Integrated In Vitro and In Silico Evaluation of the Antimicrobial and Cytotoxic Potential of Calotropis procera Leaf Ethanolic Extract: From GC-MS Profiling to Molecular Docking and Dynamics
Abstract
1. Introduction
2. Results
2.1. Solubility Test for the Ethanolic Extract of C. procera Leaves
2.2. Phytochemical Test of the Ethanolic Extract of C. procera Leaves
2.3. High-Performance Liquid Chromatography Coupled to Electrospray Ionization and Quadrupole Time-of-Flight Mass Spectrometry (HPLC-ESI-QTOF-MS)
2.4. Antibiogram Tests on the Ethanolic Extract of C. procera Leaves
2.4.1. Disk Diffusion Test: Staphylococcus aureus and Escherichia coli
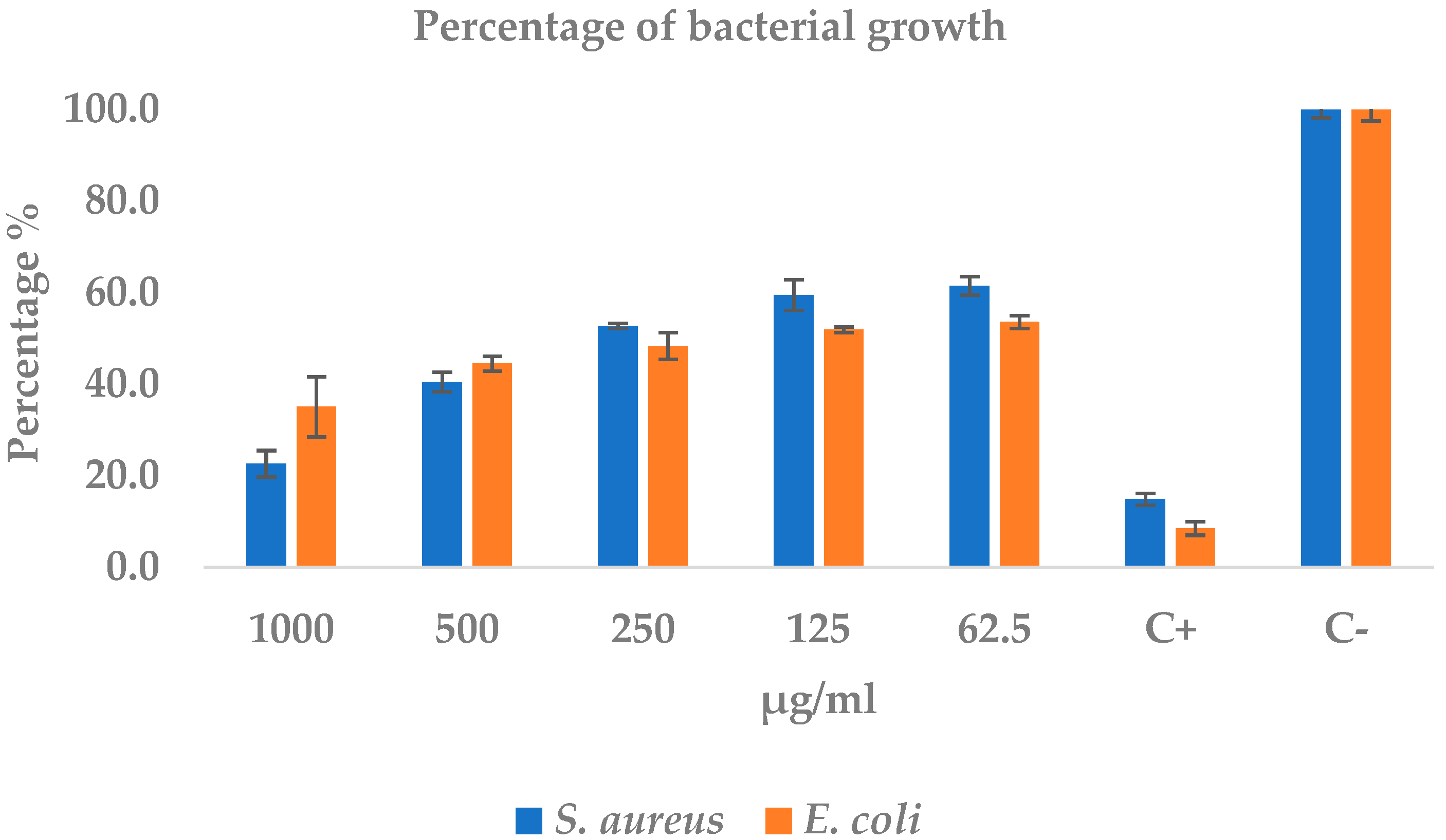
2.4.2. Minimum Inhibitory Concentration Test of S. aureus and E. coli
2.5. Evaluation of the Hemolytic Activity of EEHCP
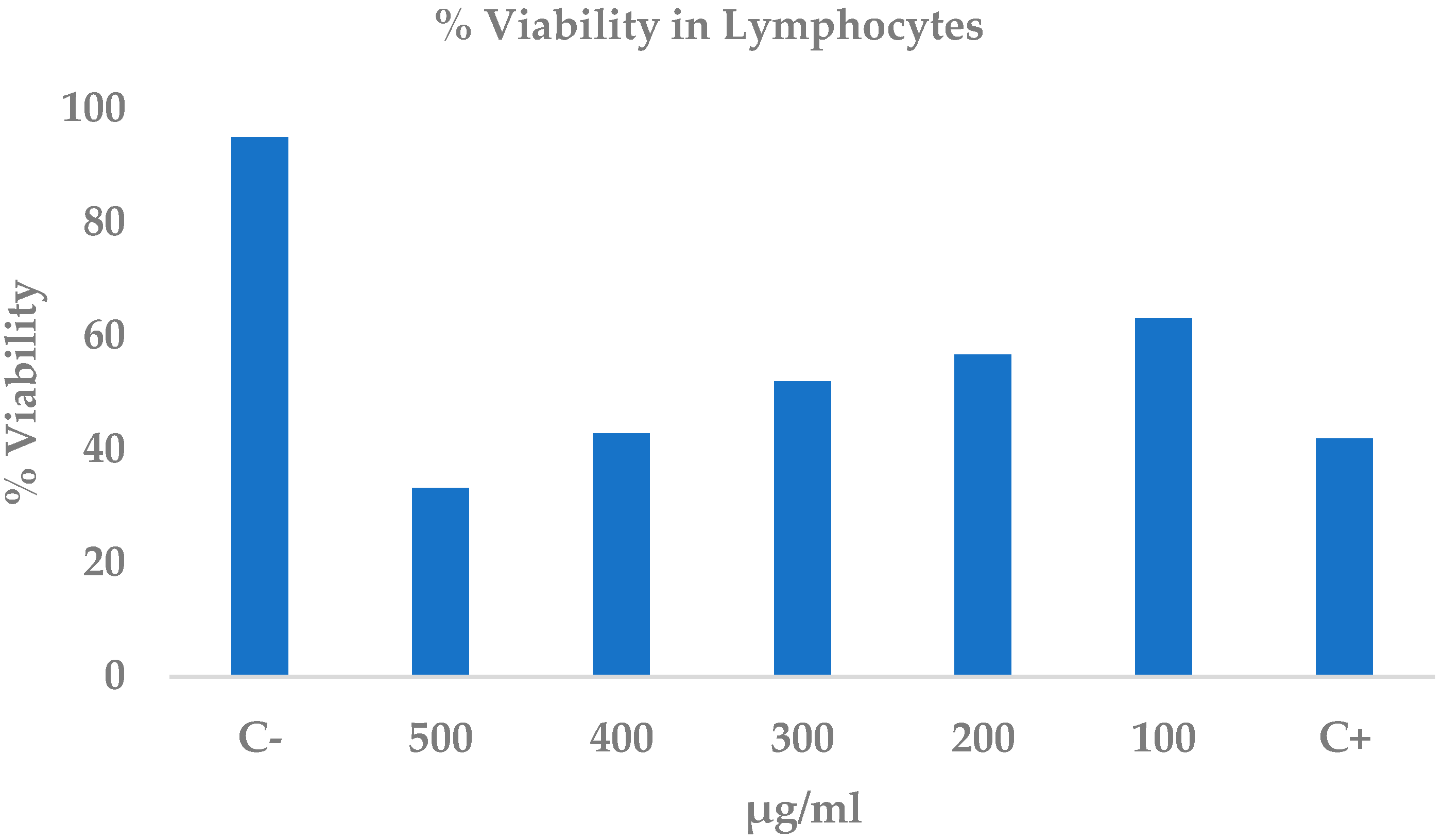
2.6. Cytotoxicity of the Ethanolic Extract of C. procera Leaves on Lymphocytes
2.7. Cytotoxicity of Ethanolic Extract of C. procera Leaves on Normal Cell Line Baby Hamster Kidney-21 Cells (BHK-21)
2.8. Data Collection
2.9. Target Identification
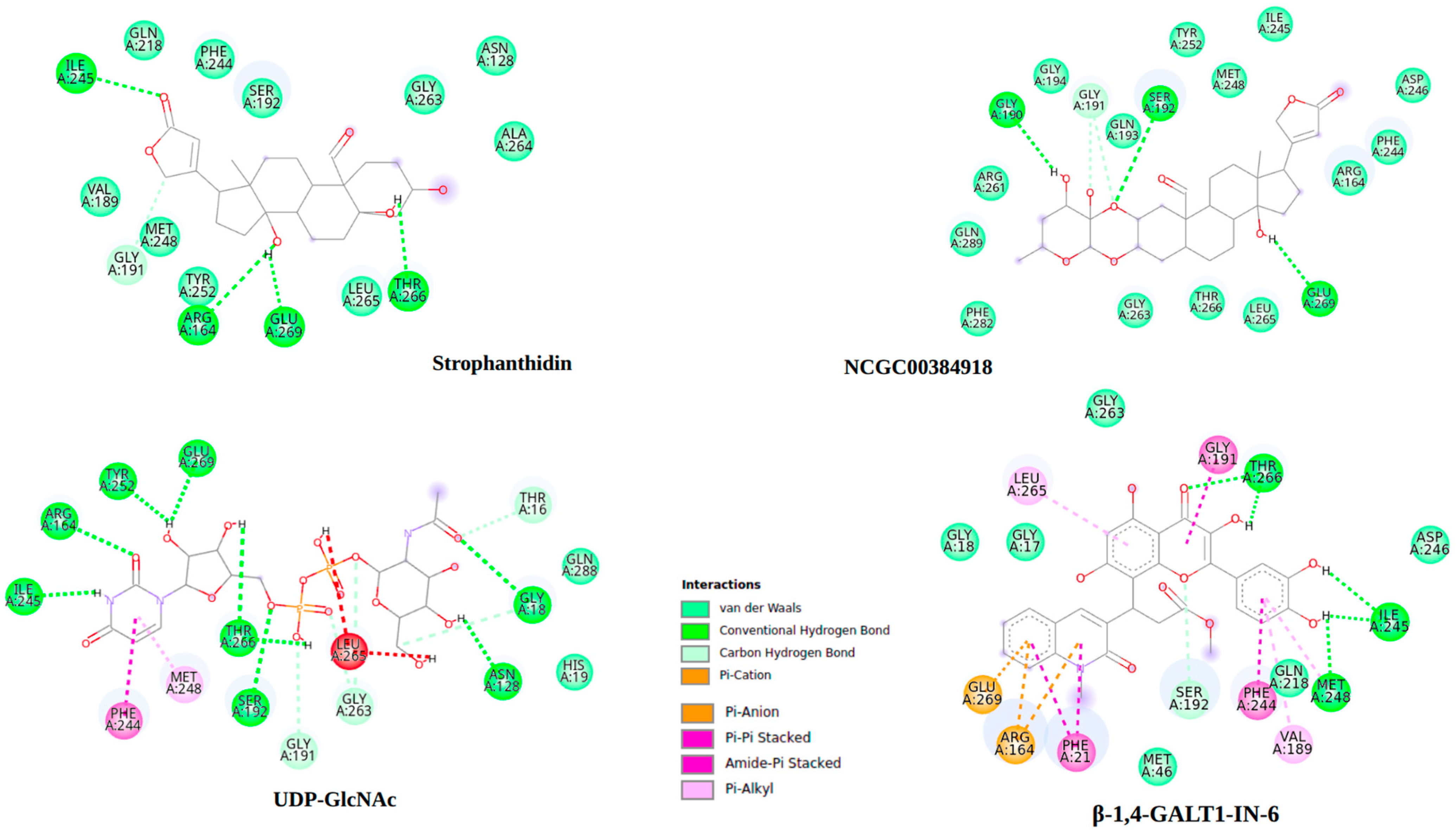
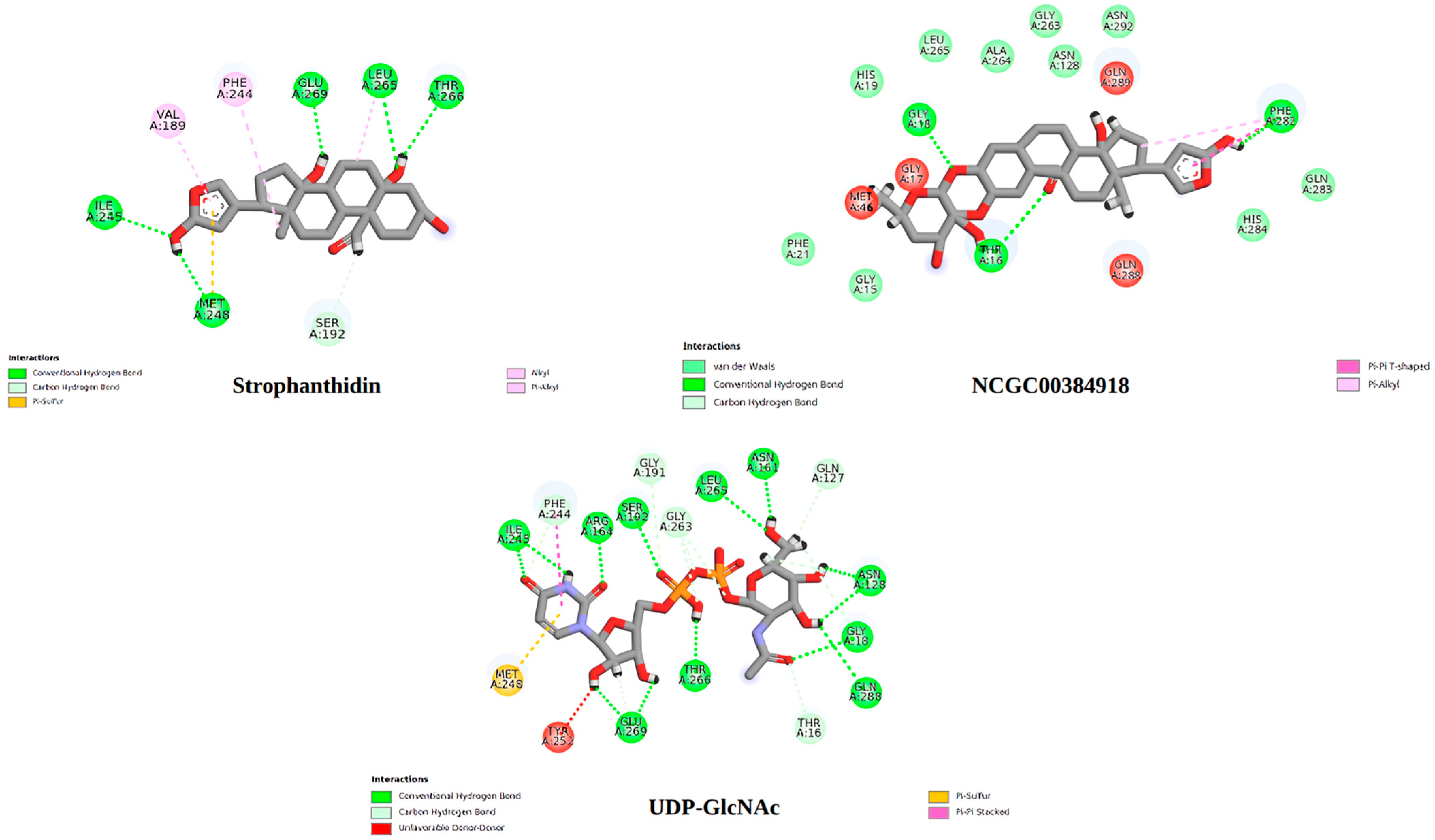
2.10. Molecular Docking
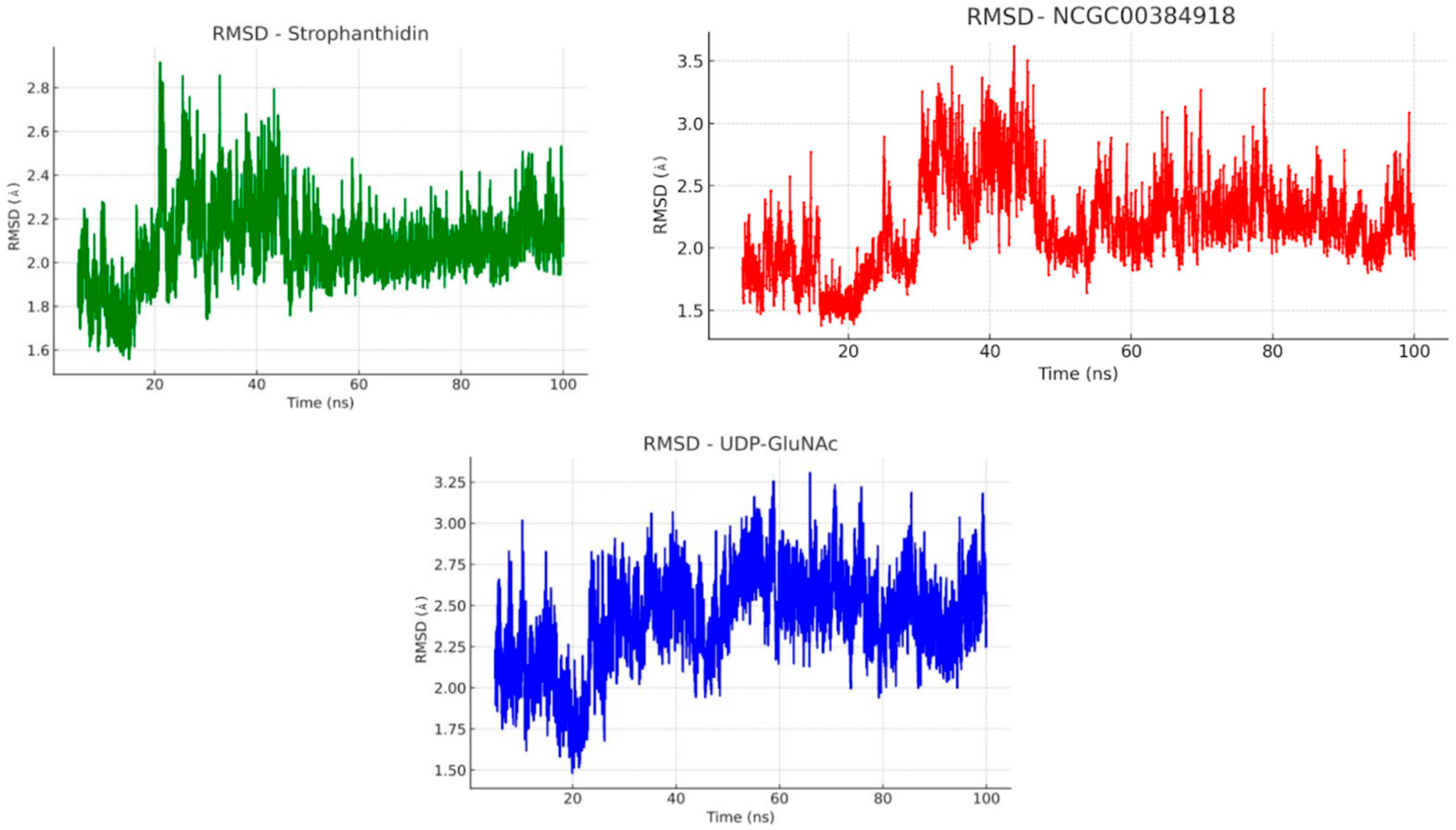
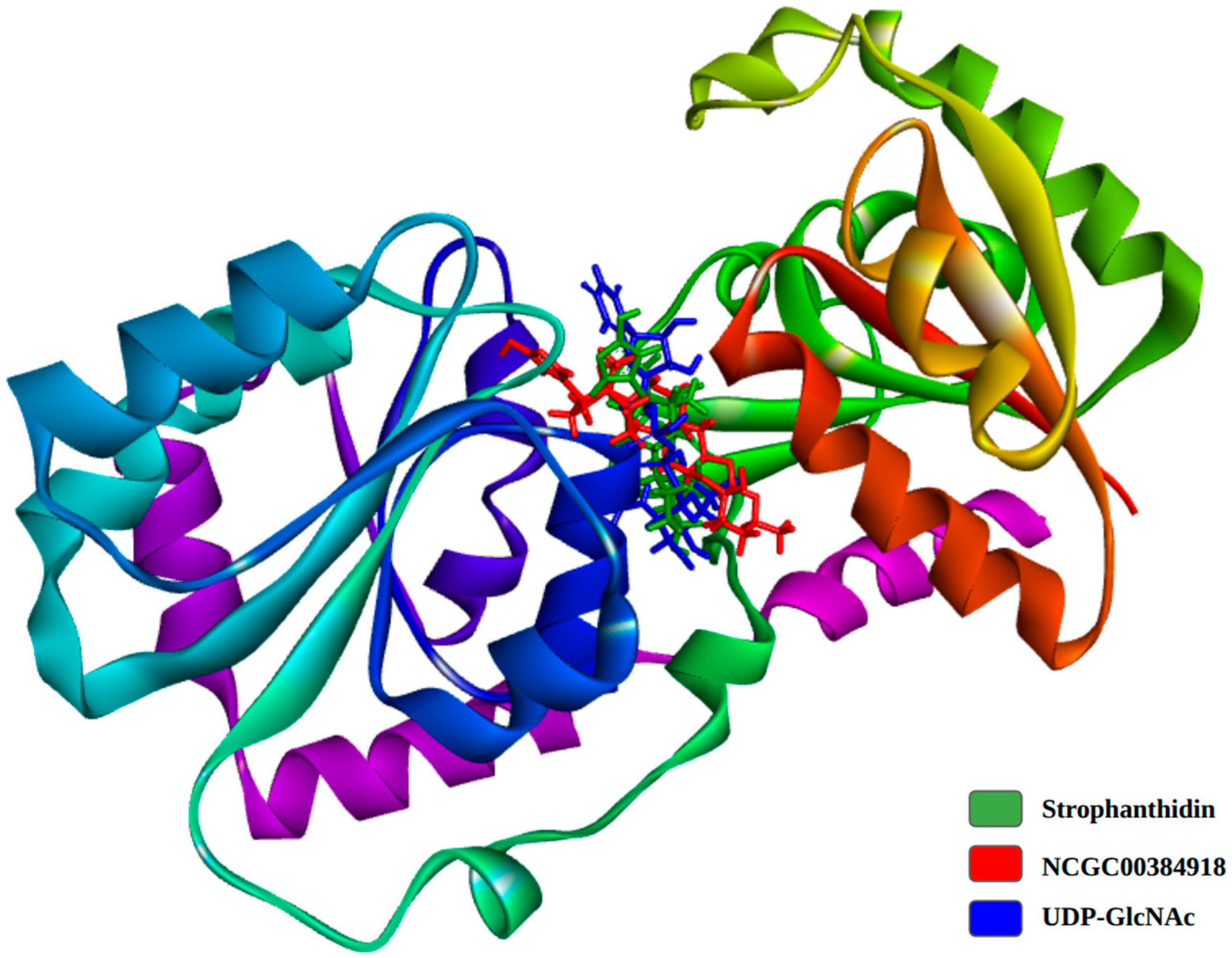
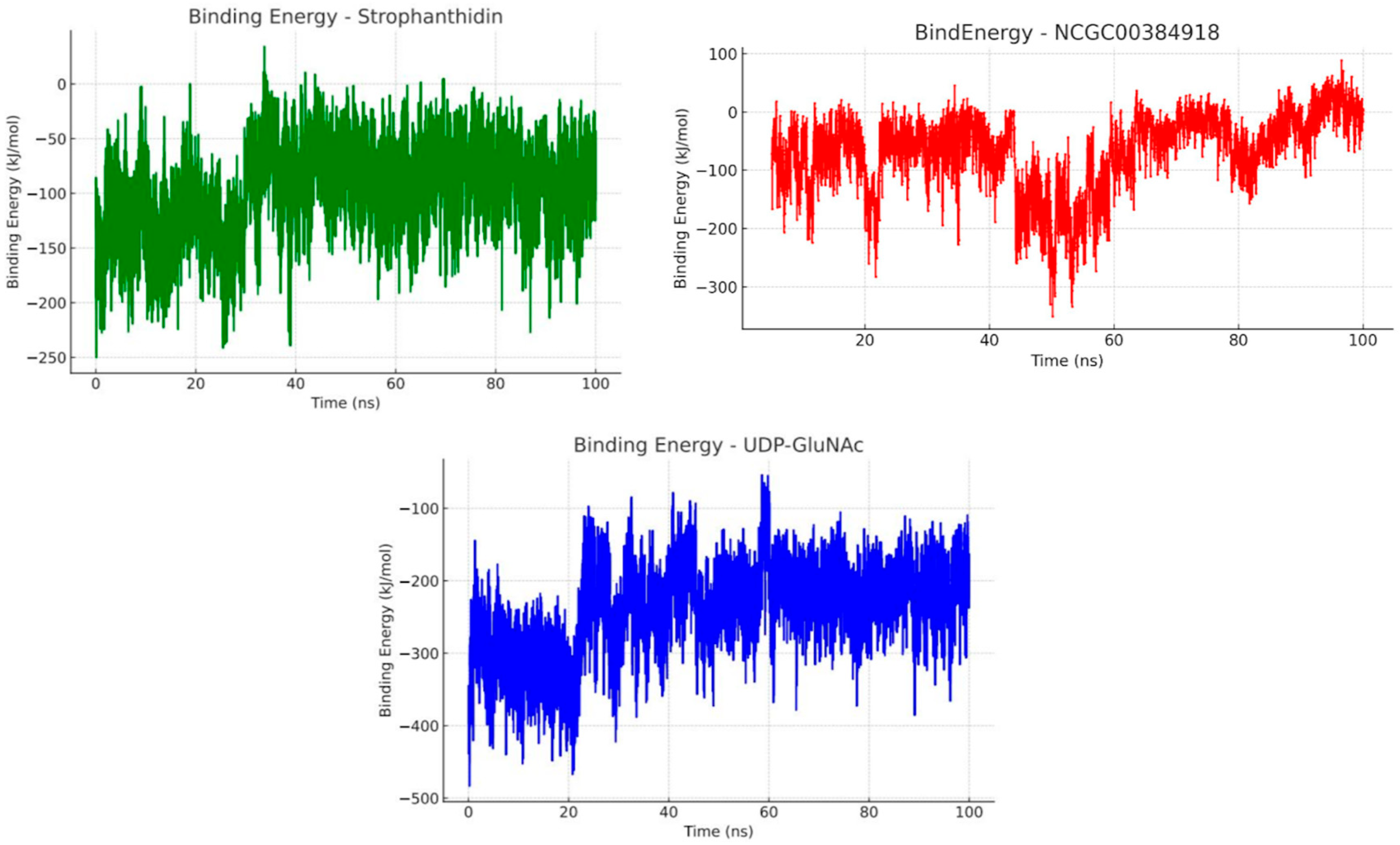
2.11. Molecular Dynamics
3. Discussion
4. Materials and Methods
4.1. Preparation of the Ethanolic Extract of Calotropis procera
4.2. Disk Diffusion Assay
4.3. Minimum Inhibitory Concentration (MIC)
4.4. Cytotoxicity in Human Lymphocytes
4.5. Cytotoxicity of the Extract on the BHK-21 Cell Line
4.6. Hemolytic Activity
4.7. Data Collection
4.8. Target Identification
4.9. ADME-Tox Properties
4.10. Docking Protocol
4.11. Molecular Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, A.; Batish, D.R.; Kaur, S.; Chauhan, B.S. An Overview of the Characteristics and Potential of Calotropis procera From Botanical, Ecological, and Economic Perspectives. Front. Plant Sci. 2021, 12, 690806. [Google Scholar] [CrossRef]
- Velmurugan, S.; Viji, V.T.; Babu, M.M.; Punitha, M.J.; Citarasu, T. Antimicrobial Effect of Calotropis procera Active Principles against Aquatic Microbial Pathogens Isolated from Shrimp and Fishes. Asian Pac. J. Trop. Biomed. 2012, 2, S812–S817. [Google Scholar] [CrossRef]
- Ahmad Nejhad, A.; Alizadeh Behbahani, B.; Hojjati, M.; Vasiee, A.; Mehrnia, M.A. Identification of Phytochemical, Antioxidant, Anticancer and Antimicrobial Potential of Calotropis procera Leaf Aqueous Extract. Sci. Rep. 2023, 13, 14716. [Google Scholar] [CrossRef] [PubMed]
- Saddiq, A.A.; Tag, H.M.; Doleib, N.M.; Salman, A.S.; Hagagy, N. Antimicrobial, Antigenotoxicity, and Characterization of Calotropis procera and Its Rhizosphere-Inhabiting Actinobacteria: In Vitro and In Vivo Studies. Molecules 2022, 27, 3123. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, P.K.; Kar, D.; Chhatoi, H.; Shahbazi, S.; Ghosh, G.; Kuanar, A. Chemometric Profile & Antimicrobial Activities of Leaf Extract of Calotropis procera and Calotropis Gigantea. Nat. Prod. Res. 2017, 31, 1954–1957. [Google Scholar] [CrossRef]
- Olu, J.; Yusuf, H.O.; Dantanko, F.; Adetunji, O.A.; Ajenifujah-Solebo, S.O. Calotropis procera Leaves and Fruits Phytochemical and GCMS Analysis and Evaluation of Their Methanolic Extracts Antimicrobial Effect on Isolated Bacteria. Plant Sci. J. 2022, 1, 16–31. [Google Scholar] [CrossRef]
- Falana, M.B.; Nurudeen, Q.O. Evaluation of Phytochemical Constituents and in Vitro Antimicrobial Activities of Leaves Extracts of Calotropis procera against Certain Human Pathogens. Not. Sci. Biol. 2020, 12, 208–221. [Google Scholar] [CrossRef]
- Shobowale, O.O.; Ogbulie, N.J.; Itoandon, E.E.; Oresegun, M.O.; Olatope, S.O.A. Phytochemical and Antimicrobial Evaluation of Aqueous and Organic Extracts of Calotropis procera Ait Leaf and Latex. Niger. Food J. 2013, 31, 77–82. [Google Scholar] [CrossRef]
- Amini, M.H.; Ashraf, K.; Salim, F.; Meng Lim, S.; Ramasamy, K.; Manshoor, N.; Sultan, S.; Ahmad, W. Important Insights from the Antimicrobial Activity of Calotropis procera. Arab. J. Chem. 2021, 14, 103181. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Mayaki, A.M.; Ogungbe, B.F.; Ojekale, A.B. In-Vitro Studies on Calotropis procera Leaf Extracts as Inhibitors of Key Enzymes Linked to Diabetes Mellitus. Iran. J. Pharm. Res. 2016, 15, 37. [Google Scholar]
- Ihegboro, G.O.; Owolarafe, T.A.; Ononamadu, C.J.; Bello, H.; Kufre-Akpan, M. Calotropis procera Root Extract’s Anti-Diabetic and Hepatoprotective Therapeutic Activity in Alloxan-Induced Pancreatic Toxicity in Wistar Rats. Iran. J. Toxicol. 2022, 16, 285–296. [Google Scholar] [CrossRef]
- Chundattu, S.J.; Agrawal, V.K.; Ganesh, N.; Chundattu, S.J.; Agrawal, V.K.; Ganesh, N. Phytochemical Investigation of Calotropis procera. Arab. J. Chem. 2011, 9, S230–S234. [Google Scholar] [CrossRef]
- Wadhwani, B.D.; Mali, D.; Vyas, P.; Nair, R.; Khandelwal, P. A Review on Phytochemical Constituents and Pharmacological Potential of Calotropis procera. RSC Adv. 2021, 11, 35854–35878. [Google Scholar] [CrossRef]
