Characterizing the Long Non-Coding RNA Profile of Endometrial Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Anti-Inflammatory Role in Osteoarthritis
Abstract
1. Introduction
2. Results and Discussion
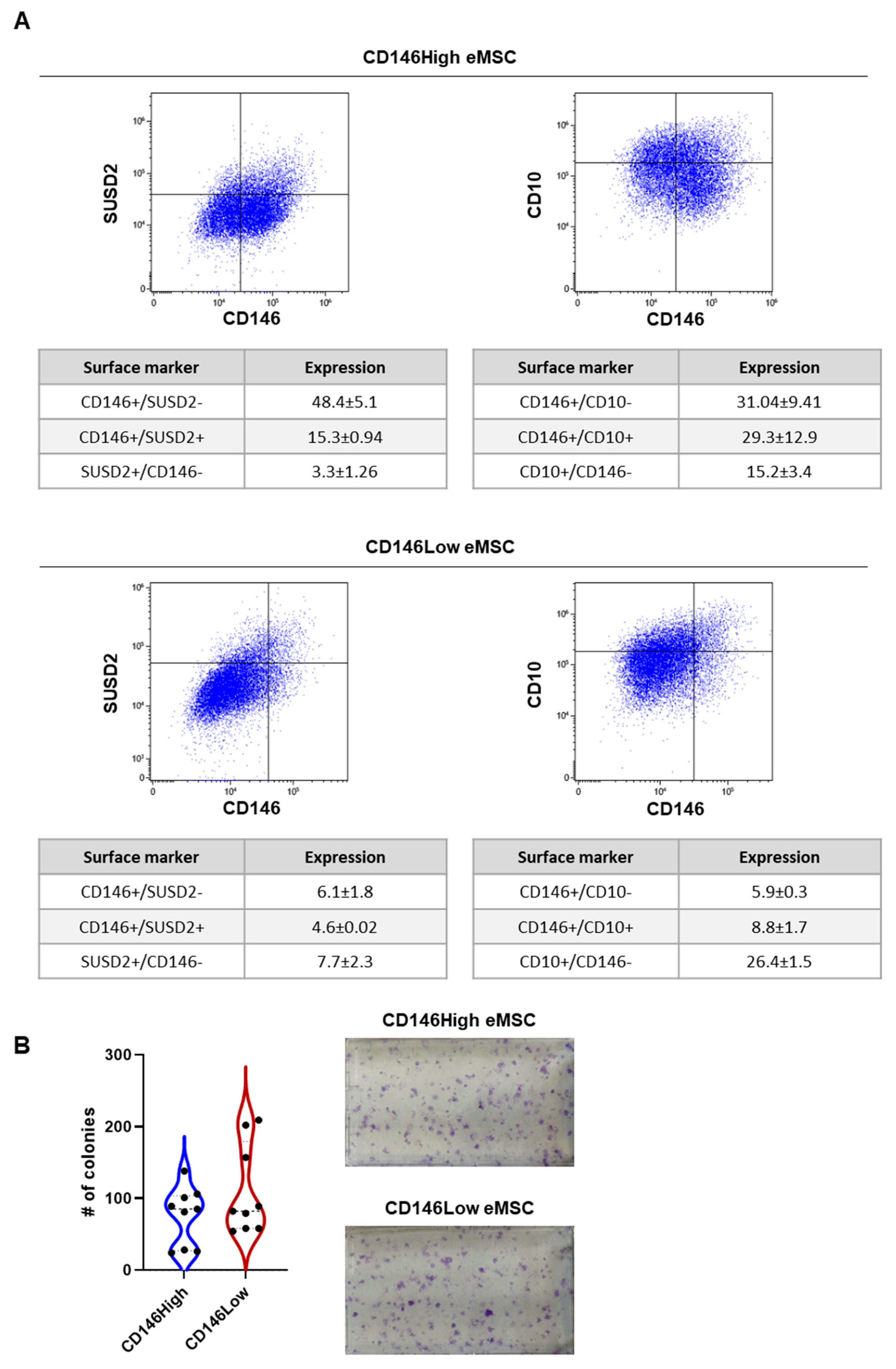
2.1. CD146High and CD146Low eMSC Characterization
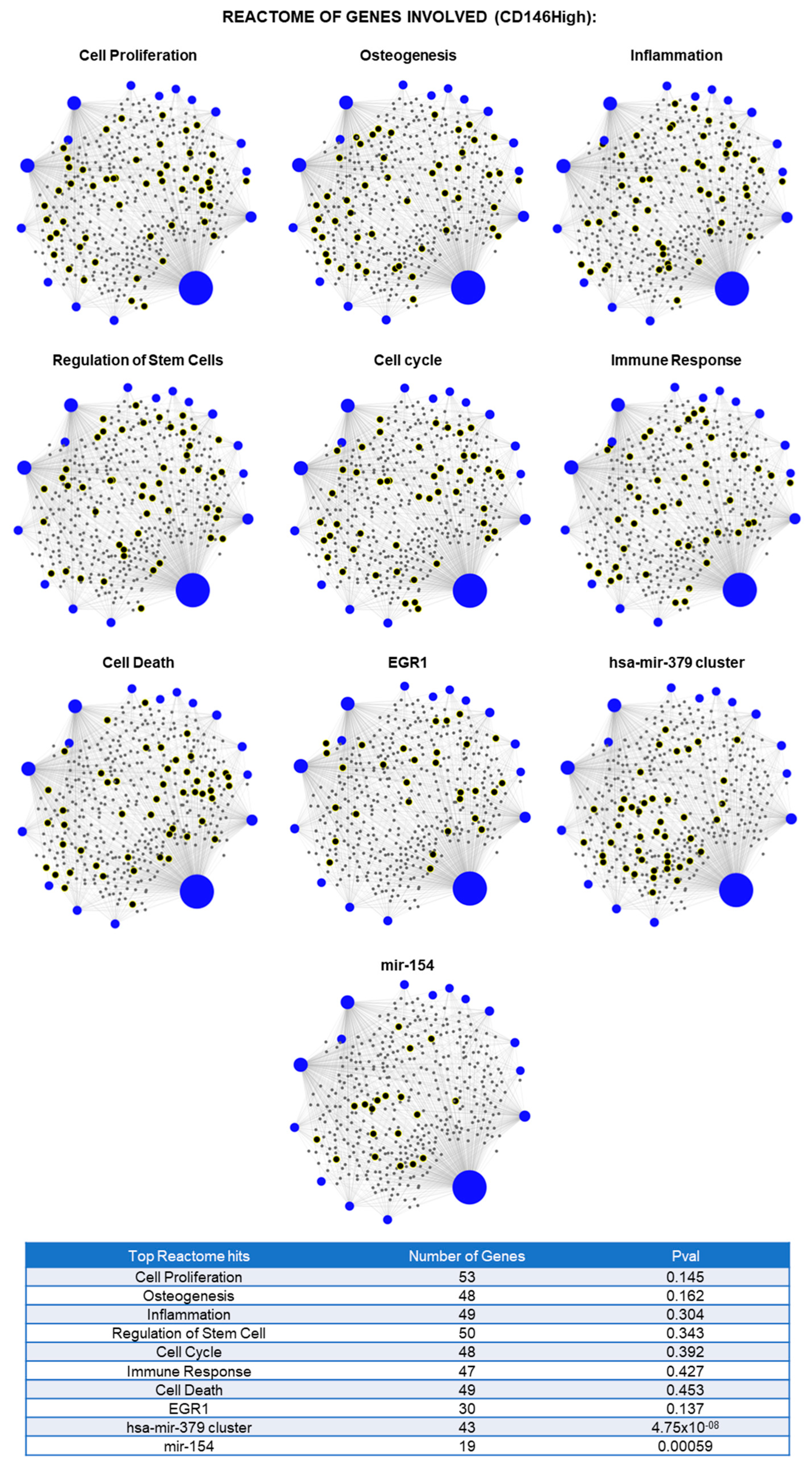
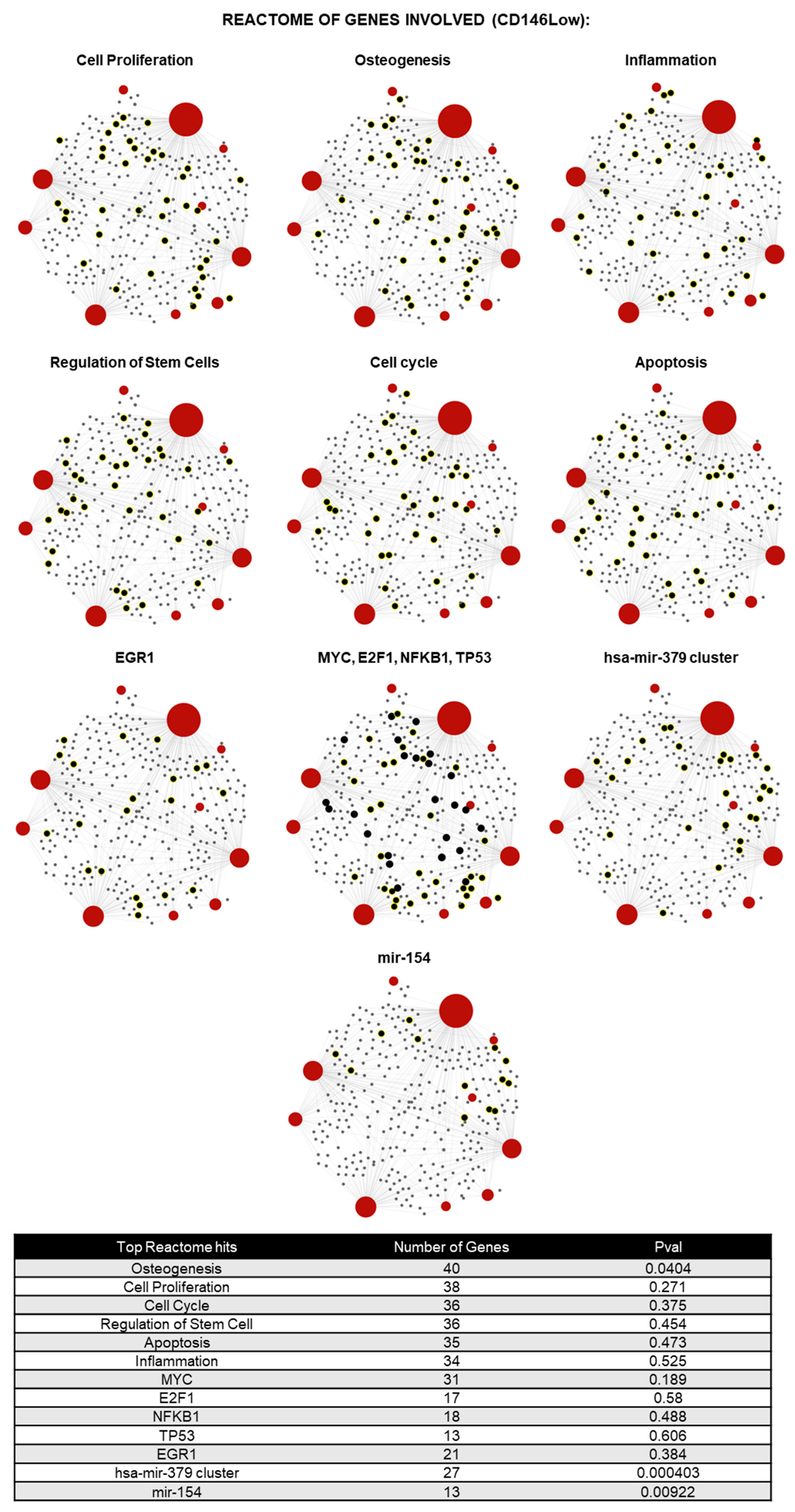
2.2. CD146High and CD146Low eMSC-EVs Possess Immunomodulatory/Angiogenic lncRNA Cargo
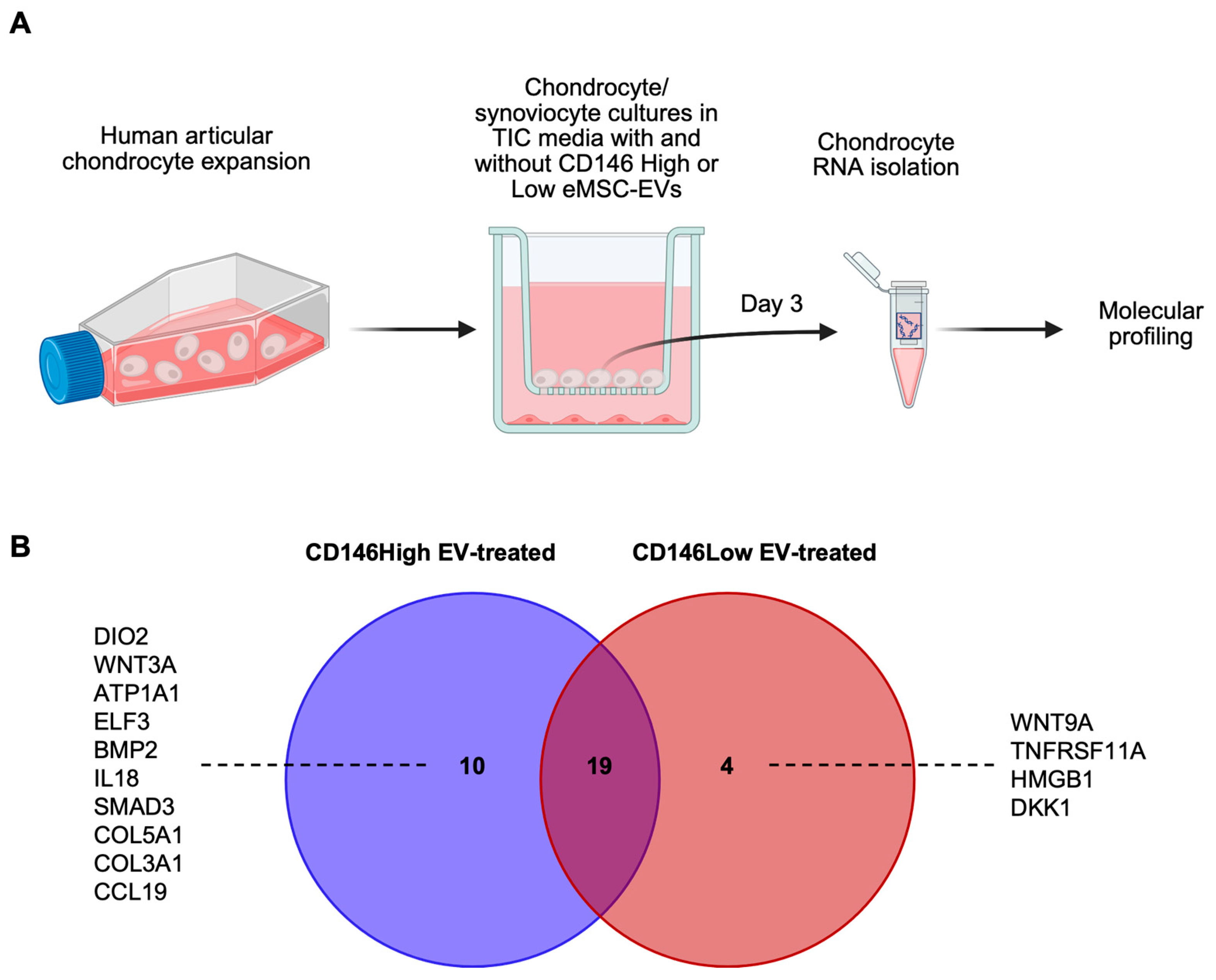
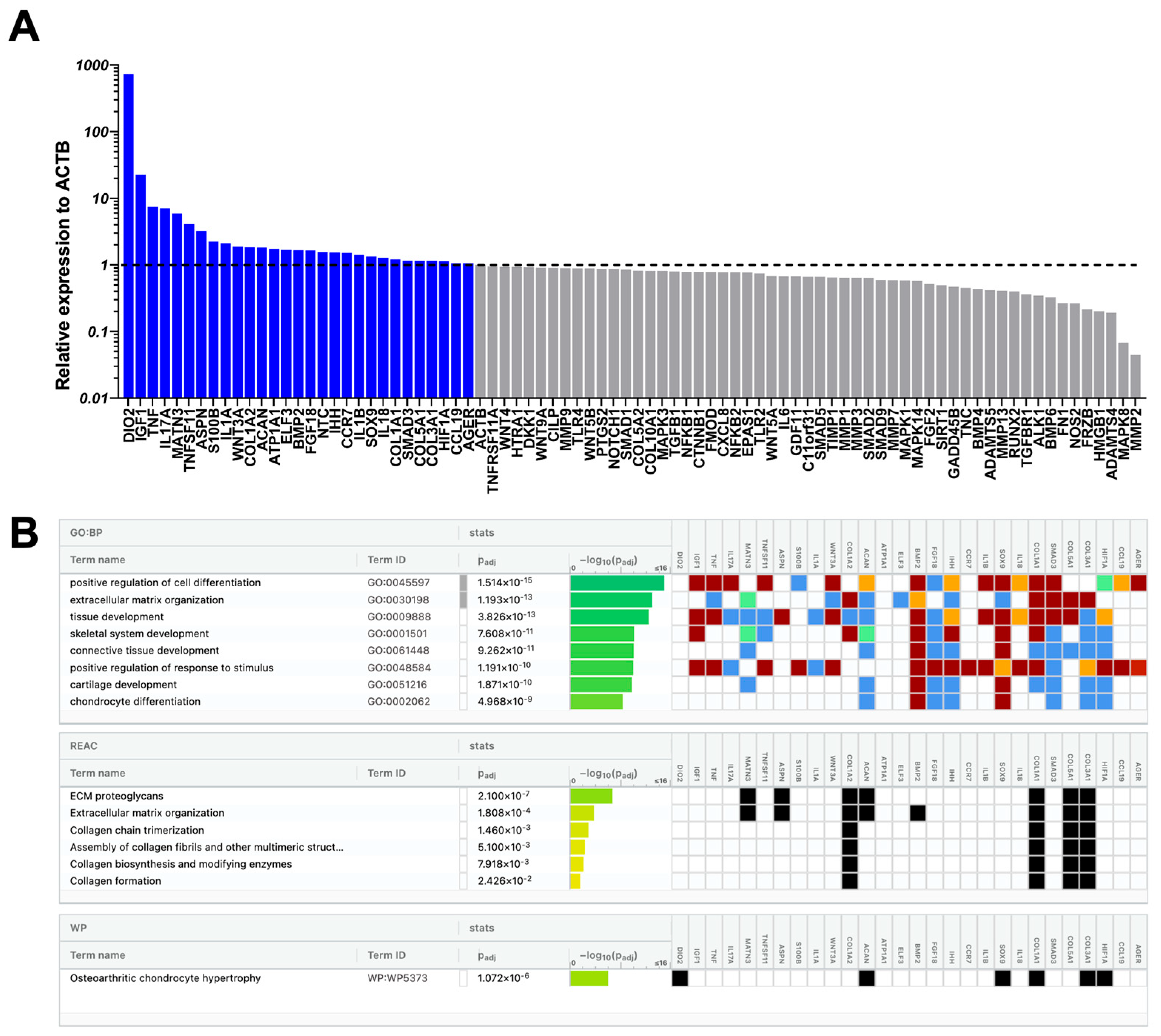
2.3. Molecular Profile of Chondrocytes Exposed to CD146High or CD146Low eMSC-EVs Under Inflammatory Conditions In Vitro
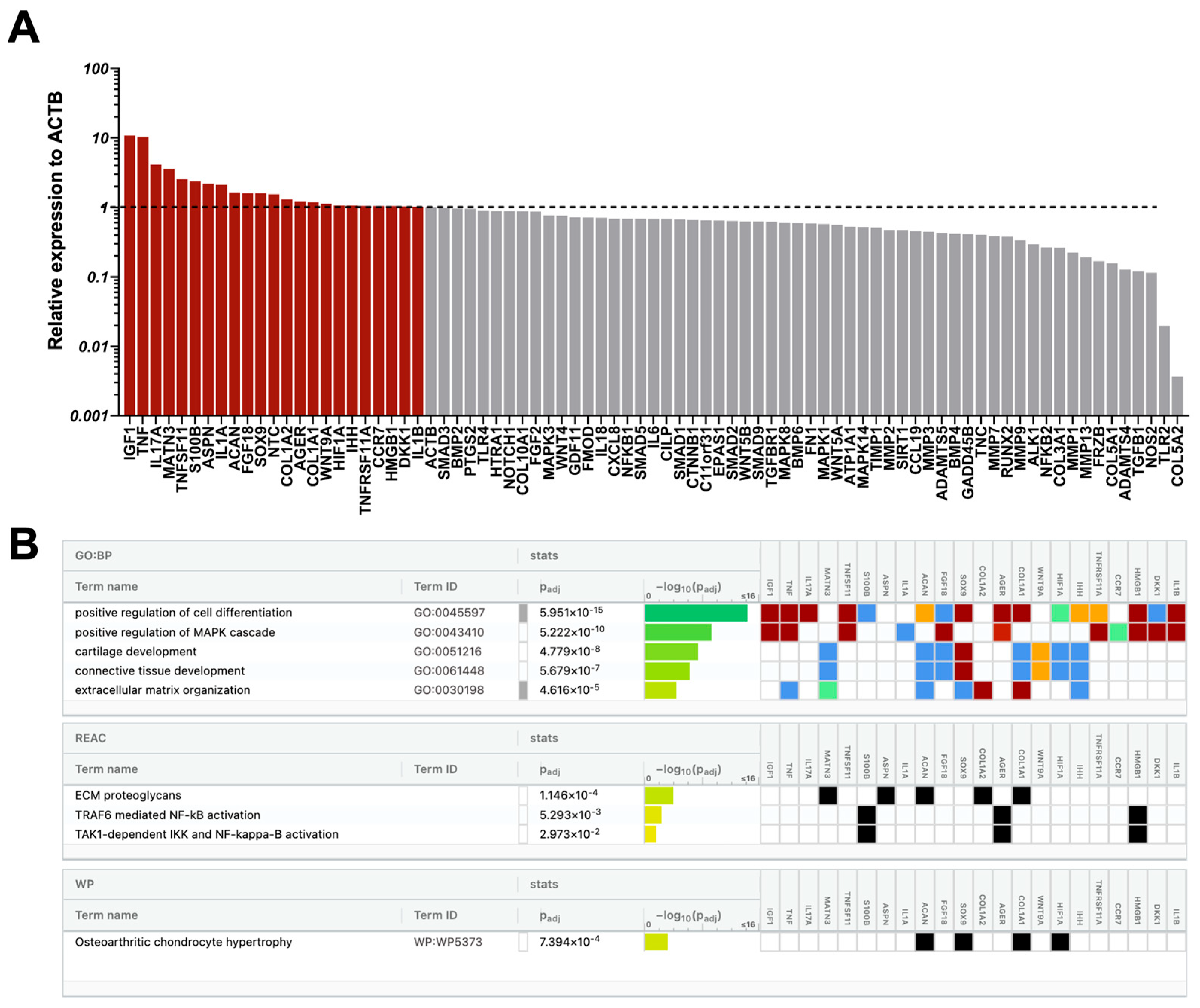
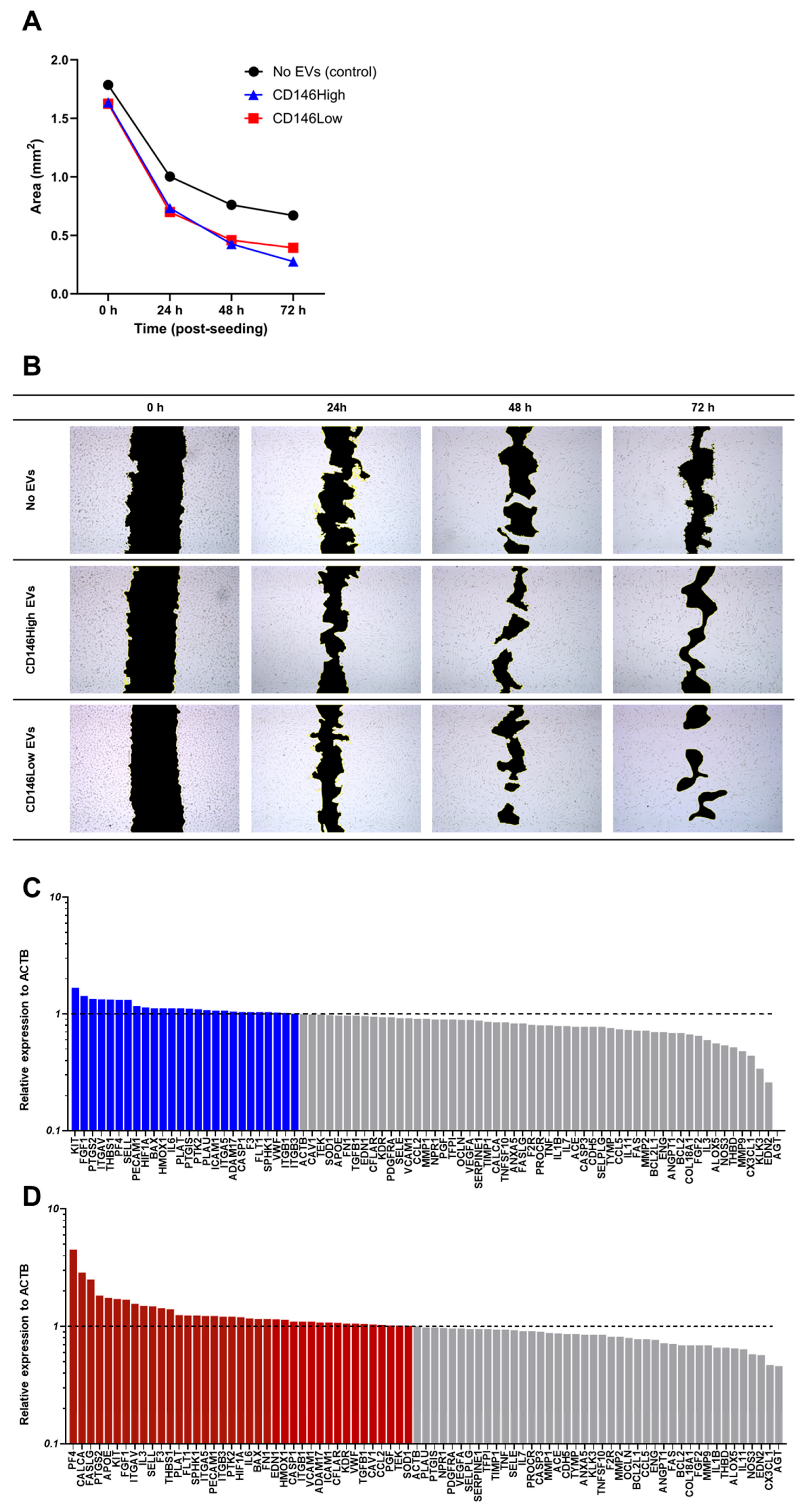
2.4. CD146High and CD146Low eMSC-EVs Effect on Endothelial Cells Under Inflammatory Conditions In Vitro
3. Materials and Methods
3.1. Isolation, Culture, and Expansion of eMSCs
3.2. CD146High and CD146Low eMSC Selection
3.3. Immunophenotype of eMSCs
3.4. Clonogenic Assay of eMSCSs
3.5. Isolation and Validation of CD146High and CD146Low eMSC-EVs
3.6. lncRNA Profile of CD146High and CD146Low eMSC-EVs
3.7. Chondrocytes/Synoviocytes Co-Culture Assay
3.8. Angiogenesis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Nguyen, H.P.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Canosa, S.; Mareschi, K.; Marini, E.; Carosso, A.R.; Castiglia, S.; Rustichelli, D.; Ferrero, I.; Gennarelli, G.; Bussolati, B.; Nocifora, A.; et al. A Novel Xeno-Free Method to Isolate Human Endometrial Mesenchymal Stromal Cells (E-MSCs) in Good Manufacturing Practice (GMP) Conditions. Int. J. Mol. Sci. 2022, 23, 1931. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Zillwood, R.M.; Nguyen, H.P.; Wu, D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 2009, 80, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.S. Endometrial Stem Cells: Orchestrating Dynamic Regeneration of Endometrium and Their Implications in Diverse Endometrial Disorders. Int. J. Biol. Sci. 2024, 20, 864–879. [Google Scholar] [CrossRef]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Bowles, A.C.; Kouroupis, D.; Willman, M.A.; Perucca Orfei, C.; Agarwal, A.; Correa, D. Signature quality attributes of CD146+ mesenchymal stem/stromal cells correlate with high therapeutic and secretory potency. Stem Cells 2020, 38, 1034–1049. [Google Scholar] [CrossRef]
- Hilage, P.; Birajdar, A.; Marsale, T.; Patil, D.; Patil, A.M.; Telang, G.; Somasundaram, I.; Sharma, R.K.; Joshi, M.G. Characterization and angiogenic potential of CD146(+) endometrial stem cells. Stem Cell Res. Ther. 2024, 15, 330. [Google Scholar] [CrossRef]
- Leñero, C.; Kaplan, L.D.; Best, T.M.; Kouroupis, D. CD146+ Endometrial-Derived Mesenchymal Stem/Stromal Cell Subpopulation Possesses Exosomal Secretomes with Strong Immunomodulatory miRNA Attributes. Cells 2022, 11, 4002. [Google Scholar] [CrossRef]
