Oxylipins in Atherosclerosis: Their Role in Inflammation, Diagnosis, and Therapeutic Perspectives
Abstract
1. Introduction
2. Pathogenesis of Atherosclerosis: A Cellular Perspective
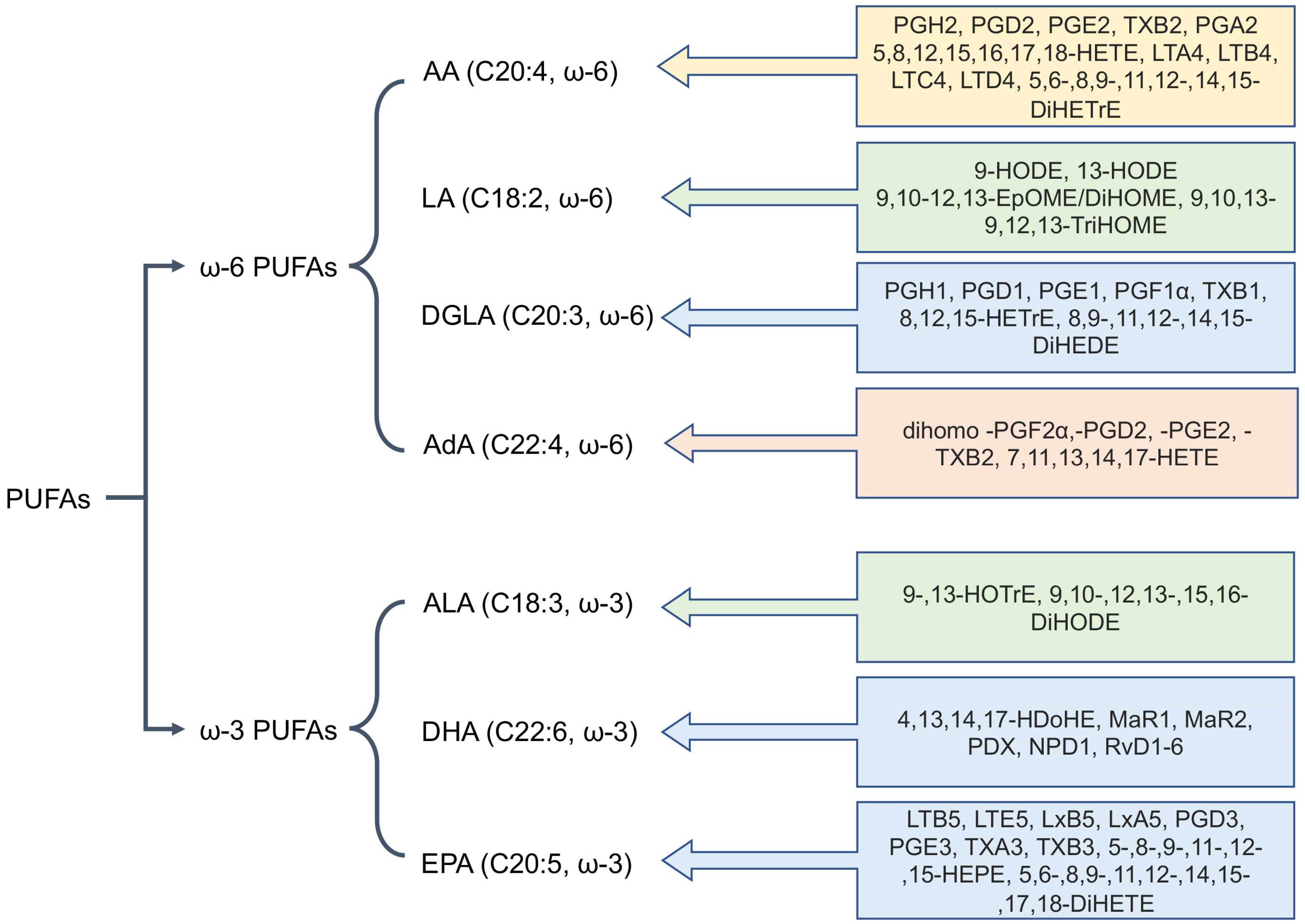
3. Biosynthesis of Oxylipins: Metabolic Pathways and Key Enzymes
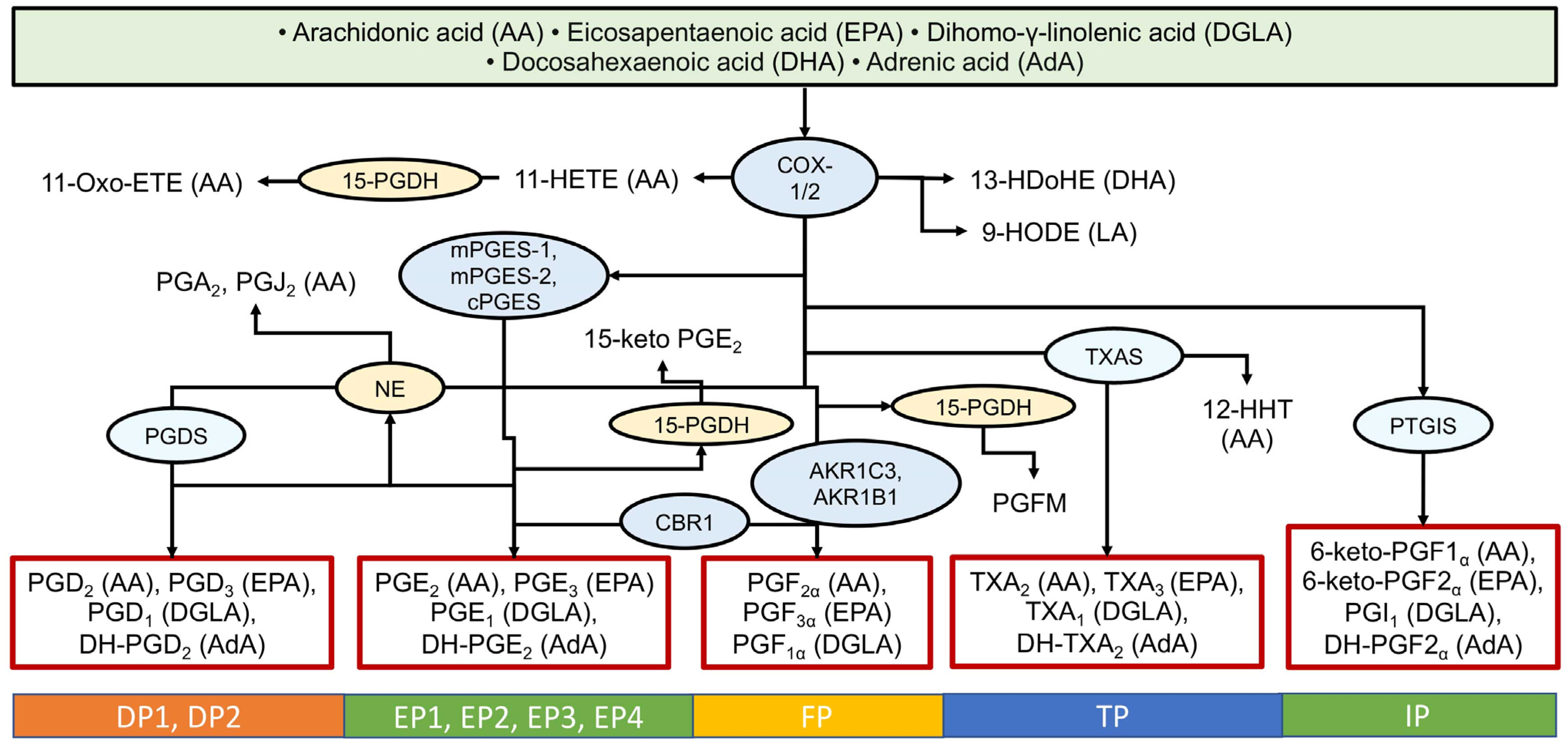
3.1. The Cyclooxygenase (COX) Pathway
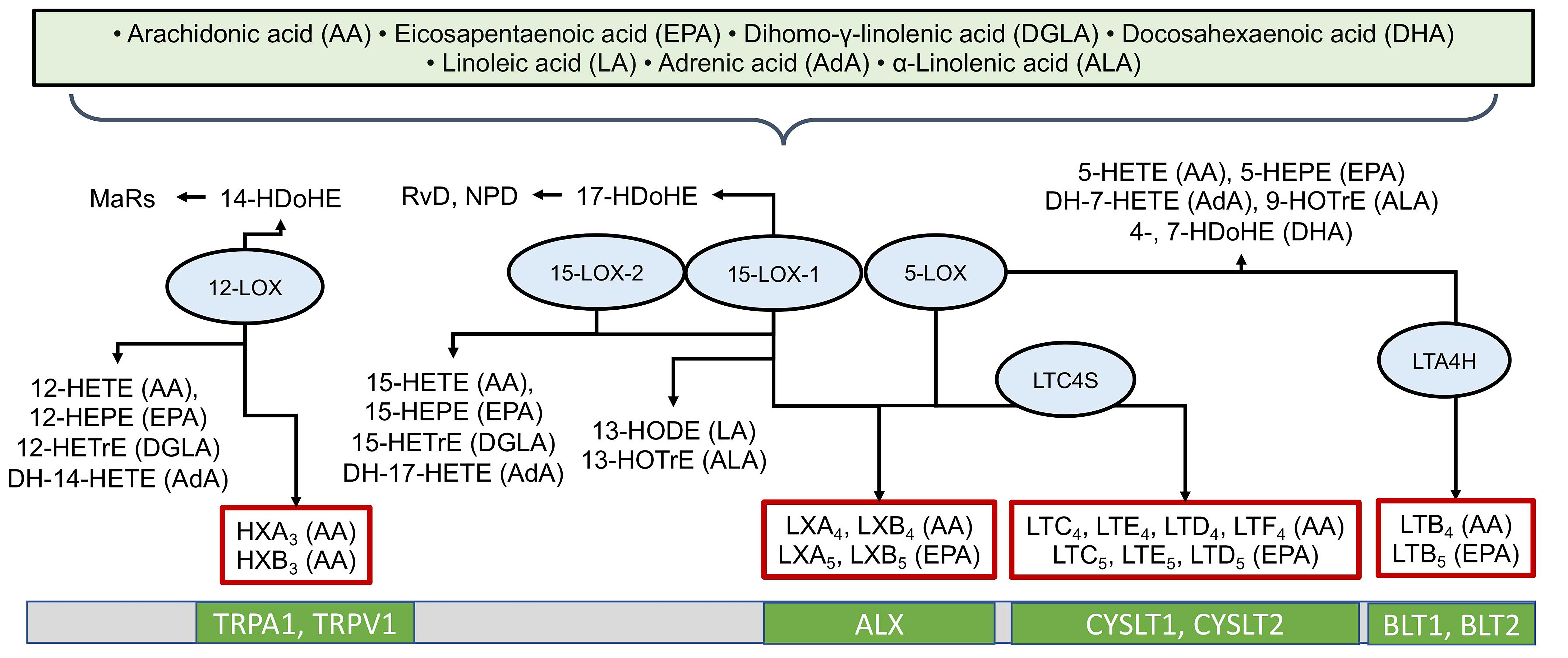
3.2. The Lipoxygenase (LOX) Pathway
3.3. The Cytochrome P450 (CYP) Pathway
3.4. The Anandamide Pathway
3.5. Non-Enzymatic Oxidation
3.6. Challenges in the Oxylipins System Investigations
4. Oxylipin Profiles as Biomarkers of Atherosclerosis: Diagnostic and Prognostic Potential
5. Challenges in the Clinical Analysis of Oxylipins
5.1. Core Problem: Lack of Method Standardization and Harmonization
5.2. Critically Important Preanalytical Factors
5.3. Analytical Challenges and Technical Limitations
5.4. Economic Barriers and Clinical Utility
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansson, G.K.; Hermansson, A. The Immune System in Atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and Its Resolution in Atherosclerosis: Mediators and Therapeutic Opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in Atherosclerosis: Pathophysiology and Mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523, Erratum in Nat. Rev. Immunol. 2015, 15, 724. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Astakhova, A.A.; Sergeeva, M.G. Resolution of Inflammation and Mood Disorders. Exp. Mol. Pathol. 2018, 105, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, N.V.; Sergeeva, M.G.; Dudek, S.M.; Verin, A.D. Arachidonic Acid Cascade in Endothelial Pathobiology. Microvasc. Res. 2005, 69, 107–127. [Google Scholar] [CrossRef]
- Parchem, K.; Letsiou, S.; Petan, T.; Oskolkova, O.; Medina, I.; Kuda, O.; O’Donnell, V.B.; Nicolaou, A.; Fedorova, M.; Bochkov, V.; et al. Oxylipin Profiling for Clinical Research: Current Status and Future Perspectives. Prog. Lipid Res. 2024, 95, 101276. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Coras, R.; Alperi-López, M.; López, P.; Ulloa, C.; Ballina-García, F.J.; Armando, A.M.; Quehenberger, O.; Guma, M.; Suárez, A. Profiling of Serum Oxylipins During the Earliest Stages of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 401–413, Erratum in Arthritis Rheumatol. 2021, 73, 799. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Pietzner, A.; Rohwer, N.; Jung, A.; Rothe, M.; Weylandt, K.H.; Elbelt, U. Bioactive Oxylipins in Type 2 Diabetes Mellitus Patients with and without Hypertriglyceridemia. Front. Endocrinol. 2023, 14, 1195247. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Kovalenko, L.V.; Donnikov, M.Y.; Sergeeva, M.G. Blood Oxylipin Profiles as Markers of Oncological Diseases. Biochemistry 2023, 88, 621–629. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Diniz, L.S.; Santos, R.S.; Lima, R.S.; Oreliana, H.; Pinto, I.F.D.; Dantas, L.S.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; et al. Plasma Oxylipin Profiling by High Resolution Mass Spectrometry Reveal Signatures of Inflammation and Hypermetabolism in Amyotrophic Lateral Sclerosis. Free Radic. Biol. Med. 2023, 208, 285–298. [Google Scholar] [CrossRef]
- Azbukina, N.V.; Chistyakov, D.V.; Goriainov, S.V.; Kotelin, V.I.; Fedoseeva, E.V.; Petrov, S.Y.; Sergeeva, M.G.; Iomdina, E.N.; Zernii, E.Y. Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma. Biology 2021, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Azbukina, N.V.; Lopachev, A.V.; Chistyakov, D.V.; Goriainov, S.V.; Astakhova, A.A.; Poleshuk, V.V.; Kazanskaya, R.B.; Fedorova, T.N.; Sergeeva, M.G. Oxylipin Profiles in Plasma of Patients with Wilson’s Disease. Metabolites 2020, 10, 222. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhu, J.; Zou, M.; Zhang, Y.; Wu, H.; Jin, T. Specialized Pro-Resolving Lipid Mediators: A Key Player in Resolving Inflammation in Autoimmune Diseases. Sci. Bull. 2025, 70, 778–794. [Google Scholar] [CrossRef]
- Diniz, L.R.; Santos, R.S.; Oreliana, H.; Zucão, A.C.A.; Yukuyama, M.N.; Resende, G.R.S.; Iijima, T.S.; Viviani, L.G.; Miyamoto, S. Mapping Oxylipin Signatures in Human Diseases Using LC-MS/MS. Redox Biochem. Chem. 2025, 14, 100063. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yeşildemir, Ö.; Koçyiğit, E.; Koçak, T.; Özen Ünaldı, B.; Ayakdaş, G.; Budán, F. Oxylipins Derived from PUFAs in Cardiometabolic Diseases: Mechanism of Actions and Possible Nutritional Interactions. Nutrients 2024, 16, 3812. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V. The Endocannabinoidomes: Pharmacological Redundancy and Promiscuity, and Multi-Kingdom Variety of Sources and Molecular Targets. Pharmacol. Rev. 2025, 77, 100070. [Google Scholar] [CrossRef]