- Aljamal, M.A.; Hasan, H.M.; Sonosy, H.A.A. Antibacterial Activity Investigation and Anti-Biotic Sensitive’s for Different Solvents (Ethanol, Propanol, DMSO and di Ethel Ether) Extracts of Seeds, Leafs and Stems of (Laurus azorica and Avena sterilis) Plants. Int. J. Curr. Microbiol. Appl. Sci. 2024, 13, 175–190. [Google Scholar] [CrossRef]
- Adebisi, A.O.; Kaialy, W.; Hussain, T.; Al-Hamidi, H.; Nokhodchi, A.; Conway, B.R.; Asare-Addo, K. Freeze-Dried Crystalline Dispersions: Solid-State, Triboelectrification and Simultaneous Dissolution Improvements. J. Drug Deliv. Sci. Technol. 2021, 61, 102173. [Google Scholar] [CrossRef]
- Hemrajani, C.; Negi, P.; Parashar, A.; Gupta, G.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; Dua, K. Overcoming Drug Delivery Barriers and Challenges in Topical Therapy of Atopic Dermatitis: A Nanotechnological Perspective. Biomed. Pharmacother. 2022, 147, 112633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, M.; Li, Y.; Zhou, M.; Wu, T.; Cheng, Z. TLC–MS Identification of Alkaloids in Leonuri Herba and Leonuri Fructus Aided by a Newly Developed Universal Derivatisation Reagent Optimised by the Response Surface Method. Phytochem. Anal. 2021, 32, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Habert, G.; Brumaud, C. Influence of Tannin and Iron Ions on the Water Resistance of Clay Materials. Constr. Build. Mater. 2022, 323, 126571. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Fang, C.; Zheng, X.; Liu, C.; Huang, Q. Extraction and identification of new flavonoid compounds in dandelion Taraxacum mongolicum Hand.-Mazz. with evaluation of antioxidant activities. Sci. Rep. 2023, 13, 2166. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Patel, S. Plant-Derived Cardiac Glycosides: Role in Heart Ailments and Cancer Management. Biomed. Pharmacother. 2016, 84, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Góral, I.; Wojciechowski, K. Surface Activity and Foaming Properties of Saponin-Rich Plants Extracts. Adv. Colloid Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, K.; Atay-Kadiri, Z.; Ghaout, S. Biological Effects of Alkaloids Extracted from Three Plants of Moroccan Arid Areas on the Desert Locust. Physiol. Entomol. 2003, 28, 232–236. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Hamed, M.M.; Ahmed, W.S.; Abdou, A.M. Antioxidant and Cytotoxic Flavonols from Calotropis procera. Z. Naturforschung Sect. C J. Biosci. 2011, 66, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization Method for Phenolic Compounds Extraction from Medicinal Plant (Juniperus procera) and Phytochemicals Screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef]
- Nenaah, G.E. Potential of Using Flavonoids, Latex and Extracts from Calotropis procera (Ait.) as Grain Protectants against Two Coleopteran Pests of Stored Rice. Ind. Crops Prod. 2013, 45, 327–334. [Google Scholar] [CrossRef]
- Crout, D.H.G.; Curtis, R.F.; Hassall, C.H.; Jones, T.L. The Cardiac Glycosides of Calotropis procera. Tetrahedron Lett. 1963, 4, 63–67. [Google Scholar] [CrossRef]
- Moustafa, A.M.Y.; Ahmed, S.H.; Nabil, Z.I.; Hussein, A.A.; Omran, M.A. Extraction and Phytochemical Investigation of Calotropis procera: Effect of Plant Extracts on the Activity of Diverse Muscles. Pharm. Biol. 2010, 48, 1080–1190. [Google Scholar] [CrossRef]
- Johnson, I.T.; Gee, J.M.; Price, K.; Curl, C.; Fenwick, G.R. Influence of Saponins on Gut Permeability and Active Nutrient Transport in Vitro. J. Nutr. 1986, 116, 2270–2277. [Google Scholar] [CrossRef]
- Nchimbi, H.Y. Ethnopharmacology of Calotropis procera: Traditional Knowledge and Scientific Insights from Dodoma Region, Tanzania. Adv. Integr. Med. 2025, 12, 100535. [Google Scholar] [CrossRef]
- Bou Malhab, L.J.; Bajbouj, K.; Shehab, N.G.; Elayoty, S.M.; Sinoj, J.; Adra, S.; Taneera, J.; Saleh, M.A.; Abdel-Rahman, W.M.; Semreen, M.H.; et al. Potential Anticancer Properties of Calotropis procera: An Investigation on Breast and Colon Cancer Cells. Heliyon 2023, 9, e16706. [Google Scholar] [CrossRef]
- Tian, X.; Gu, L.; Zeng, F.; Liu, X.; Zhou, Y.; Dou, Y.; Han, J.; Zhao, Y.; Zhang, Y.; Luo, Q.; et al. Strophanthidin Induces Apoptosis of Human Lung Adenocarcinoma Cells by Promoting TRAIL-DR5 Signaling. Molecules 2024, 29, 877. [Google Scholar] [CrossRef]
- Van Quaquebeke, E.; Simon, G.; André, A.; Dewelle, J.; El Yazidi, M.; Bruyneel, F.; Tuti, J.; Nacoulma, O.; Guissou, P.; Decaestecker, C.; et al. Identification of a Novel Cardenolicle (2′-Oxovoruscharin) from Calotropis procera and the Hemisynthesis of Novel Derivatives Displaying Potent in Vitro Antitumor Activities and High in Vivo Tolerance: Structure-Activity Relationship Analyses. J. Med. Chem. 2005, 48, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, J.; Novakovic, R.; Grujic-Milanovic, J.; Ydyrys, A.; Ablaikhanova, N.; Calina, D.; Sharifi-Rad, J.; Al-Omari, B. An Updated Pharmacological Insight into Calotropin as a Potential Therapeutic Agent in Cancer. Front. Pharmacol. 2023, 14, 1160616. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Ponce, A.; Flores-Maldonado, C.; Contreras, R.G. Cardiac Glycosides: From Natural Defense Molecules to Emerging Therapeutic Agents. Biomolecules 2025, 15, 885. [Google Scholar] [CrossRef] [PubMed]