- Namini, M.S.; Beheshtizadeh, N.; Ebrahimi-Barough, S.; Ai, J. Human endometrial stem cell-derived small extracellular vesicles enhance neurite outgrowth and peripheral nerve regeneration through activating the PI3K/AKT signaling pathway. J. Transl. Med. 2025, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef]
- Tang, T.T.; Wang, B.; Lv, L.L.; Liu, B.C. Extracellular vesicle-based Nanotherapeutics: Emerging frontiers in anti-inflammatory therapy. Theranostics 2020, 10, 8111–8129. [Google Scholar] [CrossRef]
- Blázquez, R.; Sánchez-Margallo, F.M.; Álvarez, V.; Matilla, E.; Hernández, N.; Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Casado, J.G.; Macías-García, B. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS ONE 2018, 13, e0196080. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, F.; Macías-García, B.; Sánchez-Margallo, F.M.; Blázquez, R.; Álvarez, V.; Matilla, E.; Hernández, N.; Gómez-Serrano, M.; Jorge, I.; Vázquez, J.; et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine model. Biol. Reprod. 2019, 100, 1180–1192. [Google Scholar] [CrossRef]
- Álvarez, V.; Sánchez-Margallo, F.M.; Macías-García, B.; Gómez-Serrano, M.; Jorge, I.; Vázquez, J.; Blázquez, R.; Casado, J.G. The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFbeta. J. Tissue Eng. Regen. Med. 2018, 12, 2088–2098. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Leñero, C.; Bowles, A.C.; Correa, D.; Kouroupis, D. Characterization and response to inflammatory stimulation of human endometrial-derived mesenchymal stem/stromal cells. Cytotherapy 2022, 24, 124–136. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, H.W.; Nam, J.W. The small peptide world in long noncoding RNAs. Brief. Bioinform. 2019, 20, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Atianand, M.K.; Hu, W.; Satpathy, A.T.; Shen, Y.; Ricci, E.P.; Alvarez-Dominguez, J.R.; Bhatta, A.; Schattgen, S.A.; McGowan, J.D.; Blin, J.; et al. A Long Noncoding RNA lincRNA-EPS Acts as a Transcriptional Brake to Restrain Inflammation. Cell 2016, 165, 1672–1685. [Google Scholar] [CrossRef]
- Shen, X.; Qin, J.; Wei, Z.; Liu, F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. Biomed. Pharmacother. 2023, 167, 115488. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: Data from the osteoarthritis initiative. PLoS ONE 2018, 13, e0191045. [Google Scholar] [CrossRef]
- Moulin, D.; Sellam, J.; Berenbaum, F.; Guicheux, J.; Boutet, M.A. The role of the immune system in osteoarthritis: Mechanisms, challenges and future directions. Nat. Rev. Rheumatol. 2025, 21, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Yu, J.; Du, J.; Guo, R.; Feng, Y.; Zhong, G.; Jiang, Y.; Lin, J. LncRNA HOXA-AS2 represses endothelium inflammation by regulating the activity of NF-κB signaling. Atherosclerosis 2019, 281, 38–46. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, J.; Du, J.; Zhong, G.; Qiao, L.; Lin, J. LncRNA HOXA-AS2 positively regulates osteogenesis of mesenchymal stem cells through inactivating NF-κB signalling. J. Cell Mol. Med. 2019, 23, 1325–1332. [Google Scholar] [CrossRef]
