Mechanisms of Mitochondrial Transfer Through TNTs: From Organelle Dynamics to Cellular Crosstalk
Abstract
1. Introduction
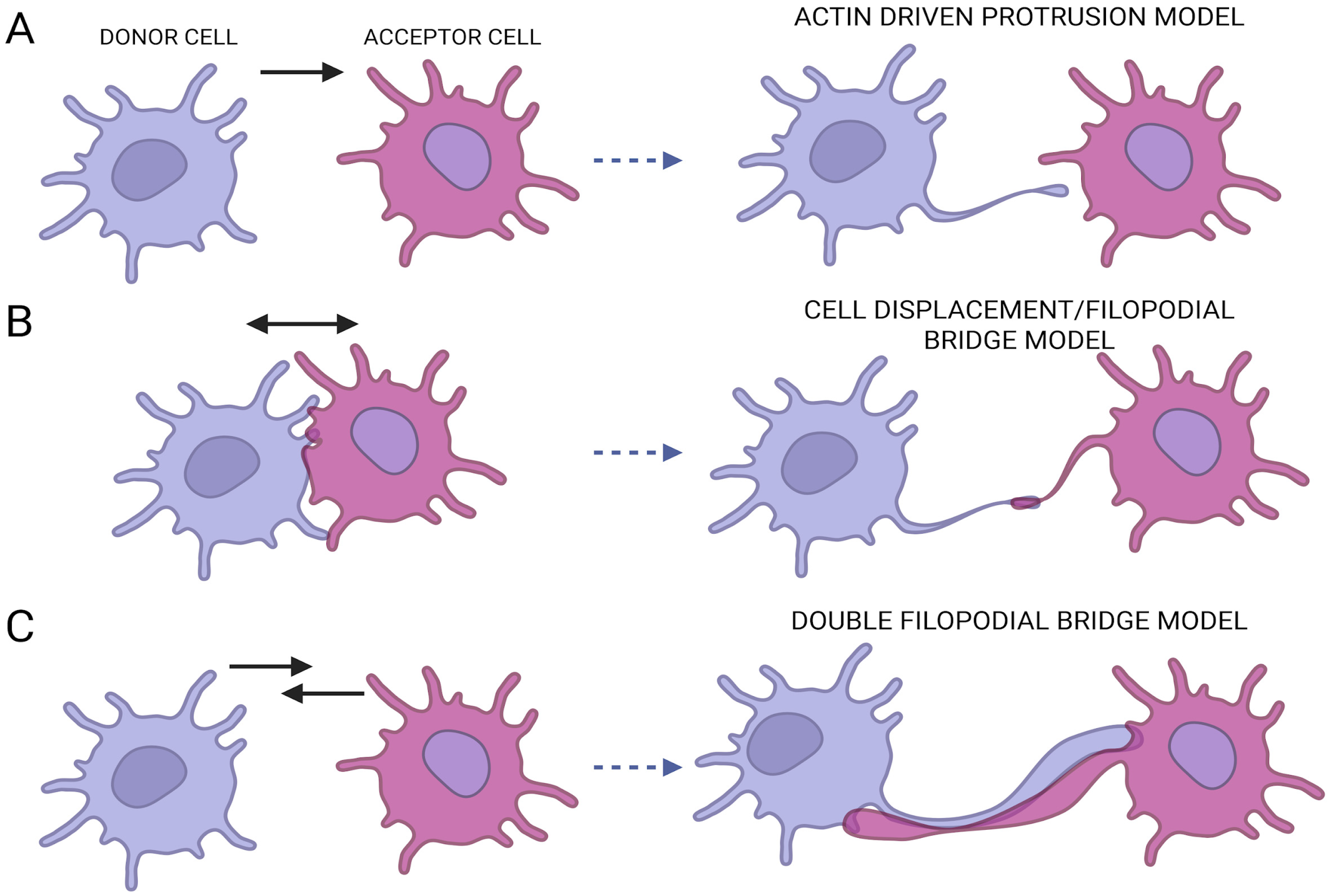
2. TNTs Biogenesis and Structure
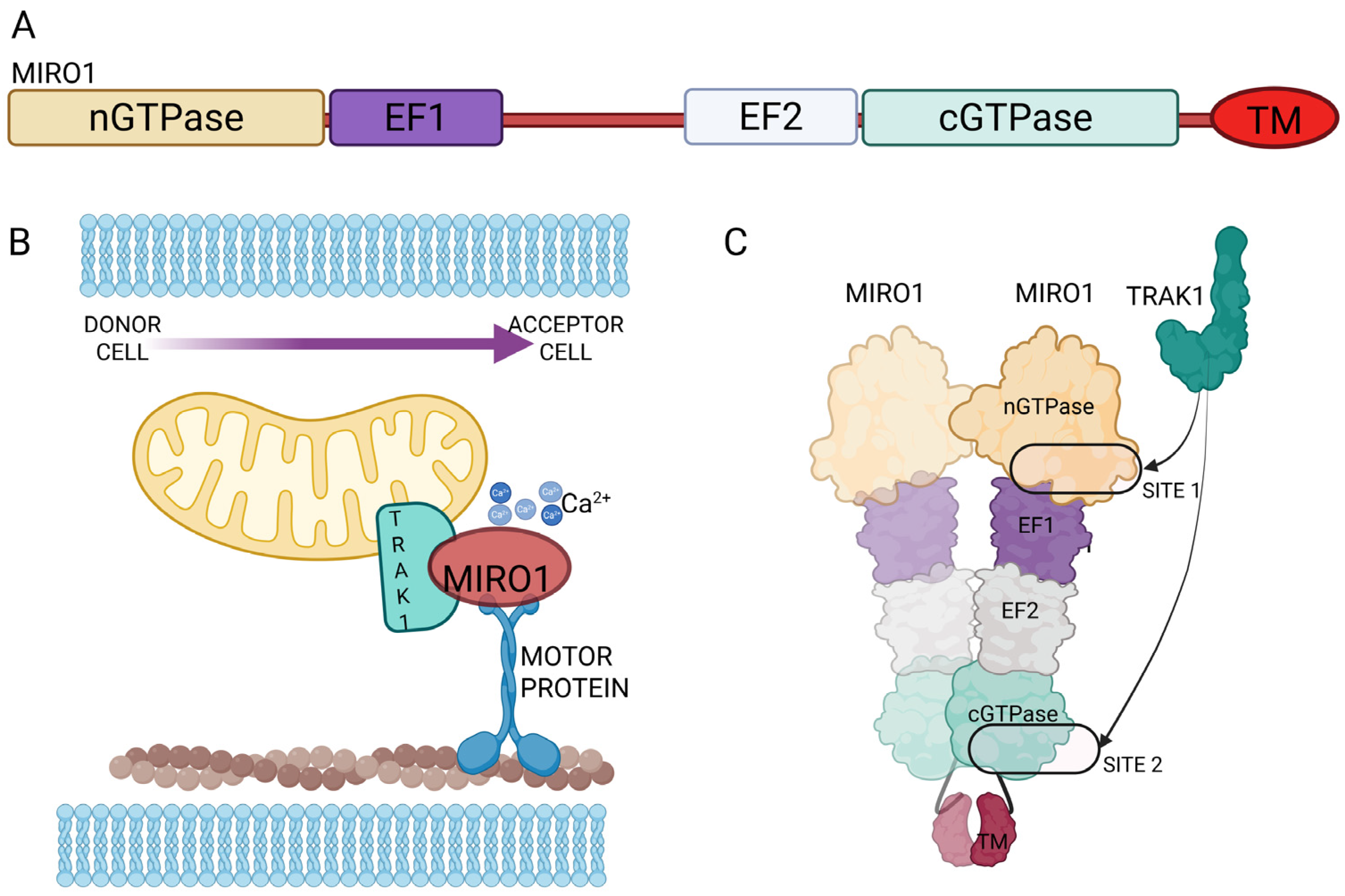
3. Mitochondrial Mobilization Along TNTs: Miro1 Mediated Transport
4. Mitochondria Shape Can Influence Their Transfer Through TNTs
5. Cytoskeletal Proteins as Regulators of TNTs-Mediated Mitochondrial Transfer
6. Tunneling Nanotubes (TNTs) and Mitochondrial Transfer in Pathological Condition
6.1. Tunneling Nanotubes and Mitochondrial Transfer in Cancer: Mechanisms of Adaptation, Resistance, and Immune Modulation Across Tumor Types
6.2. Tunneling Nanotubes and Mitochondrial Crosstalk in Cardiovascular Pathophysiology
6.3. Tunneling Nanotube-Mediated Mitochondrial Transfer in Neurodegenerative Diseases: A Double-Edged Sword
7. Translational Implication of Mitochondrial Transfer Across TNTs
Evidence and Challenges in Demonstrating TNTs In Vivo
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferenczy, L.; Maráz, A. Transfer of mitochondria by protoplast fusion in Saccharomyces cerevisiae. Nature 1977, 268, 524–525. [Google Scholar] [CrossRef]
- Perrier, Q.; Lisi, V.; Fisherwellman, K.; Lablanche, S.; Asthana, A.; Orlando, G.; Maiocchi, S. Therapeutic transplantation of mitochondria and Extracellular Vesicles: Mechanistic insights into mitochondria bioenergetics, redox signaling, and organelle dynamics in preclinical models. Free Radic. Biol. Med. 2025, 238, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Singh, K.K.; Aquilano, K.; Becker, L.B.; Berridge, M.V.; Boilard, E.; Caicedo, A.; Crewe, C.; Enríquez, J.A.; Gao, J.; et al. Recommendations for mitochondria transfer and transplantation nomenclature and characterization. Nat. Metab. 2025, 7, 53–67. [Google Scholar] [CrossRef]
- Hazan Ben-Menachem, R.; Pines, O.; Saada, A. Mitochondrial derived vesicles- Quo Vadis? FEBS J. 2024, 291, 4660–4669. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qin, A.; Liu, D.; Ruan, R.; Wang, Q.; Yuan, J.; Cheng, T.S.; Filipovska, A.; Papadimitriou, J.M.; Dai, K.; et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci. Adv. 2019, 5, eaaw7215. [Google Scholar] [CrossRef]
- Liao, P.; Chen, L.; Zhou, H.; Mei, J.; Chen, Z.; Wang, B.; Feng, J.Q.; Li, G.; Tong, S.; Zhou, J.; et al. Osteocyte mitochondria regulate angiogenesis of transcortical vessels. Nat. Commun. 2024, 15, 2529. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Khryapenkova, T.G.; Vasileva, A.K.; Marey, M.V.; Galkina, S.I.; Isaev, N.K.; Sheval, E.V.; Polyakov, V.Y.; Sukhikh, G.T.; Zorov, D.B. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J. Cell. Mol. Med. 2008, 12, 1622–1631. [Google Scholar] [CrossRef]
- Hase, K.; Kimura, S.; Takatsu, H.; Ohmae, M.; Kawano, S.; Kitamura, H.; Ito, M.; Watarai, H.; Hazelett, C.C.; Yeaman, C.; et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 2009, 11, 1427–1432. [Google Scholar] [CrossRef]
- Hanna, S.J.; McCoy-Simandle, K.; Miskolci, V.; Guo, P.; Cammer, M.; Hodgson, L.; Cox, D. The Role of Rho-GTPases and actin polymerization during Macrophage Tunneling Nanotube Biogenesis. Sci. Rep. 2017, 7, 8547. [Google Scholar] [CrossRef]