- Camunas-Alberca, S.M.; Moran-Garrido, M.; Sáiz, J.; Villaseñor, A.; Taha, A.Y.; Barbas, C. The Role of Oxylipins and Their Validation as Biomarkers in the Clinical Context. TrAC Trends Anal. Chem. 2023, 164, 117065. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef]
- Drexel, H.; Amann, F.W.; Beran, J.; Rentsch, K.; Candinas, R.; Muntwyler, J.; Luethy, A.; Gasser, T.; Follath, F. Plasma Triglycerides and Three Lipoprotein Cholesterol Fractions Are Independent Predictors of the Extent of Coronary Atherosclerosis. Circulation 1994, 90, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Xu, Y.; Wang, T. Omega-6 Polyunsaturated Fatty Acids and Their Metabolites: A Potential Targeted Therapy for Pulmonary Hypertension. Respir. Res. 2025, 26, 102. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis—From Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and Cholesterol as Predictors of Cardiovascular Events among Patients Receiving Statin Therapy: A Collaborative Analysis of Three Randomised Trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Ben-Chetrit, E.; Ridker, P.M. Low-Dose Colchicine for Atherosclerosis: Long-Term Safety. Eur. Heart J. 2024, 45, 1596–1601. [Google Scholar] [CrossRef]
- Ridker, P.M. The Time to Initiate Anti-Inflammatory Therapy for Patients With Chronic Coronary Atherosclerosis Has Arrived. Circulation 2023, 148, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Mehta, N.N.; deGoma, E.; Shapiro, M.D. IL-6 and Cardiovascular Risk: A Narrative Review. Curr. Atheroscler. Rep. 2024, 27, 12. [Google Scholar] [CrossRef]
- Moreira, D.M.; da Silva, R.L.; Vieira, J.L.; Fattah, T.; Lueneberg, M.E.; Gottschall, C.A.M. Role of Vascular Inflammation in Coronary Artery Disease: Potential of Anti-Inflammatory Drugs in the Prevention of Atherothrombosis. Inflammation and Anti-Inflammatory Drugs in Coronary Artery Disease. Am. J. Cardiovasc. Drugs 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The Role of Non-Resolving Inflammation in Atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2015, 7, a016311. [Google Scholar] [CrossRef]
- Livne-Bar, I.; Wei, J.; Liu, H.-H.; Alqawlaq, S.; Won, G.-J.; Tuccitto, A.; Gronert, K.; Flanagan, J.G.; Sivak, J.M. Astrocyte-Derived Lipoxins A4 and B4 Promote Neuroprotection from Acute and Chronic Injury. J. Clin. Investig. 2017, 2, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757, Erratum in Circulation 2003, 107, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gan, D.; Huo, S.; Chen, P. Unraveling the Discrepancies between REDUCE-IT and STRENGTH Trials with Omega-3 Fatty Acids: New Analytical Approaches. Front. Nutr. 2024, 11, 1490953. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Kapoor, K.; Dardari, Z.A.; Bhatt, D.L.; Budoff, M.J.; Nasir, K.; Miller, M.; Welty, F.K.; Miedema, M.D.; Shapiro, M.D.; et al. Omega-3 Fatty Acids, Subclinical Atherosclerosis, and Cardiovascular Events: Implications for Primary Prevention. Atherosclerosis 2022, 353, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Omega-3 (n-3) Fatty Acid-Statin Interaction: Evidence for a Novel Therapeutic Strategy for Atherosclerotic Cardiovascular Disease. Nutrients 2024, 16, 962. [Google Scholar] [CrossRef]
- Xie, X.; Liu, X.; Li, R.; Fan, L.; Huang, F. Ω-3 Fatty Acids in Atherosclerotic Cardiovascular Disease (Review). Biomed. Rep. 2024, 20, 94. [Google Scholar] [CrossRef]
- Fredman, G. Can Inflammation-Resolution Provide Clues to Treat Patients According to Their Plaque Phenotype? Front. Pharmacol. 2019, 10, 205. [Google Scholar] [CrossRef]
- Fredman, G.; Spite, M. Specialized Pro-Resolving Mediators in Cardiovascular Diseases. Mol. Aspects Med. 2017, 58, 65–71. [Google Scholar] [CrossRef]
- Salazar, J.; Pirela, D.; Nava, M.; Castro, A.; Angarita, L.; Parra, H.; Durán-Agüero, S.; Rojas-Gómez, D.M.; Galbán, N.; Añez, R.; et al. Specialized Proresolving Lipid Mediators: A Potential Therapeutic Target for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3133. [Google Scholar] [CrossRef]
