(E)-2-Benzylidenecyclanones: Part XXI—Reaction of Cyclic Chalcone Analogs with Cellular Thiols: Comparison of Reactivity of (E)-2-Arylidene-1-Indanone with -1-Tetralone and -1-Benzosuberone Analogs in Thia-Michael Reactions
Abstract
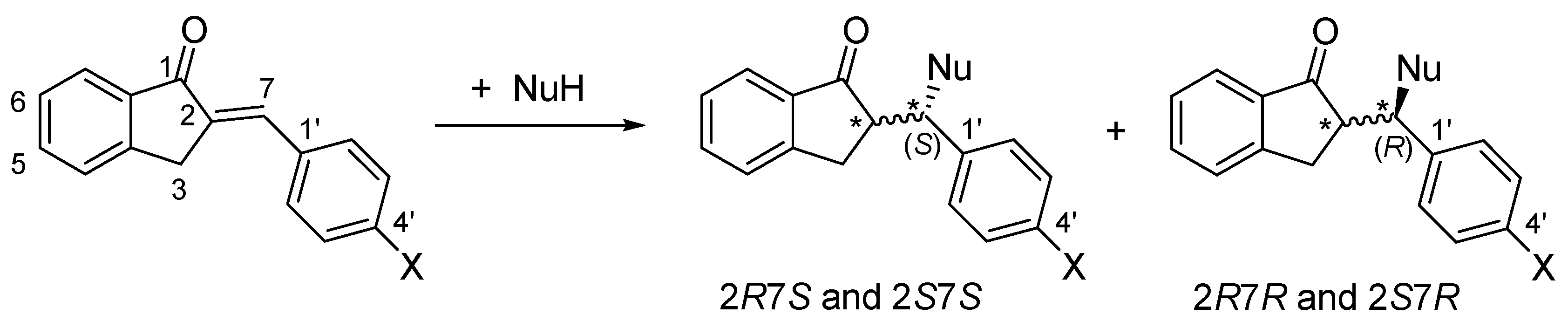
1. Introduction
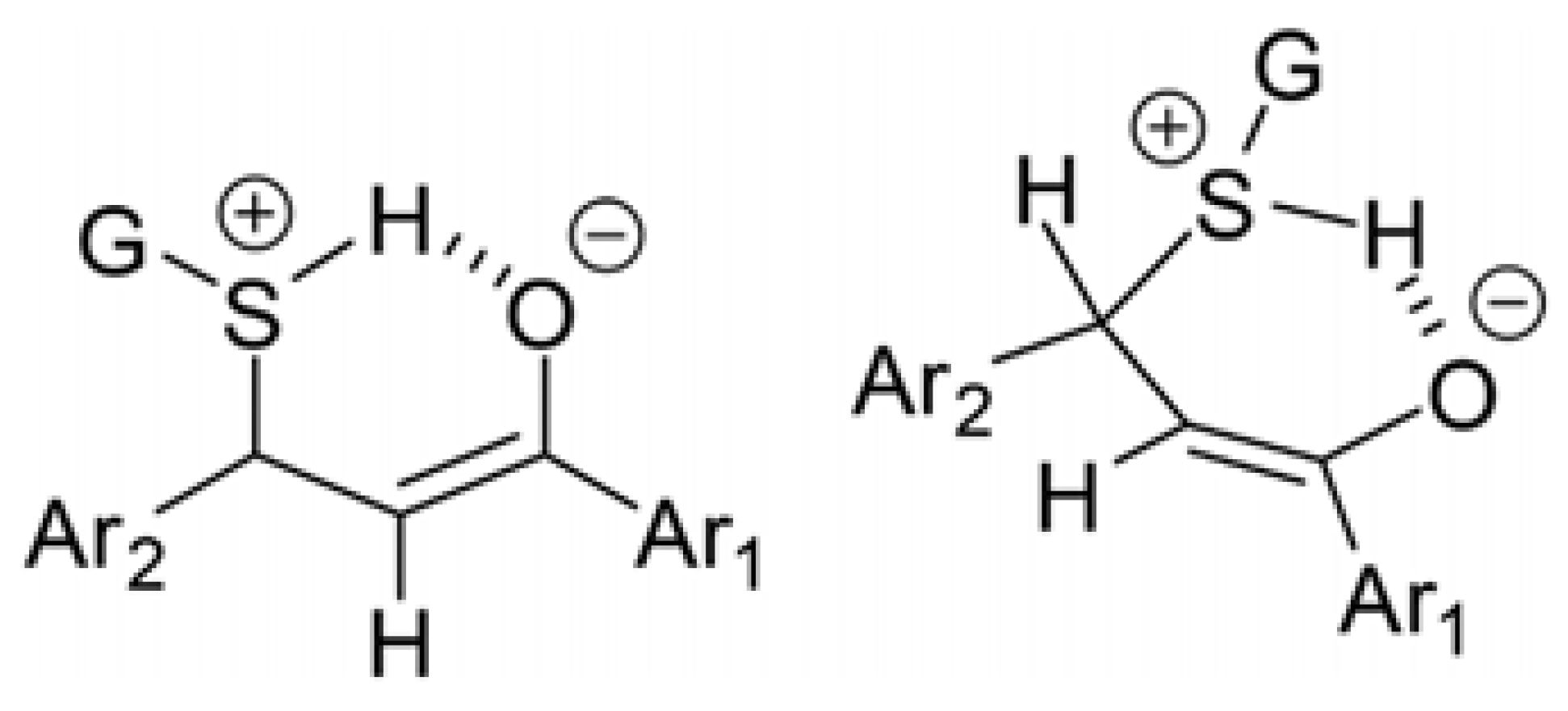
2. Results
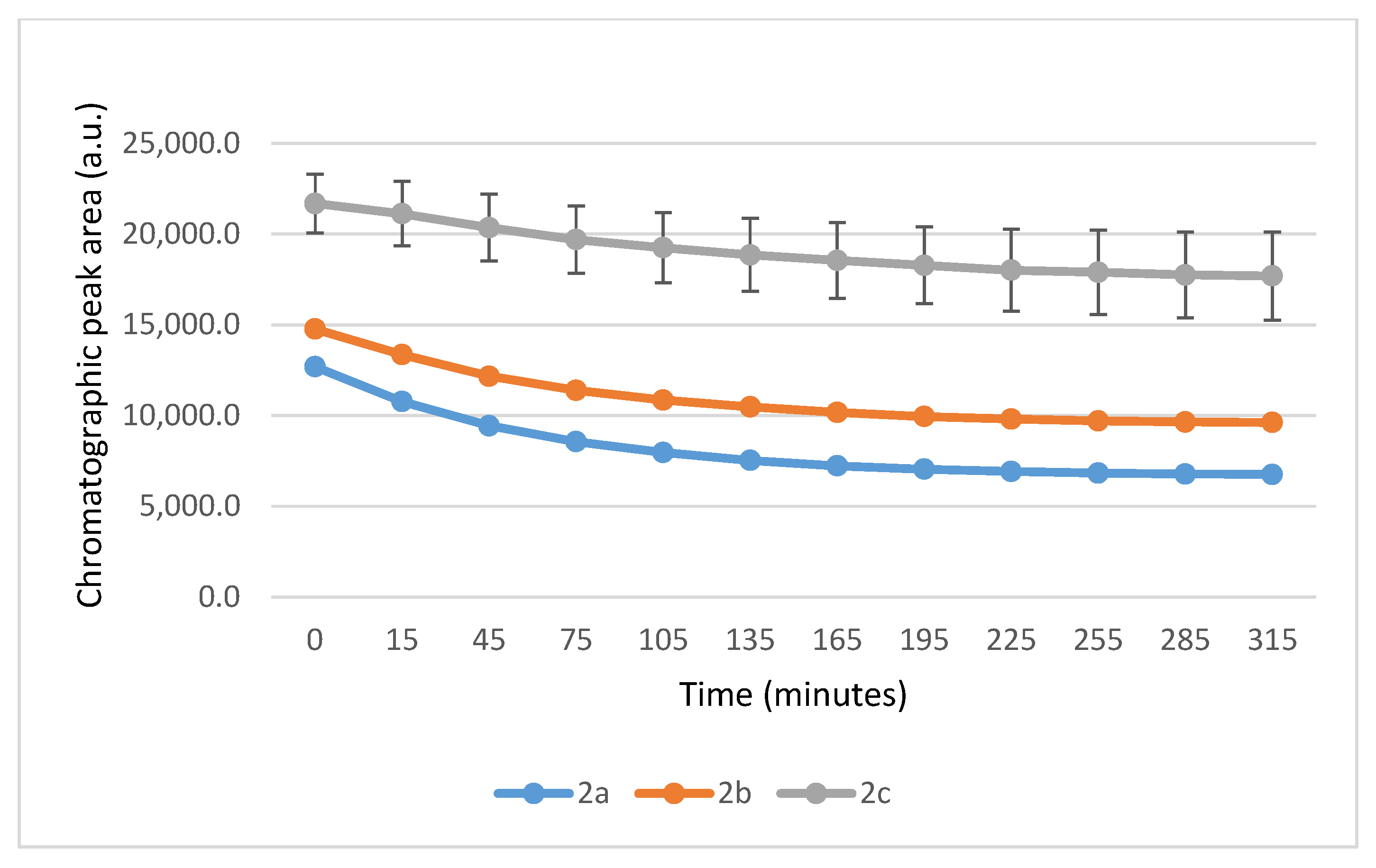
2.1. Reactions Under Slightly Basic (pH 8.0) Conditions
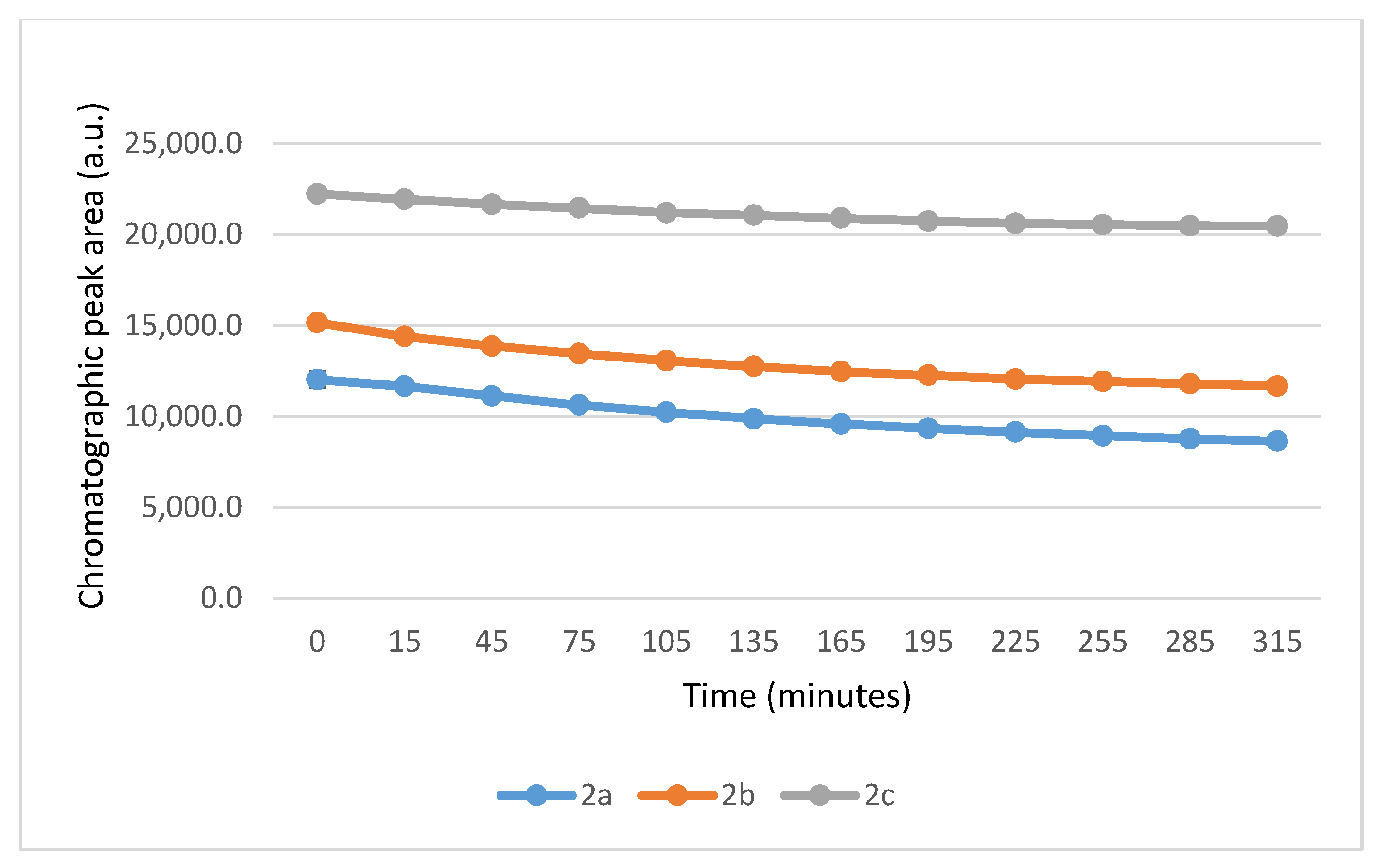
2.2. Reactions Under Slightly Acidic (pH 6.3) Conditions
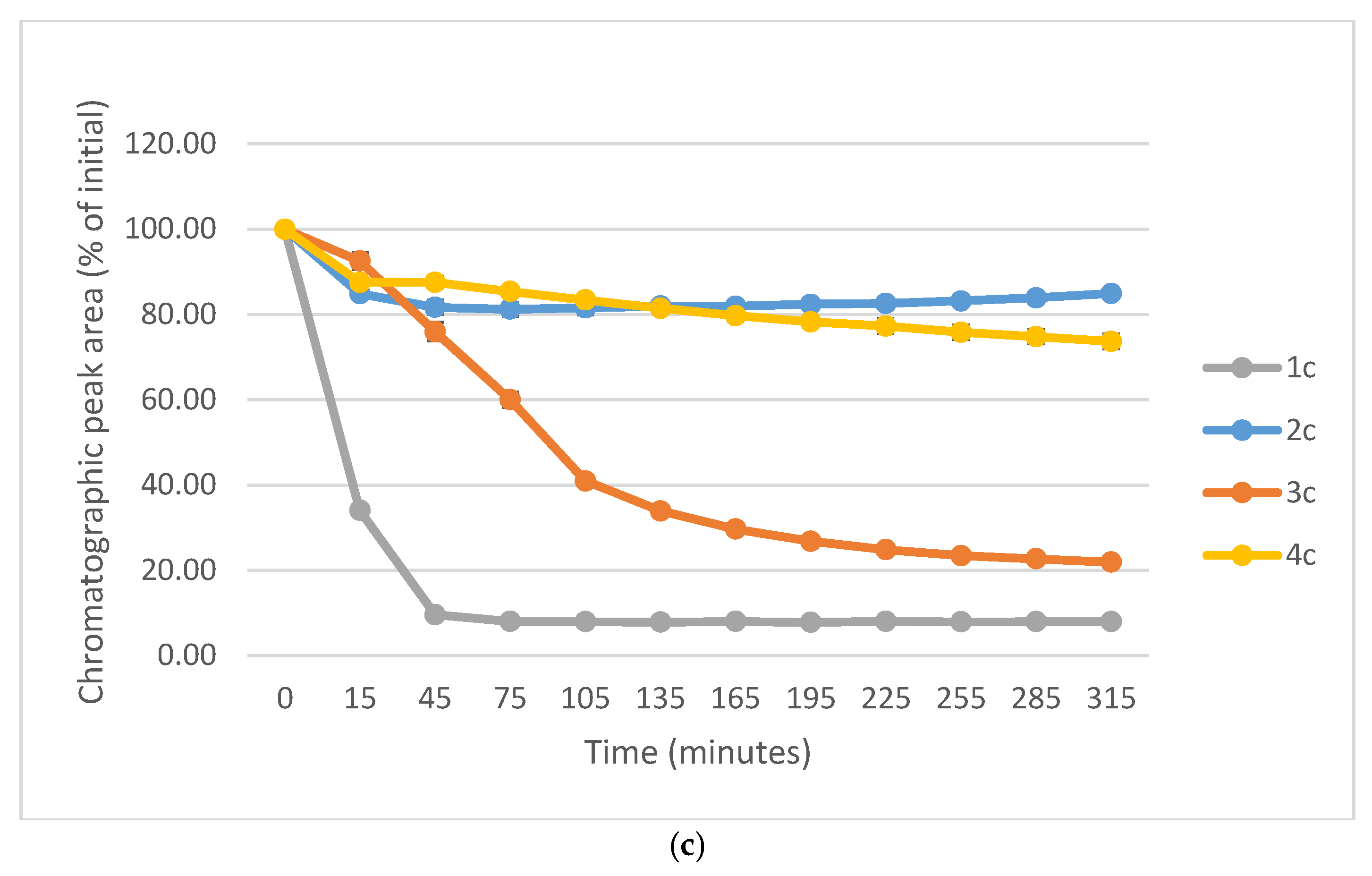
2.3. Reactions Under Acidic (pH 3.2) Conditions
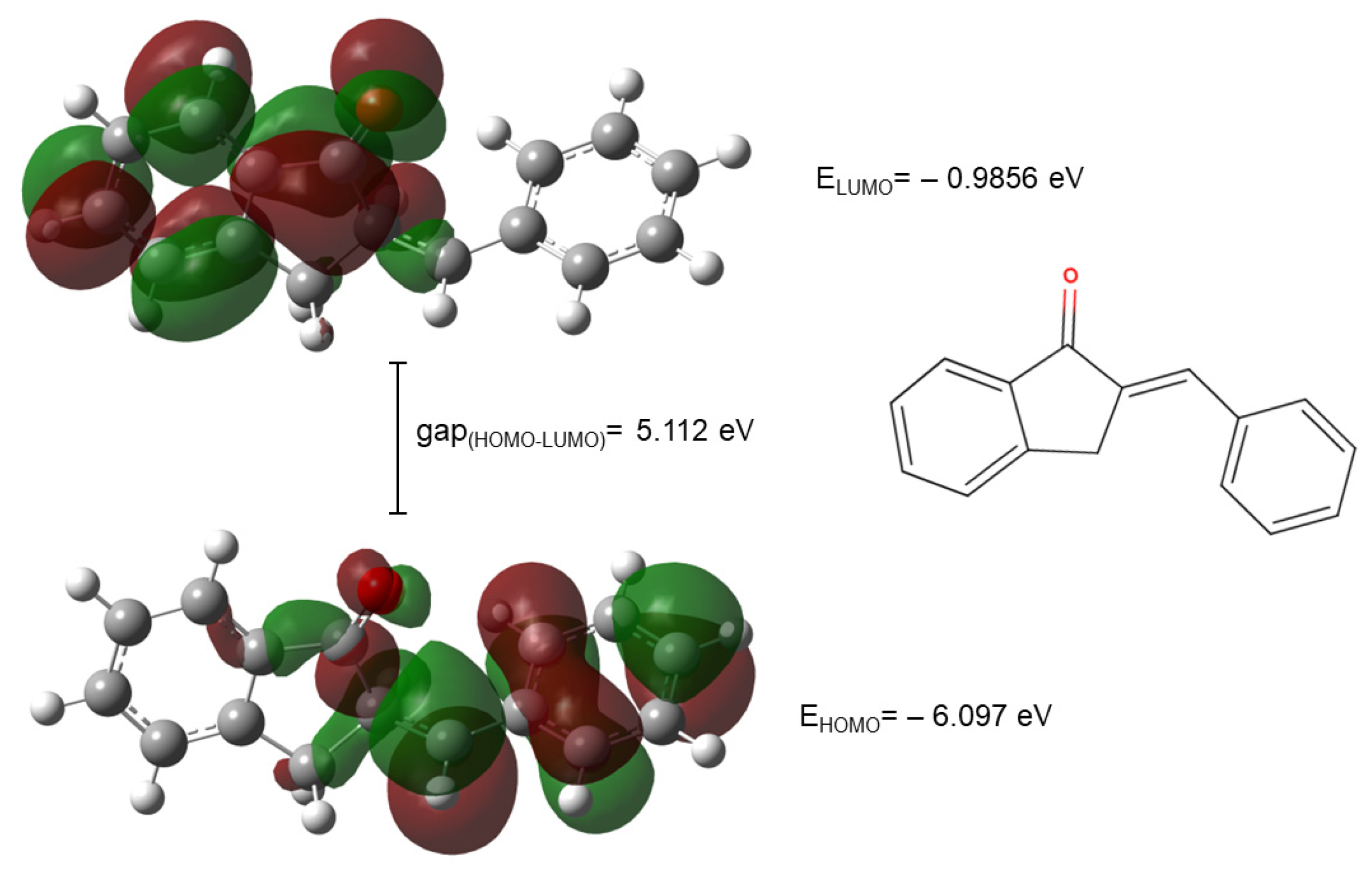
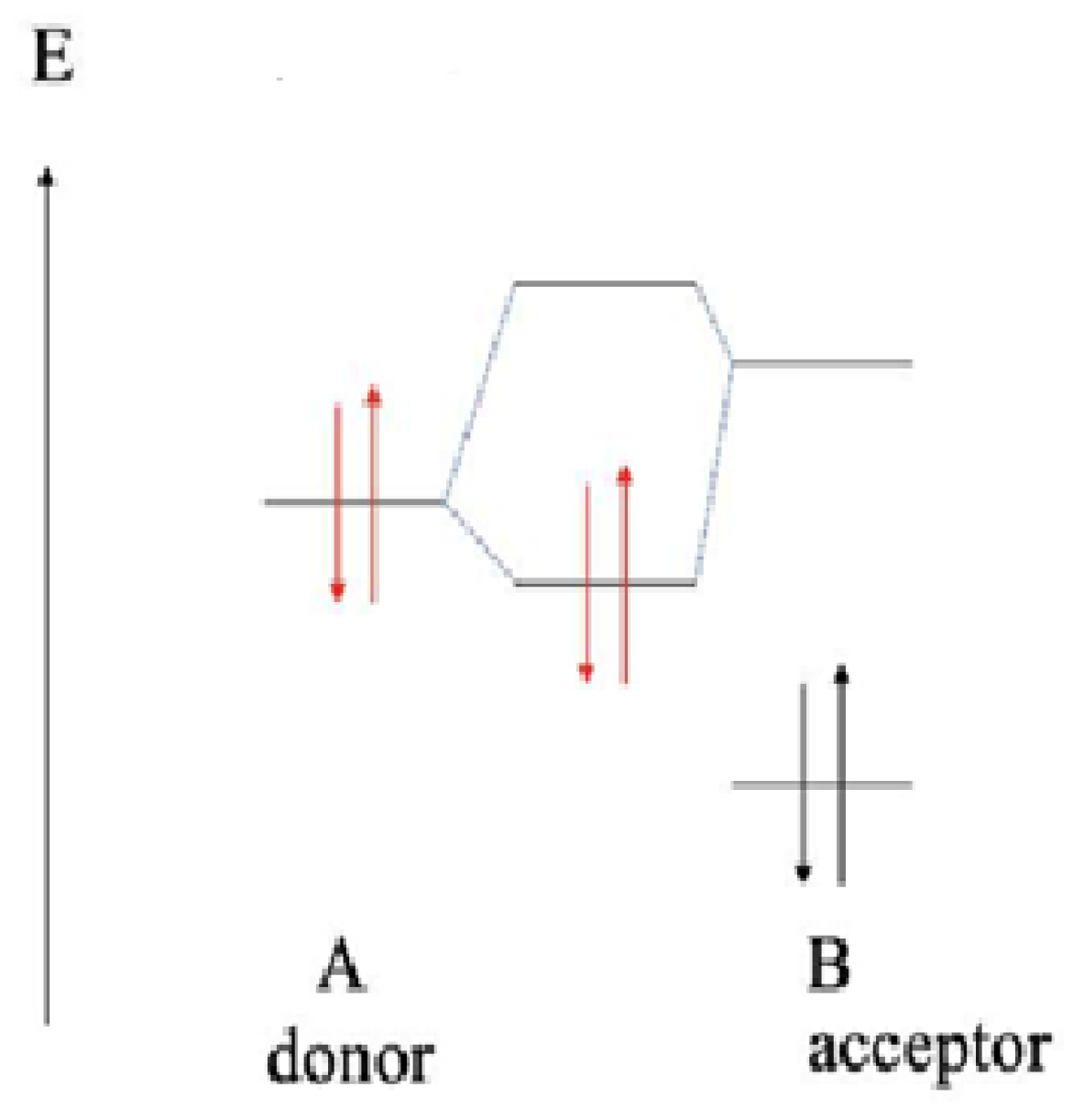
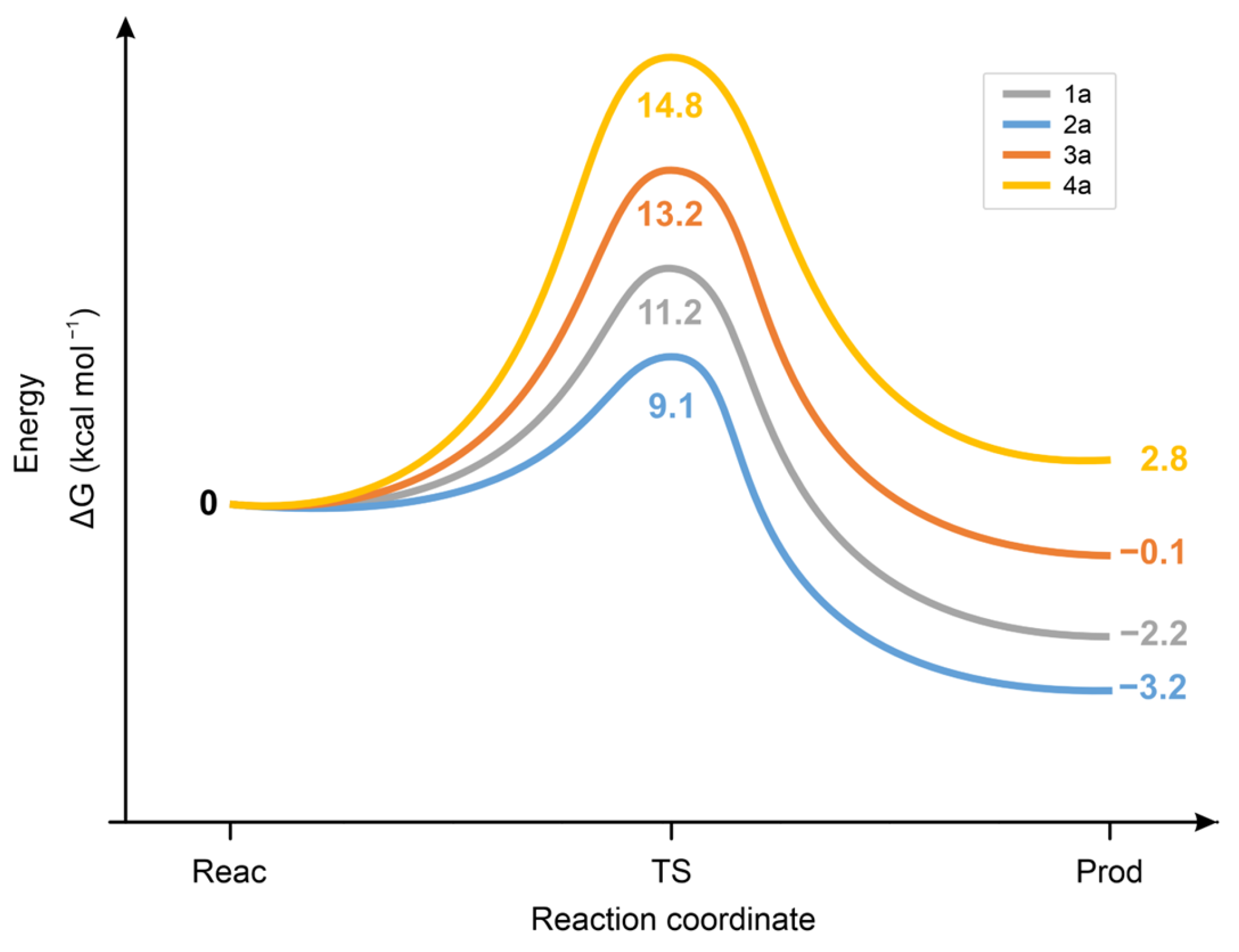
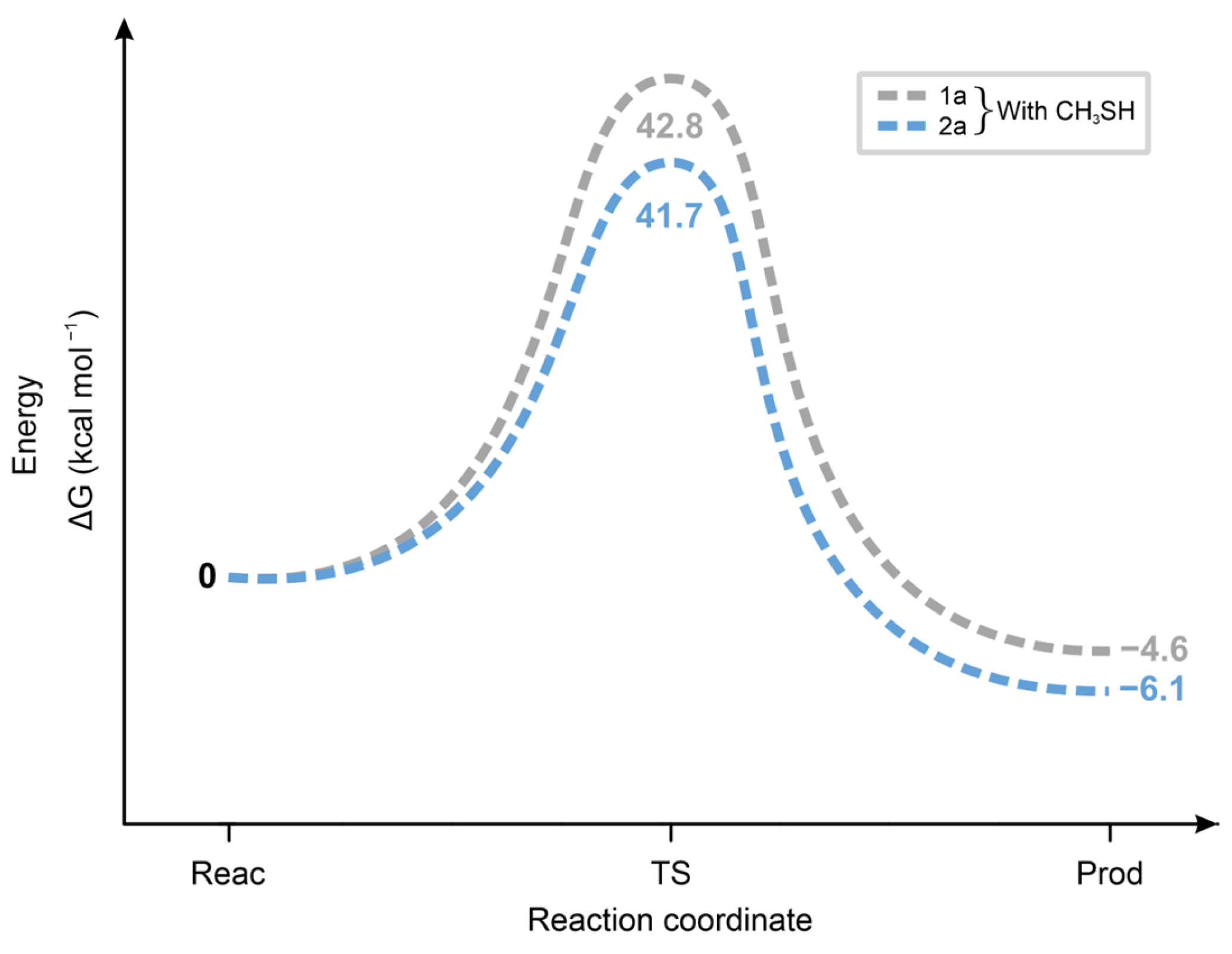
2.4. Molecular Modeling Analysis
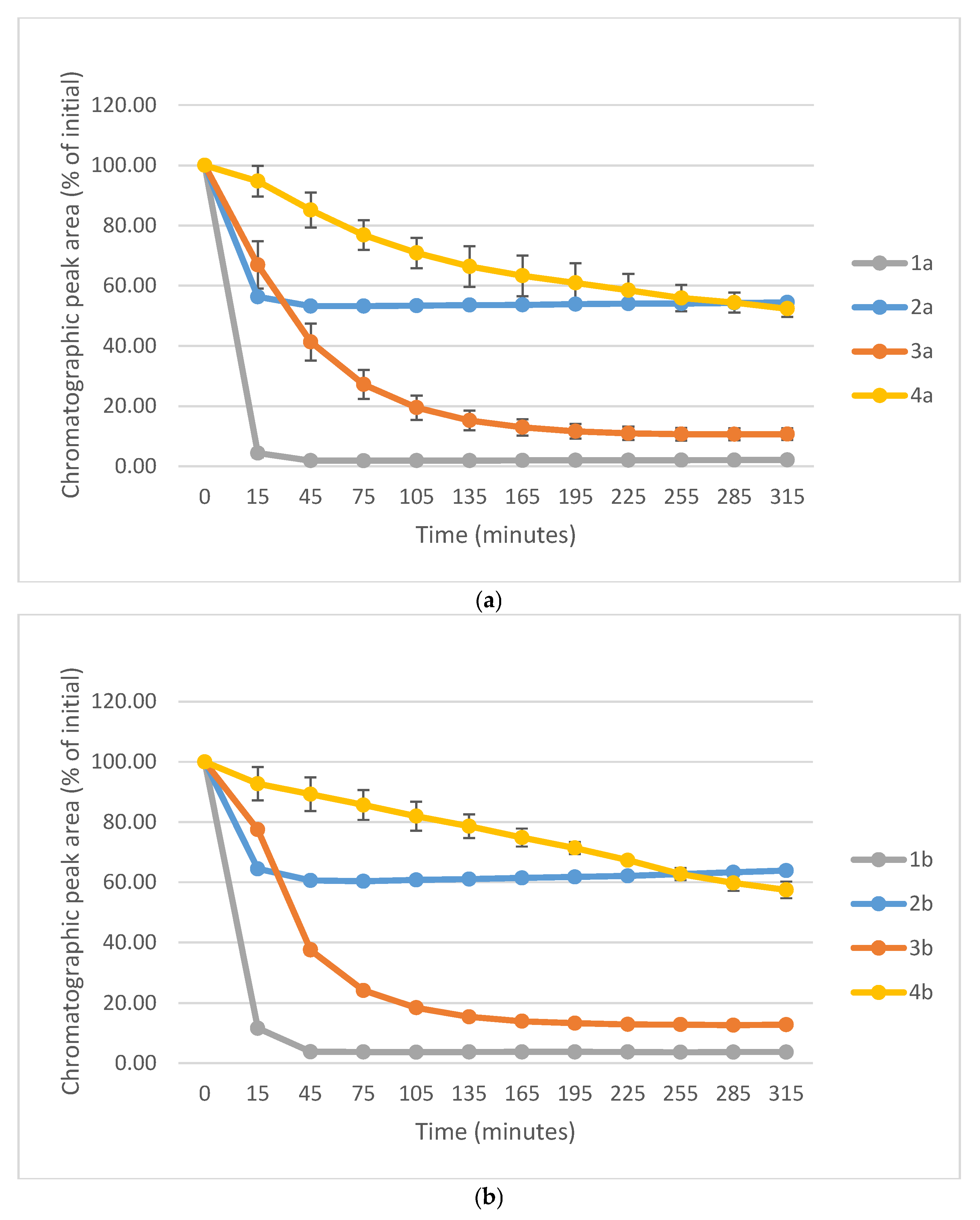
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Solutions
4.3. RP-HPLC-UV-Vis Measurements
4.4. HPLC-MS Measurements
4.5. Molecular Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bognár, G.; Kenari, F.; Pintér, Z.; Borges, I.D.; Camargo, A.J.; Oliveira, H.C.B.; Sanches-Neto, F.O.; Carvalho-Silva, V.H.; Napolitano, H.B.; Perjési, P. (E)-2-Benzylidenecyclanones: Part XX. Reaction of Cyclic Chalcone Analogs with Cellular Thiols: Unexpected Increased Reactivity of 4-Chromanone-Compared to 1-Tetralone Analogs in Thia-Michael Reactions. Molecules 2024, 29, 5493. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological properties of chalcones: A review of preclinical including molecular mechanisms and clinical evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone scaffolds, bioprecursors of flavonoids: Chemistry, bioactivities, and pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.C. Chalcone: A promising bioactive scaffold in medicinal chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Rizk, S.A.; Fahim, A.M. Synthesis, reactions and application of chalcones: A systematic review. Org. Biomol. Chem. 2023, 21, 5317–5346. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer potential of natural chalcones: In vitro and in vivo evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Zhang, L.; Sun, B.; Cui, Y.; Sang, F. Isolation and biological activity of natural chalcones based on antibacterial mechanism classification. Bioorg. Med. Chem. 2023, 93, 117454. [Google Scholar] [CrossRef]