- Sikorskaya, T.V.; Efimova, K.V.; Imbs, A.B. Lipidomes of Phylogenetically Different Symbiotic Dinoflagellates of Corals. Phytochemistry 2021, 181, 112579. [Google Scholar] [CrossRef]
- Li, H.; Xia, W.; Guo, H.; Wang, Y.; Wang, Q.; Zhang, G.; Ma, Q.; Zhou, J.; Wang, T. Auxin Inhibited Colonization of Antibiotic Resistant Bacteria in Soybean Sprouts and Spread of Resistance Genes to Endophytic Bacteria: Highlighting Energy Metabolism and Immunity Mechanism. J. Hazard. Mater. 2025, 493, 138280. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Temkin, E.; Wulffhart, L.; Pollington, T.M.; Chudasama, D.; Schechner, V.; Nutman, A.; Schwaber, M.J.; Carmeli, Y. A Benchmarking Study of Thirty-Day Mortality Following Staphylococcus aureus or Escherichia coli Bacteremia. J. Infect. 2025, 91, 106529. [Google Scholar] [CrossRef]
- Frickmann, H.; Hahn, A.; Berlec, S.; Ulrich, J.; Jansson, M.; Schwarz, N.G.; Warnke, P.; Podbielski, A. On the Etiological Relevance of Escherichia Coli and Staphylococcus aureus in Superficial and Deep Infections—A Hypothesis-Forming, Retrospective Assessment. Eur. J. Microbiol. Immunol. 2019, 9, 124–130. [Google Scholar] [CrossRef]
- Poolman, J.T.; Anderson, A.S. Escherichia coli and Staphylococcus aureus: Leading Bacterial Pathogens of Healthcare Associated Infections and Bacteremia in Older-Age Populations. Expert Rev. Vaccines 2018, 17, 607–618. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Kaku, N.; Ishige, M.; Yasutake, G.; Sasaki, D.; Ota, K.; Mitsumoto-Kaseida, F.; Kosai, K.; Hasegawa, H.; Izumikawa, K.; Mukae, H.; et al. Long-Term Impact of Molecular Epidemiology Shifts of Methicillin-Resistant Staphylococcus aureus on Severity and Mortality of Bloodstream Infection. Emerg. Microbes Infect. 2025, 14, 2449085. [Google Scholar] [CrossRef]
- Fakhri-Demeshghieh, A.; Hasannejad, H.; Khoramian, P.; Rahmanian, V.; Bahonar, A. Tetracycline Resistance among Escherichia Coli Isolates from Broilers in Iran: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2025, 54, 101–111. [Google Scholar] [CrossRef]
- Monroy, I.; Catalá-Gregori, P.; Sevilla-Navarro, S. Assessment of Antibiotic Resistance and Virulence in Escherichia Coli Strains Isolated from Poultry in Spain. Poult. Sci. 2025, 104, 104838. [Google Scholar] [CrossRef]
- Suberviola, B.; Andaluz-Ojeda, D. Antibiotic Resistance and Mortality in ICU Patients: A Retrospective Analysis of First Culture Growth Results. Antibiotics 2025, 14, 290. [Google Scholar] [CrossRef]
- Saher, U.; Ovais Omer, M.; Javeed, A.; Ahmad Anjum, A.; Rehman, K.; Awan, T. Soluble Laticifer Proteins from Calotropis procera as an Effective Candidates for Antimicrobial Therapeutics. Saudi J. Biol. Sci. 2023, 30, 103659. [Google Scholar] [CrossRef]
- Radwan, A.M.; Alghamdi, H.A.; Kenawy, S.K.M. Effect of Calotropis procera L. Plant Extract on Seeds Germination and the Growth of Microorganisms. Ann. Agric. Sci. 2019, 64, 183–187, Erratum in: Ann. Agric. Sci. 2021, 1, 96–97. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J. ADMETox: Bringing Nanotechnology Closer to Lipinski’s Rule of Five; Springer: Berlin/Heidelberg, Germany, 2020; pp. 61–74. [Google Scholar] [CrossRef]
- Kim, K.-H.; Song, D.-J.; Yu, M.-H.; Park, Y.-S.; Noh, H.-R.; Kim, H.-J.; Choi, J.-W. Hazard Classification of Household Chemical Products in Korea According to the Globally Harmonized System of Classification and Labeling of Chemicals. Ann. Occup. Environ. Med. 2013, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.E.F.; Kamel, A.M.; Clarimont, C. Improvingthe Decision-Making Process in Structural Modification of Drug Candidates: Reducing Toxicity. Drug Discov. Today 2004, 9, 1055–1064. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Elfarra, A.A. Toxicity Mechanism-Based Prodrugs: Glutathione-Dependent Bioactivation as a Strategy for Anticancer Prodrug Design. Expert Opin. Drug Discov. 2018, 13, 815–824. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, H.; Li, X.; Sheng, H.; Zhu, L. Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin. Molecules 2022, 27, 7740. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Osher, E.L.; Castillo, F.; Elumalai, N.; Waring, M.J.; Pairaudeau, G.; Tavassoli, A. A Genetically Selected Cyclic Peptide Inhibitor of BCL6 Homodimerization. Bioorg Med. Chem. 2018, 26, 3034–3038. [Google Scholar] [CrossRef]
- Nabinger, D.D.; Altenhofen, S.; Buatois, A.; Facciol, A.; Peixoto, J.V.; da Silva, J.M.K.; Chatterjee, D.; Rübensam, G.; Gerlai, R.; Bonan, C.D. Acute Administration of a Dopamine D2/D3 Receptor Agonist Alters Behavioral and Neural Parameters in Adult Zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110753. [Google Scholar] [CrossRef] [PubMed]
- Horna, G.; Ruiz, J. Type 3 Secretion System as an Anti-Pseudomonal Target. Microb. Pathog. 2021, 155, 104907. [Google Scholar] [CrossRef] [PubMed]