- Toth, P.P.; Chapman, M.J.; Parhofer, K.G.; Nelson, J.R. Differentiating EPA from EPA/DHA in Cardiovascular Risk Reduction. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 17, 100148. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H. Effects of Eicosapentaenoic Acid on Cardiovascular Events in Japanese Patients with Hypercholesterolemia: Rationale, Design, and Baseline Characteristics of the Japan EPA Lipid Intervention Study (JELIS). Am. Heart J. 2003, 146, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J. Am. Coll. Cardiol. 2019, 73, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Iwata, H.; Nishizaki, Y.; Inoue, T.; Hirayama, A.; Kimura, K.; Ozaki, Y.; Murohara, T.; Ueshima, K.; Kuwabara, Y.; et al. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid (RESPECT-EPA). Circulation 2024, 150, 425–434. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Kaur, G.; Mason, R.P.; Steg, P.G.; Bhatt, D.L. Omega-3 Fatty Acids for Cardiovascular Event Lowering. Eur. J. Prev. Cardiol. 2024, 31, 1005–1014. [Google Scholar] [CrossRef]
- Nayda, N.C.; Thomas, J.M.; Delaney, C.L.; Miller, M.D. The Effect of Omega-3 Polyunsaturated Fatty Acid Intake on Blood Levels of Omega-3s in People with Chronic Atherosclerotic Disease: A Systematic Review. Nutr. Rev. 2023, 81, 1447–1461. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, D.; Yan, X.; Shi, H.; Xian, X. Effects of ω-3 Polyunsaturated Fatty Acids on Coronary Atherosclerosis and Inflammation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 904250. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern. Med. 2016, 176, 1155–1166, Erratum in JAMA Intern. Med. 2019, 179, 457. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. Thematic Review Series: Proteomics. An Integrated Omics Analysis of Eicosanoid Biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, X.; Pei, J.; Yang, H.; Zheng, X.L. Dissecting the Role of Cannabinoids in Vascular Health and Disease. J. Cell Physiol. 2024, 239, e31373. [Google Scholar] [CrossRef] [PubMed]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.M.; Lee, J.C.Y.; Durand, T. Non-Enzymatic Lipid Oxidation Products in Biological Systems: Assessment of the Metabolites from Polyunsaturated Fatty Acids. J. Chromatogr. B 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and Functional Insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef]
- Das, U.N. Atherosclerosis and Prostaglandins. Int. J. Tissue React. 1982, 4, 127–132. [Google Scholar] [PubMed]
- Straus, D.S.; Glass, C.K. Cyclopentenone Prostaglandins: New Insights on Biological Activities and Cellular Targets. Med. Res. Rev. 2001, 21, 185–210. [Google Scholar] [CrossRef]
- Figueiredo-Pereira, M.E.; Rockwell, P.; Schmidt-Glenewinkel, T.; Serrano, P. Neuroinflammation and J2 Prostaglandins: Linking Impairment of the Ubiquitin-Proteasome Pathway and Mitochondria to Neurodegeneration. Front. Mol. Neurosci. 2015, 7, 104. [Google Scholar] [CrossRef]
- Lee, B.R.; Paing, M.H.; Sharma-Walia, N. Cyclopentenone Prostaglandins: Biologically Active Lipid Mediators Targeting Inflammation. Front. Physiol. 2021, 12, 640374. [Google Scholar] [CrossRef]
- Gong, L.; Thorn, C.F.; Bertagnolli, M.M.; Grosser, T.; Altman, R.B.; Klein, T.E. Celecoxib Pathways: Pharmacokinetics and Pharmacodynamics. Pharmacogenet Genom. 2012, 22, 310–318. [Google Scholar] [CrossRef]
- Nissen, S.E.; Yeomans, N.D.; Solomon, D.H.; Lüscher, T.F.; Libby, P.; Husni, M.E.; Graham, D.Y.; Borer, J.S.; Wisniewski, L.M.; Wolski, K.E.; et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N. Engl. J. Med. 2016, 375, 2519–2529. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Zhu, X. Insights from Pharmacovigilance and Pharmacodynamics on Cardiovascular Safety Signals of NSAIDs. Front. Pharmacol. 2024, 15, 1455212. [Google Scholar] [CrossRef]
- Patrono, C. Low-Dose Aspirin for the Prevention of Atherosclerotic Cardiovascular Disease. Eur. Heart J. 2024, 45, 2362–2376. [Google Scholar] [CrossRef]
- Berger, J.S. Aspirin for Primary Prevention-Time to Rethink Our Approach. JAMA Netw. Open 2022, 5, e2210144. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Gómez-Hernández, A.; Sánchez-Galán, E.; González, A.; Ortega, L.; Gómez-Gerique, J.A.; Tuñón, J.; Egido, J. Licofelone, a Balanced Inhibitor of Cyclooxygenase and 5-Lipoxygenase, Reduces Inflammation in a Rabbit Model of Atherosclerosis. J. Pharmacol. Exp. Ther. 2007, 320, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shan, K.; Yang, Q.; Qi, Y.; Qu, H.; Li, J.; Wang, R.; Jia, L.; Chen, W.; Feng, N.; et al. Prostaglandin E3 Attenuates Macrophage-Associated Inflammation and Prostate Tumour Growth by Modulating Polarization. J. Cell Mol. Med. 2021, 25, 5586–5601. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; Van Leyen, K. Mammalian Lipoxygenases and Their Biological Relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 308–330. [Google Scholar] [CrossRef]