- Mazumder, R.; Ichudaule; Ghosh, A.; Deb, S.; Ghosh, R. Significance of chalcone scaffolds in medicinal chemistry. Top. Curr. Chem. 2024, 382, 22. [Google Scholar] [CrossRef]
- Villa, S.M.; Heckman, J.; Bandyopadhyay, D. Medicinally privileged natural chalcones: Abundance, mechanisms of action, and clinical trials. Int. J. Mol. Sci. 2024, 25, 9623. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: A recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef]
- Folmer, F.; Blasius, R.; Morceau, F.; Tabudravu, J.; Dicato, M.; Jaspars, M.; Diederich, M. Inhibition of TNFα-induced activation of nuclear factor KB by Kava (Piper methysticum) derivatives. Biochem. Pharmacol. 2006, 71, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Laphanuwat, P.; Kongpetch, S.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Licochalcone A induces cholangiocarcinoma cell death via suppression of Nrf2 and NF-KB signaling pathways. Asian Pac. J. Cancer Prev. 2022, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Silva, M.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C.; Tarozzi, A. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Expert Rev. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Ducki, S. Antimitotic chalcones and related compounds as inhibitors of tubulin assembly. Anticancer. Agents Med. Chem. 2009, 9, 336–347. [Google Scholar] [CrossRef]
- Prakasham, A.P.; Saxena, A.K.; Luqman, S.; Chanda, D.; Kaur, T.; Gupta, A.; Yadav, D.K.; Chanotiya, C.S.; Shanker, K.; Khan, F.; et al. Synthesis and anticancer activity of 2-benzylidene indanones through inhibiting tubulin polymerization. Bioorg. Med. Chem. 2012, 20, 3049–3057. [Google Scholar] [CrossRef]
- Singh, A.; Fatima, K.; Singh, A.; Behl, A.; Mintoo, M.J.; Hasanain, M.; Ashraf, R.; Luqman, S.; Shanker, K.; Mondhe, D.M.; et al. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. Eur. J. Pharm. Sci. 2015, 76, 57–67. [Google Scholar] [CrossRef]
