(E)-N-(4-Aminobutyl)-5-(3-((4-aminobutyl)amino)-3-oxoprop-1-en-1-yl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxybenzofuran-3-carboxamide (4)
3.2. Cytotoxicity Assay
3.3. NMR Studies
3.4. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yimit, A.; Adebali, O.; Sancar, A.; Jiang, Y. Differential Damage and Repair of DNA-Adducts Induced by Anti-Cancer Drug Cisplatin across Mouse Organs. Nat. Commun. 2019, 10, 309. [Google Scholar] [CrossRef]
- Bhaduri, S.; Ranjan, N.; Arya, D.P. An Overview of Recent Advances in Duplex DNA Recognition by Small Molecules. Beilstein J. Org. Chem. 2018, 14, 1051–1086. [Google Scholar] [CrossRef]
- Montoya, S.; Soong, D.; Nguyen, N.; Affer, M.; Munamarty, S.P.; Taylor, J. Targeted Therapies in Cancer: To Be or Not to Be, Selective. Biomedicines 2021, 9, 1591. [Google Scholar] [CrossRef]
- Robinson, J.; Raguseo, F.; Nuccio, S.P.; Liano, D.; Di Antonio, M. DNA G-Quadruplex Structures: More than Simple Roadblocks to Transcription? Nucleic Acids Res. 2021, 49, 8419–8431. [Google Scholar] [CrossRef]
- Nakanishi, C.; Seimiya, H. G-Quadruplex in Cancer Biology and Drug Discovery. Biochem. Biophys. Res. Commun. 2020, 531, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.L.; Balasubramanian, S. G-Quadruplexes in Promoters throughout the Human Genome. Nucleic Acids Res. 2007, 35, 406–413. [Google Scholar] [CrossRef]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-Quadruplexes: A Promising Target for Cancer Therapy. Mol. Cancer 2021, 20, 40. [Google Scholar] [CrossRef]
- Phan, A.T.; Modi, Y.S.; Patel, D.J. Propeller-Type Parallel-Stranded G-Quadruplexes in the Human c-Myc Promoter. J. Am. Chem. Soc. 2004, 126, 8710–8716. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Thomas, S.D.; Murty, V.V.; Sedoris, K.J.; Miller, D.M. C-Myc Quadruplex-Forming Sequence Pu-27 Induces Extensive Damage in Both Telomeric and Nontelomeric Regions of DNA. J. Biol. Chem. 2014, 289, 8521–8531. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.A.; Hurley, L.H. Targeting MYC Expression through G-Quadruplexes. Genes. Cancer 2010, 1, 641–649. [Google Scholar] [CrossRef]
- Bahls, B.; Aljnadi, I.M.; Emídio, R.; Mendes, E.; Paulo, A. G-Quadruplexes in c-MYC Promoter as Targets for Cancer Therapy. Biomedicines 2023, 11, 969. [Google Scholar] [CrossRef]
- Princiotto, S.; Karelou, M.; Ioannidi, R.; Beretta, G.L.; Zaffaroni, N.; Artali, R.; Kostakis, I.K.; Mazzini, S.; Dallavalle, S. Exploring the Interaction of New Pyridoquinazoline Derivatives with G-Quadruplex in the c-MYC Promoter Region. Int. J. Mol. Sci. 2023, 24, 14346. [Google Scholar] [CrossRef] [PubMed]