- Wang, F.; Wu, D.; Chen, J.; Chen, S.; He, F.; Fu, H.; Wu, Q.; Liu, S.; Li, X.; Wang, W. Long non-coding RNA HOXA-AS2 promotes the migration, invasion and stemness of bladder cancer via regulating miR-125b/Smad2 axis. Exp. Cell Res. 2019, 375, 1–10. [Google Scholar] [CrossRef]
- Chen, C.G.; Thuillier, D.; Chin, E.N.; Alliston, T. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012, 64, 3278–3289. [Google Scholar] [CrossRef]
- Thielen, N.; Neefjes, M.; Wiegertjes, R.; van den Akker, G.; Vitters, E.; van Beuningen, H.; Blaney Davidson, E.; Koenders, M.; van Lent, P.; van de Loo, F.; et al. Osteoarthritis-Related Inflammation Blocks TGF-β’s Protective Effect on Chondrocyte Hypertrophy via (de)Phosphorylation of the SMAD2/3 Linker Region. Int. J. Mol. Sci. 2021, 22, 8124. [Google Scholar] [CrossRef]
- Tang, J.Z.; Zhao, G.Y.; Zhao, J.Z.; Di, D.H.; Wang, B. lncRNA IGF2-AS promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by sponging miR-3,126-5p to upregulate KLK4. J. Gene Med. 2021, 23, e3372. [Google Scholar] [CrossRef]
- Song, C.; Yang, Z.; Jiang, R.; Cheng, J.; Yue, B.; Wang, J.; Sun, X.; Huang, Y.; Lan, X.; Lei, C.; et al. lncRNA IGF2 AS Regulates Bovine Myogenesis through Different Pathways. Mol. Ther. Nucleic Acids 2020, 21, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, H.; Zheng, M.Q.; Dong, Q.R. LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage 2021, 13, 1274s–1284s. [Google Scholar] [CrossRef]
- Wang, A.; Hu, N.; Zhang, Y.; Chen, Y.; Su, C.; Lv, Y.; Shen, Y. MEG3 promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-361-5p/FOXO1 axis. BMC Med. Genom. 2019, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, D.; Yan, Z.; Zhan, J.; Xue, X.; Pan, X.; Yu, H. LncRNA MEG3 Protects Chondrocytes From IL-1β-Induced Inflammation via Regulating miR-9-5p/KLF4 Axis. Front. Physiol. 2021, 12, 617654. [Google Scholar] [CrossRef]
- Quintero, D.; Rodriguez, H.C.; Potty, A.G.; Kouroupis, D.; Gupta, A. Long noncoding RNAs in mesenchymal stromal/stem cells osteogenic differentiation: Implications in osteoarthritis pathogenesis. World J. Stem Cells 2022, 14, 429–434. [Google Scholar] [CrossRef]
- Kang, Y.; Song, J.; Kim, D.; Ahn, C.; Park, S.; Chun, C.H.; Jin, E.J. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J. Orthop. Res. 2016, 34, 412–418. [Google Scholar] [CrossRef]
- Zeng, G.; Deng, G.; Xiao, S.; Li, F. Fibroblast-like Synoviocytes-derived Exosomal PCGEM1 Accelerates IL-1β-induced Apoptosis and Cartilage Matrix Degradation by miR-142-5p/RUNX2 in Chondrocytes. Immunol. Investig. 2022, 51, 1284–1301. [Google Scholar] [CrossRef]
- Liu, C.; Ren, S.; Zhao, S.; Wang, Y. LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med. J. 2019, 60, 1081–1092. [Google Scholar] [CrossRef]