- He, L.-L.; Xiong, L.-T.; Wang, X.; Li, Y.-Z.; Li, J.-B.; Shi, Y.; Deng, X.; Cui, Z.-N. Application of Inhibitors Targeting the Type III Secretion System in Phytopathogenic Bacteria. Chin. Chem. Lett. 2025, 36, 110044. [Google Scholar] [CrossRef]
- Shreya; Pant, T.; Kailoo, S.; Kumar, Y. Targeting Type III Secretion System Using Salmonella: A Promising Candidate for Innovative Antibacterial Therapy. Indian J. Microbiol. 2025, 65, 898–912. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, Y.; Liu, Y.; An, H.; Deng, K.; Ashraf, M.A.; Zou, L.; Wang, J. Breaking down the Cell Wall: Still an Attractive Antibacterial Strategy. Front. Microbiol. 2022, 13, 952633. [Google Scholar] [CrossRef] [PubMed]
- Süssmuth, R.D.; Kulike-Koczula, M.; Gao, P.; Kosol, S. Fighting Antimicrobial Resistance: Innovative Drugs in Antibacterial Research. Angew. Chem. Int. Ed. 2025, 64, e202414325. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Blasco, B.; Duffey, M.; Piddock, L.J.V. Unrealized Targets in the Discovery of Antibiotics for Gram-Negative Bacterial Infections. Nat. Rev. Drug Discov. 2023, 22, 957–975. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kurushima, J.; Hashimoto, Y.; Tomita, H. Progress Overview of Bacterial Two-Component Regulatory Systems as Potential Targets for Antimicrobial Chemotherapy. Antibiotics 2020, 9, 635. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular Mechanisms of Inhibiting Glucosyltransferases for Biofilm Formation in Streptococcus Mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef]
- Shtaiwi, A.; Khan, S.U.; Khedraoui, M.; Alaraj, M.; Samadi, A.; Chtita, S. A Comprehensive Computational Study to Explore Promising Natural Bioactive Compounds Targeting Glycosyltransferase MurG in Escherichia coli for Potential Drug Development. Sci. Rep. 2024, 14, 7098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Fan, C.; Chi, Z.; Bai, M.; Sun, L.; Yang, L.; Yu, C.; Song, Z.; Yang, X.; et al. Ursolic Acid Targets Glucosyltransferase and Inhibits Its Activity to Prevent Streptococcus Mutans Biofilm Formation. Front. Microbiol. 2021, 12, 743305. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, S.; Huang, H.; Li, D.; Yang, R.; Yang, Y.; Wang, D.; Song, B.; Chen, Z. Multi-Omics Analysis Reveals That the Antimicrobial Kasugamycin Potential Targets Nitrate Reductase in Didymella Segeticola to Achieve Control of Tea Leaf Spot. Phytopathology 2022, 112, 1894–1906. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From Nitrate to NO: Potential Effects of Nitrate-Reducing Bacteria on Systemic Health and Disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef]
- Palomares-Navarro, J.J.; Bernal-Mercado, A.T.; González-Pérez, C.J.; Martínez-Tellez, M.A.; Gonzalez-Aguilar, G.A.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Inhibition of Salmonella Typhimurium Biofilm and Polysaccharide Production via Eugenol-Glucosyltransferase Interactions. Biofouling 2025, 41, 113–130. [Google Scholar] [CrossRef]
- Brinkmann, S.; Spohn, M.S.; Schäberle, T.F. Bioactive Natural Products from Bacteroidetes. Nat. Prod. Rep. 2022, 39, 1045–1065. [Google Scholar] [CrossRef] [PubMed]
- Fenn, S.; Dubern, J.F.; Cigana, C.; De Simone, M.; Lazenby, J.; Juhas, M.; Schwager, S.; Bianconi, I.; Döring, G.; Elmsley, J.; et al. Nira Is an Alternative Nitrite Reductase from Pseudomonas Aeruginosa with Potential as an Antivirulence Target. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Legood, S.; Boneca, I.G.; Buddelmeijer, N. Mode of Action of Lipoprotein Modification Enzymes—Novel Antibacterial Targets. Mol. Microbiol. 2021, 115, 356–365. [Google Scholar] [CrossRef]
- Mignani, S.; Huber, S.; Tomás, H.; Rodrigues, J.; Majoral, J.P. Compound High-Quality Criteria: A New Vision to Guide the Development of Drugs, Current Situation. Drug Discov. Today 2016, 21, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, Traditional Uses, Phytochemistry and Biological Activities of Cardamom [Elettaria cardamomum (L.) Maton]—A Critical Review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Mengin-Lecreulx, D.; Texier, L.; Rousseau, M.; Van Heijenoort, J. The MurG Gene of Escherichia Coli Codes for the UDP-N-Acetylglucosamine:N-Acetylmuramyl-(Pentapeptide) Pyrophosphoryl-Undecaprenol N-Acetylglucosamine Transferase Involved in the Membrane Steps of Peptidoglycan Synthesis. J. Bacteriol. 1991, 173, 4625–4636. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Ha, S.; Gross, B.; Falcone, B.; Walker, D.; Mokhtarzadeh, M.; Walker, S. Crystal Structure of the MurG:UDP-GlcNAc Complex Reveals Common Structural Principles of a Superfamily of Glycosyltransferases. Proc. Natl. Acad. Sci. USA 2003, 100, 845–849. [Google Scholar] [CrossRef]
- Chen, L.; Men, H.; Ha, S.; Ye, X.Y.; Brunner, L.; Hu, Y.; Walker, S. Intrinsic Lipid Preferences and Kinetic Mechanism of Escherichia Coli MurG. Biochemistry 2002, 41, 6824–6833. [Google Scholar] [CrossRef]
- Mann, P.A.; Müller, A.; Xiao, L.; Pereira, P.M.; Yang, C.; Ho Lee, S.; Wang, H.; Trzeciak, J.; Schneeweis, J.; Dos Santos, M.M.; et al. Murgocil Is a Highly Bioactive Staphylococcal-Specific Inhibitor of the Peptidoglycan Glycosyltransferase Enzyme MurG. ACS Chem. Biol. 2013, 8, 2442–2451. [Google Scholar] [CrossRef]