- Musso, L.; Mazzini, S.; Rossini, A.; Castagnoli, L.; Scaglioni, L.; Artali, R.; Di Nicola, M.; Zunino, F.; Dallavalle, S. C-MYC G-Quadruplex Binding by the RNA Polymerase I Inhibitor BMH-21 and Analogues Revealed by a Combined NMR and Biochemical Approach. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Platella, C.; Mazzini, S.; Napolitano, E.; Mattio, L.M.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Montesarchio, D.; Dallavalle, S. Plant-Derived Stilbenoids as DNA-Binding Agents: From Monomers to Dimers. Chem. A Eur. J. 2021, 27, 8832–8845. [Google Scholar] [CrossRef] [PubMed]
- Platella, C.; Raucci, U.; Rega, N.; D’Atri, S.; Levati, L.; Roviello, G.N.; Fuggetta, M.P.; Musumeci, D.; Montesarchio, D. Shedding Light on the Interaction of Polydatin and Resveratrol with G-Quadruplex and Duplex DNA: A Biophysical, Computational and Biological Approach. Int. J. Biol. Macromol. 2020, 151, 1163–1172. [Google Scholar] [CrossRef]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour Activity of Resveratrol on Human Melanoma Cells: A Possible Mechanism Related to Its Interaction with Malignant Cell Telomerase. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 2843–2851. [Google Scholar] [CrossRef]
- Dallavalle, S.; Artali, R.; Princiotto, S.; Musso, L.; Borgonovo, G.; Mazzini, S. Investigation of the Interaction between Aloe Vera Anthraquinone Metabolites and C-Myc and C-Kit G-Quadruplex DNA Structures. Int. J. Mol. Sci. 2022, 23, 16018. [Google Scholar] [CrossRef]
- van Zadelhoff, A.; Vincken, J.P.; de Bruijn, W.J.C. Exploring the Formation of Heterodimers of Barley Hydroxycinnamoylagmatines by Oxidative Enzymes. Food Chem. 2024, 446, 138898. [Google Scholar] [CrossRef]
- Ube, N.; Ishihara, A.; Yabuta, Y.; Taketa, S.; Kato, Y.; Nomura, T. Molecular Identification of a Laccase That Catalyzes the Oxidative Coupling of a Hydroxycinnamic Acid Amide for Hordatine Biosynthesis in Barley. Plant J. 2023, 115, 1037–1050. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Bernardi, D.I.; Fill, T.; Fernandes, A.A.G.; Jurberg, I.D. The Chemistry and Biology of Guanidine Secondary Metabolites. Nat. Prod. Rep. 2021, 38, 586–667. [Google Scholar] [CrossRef]
- Pandya, N.; Rani, R.; Kumar, V.; Kumar, A. Discovery of a Potent Guanidine Derivative That Selectively Binds and Stabilizes the Human BCL-2 G-Quadruplex DNA and Downregulates the Transcription. Gene 2023, 851, 146975. [Google Scholar] [CrossRef]