- Wang, Y.; Mou, Q.; Zhu, Z.; Zhao, L.; Zhu, L. MALAT1 promotes liver fibrosis by sponging miR-181a and activating TLR4-NF-κB signaling. Int. J. Mol. Med. 2021, 48, 215. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.P.; Niu, G.H.; Zhang, X.Q. Influence of lncRNA MALAT1 on septic lung injury in mice through p38 MAPK/p65 NF-κB pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1296–1304. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhang, Z.; Li, S.; Jiang, W.; Li, X.; Lv, J. LncRNA MALAT1 cessation antagonizes hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis and inflammation via the HMGB1-TLR4 axis. Mol. Immunol. 2019, 112, 22–29. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, K.; Liu, X.; Tan, Y.; Jin, W.; Li, Y.; Zhou, J.; Wang, H.; Kang, C. HOTAIR Up-Regulation Activates NF-κB to Induce Immunoescape in Gliomas. Front. Immunol. 2021, 12, 785463. [Google Scholar] [CrossRef]
- Dou, P.; Hu, R.; Zhu, W.; Tang, Q.; Li, D.; Li, H.; Wang, W. Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Pharmazie 2017, 72, 113–117. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, W.; Wang, Y.; Xia, K.; Huang, Y.; Xu, A.; Chen, Q.; Liu, B.; Tao, H.; Li, F.; et al. lncRNAs: Function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Res. Ther. 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.P.; Ding, J.B.; Liu, Z.H.; Zhou, G.J. LncRNA TUG1 promotes osteoarthritis-induced degradation of chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8574–8581. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Yang, J. TUG1 knockdown promoted viability and inhibited apoptosis and cartilage ECM degradation in chondrocytes via the miR-17-5p/FUT1 pathway in osteoarthritis. Exp. Ther. Med. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Hong, H.; Zeng, K.; Zhou, C.; Chen, X.; Xu, Z.; Li, M.; Liu, L.; Zeng, Q.; Tao, Q.; Wei, X. The pluripotent factor OCT4A enhances the self-renewal of human dental pulp stem cells by targeting lncRNA FTX in an LPS-induced inflammatory microenvironment. Stem Cell Res. Ther. 2023, 14, 109. [Google Scholar] [CrossRef]
- Wevrick, R.; Kerns, J.A.; Francke, U. Identification of a novel paternally expressed gene in the Prader-Willi syndrome region. Hum. Mol. Genet. 1994, 3, 1877–1882. [Google Scholar] [CrossRef]
- Stelzer, Y.; Sagi, I.; Yanuka, O.; Eiges, R.; Benvenisty, N. The noncoding RNA IPW regulates the imprinted DLK1-DIO3 locus in an induced pluripotent stem cell model of Prader-Willi syndrome. Nat. Genet. 2014, 46, 551–557. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Boissy, P.; Tan, Q.; Dahlgaard, J.; Traustadottir, G.A.; Kupisiewicz, K.; Laborda, J.; Delaisse, J.M.; Kassem, M. dlk1/FA1 regulates the function of human bone marrow mesenchymal stem cells by modulating gene expression of pro-inflammatory cytokines and immune response-related factors. J. Biol. Chem. 2007, 282, 7339–7351. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ditzel, N.; Mahmood, A.; Isa, A.; Traustadottir, G.A.; Schilling, A.F.; Ruiz-Hidalgo, M.J.; Laborda, J.; Amling, M.; Kassem, M. DLK1 is a novel regulator of bone mass that mediates estrogen deficiency-induced bone loss in mice. J. Bone Miner. Res. 2011, 26, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; He, H.; Shao, F.; Gao, Y.; He, J. Systemic Analyses of Cuproptosis-Related lncRNAs in Pancreatic Adenocarcinoma, with a Focus on the Molecular Mechanism of LINC00853. Int. J. Mol. Sci. 2023, 24, 7923. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, Y.; Gong, X.; Li, H. Long noncoding RNAs in head and neck cancer. Oncotarget 2017, 8, 10726–10740. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Wang, C.; Zhang, Q.; Zeng, A.; Song, L. Autophagy-related lncRNAs in tumor progression and drug resistance: A double-edged sword. Genes. Dis. 2024, 11, 367–381. [Google Scholar] [CrossRef] [PubMed]
- De Roover, A.; Escribano-Núñez, A.; Monteagudo, S.; Lories, R. Fundamentals of osteoarthritis: Inflammatory mediators in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1303–1311. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Chang, S.F.; Hsieh, R.Z.; Huang, K.C.; Chang, C.A.; Chiu, F.Y.; Kuo, H.C.; Chen, C.N.; Su, Y.P. Upregulation of Bone Morphogenetic Protein-2 Synthesis and Consequent Collagen II Expression in Leptin-stimulated Human Chondrocytes. PLoS ONE 2015, 10, e0144252. [Google Scholar] [CrossRef]
- Iijima, H.; Ito, A.; Nagai, M.; Tajino, J.; Yamaguchi, S.; Kiyan, W.; Nakahata, A.; Zhang, J.; Wang, T.; Aoyama, T.; et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthr. Cartil. 2017, 25, 964–975. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Xu, X.; Li, C.; Huang, C.; Deng, C.X. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 2001, 153, 35–46. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Stokes, D.G.; Jimenez, S.A. Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthr. Cartil. 2007, 15, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Darowish, M.; Zuscik, M.J.; Chen, D.; Schwarz, E.M.; Rosier, R.N.; Drissi, H.; O’Keefe, R.J. Smad3-Deficient Chondrocytes Have Enhanced BMP Signaling and Accelerated Differentiation. J. Bone Miner. Res. 2009, 21, 4–16. [Google Scholar] [CrossRef]
- Nalesso, G.; Sherwood, J.; Bertrand, J.; Pap, T.; Ramachandran, M.; De Bari, C.; Pitzalis, C.; Dell’accio, F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J. Cell Biol. 2011, 193, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.L.; Eldridge, S.E.; Nosrati, B.; Alvarez, M.; Thorup, A.S.; Nalesso, G.; Caxaria, S.; Barawi, A.; Nicholson, J.G.; Perretti, M.; et al. WNT3A-loaded exosomes enable cartilage repair. J. Extracell. Vesicles 2021, 10, e12088. [Google Scholar] [CrossRef] [PubMed]
- Hosseininia, S.; Weis, M.A.; Rai, J.; Kim, L.; Funk, S.; Dahlberg, L.E.; Eyre, D.R. Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthr. Cartil. 2016, 24, 1029–1035. [Google Scholar] [CrossRef]