- Liu, Y.; Breukink, E. The Membrane Steps of Bacterial Cell Wall Synthesis as Antibiotic Targets. Antibiotics 2016, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Eggert, U.S.; Ruiz, N.; Falcone, B.V.; Branstrom, A.A.; Goldman, R.C.; Silhavy, T.J.; Kahne, D. Genetic Basis for Activity Differences Between Vancomycin and Glycolipid Derivatives of Vancomycin. Science 2001, 294, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, J.; Tomašič, T.; Pajk, S.; Brinc, M.; Pišlar, A.; Anderluh, M. New Inhibitors of β-1,4-Galactosyltransferase I Discovered by Virtual Screening. ChemMedChem 2025, 20, e202400896. [Google Scholar] [CrossRef]
- Mohammadi, T.; Karczmarek, A.; Crouvoisier, M.; Bouhss, A.; Mengin-Lecreulx, D.; Den Blaauwen, T. The Essential Peptidoglycan Glycosyltransferase MurG Forms a Complex with Proteins Involved in Lateral Envelope Growth as Well as with Proteins Involved in Cell Division in Escherichia coli. Mol. Microbiol. 2007, 65, 1106–1121. [Google Scholar] [CrossRef]
- Yunta, M.J.R. Using Molecular Modelling to Study Interactions Between Molecules with Biological Activity. In Bioinformatics; Oxford University Press: Oxford, UK, 2012; Volume 92. [Google Scholar] [CrossRef]
- Nazari, S.; Abdelrasoul, A. Simulation-Based Assessment of Zwitterionic Pendant Group Variations on the Hemocompatibility of Polyethersulfone Membranes. Funct. Compos. Mater. 2024, 5, 12. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Shen, Q.; Liu, X.; Lu, J.; Chen, Y.; Lu, T.; Luo, C.; Luo, X.; Zheng, M.; et al. Non-Covalent Interactions with Aromatic Rings: Current Understanding and Implications for Rational Drug Design. Curr. Pharm. Des. 2013, 19, 6522–6533. [Google Scholar] [CrossRef]
- Arcon, J.P.; Defelipe, L.A.; Modenutti, C.P.; López, E.D.; Alvarez-Garcia, D.; Barril, X.; Turjanski, A.G.; Martí, M.A. Molecular Dynamics in Mixed Solvents Reveals Protein-Ligand Interactions, Improves Docking, and Allows Accurate Binding Free Energy Predictions. J. Chem. Inf. Model. 2017, 57, 846–863, Erratum in: J. Chem. Inf. Model. 2018, 58, 1312. [Google Scholar] [CrossRef]
- Maruyama, Y.; Igarashi, R.; Ushiku, Y.; Mitsutake, A. Analysis of Protein Folding Simulation with Moving Root Mean Square Deviation. J. Chem. Inf. Model. 2023, 63, 1529–1541. [Google Scholar] [CrossRef]
- Srivastava, H.K.; Sastry, G.N. Molecular Dynamics Investigation on a Series of HIV Protease Inhibitors: Assessing the Performance of MM-PBSA and MM-GBSA Approaches. J. Chem. Inf. Model. 2012, 52, 3088–3098. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA Methods to Estimate Ligand-Binding Affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Rafi, S.B.; Cui, G.; Song, K.; Cheng, X.; Tonge, P.J.; Simmerling, C. Insight through Molecular Mechanics Poisson-Boltzmann Surface Area Calculations into the Binding Affinity of Triclosan and Three Analogues for FabI, the E. Coli Enoyl Reductase. J. Med. Chem. 2006, 49, 4574–4580. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Sierra-Hernandez, O.; Saurith-Coronell, O.; Rodríguez-Macías, J.; Márquez, E.; Mora, J.R.; Paz, J.L.; Flores-Sumoza, M.; Mendoza-Mendoza, A.; Flores-Morales, V.; Marrero-Ponce, Y.; et al. In Silico Identification of Potential Clovibactin-like Antibiotics Binding to Unique Cell Wall Precursors in Diverse Gram-Positive Bacterial Strains. Int. J. Mol. Sci. 2025, 26, 1724. [Google Scholar] [CrossRef]
- van Gunsteren, W.F.; Daura, X.; Fuchs, P.F.J.; Hansen, N.; Horta, B.A.C.; Hünenberger, P.H.; Mark, A.E.; Pechlaner, M.; Riniker, S.; Oostenbrink, C. On the Effect of the Various Assumptions and Approximations Used in Molecular Simulations on the Properties of Bio-Molecular Systems: Overview and Perspective on Issues. ChemPhysChem 2021, 22, 264–282. [Google Scholar] [CrossRef]
- Bentley, P.J. Methods for Improving Simulations of Biological Systems: Systemic Computation and Fractal Proteins. J. R. Soc. Interface 2009, 6, S451–S466. [Google Scholar] [CrossRef][Green Version]
- Kundu, P.; Das, S.; Chattopadhyay, N. Managing Efficacy and Toxicity of Drugs: Targeted Delivery and Excretion. Int. J. Pharm. 2019, 565, 378–390. [Google Scholar] [CrossRef]
- Westhouse, R.A. Safety Assessment Considerations and Strategies for Targeted Small Molecule Cancer Therapeutics in Drug Discovery. Toxicol. Pathol. 2010, 38, 165–168. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Mendes, K.R.; Ramos, M.V.; Santos, J.N.B.; Youssef, D.T.A.; Pereira, J.D.; Endres, L.; Jarma-Orozco, A.; Solano-Gomes, R.; Jarma-Arroyo, B.; et al. Mesophyll Thickness and Sclerophylly among Calotropis procera Morphotypes Reveal Water-Saved Adaptation to Environments. J. Arid. Land. 2019, 11, 795–810. [Google Scholar] [CrossRef]
- Gonçalves-Oliveira, R.C.; Rodrigues, H.B.; Benko-Iseppon, A.M. Range Distribution of the Invasive Alien Species Calotropis procera in South America Dry Environments under Climatic Change Scenarios. J. Arid. Environ. 2022, 205, 104819. [Google Scholar] [CrossRef]