- Archambault, A.S.; Turcotte, C.; Martin, C.; Provost, V.; Larose, M.C.; Laprise, C.; Chakir, J.; Bissonnette, É.; Laviolette, M.; Bossé, Y.; et al. Comparison of Eight 15-Lipoxygenase (LO) Inhibitors on the Biosynthesis of 15-LO Metabolites by Human Neutrophils and Eosinophils. PLoS ONE 2018, 13, e0202424. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.T.; Thomas, C.P.; Kühn, H.; O’Donnell, V.B. Thrombin-Activated Human Platelets Acutely Generate Oxidized Docosahexaenoic-Acid-Containing Phospholipids via 12-Lipoxygenase. Biochem. J. 2010, 431, 141–148. [Google Scholar] [CrossRef]
- Picq, M.; Chen, P.; Perez, M.; Michaud, M.; Véricel, E.; Guichardant, M.; Lagarde, M. DHA Metabolism: Targeting the Brain and Lipoxygenation. Mol. Neurobiol. 2010, 42, 48–51. [Google Scholar] [CrossRef]
- Guichardant, M.; Véricel, E.; Lagarde, M. Biological Relevance of Double Lipoxygenase Products of Polyunsaturated Fatty Acids, Especially within Blood Vessels and Brain. Biochimie 2019, 159, 55–58. [Google Scholar] [CrossRef]
- Pickens, C.A.; Sordillo, L.M.; Zhang, C.; Fenton, J.I. Obesity Is Positively Associated with Arachidonic Acid-Derived 5- and 11-Hydroxyeicosatetraenoic Acid (HETE). Metabolism 2017, 70, 177–191. [Google Scholar] [CrossRef]
- Leung, K.S.; Chan, H.F.; Leung, H.H.; Galano, J.M.; Oger, C.; Durand, T.; Lee, J.C.Y. Short-Time UVA Exposure to Human Keratinocytes Instigated Polyunsaturated Fatty Acid without Inducing Lipid Peroxidation. Free Radic. Res. 2017, 51, 269–280. [Google Scholar] [CrossRef]
- Le, D.E.; García-Jaramillo, M.; Bobe, G.; Alcazar Magana, A.; Vaswani, A.; Minnier, J.; Jump, D.B.; Rinkevich, D.; Alkayed, N.J.; Maier, C.S.; et al. Plasma Oxylipins: A Potential Risk Assessment Tool in Atherosclerotic Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 645786. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; He, S.; Liu, J.; Zhuang, W.; Li, H.; Lin, C.; Wang, L.; Feng, J.; Wang, L. Metabolomics Unveils the Exacerbating Role of Arachidonic Acid Metabolism in Atherosclerosis. Front. Mol. Biosci. 2024, 11, 1297437. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Ma, J.; Liu, Y.; Li, Q.; Niu, S.; Shen, Z.; Zhang, L.; Pan, Z.; Zhu, D. Key Role of 15-Lipoxygenase/15-Hydroxyeicosatetraenoic Acid in Pulmonary Vascular Remodeling and Vascular Angiogenesis Associated with Hypoxic Pulmonary Hypertension. Hypertension 2011, 58, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Van Den Borne, P.; Van Der Laan, S.W.; Bovens, S.M.; Koole, D.; Kowala, M.C.; Michael, L.F.; Schoneveld, A.H.; Van De Weg, S.M.; Velema, E.; De Vries, J.P.; et al. Leukotriene B4 Levels in Human Atherosclerotic Plaques and Abdominal Aortic Aneurysms. PLoS ONE 2014, 9, e86522. [Google Scholar] [CrossRef]
- Wang, X.; Baskaran, L.; Chan, M.; Boisvert, W.; Hausenloy, D.J. Targeting Leukotriene Biosynthesis to Prevent Atherosclerotic Cardiovascular Disease. Cond. Med. 2023, 6, 33. [Google Scholar]
- Tardif, J.C.; L’Allier, P.L.; Ibrahim, R.; Grégoire, J.C.; Nozza, A.; Cossette, M.; Kouz, S.; Lavoie, M.A.; Paquin, J.; Brotz, T.M.; et al. Treatment with 5-Lipoxygenase Inhibitor VIA-2291 (Atreleuton) in Patients with Recent Acute Coronary Syndrome. Circ. Cardiovasc. Imaging 2010, 3, 298–307. [Google Scholar] [CrossRef]
- Gaztanaga, J.; Farkouh, M.; Rudd, J.H.F.; Brotz, T.M.; Rosenbaum, D.; Mani, V.; Kerwin, T.C.; Taub, R.; Tardif, J.C.; Tawakol, A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study of the Effect of VIA-2291, a 5-Lipoxygenase Inhibitor, on Vascular Inflammation in Patients after an Acute Coronary Syndrome. Atherosclerosis 2015, 240, 53–60. [Google Scholar] [CrossRef]
- Ericsson, H.; Nelander, K.; Lagerstrom-Fermer, M.; Balendran, C.; Bhat, M.; Chialda, L.; Gan, L.M.; Heijer, M.; Kjaer, M.; Lambert, J.; et al. Initial Clinical Experience with AZD5718, a Novel Once Daily Oral 5-Lipoxygenase Activating Protein Inhibitor. Clin. Transl. Sci. 2018, 11, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, D.; Broddefalk, J.; Emtenäs, H.; Hayes, M.A.; Lemurell, M.; Swanson, M.; Ulander, J.; Whatling, C.; Amilon, C.; Ericsson, H.; et al. Discovery and Early Clinical Development of an Inhibitor of 5-Lipoxygenase Activating Protein (AZD5718) for Treatment of Coronary Artery Disease. J. Med. Chem. 2019, 62, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Dahlke, P.; Peltner, L.K.; Jordan, P.M.; Werz, O. Differential Impact of 5-Lipoxygenase-Activating Protein Antagonists on the Biosynthesis of Leukotrienes and of Specialized pro-Resolving Mediators. Front. Pharmacol. 2023, 14, 1219160. [Google Scholar] [CrossRef]