- Mak, K.M.; Png, C.Y.; Lee, D.J. Type V Collagen in Health, Disease, and Fibrosis. Anat. Rec. 2016, 299, 613–629. [Google Scholar] [CrossRef]
- Mobasheri, A.; Hall, A.C.; Urban, J.P.; France, S.J.; Smith, A.L. Immunologic and autoradiographic localisation of the Na+, K(+)-ATPase in articular cartilage: Upregulation in response to changes in extracellular Na+ concentration. Int. J. Biochem. Cell Biol. 1997, 29, 649–657. [Google Scholar] [CrossRef]
- Mobasheri, A.; Trujillo, E.; Arteaga, M.F.; Martín-Vasallo, P. Na(+), K(+)-ATPase subunit composition in a human chondrocyte cell line; evidence for the presence of α1, α3, β1, β2 and β3 isoforms. Int. J. Mol. Sci. 2012, 13, 5019–5034. [Google Scholar] [CrossRef]
- Bomer, N.; den Hollander, W.; Ramos, Y.F.; Bos, S.D.; van der Breggen, R.; Lakenberg, N.; Pepers, B.A.; van Eeden, A.E.; Darvishan, A.; Tobi, E.W.; et al. Underlying molecular mechanisms of DIO2 susceptibility in symptomatic osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1571–1579. [Google Scholar] [CrossRef]
- Wondimu, E.B.; Culley, K.L.; Quinn, J.; Chang, J.; Dragomir, C.L.; Plumb, D.A.; Goldring, M.B.; Otero, M. Elf3 Contributes to Cartilage Degradation in vivo in a Surgical Model of Post-Traumatic Osteoarthritis. Sci. Rep. 2018, 8, 6438. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, X.; Hu, X.; Liu, Q.; Man, Z.; Huang, H.; Meng, Q.; Zhou, C.; Ao, Y. Silencing of miR-101 Prevents Cartilage Degradation by Regulating Extracellular Matrix-related Genes in a Rat Model of Osteoarthritis. Mol. Ther. 2015, 23, 1331–1340. [Google Scholar] [CrossRef]
- Teufel, S.; Wolff, L.; König, U.; Kobayashi, A.; Behringer, R.; Hartmann, C. Mice Lacking Wnt9a or Wnt4 Are Prone to Develop Spontaneous Osteoarthritis With Age and Display Alteration in Either the Trabecular or Cortical Bone Compartment. J. Bone Miner. Res. 2022, 37, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Später, D.; Hill, T.P.; O’Sullivan R, J.; Gruber, M.; Conner, D.A.; Hartmann, C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 2006, 133, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tao, C.; Mao, M.; Zhu, K. The Nrf2/HMGB1/NF-κB axis modulates chondrocyte apoptosis and extracellular matrix degradation in osteoarthritis. Acta Biochim. Biophys. Sin. 2023, 55, 818–830. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, J.; Zhuang, Y.; Zhang, K.; Lu, D. Overexpression of HMGB1 A-box reduced IL-1β-induced MMP expression and the production of inflammatory mediators in human chondrocytes. Exp. Cell Res. 2016, 349, 184–190. [Google Scholar] [CrossRef]
- Palumbo, A.; Atzeni, F.; Murdaca, G.; Gangemi, S. The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33. Int. J. Mol. Sci. 2023, 24, 12143. [Google Scholar] [CrossRef]