- Alam, M.S.; Rajhi, B.R.; Alam, M.F.; Hamzi, M.M.; Albratty, M.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Rehman, Z.; Moni, S.S. Spectral Characterization, Analgesic, and Anti-Inflammatory Effects of Ethanolic Extract of Calotropis procera Leaf and Dry Latex from Jazan, Kingdom of Saudi Arabia. Not. Bot. Horti Agrobot. Cluj. Napoca 2024, 52, 13626. [Google Scholar] [CrossRef]
- Habeeb, A.; Ramesh, S.; Shanmugam, R. Calotropis procera and the Pharmacological Properties of Its Aqueous Leaf Extract: A Review. Cureus 2024, 16, e60354. [Google Scholar] [CrossRef]
- Joshi, T.; Pandey, N. Phytochemical and Antibacterial Screening of Leaves and Latex of Calotropis procera: A Comparison. Univ. J. Phytochem. Ayurvedic Heights 2024, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Andrew, O.E.; Umeh, E.U.; Elabor, I.; Zakari, S.; Samuel, E.; Anna, S.F. Antibacterial Potential and Time-Kill Kinetics of Calotropis procera Extracts. UMYU Sci. 2024, 3, 459–468. [Google Scholar] [CrossRef]
- Kastrat, E.; Cheng, H.P. Escherichia Coli Has an Undiscovered Ability to Inhibit the Growth of Both Gram-Negative and Gram-Positive Bacteria. Sci. Rep. 2024, 14, 7420. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Henneberg, K.Å.; Wang, H.; Stavnsbjerg, C.; Bjarnsholt, T.; Ciofu, O.; Johansen, U.R.; Sams, T. Formation of Pseudomonas Aeruginosa Inhibition Zone during Tobramycin Disk Diffusion Is Due to Transition from Planktonic to Biofilm Mode of Growth. Int. J. Antimicrob. Agents 2019, 53, 564–573. [Google Scholar] [CrossRef]
- Melo, R.S.; Azevedo, Á.M.A.; Pereira, A.M.G.; Rocha, R.R.; Cavalcante, R.M.B.; Matos, M.N.C.; Lopes, P.H.R.; Gomes, G.A.; Rodrigues, T.H.S.; Dos Santos, H.S.; et al. Chemical Composition and Antimicrobial Effectiveness of Ocimum Gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24, 3864. [Google Scholar] [CrossRef]
- Chometon, T.Q.; Da Silva Siqueira, M.; Sant’anna, J.C.; Almeida, M.R.; Gandini, M.; De Almeida Nogueira, A.C.M.; Antas, P.R.Z. A Protocol for Rapid Monocyte Isolation and Generation of Singular Human Monocyte-Derived Dendritic Cells. PLoS ONE 2020, 15, e0231132. [Google Scholar] [CrossRef]
- Grievink, H.W.; Luisman, T.; Kluft, C.; Moerland, M.; Malone, K.E. Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv Biobank 2016, 14, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Supandi, S.K.; Michael, Y.C.; Bargowo, L. Viability Testof Lemongrass Extract (Cymbopogon Citratus) on BHK-21 Fibroblast Cell Culture. Med. Leg. Update 2020, 20, 820–824. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; González, M.C.; De Moya, Y.S.; Rodríguez, J.D. Volatile Metabolites of Piper Eriopodon (Miq.) C.DC. from Northern Region of Colombia and Assessment of In Vitro Bioactivities of the Leaf Essential Oil. Molecules 2023, 28, 2594. [Google Scholar] [CrossRef]
- Ertl, P. Molecular Structure Input on the Web. J. Cheminform 2010, 2, 1. [Google Scholar] [CrossRef]
- Bienfait, B.; Ertl, P. JSME: A Free Molecule Editor in JavaScript. J. Cheminform 2013, 5, 24. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef]
- Vainio, M.J.; Johnson, M.S. Generating Conformer Ensembles Using a Multiobjective Genetic Algorithm. J. Chem. Inf. Model. 2007, 47, 2462–2474. [Google Scholar] [CrossRef]
- Cannady, E.; Katyayan, K.; Patel, N. ADME Principles in Small Molecule Drug Discovery and Development: An Industrial Perspective. Toxicol. Pathol. 2022, 1, 51–76. [Google Scholar] [CrossRef]
- Joshi, N.; Arya, R.K.K.; Bisht, D.; Joshi, D.C. Role of Drug Metabolism & Disposition in Discovery and Development of New Drugs. World J. Pharm. Sci. 2021, 9, 180–190. [Google Scholar] [CrossRef]
- Saunders, A.; Harrington, P. de B. Advances in Activity/Property Prediction from Chemical Structures. Crit. Rev. Anal. Chem. 2024, 54, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Segall, M.D.; Barber, C. Addressing Toxicity Risk When Designing and Selecting Compounds in Early Drug Discovery. Drug Discov. Today 2014, 19, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Ferreira, R.S.; Irwin, J.J.; Shoichet, B.K. Docking and Chemoinformatic Screens for New Ligands and Targets. Curr. Opin. Biotechnol. 2009, 20, 429–436. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, W. Molecular Docking for Drug Discovery and Development: A Widely Used Approach but Far from Perfect. Future Med. Chem. 2016, 8, 1710. [Google Scholar] [CrossRef]
- Santos-Martins, D.; Solis-Vasquez, L.; Tillack, A.F.; Sanner, M.F.; Koch, A.; Forli, S. Accelerating A Uto D Ock 4 with GPUs and Gradient-Based Local Search. J. Chem. Theory Comput. 2021, 17, 1060–1073. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software News and Updates AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Salo-Ahen, O.M.H.; Alanko, I.; Bhadane, R.; Alexandre, A.M.; Honorato, R.V.; Hossain, S.; Juffer, A.H.; Kabedev, A.; Lahtela-Kakkonen, M.; Larsen, A.S.; et al. Molecular Dynamics Simulations in Drug Discovery and Pharmaceutical Development. Processes 2020, 9, 71. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of Molecular Docking Analysis and Molecular Dynamics Simulations for Studying Food Proteins and Bioactive Peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef]