- Henderson, J.M.; Ljubojevic, N.; Belian, S.; Chaze, T.; Castaneda, D.; Battistella, A.; Gianetto, Q.G.; Matondo, M.; Descroix, S.; Bassereau, P.; et al. Tunnelling nanotube formation is driven by Eps8/IRSp53-dependent linear actin polymerization. EMBO J. 2023, 42, e113761. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, H.H.; Carvalho, R.N. Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 2008, 20, 470–475. [Google Scholar] [CrossRef]
- Chang, M.; Lee O-c Bu, G.; Oh, J.; Yunn, N.-O.; Ryu, S.H.; Kwon, H.-B.; Kolomeisky, A.B.; Shim, S.-H.; Doh, J.; Jeon, J.H.; et al. Formation of cellular close-ended tunneling nanotubes through mechanical deformation. Sci. Adv. 2022, 8, eabj3995. [Google Scholar] [CrossRef]
- Belian, S.; Korenkova, O.; Zurzolo, C. Actin-based protrusions at a glance. J. Cell Sci. 2023, 136, jcs261156. [Google Scholar] [CrossRef]
- Sahinbegovic, H.; Jelinek, T.; Hrdinka, M.; Bago, J.R.; Turi, M.; Sevcikova, T.; Kurtovic-Kozaric, A.; Hajek, R.; Simicek, M. Intercellular Mitochondrial Transfer in the Tumor Microenvironment. Cancers 2020, 12, 1787. [Google Scholar] [CrossRef]
- Fransson, S.; Ruusala, A.; Aspenström, P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 2006, 344, 500–510. [Google Scholar] [CrossRef]
- Wang, X.; Winter, D.; Ashrafi, G.; Schlehe, J.; Wong, Y.L.; Selkoe, D.; Rice, S.; Steen, J.; LaVoie, M.J.; Schwarz, T.L. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 2011, 147, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Birsa, N.; Norkett, R.; Wauer, T.; Mevissen, T.E.; Wu, H.C.; Foltynie, T.; Bhatia, K.; Hirst, W.D.; Komander, D.; Plun-Favreau, H.; et al. Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J. Biol. Chem. 2014, 289, 14569–14582. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Singh, S.; Rehman, R.; Tiwari, B.K.; A Jha, K.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Las, G.; Shirihai, O.S. Miro1: New wheels for transferring mitochondria. EMBO J. 2014, 33, 939–941. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Jiang, D.; Liang, X.; Liao, S.; Zhang, Z.; Yue, W.; Li, X.; Chiu, S.-M.; Chai, Y.-H.; et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Rep. 2016, 7, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Zaninello, M.; Bean, C. Highly Specialized Mechanisms for Mitochondrial Transport in Neurons: From Intracellular Mobility to Intercellular Transfer of Mitochondria. Biomolecules 2023, 13, 938. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 2018, 23, 687. [Google Scholar] [CrossRef]
- Boukelmoune, N.; Chiu, G.S.; Kavelaars, A.; Heijnen, C.J. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol. Commun. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Shepherd, A.; Uzor, N.E.; Trinh, R.; Kavelaars, A.; Heijnen, C.J. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol. Commun. 2020, 8, 36. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.; Lu, J.; Pei, G. Mitochondria Are Dynamically Transferring Between Human Neural Cells and Alexander Disease-Associated GFAP Mutations Impair the Astrocytic Transfer. Front. Cell Neurosci. 2019, 13, 316. [Google Scholar] [CrossRef]