- Menezes, J.C. Arylidene indanone scaffold: Medicinal chemistry and structure–activity relationship view. RSC Adv. 2017, 7, 9357–9372. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Patil, S.A. Recent developments in biological activities of indanones. Eur. J. Med. Chem. 2017, 138, 182–198. [Google Scholar] [CrossRef]
- Özdemir, A.; Gökbulut, S.; Sever, B.; Ciftci, G.A.; Altintop, M.D. Synthesis and evaluation of a new series of arylidene indanones as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2018, 18, 1394–1404. [Google Scholar] [CrossRef]
- Srivastava, A.; Fatima, K.; Fatima, E.; Singh, A.; Singh, A.; Shukla, A.; Luqman, S.; Shanker, K.; Chanda, D.; Khan, F.; et al. Fluorinated benzylidene indanone exhibits antiproliferative activity through modulation of microtubule dynamics and antiangiogenic activity. Eur. J. Pharm. Sci. 2020, 154, 105513. [Google Scholar] [CrossRef]
- Lévai, A. Synthesis of exocyclic α,β-unsaturated ketones. Arkivoc 2004, vii, 15–33. [Google Scholar]
- Dimmock, J.R.; Kandepu, N.M.; Nazarali, A.J.; Kowalchuk, T.P.; Motaganahalli, N.; Quail, J.W.; Mykytiuk, P.A.; Audette, G.F.; Prasad, L.; Perjési, P.; et al. Conformational and quantitative structure−activity relationship study of cytotoxic 2-arylidenebenzocycloalkanones. J. Med. Chem. 1999, 42, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Zello, G.A.; Oloo, E.O.; Quail, J.W.; Kraatz, H.-B.; Perjési, P.; Aradi, F.; Takács-Novák, K.; Allen, T.M.; Santos, C.L.; et al. Correlations between cytotoxicity and topography of some 2-arylidenebenzocycloalkanones determined by X-ray crystallography. J. Med. Chem. 2002, 45, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Kenari, F.; Molnár, S.; Perjési, P. Reaction of chalcones with cellular thiols. The effect of the 4-substitution of chalcones and protonation state of the thiols on the addition process. Diastereoselective thiol addition. Molecules 2021, 26, 4332. [Google Scholar] [CrossRef]
- Kenari, F.; Molnár, S.; Borges, I.D.; Napolitano, H.B.; Perjési, P. (E)-2-Benzylidenecyclanones: Part XVIII. Study the possible link between glutathione reactivity and cancer cell cytotoxic effects of some cyclic chalcone analogs a comparison of the reactivity of the open-chain and the seven-membered homologs. Int. J. Mol. Sci. 2023, 24, 8557. [Google Scholar] [CrossRef]