- Gomes, A.R.; Varela, C.L.; Pires, A.S.; Tavares-da-Silva, E.J.; Roleira, F.M.F. Synthetic and Natural Guanidine Derivatives as Antitumor and Antimicrobial Agents: A Review. Bioorg Chem. 2023, 138, 106600. [Google Scholar] [CrossRef]
- Pantos, A.; Tsogas, I.; Paleos, C.M. Guanidinium Group: A Versatile Moiety Inducing Transport and Multicompartmentalization in Complementary Membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Gao, R.; Piotrowski, J.S.; Kabbage, M.; Lu, F.; Ralph, J. Scaled-up Production of Poacic Acid, a Plant-Derived Antifungal Agent. Ind. Crops Prod. 2017, 103, 240–243. [Google Scholar] [CrossRef]
- Dai, J.; Carver, M.; Hurley, L.H.; Yang, D. Solution Structure of a 2:1 Quindoline–c-MYC G-Quadruplex: Insights into G-Quadruplex-Interactive Small Molecule Drug Design. J. Am. Chem. Soc. 2011, 133, 17673–17680. [Google Scholar] [CrossRef]
- Walz, S.; Lorenzin, F.; Morton, J.; Wiese, K.E.; von Eyss, B.; Herold, S.; Rycak, L.; Dumay-Odelot, H.; Karim, S.; Bartkuhn, M.; et al. Activation and Repression by Oncogenic MYC Shape Tumour-Specific Gene Expression Profiles. Nature 2014, 511, 483–487. [Google Scholar] [CrossRef]
- Gupta, N.; Jung, K.; Wu, C.; Alshareef, A.; Alqahtani, H.; Damaraju, S.; Mackey, J.R.; Ghosh, S.; Sabri, S.; Abdulkarim, B.S.; et al. High Myc Expression and Transcription Activity Underlies Intra-Tumoral Heterogeneity in Triple-Negative Breast Cancer. Oncotarget 2017, 8, 28101–28115. [Google Scholar] [CrossRef]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and Synthesis of New Ferulic Acid Dehydrodimers Present in Grass Cell Walls. J. Chem. Soc. Perkin Trans. 1994, 1, 3485. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Wang, W.; Sang, S. Germination under the Dark as an Efficient Method to Enrich Barley Hordatine Aglycones and to Prepare a Hordatine-Rich Fraction. Food Chem. 2025, 472, 142963. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, T.; Nitta, M.; Kasahara, K.; Chiba, T.; Yiping, Y.; Tsuji, K.; Kan, T.; Nukaya, H.; Ishiguro, M.; Koike, M.; et al. Structure-Activity Relationship Study on A1 Adrenergic Receptor Antagonists from Beer. Bioorg Med. Chem. Lett. 2009, 19, 5905–5908. [Google Scholar] [CrossRef]