- Molaie, M.; Lotfi, R.; Heidari Moghadam, R.; Rezaiemanesh, A.; Karaji, A.G.; Salari, F. Imbalanced Serum Levels of Resolvin E1 (RvE1) and Leukotriene B4 (LTB4) May Contribute to the Pathogenesis of Atherosclerosis. Prostaglandins Other Lipid Mediat. 2023, 169, 106781. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K.; Schulte, F.; Alfaddagh, A.; Elajami, T.K.; Bistrian, B.R.; Hardt, M. Regression of Human Coronary Artery Plaque Is Associated with a High Ratio of (18-Hydroxy-Eicosapentaenoic Acid + Resolvin E1) to Leukotriene B4. FASEB J. 2021, 35, e21448. [Google Scholar] [CrossRef]
- De Bartolo, A.; Romeo, N.; Angelone, T.; Rocca, C. Specialized Pro-Resolving Mediators as Emerging Players in Cardioprotection: From Inflammation Resolution to Therapeutic Potential. Acta Physiol. 2025, 241, e70062. [Google Scholar] [CrossRef]
- Millar, B.; de Gaetano, M. Posing the Rationale for Synthetic Lipoxin Mimetics as an Adjuvant Treatment to Gold Standard Atherosclerosis Therapies. Front. Pharmacol. 2023, 14, 1125858. [Google Scholar] [CrossRef]
- Fu, T.; Mohan, M.; Brennan, E.P.; Woodman, O.L.; Godson, C.; Kantharidis, P.; Ritchie, R.H.; Qin, C.X. Therapeutic Potential of Lipoxin A4 in Chronic Inflammation: Focus on Cardiometabolic Disease. ACS Pharmacol. Transl. Sci. 2020, 3, 43–55. [Google Scholar] [CrossRef]
- Weng, X.; Tan, H.; Huang, Z.; Chen, J.; Zhang, N.; Wang, Q.; Li, Q.; Gao, J.; Sun, D.; Yakufu, W.; et al. Targeted Delivery and ROS-Responsive Release of Resolvin D1 by Platelet Chimeric Liposome Ameliorates Myocardial Ischemia-Reperfusion Injury. J. Nanobiotechnol. 2022, 20, 454. [Google Scholar] [CrossRef]
- Poeckel, D.; Funk, C.D. The 5-Lipoxygenase/Leukotriene Pathway in Preclinical Models of Cardiovascular Disease. Cardiovasc. Res. 2010, 86, 243–253. [Google Scholar] [CrossRef]
- Fredman, G.; Hellmann, J.; Proto, J.D.; Kuriakose, G.; Colas, R.A.; Dorweiler, B.; Connolly, E.S.; Solomon, R.; Jones, D.M.; Heyer, E.J.; et al. An Imbalance between Specialized Pro-Resolving Lipid Mediators and pro-Inflammatory Leukotrienes Promotes Instability of Atherosclerotic Plaques. Nat. Commun. 2016, 7, 12859. [Google Scholar] [CrossRef]
- Yin, L.; Wang, L.; Shi, Z.; Ji, X.; Liu, L. The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-Translational Modification and Selective Modulators. Front. Physiol. 2022, 13, 826811. [Google Scholar] [CrossRef]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The Resolution of Inflammation through Omega-3 Fatty Acids in Atherosclerosis, Intimal Hyperplasia, and Vascular Calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- McGiff, J.C. Cytochrome P-450 Metabolism of Arachidonic Acid. Annu. Rev. Pharmacol. Toxicol. 1991, 31, 339–369. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Edin, M.L.; Maeyer, R.P.H.D.; Bystrom, J.; Newson, J.; Lih, F.B.; Stables, M.; Zeldin, D.C.; Bishop-Bailey, D. CYP450-Derived Oxylipins Mediate Inflammatory Resolution. Proc. Natl. Acad. Sci. USA 2016, 113, E3240–E3249. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Song, C.Y.; Ghafoor, K.; Ghafoor, H.U.; Khan, N.S.; Thirunavukkarasu, S.; Jennings, B.L.; Estes, A.M.; Zaidi, S.; Bridges, D.; Tso, P.; et al. Cytochrome P450 1B1 Contributes to the Development of Atherosclerosis and Hypertension in Apolipoprotein E-Deficient Mice. Hypertension 2016, 67, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hercule, H.C.; Schunck, W.H.; Gross, V.; Seringer, J.; Leung, F.P.; Weldon, S.M.; Da Costa Goncalves, A.C.; Huang, Y.; Luft, F.C.; Gollasch, M. Interaction between P450 Eicosanoids and Nitric Oxide in the Control of Arterial Tone in Mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 54–60. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Gruenloh, S.K.; Buonaccorsi, J.N.; Zhang, R.; Gross, G.J.; Falck, J.R.; Patel, P.K.; Jacobs, E.R.; Medhora, M. Multiple Antiapoptotic Targets of the PI3K/Akt Survival Pathway Are Activated by Epoxyeicosatrienoic Acids to Protect Cardiomyocytes from Hypoxia/Anoxia. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H724–H735. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Arachidonic Acid Cytochrome P450 Epoxygenase Pathway. J. Lipid Res. 2009, 50, S52–S56. [Google Scholar] [CrossRef]
- Liu, X.; Qian, Z.Y.; Xie, F.; Fan, W.; Nelson, J.W.; Xiao, X.; Kaul, S.; Barnes, A.P.; Alkayed, N.J. Functional Screening for G Protein-Coupled Receptor Targets of 14,15-Epoxyeicosatrienoic Acid. Prostaglandins Other Lipid Mediat. 2017, 132, 31–40. [Google Scholar] [CrossRef]
- Wang, Y.X.J.; Ulu, A.; Zhang, L.N.; Hammock, B. Soluble Epoxide Hydrolase in Atherosclerosis. Curr. Atheroscler. Rep. 2010, 12, 174–183. [Google Scholar] [CrossRef]