- Caccuri, A.M.; Antonini, G.; Board, P.G.; Parker, M.W.; Nicotra, M.; Bello, M.L.; Federici, G.; Ricci, G. Proton release on binding of glutathione to alpha, mu and delta class Glutathione Transferases. Biochem. J. 1999, 344, 419–425. [Google Scholar] [CrossRef]
- Rohani, N.; Hao, L.; Alexis, M.; Joughin, B.; Krismer, K.; Moufarrej, M.; Soltis, A.; Lauffenburger, D.; Yaffe, M.; Burge, C.; et al. Acidification of tumor at stromal boundaries drives transcriptome alterations associated with aggressive phenotypes. Cancer Res. 2019, 79, 1952–1966. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Perjési, P. (E)-2-Benzylidenebenzocyclanones: Part XIII. (E)/(Z)-Isomerization of some cyclic chalcone analogues. Effect of ring size on lipophilicity of geometric isomers. Monatsh. Chem. 2015, 146, 1275–1281. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Perjési, P.; Perjessy, A.; Kolehmainen, E.; Ősz, E.; Samalikova, M.; Linnanto, J.; Virtanen, E. E-2-Benzylidenebenzocycloalkanones III. Studies on transmission of substituent effects on IR carbonyl stretching frequencies and 13C NMR chemical shifts of E-2-(X-benzylidene)-1-indanones. Comparison with the IR data of E-2-(X-benzylidene)-1-indanones, -tetralones, and -benzosuberones. J. Mol. Struct. 2004, 697, 41–47. [Google Scholar]
- Pearson, R.G.; Songstad, J. Application of the principle of hard and soft acids and bases to organic chemistry. J. Am. Chem. Soc. 1967, 89, 1827–1836. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Humphlett, W.J. The thermal reversibility of the michael reaction V. The effect of the structure of certain thiol adducts on cleavage. Can. J. Chem. 1966, 44, 2315–2321. [Google Scholar] [CrossRef]
- Klopman, G. Chemical reactivity and the concept of charge- and frontier-controlled reactions. J. Am. Chem.Soc. 1967, 90, 223–234. [Google Scholar] [CrossRef]
- Ho, T.L. The hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Klopman, G. The control of chemical reactivity. J. Mol. Struct. TEOCHEM 1983, 103, 121–129. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T.; DeCaprio, A.; Barber, D.S. Application of the hard and soft, acids and bases (HSAB) theory to toxicant–target interactions. Chem. Res. Toxicol. 2012, 25, 239–251. [Google Scholar] [CrossRef]