- Ambrus, A.; Chen, D.; Dai, J.; Jones, R.A.; Yang, D. Solution Structure of the Biologically Relevant G-Quadruplex Element in the Human c-MYC Promoter. Implications for G-Quadruplex Stabilization. Biochemistry 2005, 44, 2048–2058. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Vriend, G. New Ways to Boost Molecular Dynamics Simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Murillo, R.; Robertson, J.C.; Zgarbová, M.; Šponer, J.; Otyepka, M.; Jurečka, P.; Cheatham, T.E. Assessing the Current State of Amber Force Field Modifications for DNA. J. Chem. Theory Comput. 2016, 12, 4114–4127. [Google Scholar] [CrossRef]
- BIOVIA. Discovery Studio Visualize Dassault Systèmes; BIOVIA: San Diego, CA, USA, 2019. [Google Scholar]



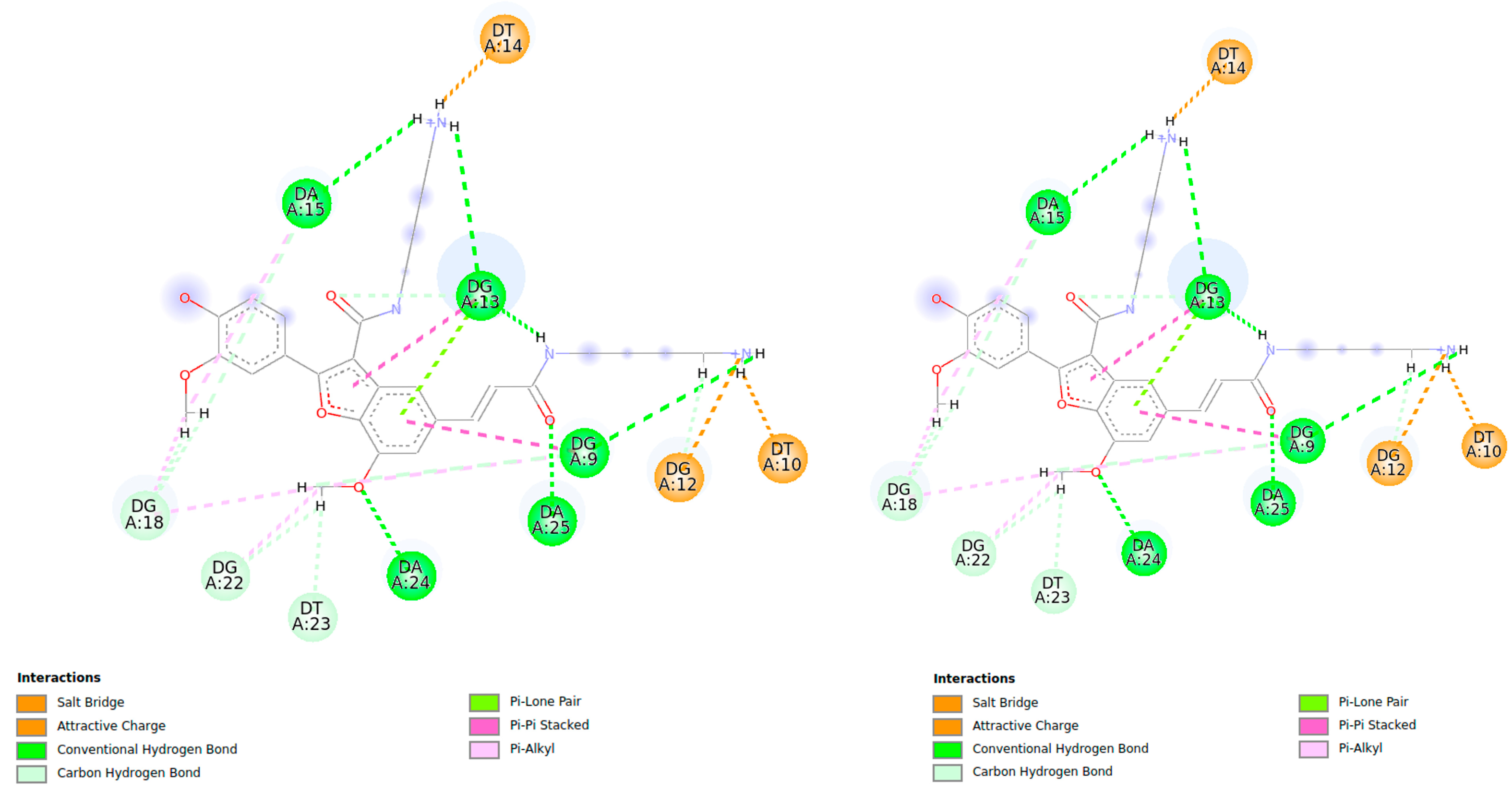
| Compound 3 | Pu22T14T23 3’-End | d(Å) from Docking Studies |
|---|---|---|
| Har (o OCH3 bf) a | NHG18 | 4.28 |
| Har (o OCH3 bf) a | NHG22 | 4.13 |
| OCH3 bf | NHG22 | 4.77 |
| Har (o OCH3 on Phe-OH) | H8G22 | 5.39 |
| Har (p OCH3 bf) | NHG13 | 3.10 |
| 5’-end | ||
| OCH3 bf | NHG7 | 3.65 |
| OCH3 | NHG20 | 4.94 |
| IC50 (µM, 72 h) | ||
|---|---|---|
| Compound | U2OS | MDA-MD-231 |
| RHPS4 | 4.7 ± 0.84 | 11.2 ± 7.4 |
| 1 | 93 ± 9 | 89 ± 5.2 |
| 2 | >100 | >100 |
| 3 | 56.9 ± 0.07 | 44 ± 5.6 |
| 4 | >100 | >100 |
| 9 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dozio, D.; Caccia, A.; Dallavalle, S.; Beretta, G.L.; Perego, P.; Artali, R.; Mazzini, S.; Princiotto, S. Exploring Guanidinium Group Involvement in Hordatine Interactions with the G-Quadruplex Motif Within the c-MYC Promoter Region. Int. J. Mol. Sci. 2025, 26, 10580. https://doi.org/10.3390/ijms262110580
Dozio D, Caccia A, Dallavalle S, Beretta GL, Perego P, Artali R, Mazzini S, Princiotto S. Exploring Guanidinium Group Involvement in Hordatine Interactions with the G-Quadruplex Motif Within the c-MYC Promoter Region. International Journal of Molecular Sciences. 2025; 26(21):10580. https://doi.org/10.3390/ijms262110580
Chicago/Turabian StyleDozio, Denise, Aziza Caccia, Sabrina Dallavalle, Giovanni Luca Beretta, Paola Perego, Roberto Artali, Stefania Mazzini, and Salvatore Princiotto. 2025. "Exploring Guanidinium Group Involvement in Hordatine Interactions with the G-Quadruplex Motif Within the c-MYC Promoter Region" International Journal of Molecular Sciences 26, no. 21: 10580. https://doi.org/10.3390/ijms262110580
APA StyleDozio, D., Caccia, A., Dallavalle, S., Beretta, G. L., Perego, P., Artali, R., Mazzini, S., & Princiotto, S. (2025). Exploring Guanidinium Group Involvement in Hordatine Interactions with the G-Quadruplex Motif Within the c-MYC Promoter Region. International Journal of Molecular Sciences, 26(21), 10580. https://doi.org/10.3390/ijms262110580