- Tseng, N.; Lambie, S.C.; Huynh, C.Q.; Sanford, B.; Patel, M.; Herson, P.S.; Ormond, D.R. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow Metab. 2021, 41, 761–770. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Liu, S.; Xiao, J.; He, Y.; Shao, Z.; Zhang, Y.; Cai, X.; Xiong, L. Tunneling Nanotube-Mediated Mitochondrial Transfer Rescues Nucleus Pulposus Cells from Mitochondrial Dysfunction and Apoptosis. Oxidative Med. Cell. Longev. 2022, 2022, 3613319. [Google Scholar] [CrossRef]
- Ding, P.; Gao, C.; Zhou, J.; Mei, J.; Li, G.; Liu, D.; Li, H.; Liao, P.; Yao, M.; Wang, B.; et al. Mitochondria from osteolineage cells regulate myeloid cell-mediated bone resorption. Nat. Commun. 2024, 15, 5094. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Corbetta, B.; Bellini, S.; Gambino, R.; Bruno, S.; Kimura, S.; Hase, K.; Ohno, H.; Gruden, G. Protective effect of mesenchymal stromal cells in diabetic nephropathy: The In vitro and In vivo role of the M-Sec-tunneling nanotubes. Clin. Sci. 2024, 138, 1537–1559. [Google Scholar] [CrossRef]
- Novak, J.; Nahacka, Z.; Oliveira, G.L.; Brisudova, P.; Dubisova, M.; Dvorakova, S.; Miklovicova, S.; Dalecka, M.; Puttrich, V.; Grycova, L.; et al. The adaptor protein Miro1 modulates horizontal transfer of mitochondria in mouse melanoma models. Cell Rep. 2025, 44, 115154. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Huang, X.; Guo, H.; Li, Z.; Fan, S.; Qin, H.; Meng, F.; Liu, P.; Wang, X.; et al. Dynamic three-dimensional culture enhances tunneling nanotubes-mediated mitochondrial transfer in mesenchymal stromal cells to accelerate wound healing. J. Nanobiotechnol. 2025, 23, 559. [Google Scholar] [CrossRef] [PubMed]
- Ravitch, E.E.; Baltrusaitis, E.E.; Perez, T.A.; Barrie, K.R.; Fenton, A.R.; Holzbaur, E.L.F.; Dominguez, R. Structural-functional characterization of the MIRO1-TRAK1 complex. Nat. Commun. 2025, 16, 6173. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Scorrano, L. Determinants and outcomes of mitochondrial dynamics. Mol. Cell 2023, 83, 857–876. [Google Scholar] [CrossRef]
- Nasoni, M.G.; Carloni, S.; Canonico, B.; Burattini, S.; Cesarini, E.; Papa, S.; Pagliarini, M.; Ambrogini, P.; Balduini, W.; Luchetti, F. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal Res. 2021, 71, e12747. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.R.; Blackstone, C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010, 1201, 34–39. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Jr Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kapur, M.; Li, M.; Choi, M.C.; Choi, S.; Kim, H.J.; Kim, I.; Lee, E.; Taylor, J.P.; Yao, T.-P. MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria. J. Cell Sci. 2014, 127 Pt 22, 4954–4963. [Google Scholar] [CrossRef]
- Pyakurel, A.; Savoia, C.; Hess, D.; Scorrano, L. Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Mol. Cell 2015, 58, 244–254. [Google Scholar] [CrossRef]
- de Brito, O.M.; Scorrano, L. Mitofusin 2: A Mitochondria-Shaping Protein with Signaling Roles Beyond Fusion. Antioxidants Redox Signal. 2008, 10, 621–634. [Google Scholar] [CrossRef]