- Hoser, A.; Kaluski, Z.; Maluszynska, H.; Orlov, V.D. 2-Benzylidene- l-indanone. Acta Cryst. 1980, B36, 1256–1258. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T.; Geohagen, B.C.; Das, S. Neurotoxic mechanisms of electrophilic type-2 alkenes: Soft–soft interactions described by quantum mechanical parameters. Toxicol. Sci. 2007, 98, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Bernáth, G.; Fülöp, F.; Kálmán, A.; Argay, G.; Sohár, P.; Pelcer, I. Stereochemical studies-75. Saturated heterocycles-62. Connection between the diastereoselectivity and the dominant conformation in the formation of condensed-skeleton 1,3-oxazines, First X-ray diffraction evidence of N-outside conformation. Tetrahedron 1984, 40, 3587–3593. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision C. 01. 2016; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241, Erratum in Theor. Chem. Acc. 2008, 119, 525. [Google Scholar]
- Zhang, G.; Musgrave, C.B. Comparison of DFT methods for molecular orbital eigenvalue calculations. J. Phys. Chem. A 2007, 111, 1554–1562. [Google Scholar] [CrossRef]
- Weiner, P.K.; Langridge, R.; Blaney, J.M.; Schaefer, R.; Kollman, P.A. Electrostatic potential molecular surfaces. Proc. Natl. Acad. Sci. USA 1982, 79, 3754–3758. [Google Scholar] [CrossRef]
- Náray-Szabó, G.; Ferenczy, G.G. Molecular electrostatics. Chem. Rev. 1995, 95, 829–847. [Google Scholar] [CrossRef]
- Sanches-Neto, F.O.; Coutinho, N.D.; Aquilanti, V.; Silva, W.A.; Carvalho-Silva, V.H. Mechanism and kinetics of the degradation of nitazoxanide and hydroxychloroquine drugs by hydroxyl radicals: Theoretical approach to ecotoxicity. J. Braz. Chem. Soc. 2023, 34, 1119–1129. [Google Scholar] [CrossRef]
- Sanches-Neto, F.O.; Coutinho, N.D.; Palazzetti, F.; Carvalho-Silva, V.H. Temperature dependence of rate constants for the H(D) + CH4 reaction in gas and aqueous phase: Deformed transition-state theory study including quantum tunneling and diffusion effects. Struct. Chem. 2020, 31, 609–617. [Google Scholar] [CrossRef]