- AlRawashdeh, S.; Barakat, K.H. Applications of Molecular Dynamics Simulations in Drug Discovery. Methods Mol. Biol. 2024, 2714, 127–141. [Google Scholar] [CrossRef]
- Wu, X.; Xu, L.Y.; Li, E.M.; Dong, G. Application of Molecular Dynamics Simulation in Biomedicine. Chem. Biol. Drug Des. 2022, 99, 789–800. [Google Scholar] [CrossRef]
- Nugraha, G.; Istyastono, E.P. Virtual Target Construction for Structure-Based Screening in the Discovery of Histamine H2 Receptor Ligands. Int. J. Appl. Pharm. 2021, 13, 239–241. [Google Scholar] [CrossRef]
- Huai, Z.; Shen, Z.; Sun, Z. Binding Thermodynamics and Interaction Patterns of Inhibitor-Major Urinary Protein-I Binding from Extensive Free-Energy Calculations: Benchmarking AMBER Force Fields. J. Chem. Inf. Model. 2021, 61, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Vögele, M.; Zhang, B.W.; Kaindl, J.; Wang, L. Is the Functional Response of a Receptor Determined by the Thermodynamics of Ligand Binding? J. Chem. Theory Comput. 2023, 19, 8414–8422. [Google Scholar] [CrossRef] [PubMed]
- Sapundzhi, F.; Popstoilov, M.; Lazarova, M. RMSD Calculations for Comparing Protein Three-Dimensional Structures. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). In Numberical Methoda and Applications; Spinger: Berlin/Heidelberg, Germany, 2023; pp. 279–288. [Google Scholar] [CrossRef]

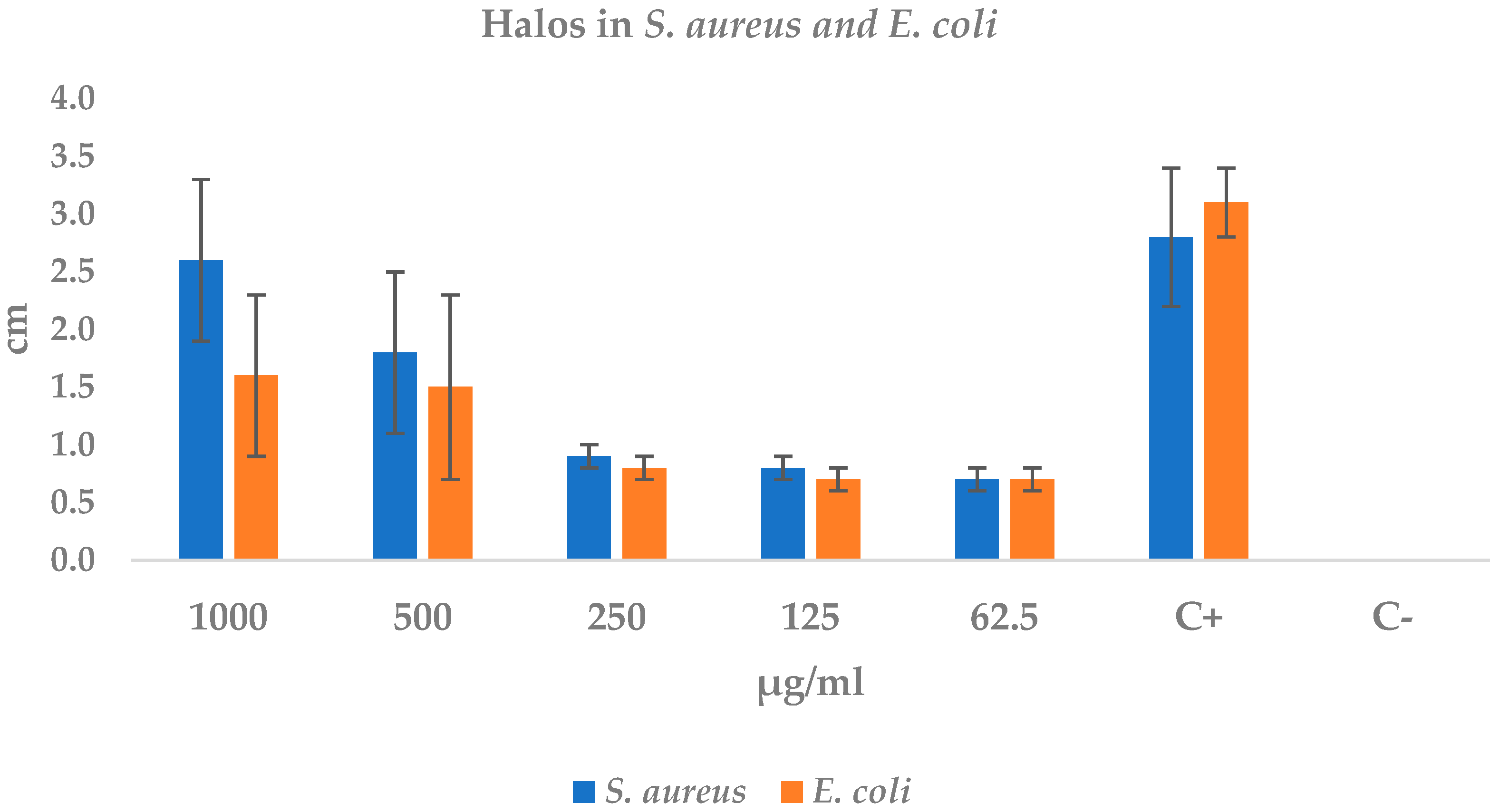

| Solvent | Result |
|---|---|
| Distilled water | Insoluble |
| Methanol | Partially soluble |
| Ethanol 96% | fully soluble |
| Ethyl acetate | Partially soluble |
| Acetone | Insoluble |
| Chloroform | Fully soluble |
| Xylol | Partially soluble |
| Heptane | Slighty soluble |
| DMSO | Fully soluble |
| Proof | Result | Methodology |
|---|---|---|
| alkaloids Wagner’s test | + | [16] |
| tannins Iron chloride (FeCl) | − | [17] |
| flavonoids Hydrochloric acid and magnesium tape | + | [18] |
| phenols Folin-Ciocalteau and sodium carbonate | + | [19] |
| cardiac glycosides Kiliani’s test | + | [20] |
| saponins Foaming | + | [21] |
| No. | tr, min | Tentative Annotation | Formula | Observed Ion | Experimental m/z | Theoretical m/z | Δ ppm |
|---|---|---|---|---|---|---|---|
| 1 | 10.902 | Strophanthidin | C23H32O6 | [M + H]+ | 405.2180 | 405.2271 | −22.6169 |
| 2 | 11.605 | Anthranilate | C7H7NO2 | [M + H]+ | 138.0540 | 138.0549 | −6.9175 |
| 3 | 11.659 | Indole-3-carboxylic acid | C9H7NO2 | [M + H]+ | 162.0530 | 162.0549 | −12.0638 |
| 4 | 12.015 | Indole-3-acetic acid | C10H9NO2 | [M + H]+ | 176.0680 | 176.0706 | −14.7952 |
| 5 | 12.523 | NCGC00384918 | C29H40O9 | [M-H2O + H]+ | 515.2510 | 515.2639 | −25.1230 |
| 6 | 12.892 | MLS001077351-01 | C14H12N2O2 | [M + H]+ | 241.0930 | 241.0971 | −17.2295 |
| 7 | 13.551 | Tryptamine-C5:0 | C15H20N2O | [M + H]+ | 245.1610 | 245.1648 | −15.6629 |
| 8 | 14.055 | Gitoxigenin | C23H34O5 | [M + H-H2O]+ | 373.2300 | 373.2373 | −19.6550 |
| 9 | 14.198 | NCGC00179704 | C15H24O3 | [M-H2O + H]+ | 235.1650 | 235.1692 | −18.0976 |
| 10 | 21.226 | 10S-Hydroxypheophorbide a | C35H36N4O6 | [M + H]+ | 609.2540 | 609.2707 | −27.5099 |
| 11 | 24.474 | Pyropheophorbide a | C33H34N4O3 | [M + H]+ | 535.2570 | 535.2703 | −24.9724 |
| Treatments (µg/mL) | S. aureus | E. coli |
|---|---|---|
| 1000 | 2.6 ± 0.7 | 1.6 ± 0.7 |
| 500 | 1.8 ± 0.7 | 1.5 ± 0.8 |
| 250 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| 125 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| 62.5 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Positive control | 2.8 ± 0.6 | 3.1 ± 0.3 |
| Negative control | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Treatments (µg/mL) | S. aureus Absorbance (Mean ± SD) | S. aureus % Growth (Mean ± SD) | E. coli Absorbance (Mean ± SD) | E. coli % Growth (Mean ± SD) |
|---|---|---|---|---|
| 1000 | 0.07 ± 0.02 | 22.61 ± 2.92 | 0.25 ± 0.05 | 35.09 ± 6.55 |
| 500 | 0.18 ± 0.02 | 40.50 ± 2.10 | 0.32 ± 0.01 | 44.49 ± 1.59 |
| 250 | 0.31 ± 0.04 | 52.71 ± 0.55 | 0.35 ± 0.02 | 48.35 ± 2.88 |
| 125 | 0.41 ± 0.03 | 59.50 ± 3.38 | 0.37 ± 0.00 | 51.93 ± 0.60 |
| 62.5 | 0.46 ± 0.02 | 61.50 ± 2.01 | 0.38 ± 0.01 | 53.61 ± 1.39 |
| Positive control | 0.12 ± 0.01 | 14.90 ± 1.28 | 0.06 ± 0.01 | 8.49 ± 1.49 |
| Negative control | 0.77 ± 0.01 | 100.00 ± 1.83 | 0.71 ± 0.02 | 100.00 ± 2.48 |
| Treatments µg/mL | % Hemolysis |
|---|---|
| Negative control | 2.2 ± 0.3 |
| 1000 | 9.3 ± 0.1 |
| l500 | 1.8 ± 1.1 |
| 250 | 1.9 ± 1.0 |
| 125 | 1.4 ± 0.6 |
| 63 | 0.8 ± 0.5 |
| 32 | 1.3 ± 0.4 |
| Positive control | 100.6 ± 1.2 |
| Treatments µg/mL | % Viability |
|---|---|
| 500 | 33.2 ± 4.5 * |
| 400 | 42.8 ± 9.4 * |
| 300 | 52.0 ± 8.8 * |
| 200 | 56.7 ± 19.5 |
| 100 | 63.1 ± 0.7 |
| Methanol | 41.9 ± 5.9 |
| PBS | 95.0 ± 3.8 |
| Treatments µg/ mL | % Vitality |
|---|---|
| Negative control | 100.5 ± 2.0 |
| 500 | 16.7 ± 2.3 * |
| 250 | 47.7 ± 3.4 * |
| 125 | 71.5 ± 8.0 * |
| 62.5 | 76.7 ± 17.0 * |
| Positive control | 6.2 ± 1.1 * |
| Compound | Iso-SMILES | Tox Level |
|---|---|---|
| UDP-GlcNAc | CC(=O)N[C@@H]1[C@H]([C@@H]([C@H](O[C@@H]1OP(=O)(O)OP(=O)(O)OC[C@@H]2[C@H]([C@H]([C@@H](O2)N3C=CC(=O)NC3=O)O)O)CO)O)O | 6 |