- Naón, D.; Hernández-Alvarez, M.I.; Shinjo, S.; Wieczor, M.; Ivanova, S.; Martins de Brito, O.; Quintana, A.; Hidalgo, J.; Palacín, M.; Aparicio, P.; et al. Splice variants of mitofusin 2 shape the endoplasmic reticulum and tether it to mitochondria. Science 2023, 380, eadh9351. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Lampe, P.A.; Stojanovski, D.; Korwitz, A.; Anand, R.; Tatsuta, T.; Langer, T. Stress-induced OMA1 activation and autocatalytic turnover regulate OPA1-dependent mitochondrial dynamics. EMBO J. 2014, 33, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, M.; Kohr, M.J.; Quirin, C.; Menabò, R.; Alanova, P.; Alan, L.; Pellattiero, A.; Murphy, E.; Di Lisa, F.; Scorrano, L. Oxidization of optic atrophy 1 cysteines occurs during heart ischemia-reperfusion and amplifies cell death by oxidative stress. Redox Biol. 2023, 63, 102755. [Google Scholar] [CrossRef]
- Jabbar, J.; Afroze, B.; Ling, N.X.Y.; Oakhill, J.S.; Rouiller, I. Lysine acetylation modulates s-OPA1 GTPase activity and oligomerization in mitochondrial membrane remodeling. Protein Sci. 2025, 34, e70179. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, F.X.; Zhou, H.; Lu, Y.Y.; Tan, H.; Yu, S.J.; Yuan, J.; Liu, H.; Meng, W.; Jin, Z.-B. Bioenergetic Crosstalk between Mesenchymal Stem Cells and various Ocular Cells through the intercellular trafficking of Mitochondria. Theranostics 2020, 10, 7260–7272. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, F.; Jiang, D.; Xu, Z.; Zhang, H.; Jin, Z.B. ROCK inhibitor enhances mitochondrial transfer via tunneling nanotubes in retinal pigment epithelium. Theranostics 2024, 14, 5762–5777. [Google Scholar] [CrossRef]
- Simone, L.; Capobianco, D.L.; Di Palma, F.; Binda, E.; Legnani, F.G.; Vescovi, A.L.; Svelto, M.; Pisani, F. GFAP serves as a structural element of tunneling nanotubes between glioblastoma cells and could play a role in the intercellular transfer of mitochondria. Front. Cell Dev. Biol. 2023, 11, 1221671. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Cheng, H.; Song, L.; Liu, J.; Caplan, S.; Zhu, L.; Wu, J.Y. MICAL2PV suppresses the formation of tunneling nanotubes and modulates mitochondrial trafficking. EMBO Rep. 2021, 22, e52006. [Google Scholar] [CrossRef] [PubMed]
- Marlein, C.R.; Piddock, R.E.; Mistry, J.J.; Zaitseva, L.; Hellmich, C.; Horton, R.H.; Zhou, Z.; Auger, M.J.; Bowles, K.M.; Rushworth, S.A. CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res. 2019, 79, 2285–2297. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Fan, X.L.; Jiang, D.; Zhang, Y.; Li, X.; Xu, Z.B.; Fang, S.-B.; Chiu, S.; Tse, H.-F.; Lian, Q.; et al. Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation. Stem Cell Rep. 2018, 11, 1120–1135. [Google Scholar] [CrossRef]
- Barutta, F.; Kimura, S.; Hase, K.; Bellini, S.; Corbetta, B.; Corbelli, A.; Fiordaliso, F.; Barreca, A.; Papotti, M.G.; Ghiggeri, G.M.; et al. Protective Role of the M-Sec-Tunneling Nanotube System in Podocytes. J. Am. Soc. Nephrol. 2021, 32, 1114–1130. [Google Scholar] [CrossRef] [PubMed]
- Kukita, T.; Takahashi, A.; Zhang, J.Q.; Kukita, A. Membrane nanotube formation in osteoclastogenesis. Methods Mol. Biol. 2015, 1313, 193–202. [Google Scholar] [CrossRef]
- Takahashi, A.; Kukita, A.; Li, Y.J.; Zhang, J.Q.; Nomiyama, H.; Yamaza, T.; Ayukawa, Y.; Koyano, K.; Kukita, T. Tunneling nanotube formation is essential for the regulation of osteoclastogenesis. J. Cell. Biochem. 2013, 114, 1238–1247. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Li, H.; Wang, Q.; Mu, D. Enhancing fat graft survival: Thymosin beta-4 facilitates mitochondrial transfer from ADSCs via tunneling nanotubes by upregulating the Rac/F-actin pathway. Free Radic. Biol. Med. 2025, 228, 281–298. [Google Scholar] [CrossRef]