- Cao, M.; Li, M.; Li, X.; Li, Y.; Chen, Y.; Drekolia, M.K.; Cheng, X.; Lagos, F.D.; Bibli, S.I.; Hu, J. Endothelial Soluble Epoxide Hydrolase Links Polyunsaturated Fatty Acid Metabolism to Oxidative Stress and Atherosclerosis Progression. Redox Biol. 2025, 85, 103730. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Peng, H.; Peng, R.; Fan, Q.; Zhao, S.; Xu, D.; Morisseau, C.; Chiamvimonvat, N.; Hammock, B.D. Inhibition of Soluble Epoxide Hydrolase in Mice Promotes Reverse Cholesterol Transport and Regression of Atherosclerosis. Atherosclerosis 2015, 239, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Vincelette, J.; Cheng, Y.; Mehra, U.; Chen, D.; Anandan, S.K.; Gless, R.; Webb, H.K.; Wang, Y.X. Inhibition of Soluble Epoxide Hydrolase Attenuated Atherosclerosis, Abdominal Aortic Aneurysm Formation, and Dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1265–1270. [Google Scholar] [CrossRef]
- Shi, Z.; He, Z.; Wang, D.W. CYP450 Epoxygenase Metabolites, Epoxyeicosatrienoic Acids, as Novel Anti-Inflammatory Mediators. Molecules 2022, 27, 3873. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Prospective for Cytochrome P450 Epoxygenase Cardiovascular and Renal Therapeutics. Pharmacol. Ther. 2018, 192, 1–19. [Google Scholar] [CrossRef]
- Imig, J.D.; Elmarakby, A.; Nithipatikom, K.; Wei, S.; Capdevila, J.H.; Tuniki, V.R.; Sangras, B.; Anjaiah, S.; Manthati, V.L.; Reddy, D.S.; et al. Development of Epoxyeicosatrienoic Acid Analogs with in Vivo Anti-Hypertensive Actions. Front. Physiol. 2010, 1, 157. [Google Scholar] [CrossRef]
- Gao, P.; Cao, Y.; Ma, L. Regulation of Soluble Epoxide Hydrolase in Renal-Associated Diseases: Insights from Potential Mechanisms to Clinical Researches. Front. Endocrinol. 2024, 15, 1304547. [Google Scholar] [CrossRef]
- Uyama, T.; Sasaki, S.; Okada-Iwabu, M.; Murakami, M. Recent Progress in N-Acylethanolamine Research: Biological Functions and Metabolism Regulated by Two Distinct N-Acyltransferases: CPLA2ε and PLAAT Enzymes. Int. J. Mol. Sci. 2025, 26, 3359. [Google Scholar] [CrossRef]
- Maccarrone, M.; Marzo, V.D.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef]
- Berger, A.; Crozier, G.; Bisogno, T.; Cavaliere, P.; Innis, S.; Di Marzo, V. Anandamide and Diet: Inclusion of Dietary Arachidonate and Docosahexaenoate Leads to Increased Brain Levels of the Corresponding N-Acylethanolamines in Piglets. Proc. Natl. Acad. Sci. USA 2001, 98, 6402–6406. [Google Scholar] [CrossRef]
- Park, Y.; Watkins, B.A. Dietary PUFAs and Exercise Dynamic Actions on Endocannabinoids in Brain: Consequences for Neural Plasticity and Neuroinflammation. Adv. Nutr. 2022, 13, 1989–2001. [Google Scholar] [CrossRef]
- Fišar, Z. Cannabinoids and Atherosclerosis. Med. Rep. 2009, 110, 5–12. [Google Scholar]
- Cunha, P.; Romão, A.M.; Mascarenhas-Melo, F.; Teixeira, H.M.; Reis, F. Endocannabinoid System in Cardiovascular Disorders—New Pharmacotherapeutic Opportunities. J. Pharm. Bioallied Sci. 2011, 3, 350–360. [Google Scholar] [CrossRef]
- Pacher, P.; Mukhopadhyay, P.; Mohanraj, R.; Godlewski, G.; Bátkai, S.; Kunos, G. Modulation of the Endocannabinoid System in Cardiovascular Disease: Therapeutic Potential and Limitations. Hypertension 2008, 52, 601–607. [Google Scholar] [CrossRef]
- Lenglet, S.; Thomas, A.; Soehnlein, O.; Montecucco, F.; Burger, F.; Pelli, G.; Galan, K.; Cravatt, B.; Staub, C.; Steffens, S. Fatty Acid Amide Hydrolase Deficiency Enhances Intraplaque Neutrophil Recruitment in Atherosclerotic Mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 215–223. [Google Scholar] [CrossRef]
- Xu, W.; Wei, Z.; Dong, J.; Duan, F.; Chen, K.; Chen, C.; Liu, J.; Yang, X.; Chen, L.; Xiao, H.; et al. Global Metabolomics Reveals the Metabolic Dysfunction in Ox-LDL Induced Macrophage-Derived Foam Cells. Front. Pharmacol. 2017, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Noriega, S.E.; Kassuha, D.E.; Fuentes, L.B.; Manucha, W. Anandamide and Endocannabinoid System: An Attractive Therapeutic Approach for Cardiovascular Disease. Ther. Adv. Cardiovasc. Dis. 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed Characterization of the Endocannabinoid System in Human Macrophages and Foam Cells, and Anti-Inflammatory Role of Type-2 Cannabinoid Receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Puhl, S.L. Cannabinoid-Sensitive Receptors in Cardiac Physiology and Ischaemia. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118462. [Google Scholar] [CrossRef]
- Teichmann, T.; Pflüger-Müller, B.; Giménez, V.M.M.; Sailer, F.; Dirks, H.; Zehr, S.; Warwick, T.; Brettner, F.; Munoz-Tello, P.; Zimmer, A.; et al. The Endocannabinoid Anandamide Mediates Anti-Inflammatory Effects through Activation of NR4A Nuclear Receptors. Br. J. Pharmacol. 2025, 182, 1164–1182. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef]