- Weng, L.H.; Wang, C.J.; Ko, J.Y.; Sun, Y.C.; Su, Y.S.; Wang, F.S. Inflammation induction of Dickkopf-1 mediates chondrocyte apoptosis in osteoarthritic joint. Osteoarthr. Cartil. 2009, 17, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.J.; Devenish, R.J.; Handley, C.J. Modulation of aggrecan and link-protein synthesis in articular cartilage. Biochem. J. 1992, 288 Pt 3, 721–726. [Google Scholar] [CrossRef]
- Jenniskens, Y.M.; Koevoet, W.; de Bart, A.C.W.; Weinans, H.; Jahr, H.; Verhaar, J.A.N.; DeGroot, J.; van Osch, G.J.V.M. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFβ2 and FGF2. Osteoarthr. Cartil. 2006, 14, 1136–1146. [Google Scholar] [CrossRef]
- Jayasuriya, C.T.; Goldring, M.B.; Terek, R.; Chen, Q. Matrilin-3 induction of IL-1 receptor antagonist is required for up-regulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res. Ther. 2012, 14, R197. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, J.; Jing, X.; Ye, Y.; Guo, J.; Sun, K.; Guo, F. Fibroblast growth factor 18 exerts anti-osteoarthritic effects through PI3K-AKT signaling and mitochondrial fusion and fission. Pharmacol. Res. 2019, 139, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Saito, T.; Chang, S.H.; Kobayashi, H.; Ladel, C.H.; Guehring, H.; Chung, U.I.; Kawaguchi, H. Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J. Biol. Chem. 2014, 289, 10192–10200. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Bendele, A.M.; Thompson, D.L.; Littau, A.; Waggie, K.S.; Reardon, B.; Ellsworth, J.L. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005, 13, 623–631. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Na, H.S.; Park, J.S.; Cho, K.H.; Kwon, J.Y.; Choi, J.; Jhun, J.; Kim, S.J.; Park, S.H.; Cho, M.L. Interleukin-1-Interleukin-17 Signaling Axis Induces Cartilage Destruction and Promotes Experimental Osteoarthritis. Front. Immunol. 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, J.Y.; Baldwin, M.J.; Cribbs, A.P.; Philpott, M.; Carr, A.J.; Dakin, S.G.; Snelling, S.J.B. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front. Immunol. 2021, 12, 676173. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Takahata, Y.; Murakami, T.; Nakamura, E.; Ohkawa, M.; Ruengsinpinya, L. Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Int. J. Mol. Sci. 2020, 21, 1340. [Google Scholar] [CrossRef]
- Wei, F.; Zhou, J.; Wei, X.; Zhang, J.; Fleming, B.C.; Terek, R.; Pei, M.; Chen, Q.; Liu, T.; Wei, L. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthr. Cartil. 2012, 20, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, G.D.; Vasquez-Pacheco, E.; Chu, X.; Seeger, W.; El Agha, E.; Bellusci, S.; Lingampally, A. Revisiting pulmonary fibrosis: Inflammatory dynamics of the lipofibroblast-to-inflammatory lipofibroblast-to-activated myofibroblast reversible switch. Front. Immunol. 2025, 16, 1609509. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, M.; Suzuki, T.; Hosono, K.; Kamata, Y.; Fukamizu, A.; Kitasato, H.; Fujita, Y.; Majima, M. Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomed. Pharmacother. 2009, 63, 627–634. [Google Scholar] [CrossRef]