- Perjési, P.; Takács, M.; Ősz, E.; Pintér, Z.; Vámos, J.; Takács-Novák, K. In-solution and on-plate light-catalyzed E/Z isomerization of cyclic chalcone analogues. Lipophilicity of E- and Z-2-(X-benzylidene)-1-benzosuberones. J. Chromatogr. Sci. 2005, 43, 289–295. [Google Scholar] [CrossRef]
- Kozurkova, M.; Tomeckova, V. Interaction of chalcone derivatives with important biomacromolecules. In Chalcones and Their Synthetic Analogs; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; Chapter 4; pp. 95–133. [Google Scholar]

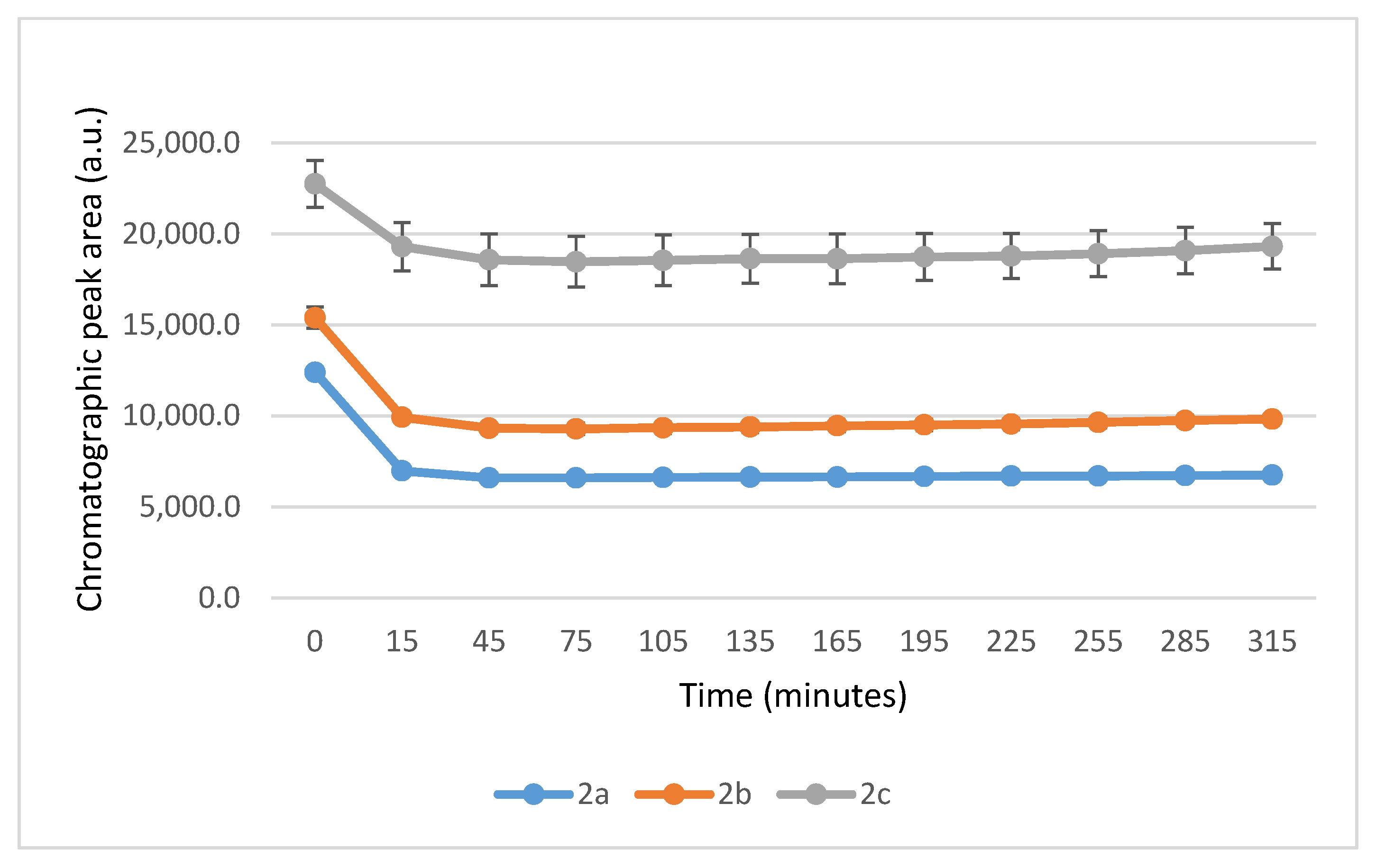

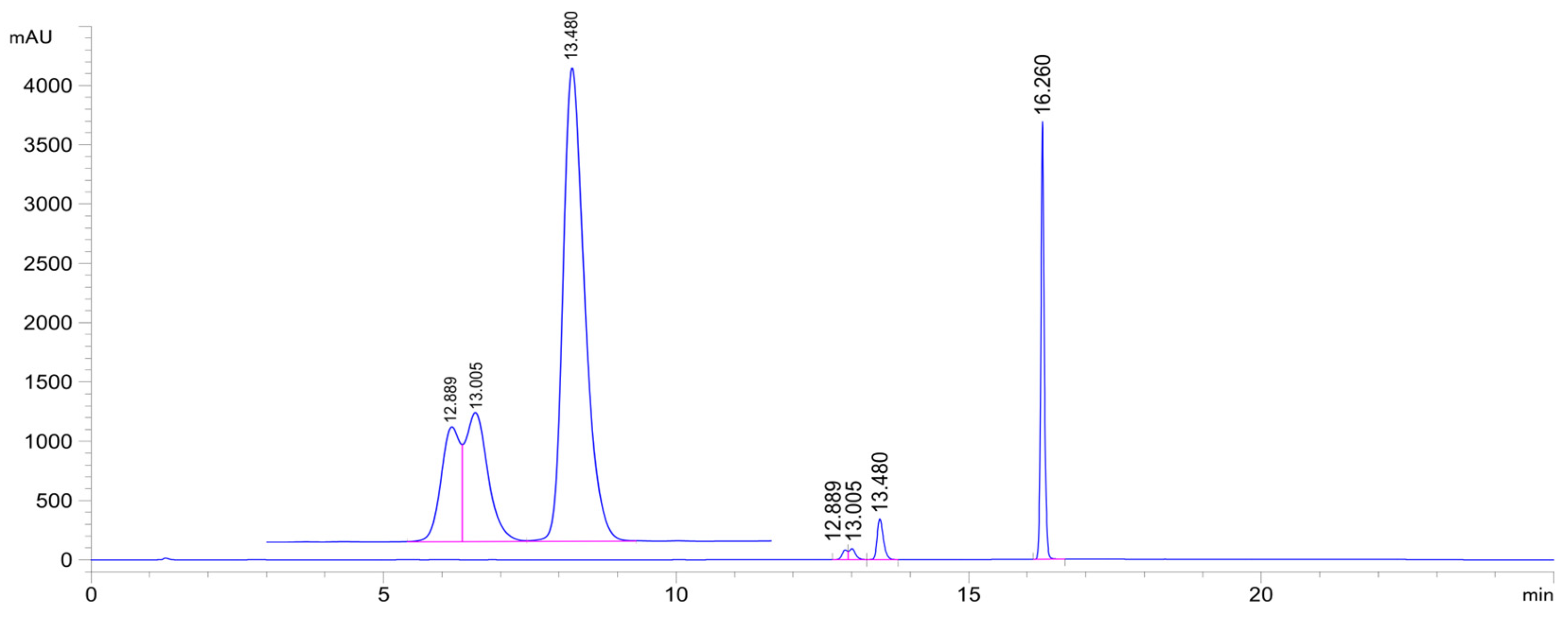
| pH 3 | Compound | tR (E)-Isomer | Area Ratio 4 A315/A0 | tR (Z)-Isomer | tR 2 GSH-1a | Area GSH-1a | tR 2 GSH-1b | Area GSH-1b | tR 2 GSH-2 | Area GSH-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 3.2 | 2a | 16.3 | 0.82 | N.D. 5 | 12.6 | 199 | 12.8 | 342 | 13.2 | 1566 |
| 3.2 | 2b | 16.6 | 0.78 | N.D. 5 | 13.8 | 122 | 14.0 | 144 | 14.4 | 986 |
| 3.2 | 2c | 16.3 | 0.92 | N.D. 5 | 12.9 | 55 | 13.0 | 86 | 13.5 | 599 |
| 6.3 | 2a | 16.3 | 0.53 | N.D. 5 | 12.6 | 1449 | 12.8 | 1556 | 13.3 | 5727 |
| 6.3 | 2b | 16.6 | 0.65 | N.D. 5 | 13.8 | 1001 | 14.0 | 1143 | 14.3 | 4171 |
| 6.3 | 2c | 16.2 | 0.82 | N.D. 5 | 12.7 | 614 | 12.9 | 820 | 13.3 | 2868 |
| 8.0 | 2a | 16.3 | 0.55 | N.D. 5 | 12.7 | 1597 | 12.9 | 1834 | 13.3 | 5623 |
| 8.0 | 2b | 16.6 | 0.64 | N.D. 5 | 13.8 | 1192 | 14.0 | 1406 | 14.3 | 4073 |
| 8.0 | 2c | 16.2 | 0.85 | N.D. 5 | 12.7 | 743 | 12.9 | 1047 | 13.4 | 2699 |
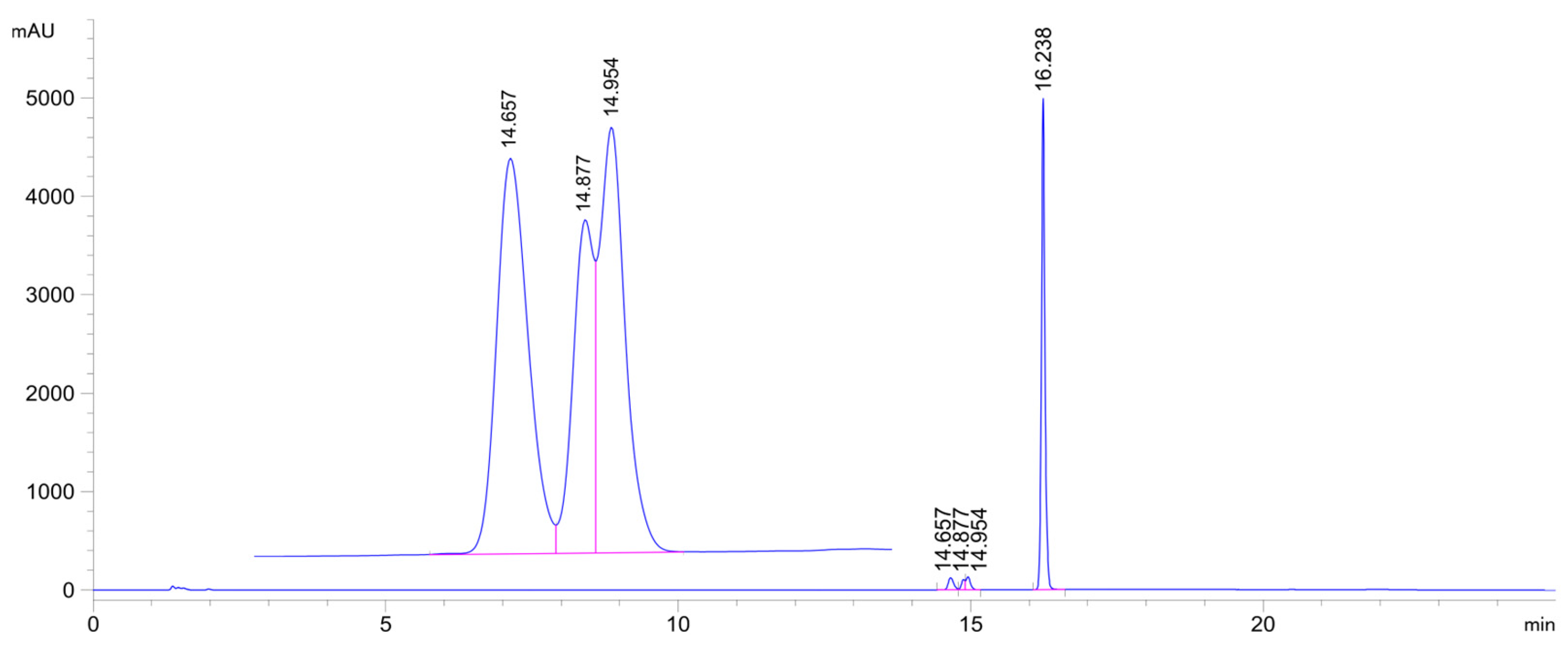
| pH 3 | Com-pound | tR (E)-Isomer | Area Ratio 4 A315/A0 | tR 2 (Z)-Isomer | tR NAC-1 | Area 2 NAC-1 | tR NAC-2a | Area 2 NAC-2a | tR NAC-2b | Area 2 NAC-2b |
|---|---|---|---|---|---|---|---|---|---|---|
| 3.2 | 2a | 16.3 | 0.67 | N.D. 5 | 14.7 | 1069 | 14.9 | 119 | 15.0 | 286 |
| 3.2 | 2b | 16.5 | 0.89 | N.D. 5 | 15.3 | 700 | 15.5 | 87 | 15.5 | 212 |
| 3.2 | 2c | 16.3 | 0.95 | N.D. 5 | 14.7 | 361 | 14.9 | 47 | 15.0 | 134 |
| 6.3 | 2a | 16.2 | 0.72 | N.D. 5 | 14.7 | 2171 | 14.9 | 1165 | 15.0 | 1666 |
| 6.3 | 2b | 16.5 | 0.77 | N.D. 5 | 15.3 | 1318 | 15.4 | 751 | 15.5 | 1112 |
| 6.3 | 2c | 16.2 | 0.92 | N.D. 5 | 14.7 | 816 | 14.9 | 484 | 15.0 | 763 |
| 8.0 | 2a | 16.2 | 0.59 | N.D. 5 | 14.7 | 2849 | 14.9 | 1788 | 15.0 | 2375 |
| 8.0 | 2b | 16.6 | 0.71 | N.D. 5 | 15.3 | 1894 | 15.5 | 1212 | 15.6 | 1588 |