- Baldwin, J.G.; Heuser-Loy, C.; Saha, T.; Schelker, R.C.; Slavkovic-Lukic, D.; Strieder, N.; Hernandez-Lopez, I.; Rana, N.; Barden, M.; Mastrogiovanni, F.; et al. Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy. Cell 2024, 187, 6614–6630.e21. [Google Scholar] [CrossRef]
- Civita, P.; Leite, D.M.; Pilkington, G.J. Pre-Clinical Drug Testing in 2D and 3D Human In Vitro Models of Glioblastoma Incorporating Non-Neoplastic Astrocytes: Tunneling Nano Tubules and Mitochondrial Transfer Modulates Cell Behavior and Therapeutic Respons. Int. J. Mol. Sci. 2019, 20, 6017. [Google Scholar] [CrossRef]
- Valdebenito, S.; Audia, A.; Bhat, K.P.L.; Okafo, G.; Eugenin, E.A. Tunneling Nanotubes Mediate Adaptation of Glioblastoma Cells to Temozolomide and Ionizing Radiation Treatment. iScience 2020, 23, 101450. [Google Scholar] [CrossRef]
- Marlein, C.R.; Zaitseva, L.; Piddock, R.E.; Robinson, S.D.; Edwards, D.R.; Shafat, M.S.; Zhou, Z.; Lawes, M.; Bowles, K.M.; Rushworth, S.A. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood 2017, 130, 1649–1660. [Google Scholar] [CrossRef]
- Liu, R.; Shan, W.; Wang, Z.; Wang, H.; Li, C.; Yang, L.; Guo, R. Unveiling mitochondrial transfer in tumor immune evasion: Mechanisms, challenges, and clinical implications. Front. Immunol. 2025, 16, 1625814. [Google Scholar] [CrossRef]
- Jing, M.; Xiong, X.; Mao, X.; Song, Q.; Zhang, L.; Ouyang, Y.; Pang, Y.; Fu, Y.; Yan, W. HMGB1 promotes mitochondrial transfer between hepatocellular carcinoma cells through RHOT1 and RAC1 under hypoxia. Cell Death Dis. 2024, 15, 155. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, X.; Li, F.; Yu, Y.; Chen, Z.; Liu, Z.; Wang, Z.; Xu, H.; Yang, W. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget 2017, 8, 15539–15552. [Google Scholar] [CrossRef]
- Sáenz-de-Santa-María, I.; Bernardo-Castiñeira, C.; Enciso, E.; García-Moreno, I.; Chiara, J.L.; Suarez, C.; Chiara, M.-D. Control of long-distance cell-to-cell communication and autophagosome transfer in squamous cell carcinoma via tunneling nanotubes. Oncotarget 2017, 8, 20939–20960. [Google Scholar] [CrossRef]
- Patheja, P.; Sahu, K. Macrophage conditioned medium induced cellular network formation in MCF-7 cells through enhanced tunneling nanotube formation and tunneling nanotube mediated release of viable cytoplasmic fragments. Exp. Cell Res. 2017, 355, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, V.; Rehman, A.; Panda, S.K.; Torsiello, M.; Marigliano, M.; Nicoletti, M.M.; Ferraro, G.A.; De Falco, V.; Lappano, R.; Lieto, E.; et al. Mitochondrial transfer from Adipose stem cells to breast cancer cells drives multi-drug resistance. J. Exp. Clin. Cancer Res. 2024, 43, 166. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Mileo, A.M.; Matarrese, P. Microtubule-Based Mitochondrial Dynamics as a Valuable Therapeutic Target in Cancer. Cancers 2021, 13, 5812. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Wang, L.-L.; Chen, Y.-Y. Mitochondrial Transfer in Cardiovascular Disease: From Mechanisms to Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 771298. [Google Scholar] [CrossRef]