- Sim, S.L.; Kumari, S.; Kaur, S.; Khosrotehrani, K. Macrophages in Skin Wounds: Functions and Therapeutic Potential. Biomolecules 2022, 12, 1659. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Koistinen, H.; Künnapuu, J.; Jeltsch, M. KLK3 in the Regulation of Angiogenesis-Tumorigenic or Not? Int. J. Mol. Sci. 2021, 22, 13545. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Yu, H.; Williams, J.; Smallwood, P.M.; Nathans, J. Endothelin-2 signaling in the neural retina promotes the endothelial tip cell state and inhibits angiogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E3830–E3839. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Fila, M.; Chojnacki, J.; Sobczuk, P.; Chojnacki, C.; Blasiak, J. Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine. Nutrients 2023, 15, 289. [Google Scholar] [CrossRef]
- Toda, M.; Suzuki, T.; Hosono, K.; Kurihara, Y.; Kurihara, H.; Hayashi, I.; Kitasato, H.; Hoka, S.; Majima, M. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed. Pharmacother. 2008, 62, 352–359. [Google Scholar] [CrossRef]
- Leone, P.; Malerba, E.; Susca, N.; Favoino, E.; Perosa, F.; Brunori, G.; Prete, M.; Racanelli, V. Endothelial cells in tumor microenvironment: Insights and perspectives. Front. Immunol. 2024, 15, 1367875. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conforti, C.; Kimbrough, D.W.; Patel, N.; Ley, M.B.R.G.; Medina Flores, J.; Correa, D.; Kaplan, L.D.; Best, T.M.; Kouroupis, D. Characterizing the Long Non-Coding RNA Profile of Endometrial Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Anti-Inflammatory Role in Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 10567. https://doi.org/10.3390/ijms262110567
Conforti C, Kimbrough DW, Patel N, Ley MBRG, Medina Flores J, Correa D, Kaplan LD, Best TM, Kouroupis D. Characterizing the Long Non-Coding RNA Profile of Endometrial Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Anti-Inflammatory Role in Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(21):10567. https://doi.org/10.3390/ijms262110567
Chicago/Turabian StyleConforti, Cole, Darden Wood Kimbrough, Neep Patel, Michelle B. R. G. Ley, Jose Medina Flores, Diego Correa, Lee D. Kaplan, Thomas M. Best, and Dimitrios Kouroupis. 2025. "Characterizing the Long Non-Coding RNA Profile of Endometrial Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Anti-Inflammatory Role in Osteoarthritis" International Journal of Molecular Sciences 26, no. 21: 10567. https://doi.org/10.3390/ijms262110567
APA StyleConforti, C., Kimbrough, D. W., Patel, N., Ley, M. B. R. G., Medina Flores, J., Correa, D., Kaplan, L. D., Best, T. M., & Kouroupis, D. (2025). Characterizing the Long Non-Coding RNA Profile of Endometrial Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Anti-Inflammatory Role in Osteoarthritis. International Journal of Molecular Sciences, 26(21), 10567. https://doi.org/10.3390/ijms262110567