| 8.0 | 2c | 16.3 | 0.88 | N.D. 5 | 14.7 | 1314 | 14.9 | 775 | 15.0 | 1165 |
| Compound | pH | 15 min | 45 min | 75 min | 165 min | 255 min | 315 min |
|---|---|---|---|---|---|---|---|
| 2a | 8.0 | 2.04 | 1.70 | 1.66 | 1.65 | 1.64 | 1.64 |
| 6.3 | 2.71 | 2.61 | 2.48 | 2.19 | 2.05 | 1.91 | |
| 3.2 | 2.45 | 2.56 | 2.72 | 2.89 | 2.92 | 2.90 | |
| 2b | 8.0 | 2.20 | 1.69 | 1.60 | 1.58 | 1.57 | 1.57 |
| 6.3 | 3.19 | 2.89 | 2.69 | 2.31 | 2.06 | 1.95 | |
| 3.2 | 2.77 | 3.28 | 3.41 | 3.60 | 3.68 | 3.71 | |
| 2c | 8.0 | 2.19 | 1.63 | 1.54 | 1.51 | 1.51 | 1.51 |
| 6.3 | 3.42 | 3.14 | 2.93 | 2.46 | 2.16 | 2.0 | |
| 3.2 | 2.36 | 3.31 | 3.68 | 4.17 | 4.23 | 4.25 |
| Compound | pH | 15 min | 45 min | 75 min | 165 min | 255 min | 315 min |
|---|---|---|---|---|---|---|---|
| 2a | 8.0 | 3.07 | 2.03 | 1.69 | 1.48 | 1.47 | 1.46 |
| 6.3 | 1.16 | 1.10 | 1.12 | 1.20 | 1.26 | 1.30 | |
| 3.2 | 0.32 | 0.32 | 0.35 | 0.35 | 0.36 | 0.38 | |
| 2b | 8.0 | 3.68 | 2.72 | 2.21 | 1.65 | 1.51 | 1.48 |
| 6.3 | 0.90 | 1.01 | 1.08 | 1.24 | 1.35 | 1.41 | |
| 3.2 | 0.35 | 0.36 | 0.36 | 0.38 | 0.41 | 0.43 | |
| 2c | 8.0 | 3.86 | 2.88 | 2.34 | 1.70 | 1.52 | 1.48 |
| 6.3 | 1.44 | 1.44 | 1.44 | 1.48 | 1.51 | 1.53 | |
| 3.2 | 0.39 | 0.41 | 0.43 | 0.45 | 0.50 | 0.50 |
| Descriptor | 1a eV | 2a eV | 3a eV | 4a eV | CH3SH eV | CH3S− eV |
|---|---|---|---|---|---|---|
| EHOMO | −7.89 | −6.09 | −7.76 | −7.88 | −7.98 | −5.86 |
| ELUMO | −1.70 | −0.98 | −1.41 | −0.96 | 0.091 | 0.30 |
| ΔEHOMO-LUMO | 6.38 | 7.08 | 9.17 | 8.84 | 8.07 | 6.155 |
| Series | P388 | L1210 | Molt 4/C8 | CEM |
|---|---|---|---|---|
| 2 | 37.0 | 174.0 | 255.0 | 63.0 |
| 3 | 17.6 | 94.0 | 96.9 | 68.9 |
| 4 | 12.8 | 103 | 88 | 56.7 |
| Descriptor | 1a eV | 2a eV | 3a eV | 4a eV |
|---|---|---|---|---|
| EHOMO (CH3S−) | −5.86 | −5.86 | −5.86 | −5.86 |
| EHOMO (CH3SH) | −7.98 | −7.98 | −7.98 | −7.98 |
| ELUMO(Chalcone) | −1.70 | −0.98 | −1.41 | −0.96 |
| ΔEHOMO-LUMO(CH3S−) | 4.16 | 4.87 | 4.45 | 4.90 |
| ΔEHOMO-LUMO(CH3SH) | 6.28 | 6.99 | 6.57 | 7.02 |
| [CH3S−] (mol/L) | 1a (s) | 2a (s) | 3a (s) | 4a (s) |
|---|---|---|---|---|
| 0.049 | 3.70 × 10−4 | 1.09 × 10−5 | 1.10 × 10−2 | 1.50 × 10−1 |