- Martins-Marques, T. Connecting different heart diseases through intercellular communication. Biol. Open 2021, 10, bio058777. [Google Scholar] [CrossRef] [PubMed]
- Di Lisa, F.; Canton, M.; Carpi, A.; Kaludercic, N.; Menabò, R.; Menazza, S.; Semenzato, M. Mitochondrial injury and protection in ischemic pre- and postconditioning. Antioxid. Redox Signal. 2011, 14, 881–891. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Zhang, X.; Wan, S.; An, P.; Zhu, Y.; Luo, Y.; Luo, J. Mitochondrial cardiovascular diseases: Molecular mechanisms, multi-omics exploration and therapeutic strategies. J. Adv. Res. 2025. [CrossRef]
- Actis Dato, V.; Lange, S.; Cho, Y. Metabolic Flexibility of the Heart: The Role of Fatty Acid Metabolism in Health, Heart Failure, and Cardiometabolic Diseases. Int. J. Mol. Sci. 2024, 25, 1211. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Brandes, R.P.; Haendeler, J.; Zeiher, A.M.; Dimmeler, S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: A novel mechanism for cell fate changes? Circ. Res. 2005, 96, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ellman, D.G.; Fang, S.; Bak, S.T.; Nørgård, M.Ø.; Svenningsen, P.; Andersen, D.C. Transfer of cardiomyocyte-derived extracellular vesicles to neighboring cardiac cells requires tunneling nanotubes during heart development. Theranostics 2024, 14, 3843–3858. [Google Scholar] [CrossRef]
- Miao, L.; Lu, Y.; Nusrat, A.; Fan, G.; Zhang, S.; Zhao, L.; Wu, C.-L.; Guo, H.; Huyen, T.L.N.; Zheng, Y.; et al. Tunneling nanotube–like structures regulate distant cellular interactions during heart formation. Science 2025, 387, eadd3417. [Google Scholar] [CrossRef]
- He, K.; Shi, X.; Zhang, X.; Dang, S.; Ma, X.; Liu, F.; Xu, M.; Lv, Z.; Han, D.; Fang, X.; et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc. Res. 2011, 92, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hu, J.; Yan, Q.; Zhu, J.; Zhu, Z.; Chen, Y.; Sun, J.; Zhang, R. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 2016, 13, 1517–1524. [Google Scholar] [CrossRef]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Vallabhaneni, K.C.; Haller, H.; Dumler, I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2012, 21, 3104–3113. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Liu, S.; Xian, Z.; Zhao, L.; Li, Y.; Lu, W.; Shao, C.; Chai, S. Bone marrow mesenchymal stem cells transport connexin43 via tunneling nanotubes to alleviate isopreterenol-induced myocardial hypertrophy. Stem Cell Res. Ther. 2025, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Li, X.; Xu, D.; Zhao, L.; Yang, Y.; Luan, Y.; Zhang, B. Targeting mitochondrial transfer: A new horizon in cardiovascular disease treatment. J. Transl. Med. 2024, 22, 1160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Sun, H.; Chen, N.-H.; Yuan, Y.-H. Tunneling nanotubes: A novel pharmacological target for neurodegenerative diseases? Pharmacol. Res. 2021, 170, 105541. [Google Scholar] [CrossRef]
- Palese, F.; Rakotobe, M.; Zurzolo, C. Transforming the concept of connectivity: Unveiling tunneling nanotube biology and their roles in brain development and neurodegeneration. Physiol. Rev. 2025, 105, 1823–1865. [Google Scholar] [CrossRef]