- Archambault, A.S.; Tinto, F.; Dumais, É.; Rakotoarivelo, V.; Kostrzewa, M.; Plante, P.L.; Martin, C.; Simard, M.; Silvestri, C.; Pouliot, R.; et al. Biosynthesis of the Novel Endogenous 15-Lipoxygenase Metabolites N-13-Hydroxy-Octodecadienoyl-Ethanolamine and 13-Hydroxy-Octodecadienoyl-Glycerol by Human Neutrophils and Eosinophils. Cells 2021, 10, 2322. [Google Scholar] [CrossRef]
- Balvers, M.G.J.; Verhoeckx, K.C.M.; Plastina, P.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F. Docosahexaenoic Acid and Eicosapentaenoic Acid Are Converted by 3T3-L1 Adipocytes to N-Acyl Ethanolamines with Anti-Inflammatory Properties. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1107–1114. [Google Scholar] [CrossRef]
- Meyer-Lindemann, U.; Moggio, A.; Dutsch, A.; Kessler, T.; Sager, H.B. The Impact of Exercise on Immunity, Metabolism, and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 3394. [Google Scholar] [CrossRef]
- Tani, S.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A. Association of Higher N-3 Polyunsaturated Fatty Acid Consumption and Aerobic Exercise with Lower Neutrophil-to-Lymphocyte Ratio: Implications of Anti-Atherosclerotic Effect of Fish Consumption. Ann. Nutr. Metab. 2024, 80, 101–108. [Google Scholar] [CrossRef]
- Praticò, D.; Iuliano, L.; Mauriello, A.; Spagnoli, L.; Lawson, J.A.; Maclouf, J.; Violi, F.; FitzGerald, G.A. Localization of Distinct F2-Isoprostanes in Human Atherosclerotic Lesions. J. Clin. Investig. 1997, 100, 2028–2034. [Google Scholar] [CrossRef]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic Acids: Novel Regulators of Macrophage Differentiation and Atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef]
- Abramova, A.; Bride, J.; Oger, C.; Demion, M.; Galano, J.M.; Durand, T.; Roy, J. Metabolites Derived from Radical Oxidation of PUFA: NEO-PUFAs, Promising Molecules for Health? Atherosclerosis 2024, 398, 118600. [Google Scholar] [CrossRef]
- Lefort, B.; Gélinas, R.; Forest, A.; Bouchard, B.; Daneault, C.; Robillard Frayne, I.; Roy, J.; Oger, C.; Greffard, K.; Galano, J.M.; et al. Remodeling of Lipid Landscape in High Fat Fed Very-Long Chain Acyl-CoA Dehydrogenase Null Mice Favors pro-Arrhythmic Polyunsaturated Fatty Acids and Their Downstream Metabolites. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166843. [Google Scholar] [CrossRef]
- Gladine, C.; Newman, J.W.; Durand, T.; Pedersen, T.L.; Galano, J.M.; Demougeot, C.; Berdeaux, O.; Pujos-Guillot, E.; Mazur, A.; Comte, B. Lipid Profiling Following Intake of the Omega 3 Fatty Acid DHA Identifies the Peroxidized Metabolites F4-Neuroprostanes as the Best Predictors of Atherosclerosis Prevention. PLoS ONE 2014, 9, e89393. [Google Scholar] [CrossRef]
- Zhang, Z.J. Systematic Review on the Association between F2-Isoprostanes and Cardiovascular Disease. Ann. Clin. Biochem. 2013, 50, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; Roberts, L.J. F2-Isoprostanes as an Indicator and Risk Factor for Coronary Heart Disease. Free Radic. Biol. Med. 2011, 50, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef]
- Perveen, N.; Kishore, U.; Al Aiyan, A.; Willingham, A.L.; Mohteshamuddin, K. Exploring the Innate Immunity in Invertebrates. Adv. Exp. Med. Biol. 2025, 1476, 411–423. [Google Scholar] [CrossRef]
- Kulterer, O.C.; Niederstaetter, L.; Herz, C.T.; Haug, A.R.; Bileck, A.; Pils, D.; Kautzky-Willer, A.; Gerner, C.; Kiefer, F.W. The Presence of Active Brown Adipose Tissue Determines Cold-Induced Energy Expenditure and Oxylipin Profiles in Humans. J. Clin. Endocrinol. Metab. 2020, 105, 2203–2216. [Google Scholar] [CrossRef]
- Buckner, T.; Johnson, R.K.; Vanderlinden, L.A.; Carry, P.M.; Romero, A.; Onengut-Gumuscu, S.; Chen, W.M.; Kim, S.; Fiehn, O.; Frohnert, B.I.; et al. Genome-Wide Analysis of Oxylipins and Oxylipin Profiles in a Pediatric Population. Front. Nutr. 2023, 10, 1040993. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, S.; Maurer, S.; Fromme, T.; Colson, C.; Virtanen, K.A.; Amri, E.Z.; Klingenspor, M. Fatty Acid Metabolite Profiling Reveals Oxylipins as Markers of Brown but Not Brite Adipose Tissue. Front. Endocrinol. 2020, 11, 73. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Grabeklis, S.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G.; Reiser, G. Astrocytes Synthesize Primary and Cyclopentenone Prostaglandins That Are Negative Regulators of Their Proliferation. Biochem. Biophys. Res. Commun. 2018, 500, 204–210. [Google Scholar] [CrossRef]