| 0.01 | 1.81 × 10−3 | 5.32 × 10−5 | 5.37 × 10−2 | 7.37 × 10−1 |
| 0.1 | 1.81 × 10−4 | 5.32 × 10−6 | 5.37 × 10−3 | 7.37 × 10−2 |
| [CH3SH] (mol/L) | 1a (yr) | 2a (yr) |
|---|---|---|
| 0.049 | 1.60 × 1012 | 2.60 × 1011 |
| 0.01 | 7.86 × 1012 | 1.28 × 1012 |
| 0.1 | 7.86 × 1011 | 1.28 × 1011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadlecsik, C.; Bognár, G.; Kenari, F.; Pintér, Z.; Ribeiro, J.C.d.O.; Envall, M.G.; Carvalho-Silva, V.H.; Napolitano, H.B.; Perjési, P. (E)-2-Benzylidenecyclanones: Part XXI—Reaction of Cyclic Chalcone Analogs with Cellular Thiols: Comparison of Reactivity of (E)-2-Arylidene-1-Indanone with -1-Tetralone and -1-Benzosuberone Analogs in Thia-Michael Reactions. Int. J. Mol. Sci. 2025, 26, 10573. https://doi.org/10.3390/ijms262110573
Kadlecsik C, Bognár G, Kenari F, Pintér Z, Ribeiro JCdO, Envall MG, Carvalho-Silva VH, Napolitano HB, Perjési P. (E)-2-Benzylidenecyclanones: Part XXI—Reaction of Cyclic Chalcone Analogs with Cellular Thiols: Comparison of Reactivity of (E)-2-Arylidene-1-Indanone with -1-Tetralone and -1-Benzosuberone Analogs in Thia-Michael Reactions. International Journal of Molecular Sciences. 2025; 26(21):10573. https://doi.org/10.3390/ijms262110573
Chicago/Turabian StyleKadlecsik, Csaba, Gábor Bognár, Fatemeh Kenari, Zoltán Pintér, Júlio César de Oliveira Ribeiro, Mário G. Envall, Valter H. Carvalho-Silva, Hamilton B. Napolitano, and Pál Perjési. 2025. "(E)-2-Benzylidenecyclanones: Part XXI—Reaction of Cyclic Chalcone Analogs with Cellular Thiols: Comparison of Reactivity of (E)-2-Arylidene-1-Indanone with -1-Tetralone and -1-Benzosuberone Analogs in Thia-Michael Reactions" International Journal of Molecular Sciences 26, no. 21: 10573. https://doi.org/10.3390/ijms262110573
APA StyleKadlecsik, C., Bognár, G., Kenari, F., Pintér, Z., Ribeiro, J. C. d. O., Envall, M. G., Carvalho-Silva, V. H., Napolitano, H. B., & Perjési, P. (2025). (E)-2-Benzylidenecyclanones: Part XXI—Reaction of Cyclic Chalcone Analogs with Cellular Thiols: Comparison of Reactivity of (E)-2-Arylidene-1-Indanone with -1-Tetralone and -1-Benzosuberone Analogs in Thia-Michael Reactions. International Journal of Molecular Sciences, 26(21), 10573. https://doi.org/10.3390/ijms262110573