- Tarasiuk, O.; Scuteri, A. Role of Tunneling Nanotubes in the Nervous System. Int. J. Mol. Sci. 2022, 23, 12545. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Romeo, F.; Inoue, S.; Niklison-Chirou, M.V.; Elia, A.J.; Dinsdale, D.; Morone, N.; Knight, R.A.; Mak, T.W.; Melino, G. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 2016, 23, 1502–1514. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Keeney, P.M.; Bennett Jr, J.P. Differentiation of human neural stem cells into motor neurons stimulates mitochondrial biogenesis and decreases glycolytic flux. Stem Cells Dev. 2015, 24, 1984–1994. [Google Scholar] [CrossRef]
- Zheng, X.; Boyer, L.; Jin, M.; Mertens, J.; Kim, Y.; Ma, L.; Hamm, M.; Gage, F.H.; Hunter, T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 2016, 5, e13374. [Google Scholar] [CrossRef]
- Iwata, R.; Vanderhaeghen, P. Regulatory roles of mitochondria and metabolism in neurogenesis. Curr. Opin. Neurobiol. 2021, 69, 231–240. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Xie, C.; Tan, Z.; Tian, Q.; Zhu, D.; Liu, M.; Guan, Y. Rescue of Brain Function Using Tunneling Nanotubes Between Neural Stem Cells and Brain Microvascular Endothelial Cells. Mol. Neurobiol. 2016, 53, 2480–2488. [Google Scholar] [CrossRef]
- Anoar, S.; Woodling, N.S.; Niccoli, T. Mitochondria Dysfunction in Frontotemporal Dementia/Amyotrophic Lateral Sclerosis: Lessons From Drosophila Models. Front. Neurosci. 2021, 15, 786076. [Google Scholar] [CrossRef]
- Pisani, F.; Castagnola, V.; Simone, L.; Loiacono, F.; Svelto, M.; Benfenati, F. Role of pericytes in blood-brain barrier preservation during ischemia through tunneling nanotubes. Cell Death Dis. 2022, 13, 582. [Google Scholar] [CrossRef]
- Scheiblich, H.; Eikens, F.; Wischhof, L.; Opitz, S.; Jüngling, K.; Cserép, C.; Schmidt, S.V.; Lambertz, J.; Bellande, T.; Pósfai, B.; et al. Microglia rescue neurons from aggregate-induced neuronal dysfunction and death through tunneling nanotubes. Neuron 2024, 112, 3106–3125.e8. [Google Scholar] [CrossRef]
- Chang, J.C.; Wu, S.L.; Liu, K.H.; Chen, Y.H.; Chuang, C.S.; Cheng, F.C.; Su, H.-L.; Wei, Y.-H.; Kuo, S.-J.; Liu, C.-S. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: Restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl. Res. 2016, 170, 40–56.e3. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, M.; Fu, C.; Fu, A. Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 2017, 34, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Belian, S.; Zurzolo, C. Hijacking intercellular trafficking for the spread of protein aggregates in neurodegenerative diseases: A focus on tunneling nanotubes (TNTs). Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Tardivel, M.; Bégard, S.; Bousset, L.; Dujardin, S.; Coens, A.; Melki, R.; Buée, L.; Colin, M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 2016, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Abounit, S.; Wu, J.W.; Duff, K.; Victoria, G.S.; Zurzolo, C. Tunneling nanotubes: A possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 2016, 10, 344–351. [Google Scholar] [CrossRef]