| β-1,4-GALT1-IN-6 | [H]Oc1c([H])c([H])c(-c2oc3c(C@@(c4c([H])c5c([H])c([H])c([H])c([H])c5n(C([H])([H])[H])c4=O)C([H])([H])C(=O)OC([H])([H])[H])c(O[H])c([H])c(O[H])c3c(=O)c2O[H])c([H])c1O[H] | 5 |
| Alendronate | [H]OC(C([H])([H])C([H])([H])C([H])([H])N([H])[H])(P@TB1(O[H])O[H])P@SP3(O[H])O[H] | 4 |
| C6 | [H]O[C@@H]1C@HOC@(C([H])([H])OP@SP3([O-])OP@SP3([O-])[O-])[C@@H]1O[H] | 6 |
| Strophanthidin | [H]OC12C([H])([H])C([H])([H])[C@]3([H])C@@(C([H])([H])C([H])([H])[C@]4(C([H])([H])[H])[C@@]3(O[H])C([H])([H])C([H])([H])[C@@]4([H])C3=C([H])C(=O)OC3([H])[H])[C@]1(C([H])=O)C([H])([H])C([H])([H])C@@(O[H])C2([H])[H] | 2 |
| Anthranilate | [H]OC(=O)c1c([H])c([H])c([H])c([H])c1N([H])[H] | 4 |
| Tryptamine-C5 | [H]c1c([H])c([H])c2c(c1[H])c(C([H])([H])C([H])([H])N([H])C(=O)C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])c([H])n2[H] | 3 |
| NCGC00384918 | [H]O[C@@]12O[C@@]3([H])C([H])([H])[C@@]4(C([H])=O)C@@(C([H])([H])C([H])([H])[C@@]5([H])[C@]4([H])C([H])([H])C([H])([H])[C@@]4(C([H])([H])[H])[C@]5(O[H])C([H])([H])C([H])([H])[C@]4([H])C4=C([H])C(=O)OC4([H])[H])C([H])([H])[C@@]3([H])O[C@@]1([H])OC@@(C([H])([H])[H])C([H])([H])[C@@]2([H])O[H] | 2 |
| MLS001077351-01 | [H]c1c([H])c([H])c2c3c([H])c(C(=O)OC([H])([H])C([H])([H])[H])n([H])c([H])c-3nc2c1[H] | 4 |
| Indole-3-carboxylic acid | [H]OC(=O)c1c([H])n([H])c2c([H])c([H])c([H])c([H])c12 | 5 |
| NCGC00179704 | [H]OC(=O)C(=C([H])[H])[C@]1([H])C([H])([H])C([H])([H])[C@@]2(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C@(C([H])([H])[H])[C@]2([H])C1([H])[H] | 5 |
| Gitoxigenin | [H]O[C@@]1([H])C([H])([H])C([H])([H])[C@@]2(C([H])([H])[H])C@@(C([H])([H])C([H])([H])[C@@]3([H])[C@]2([H])C([H])([H])C([H])([H])[C@]2(C([H])([H])[H])C@@(C4=C([H])C(=O)OC4([H])[H])C@@(O[H])C([H])([H])[C@]32O[H])C1([H])[H] | 2 |
| Indole-3-acetic acid | [H]OC(=O)C([H])([H])c1c([H])n([H])c2c([H])c([H])c([H])c([H])c12 | 4 |
| PDB | Organism | Function | Score | PDB Code | Organism | Function | Score |
|---|---|---|---|---|---|---|---|
| NCGC00384918 | Strophanthidin | ||||||
| 3qfw | R. palustris | Unknown function | 0.996 | 1lvl | P. putida | Lipoamino dehydrogenase | 0.929 |
| 2p67 | E. coli K12 | Transport system kinase | 0.952 | 1nlm | E. coli | Essential Glucosyltransferasa | 0.933 |
| 2zm5 | E. coli K12 | Transferase | 0.951 | 1o9y | P. syringae | T3SS | 0.921 |
| 1ghh | E. coli | DNA repair | 0.951 | 2p67 | E. coli K12 | Transport system kinase | 0.92 |
| 1o9y | P. syringae | T3SS | 0.947 | 2zm5 | E. coli K12 | Transferase | 0.916 |
| 1tu1 | P. aeruginosa | Unknown function | 0.946 | 1ghh | E. coli | DNA repair | 0.916 |
| 2nya | E. coli K12 | Nitrate reductase | 0.929 | 1tu1 | P. aeruginosa | Unknown function | 0.909 |
| 1nlm | E. coli | Essential Glucosyltransferase | 0.908 | 2nya | E. coli K12 | Nitrate reductase | 0.886 |
| Ligand | Binding Energy (Kcal/mol) |
|---|---|
| UDP-GlcNAc | −8.41 |
| Strophanthidin | −9.43 |
| NCGC00384918 | −9.12 |
| β-1,4-GALT1-IN-6 | −9.29 |
| Alendronate | −3.99 |
| Compound 6 | −6.30 |
| Complex Compound-MurG | Binding Energy (kJ/mol) |
|---|---|
| UDP-GlcNAc | −237.56 |
| Strophantidin | −96.44 |
| NCGC00394918 | −49.34 |
| Target | X Center | X Size | Y Center | Y Size | Z Center | Z Size |
|---|---|---|---|---|---|---|
| MurG | 38 | 50 | −4 | 50 | 21 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Macías, J.D.; Saurith-Coronell, O.; Martínez Parra, L.; Carrascal-Hernández, D.C.; Fuentes-Gandara, F.; Insuasty, D.; Márquez-Brazón, E.A. Integrated In Vitro and In Silico Evaluation of the Antimicrobial and Cytotoxic Potential of Calotropis procera Leaf Ethanolic Extract: From GC-MS Profiling to Molecular Docking and Dynamics. Int. J. Mol. Sci. 2025, 26, 10574. https://doi.org/10.3390/ijms262110574
Rodríguez-Macías JD, Saurith-Coronell O, Martínez Parra L, Carrascal-Hernández DC, Fuentes-Gandara F, Insuasty D, Márquez-Brazón EA. Integrated In Vitro and In Silico Evaluation of the Antimicrobial and Cytotoxic Potential of Calotropis procera Leaf Ethanolic Extract: From GC-MS Profiling to Molecular Docking and Dynamics. International Journal of Molecular Sciences. 2025; 26(21):10574. https://doi.org/10.3390/ijms262110574
Chicago/Turabian StyleRodríguez-Macías, Juan David, Oscar Saurith-Coronell, Laura Martínez Parra, Domingo César Carrascal-Hernández, Fabio Fuentes-Gandara, Daniel Insuasty, and Edgar A. Márquez-Brazón. 2025. "Integrated In Vitro and In Silico Evaluation of the Antimicrobial and Cytotoxic Potential of Calotropis procera Leaf Ethanolic Extract: From GC-MS Profiling to Molecular Docking and Dynamics" International Journal of Molecular Sciences 26, no. 21: 10574. https://doi.org/10.3390/ijms262110574
APA StyleRodríguez-Macías, J. D., Saurith-Coronell, O., Martínez Parra, L., Carrascal-Hernández, D. C., Fuentes-Gandara, F., Insuasty, D., & Márquez-Brazón, E. A. (2025). Integrated In Vitro and In Silico Evaluation of the Antimicrobial and Cytotoxic Potential of Calotropis procera Leaf Ethanolic Extract: From GC-MS Profiling to Molecular Docking and Dynamics. International Journal of Molecular Sciences, 26(21), 10574. https://doi.org/10.3390/ijms262110574