- Guryleva, M.V.; Chistyakov, D.V.; Lopachev, A.V.; Goriainov, S.V.; Astakhova, A.A.; Timoshina, Y.A.; Khutorova, A.V.; Fedorova, T.N.; Sergeeva, M.G. Modulation of the Primary Astrocyte-Enriched Cultures’ Oxylipin Profiles Reduces Neurotoxicity. Metabolites 2021, 11, 498. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Azbukina, N.V.; Lopachev, A.V.; Goriainov, S.V.; Astakhova, A.A.; Ptitsyna, E.V.; Klimenko, A.S.; Poleshuk, V.V.; Kazanskaya, R.B.; Fedorova, T.N.; et al. Plasma Oxylipin Profiles Reflect Parkinson’s Disease Stage. Prostaglandins Other Lipid Mediat. 2024, 171, 106788. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chang, M.T.; Leu, H.B.; Yin, W.H.; Tseng, W.K.; Wu, Y.W.; Lin, T.H.; Yeh, H.I.; Chang, K.C.; Wang, J.H.; et al. Association of Arachidonic Acid-Derived Lipid Mediators with Subsequent Onset of Acute Myocardial Infarction in Patients with Coronary Artery Disease. Sci. Rep. 2020, 10, 8105. [Google Scholar] [CrossRef] [PubMed]
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Methods of the Analysis of Oxylipins in Biological Samples. Molecules 2020, 25, 349. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yin, H.-H.; Wu, M.-J.; He, X.; Jiang, Q.; Zhang, L.-T.; Liu, J.-Y. High Sensitivity and Wide Linearity LC-MS/MS Method for Oxylipin Quantification in Multiple Biological Samples. J. Lipid Res. 2022, 63, 100302. [Google Scholar] [CrossRef]
- Burla, B.; Arita, M.; Arita, M.; Bendt, A.K.; Cazenave-Gassiot, A.; Dennis, E.A.; Ekroos, K.; Han, X.; Ikeda, K.; Liebisch, G.; et al. MS-Based Lipidomics of Human Blood Plasma: A Community-Initiated Position Paper to Develop Accepted Guidelines. J. Lipid Res. 2018, 59, 2001–2017. [Google Scholar] [CrossRef]
- Huynh, K.; Barlow, C.K.; Jayawardana, K.S.; Weir, J.M.; Mellett, N.A.; Cinel, M.; Magliano, D.J.; Shaw, J.E.; Drew, B.G.; Meikle, P.J. High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chem. Biol. 2019, 26, 71–84.e4. [Google Scholar] [CrossRef]
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing Lipidomics: NIST Interlaboratory Comparison Exercise for Lipidomics Using SRM 1950-Metabolites in Frozen Human Plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef]
- Mainka, M.; Dalle, C.; Pétéra, M.; Dalloux-Chioccioli, J.; Kampschulte, N.; Ostermann, A.I.; Rothe, M.; Bertrand-Michel, J.; Newman, J.W.; Gladine, C.; et al. Harmonized Procedures Lead to Comparable Quantification of Total Oxylipins across Laboratories. J. Lipid Res. 2020, 61, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Rund, K.M.; Nolte, F.; Doricic, J.; Greite, R.; Schott, S.; Lichtinghagen, R.; Gueler, F.; Schebb, N.H. Clinical Blood Sampling for Oxylipin Analysis—Effect of Storage and Pneumatic Tube Transport of Blood on Free and Total Oxylipin Profile in Human Plasma and Serum. Analyst 2020, 145, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Mainka, M.; Dalle, C.; Ostermann, A.I.; Rund, K.M.; Kutzner, L.; Froehlich, L.F.; Bertrand-Michel, J.; Gladine, C.; Schebb, N.H. Stability of Oxylipins during Plasma Generation and Long-Term Storage. Talanta 2020, 217, 121074. [Google Scholar] [CrossRef]
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Modern Methods of Sample Preparation for the Analysis of Oxylipins in Biological Samples. Molecules 2019, 24, 1639. [Google Scholar] [CrossRef]
- Pedersen, T.L.; Gray, I.J.; Newman, J.W. Plasma and Serum Oxylipin, Endocannabinoid, Bile Acid, Steroid, Fatty Acid and Nonsteroidal Anti-Inflammatory Drug Quantification in a 96-Well Plate Format. Anal. Chim. Acta 2021, 1143, 189–200. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistyakov, D.V.; Chistyakov, V.V.; Sergeeva, M.G. Oxylipins in Atherosclerosis: Their Role in Inflammation, Diagnosis, and Therapeutic Perspectives. Int. J. Mol. Sci. 2025, 26, 10577. https://doi.org/10.3390/ijms262110577
Chistyakov DV, Chistyakov VV, Sergeeva MG. Oxylipins in Atherosclerosis: Their Role in Inflammation, Diagnosis, and Therapeutic Perspectives. International Journal of Molecular Sciences. 2025; 26(21):10577. https://doi.org/10.3390/ijms262110577
Chicago/Turabian StyleChistyakov, Dmitry V., Vasiliy V. Chistyakov, and Marina G. Sergeeva. 2025. "Oxylipins in Atherosclerosis: Their Role in Inflammation, Diagnosis, and Therapeutic Perspectives" International Journal of Molecular Sciences 26, no. 21: 10577. https://doi.org/10.3390/ijms262110577
APA StyleChistyakov, D. V., Chistyakov, V. V., & Sergeeva, M. G. (2025). Oxylipins in Atherosclerosis: Their Role in Inflammation, Diagnosis, and Therapeutic Perspectives. International Journal of Molecular Sciences, 26(21), 10577. https://doi.org/10.3390/ijms262110577