- Chastagner, P.; Loria, F.; Vargas, J.Y.; Tois, J.; Diamond, M.I.; Okafo, G.; Brou, C.; Zurzolo, C. Fate and propagation of endogenously formed Tau aggregates in neuronal cells. EMBO Mol. Med. 2020, 12, e12025. [Google Scholar] [CrossRef]
- Dilna, A.; Deepak, K.V.; Damodaran, N.; Kielkopf, C.S.; Kagedal, K.; Ollinger, K.; Nath, S. Amyloid-β induced membrane damage instigates tunneling nanotube-like conduits by p21-activated kinase dependent actin remodulation. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166246. [Google Scholar] [CrossRef]
- Costanzo, M.; Zurzolo, C. The cell biology of prion-like spread of protein aggregates: Mechanisms and implication in neurodegeneration. Biochem. J. 2013, 452, 1–17. [Google Scholar] [CrossRef]
- Sharma, M.; Subramaniam, S. Rhes travels from cell to cell and transports Huntington disease protein via TNT-like protrusion. J. Cell Biol. 2019, 218, 1972–1993. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Im, G.B.; Luo, A.C.; Zhu, Y.; Hong, X.; Neumeyer, J.; Tang, H.-W.; Perrimon, N.; Melero-Martin, J.M. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 2024, 629, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Zheng, Q.W.; Zheng, J.; Zhang, J.B.; Liu, H.; Tie, J.J.; Zhang, K.-L.; Wu, F.-F.; Li, X.-D.; Zhang, S.; et al. Mitochondria transplantation transiently rescues cerebellar neurodegeneration improving mitochondrial function and reducing mitophagy in mice. Nat. Commun. 2025, 16, 2839. [Google Scholar] [CrossRef]
- Emani, S.M.; McCully, J.D. Mitochondrial transplantation: Applications for pediatric patients with congenital heart disease. Transl. Pediatr. 2018, 7, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef]
- Rossi, A.; Asthana, A.; Riganti, C.; Sedrakyan, S.; Byers, L.N.; Robertson, J.V.; Senger, R.S.; Montali, F.; Grange, C.; Dalmasso, A.; et al. Mitochondria Transplantation Mitigates Damage in an In Vitro Model of Renal Tubular Injury and in an Ex Vivo Model of DCD Renal Transplantation. Ann. Surg. 2023, 278, e1313-e26. [Google Scholar] [CrossRef]
- Mukkala, A.N.; David, B.A.; Ailenberg, M.; Liang, J.; Vaswani, C.M.; Karakas, D.B.; Goldfarb, R.B.; Barbour, W.B.; Gasner, A.B.; Wu, R.S.B.; et al. Mitochondrial Transplantation: A Novel Therapy for Liver Ischemia/Reperfusion Injury. Ann. Surg. 2025, 281, 1032–1047. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamberlan, M.; Semenzato, M. Mechanisms of Mitochondrial Transfer Through TNTs: From Organelle Dynamics to Cellular Crosstalk. Int. J. Mol. Sci. 2025, 26, 10581. https://doi.org/10.3390/ijms262110581
Zamberlan M, Semenzato M. Mechanisms of Mitochondrial Transfer Through TNTs: From Organelle Dynamics to Cellular Crosstalk. International Journal of Molecular Sciences. 2025; 26(21):10581. https://doi.org/10.3390/ijms262110581
Chicago/Turabian StyleZamberlan, Margherita, and Martina Semenzato. 2025. "Mechanisms of Mitochondrial Transfer Through TNTs: From Organelle Dynamics to Cellular Crosstalk" International Journal of Molecular Sciences 26, no. 21: 10581. https://doi.org/10.3390/ijms262110581
APA StyleZamberlan, M., & Semenzato, M. (2025). Mechanisms of Mitochondrial Transfer Through TNTs: From Organelle Dynamics to Cellular Crosstalk. International Journal of Molecular Sciences, 26(21), 10581. https://doi.org/10.3390/ijms262110581







