2.1. ABCL Cytotoxicity
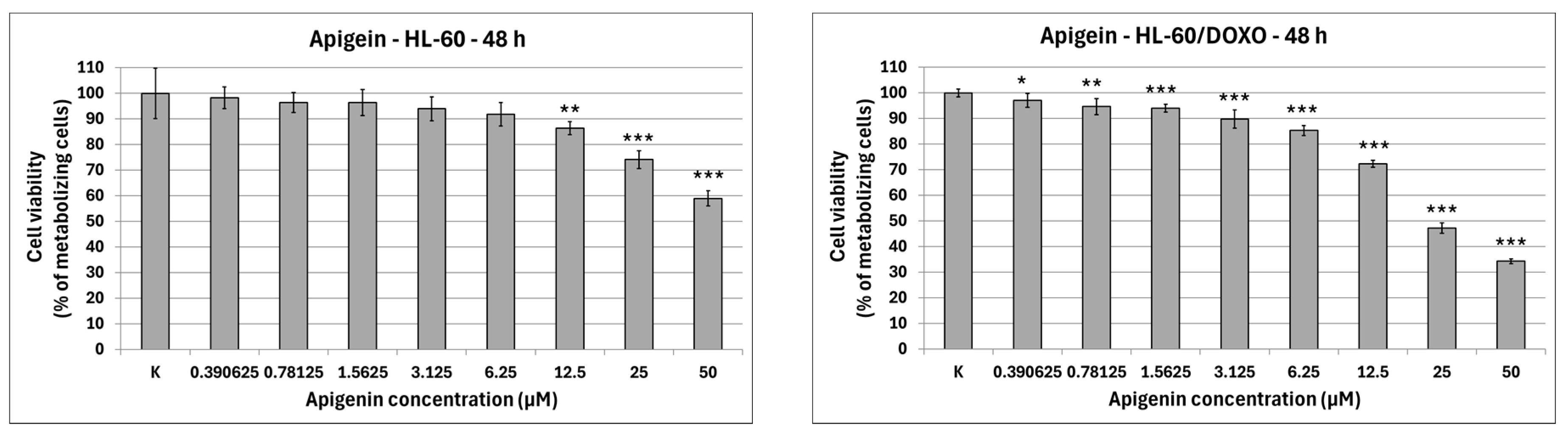
Apigenin is moderately cytotoxic towards doxorubicin-sensitive HL-60 cells with an IC
50 at 40.15 µM, significantly reducing the percentage of metabolizing cells to 86.42 ± 2.56% at 12.5 µM (
p < 0.01), 74.11 ± 3.50% at 25 µM (
p < 0.001), and 58.97 ± 2.98% at 50 µM (
p < 0.001), while exhibiting higher cytotoxicity towards doxorubicin-resistant HL-60/DOXO cells, with an IC
50 at 17.26 µM, significantly reducing the percentage of metabolizing cells already from the smallest concentration (
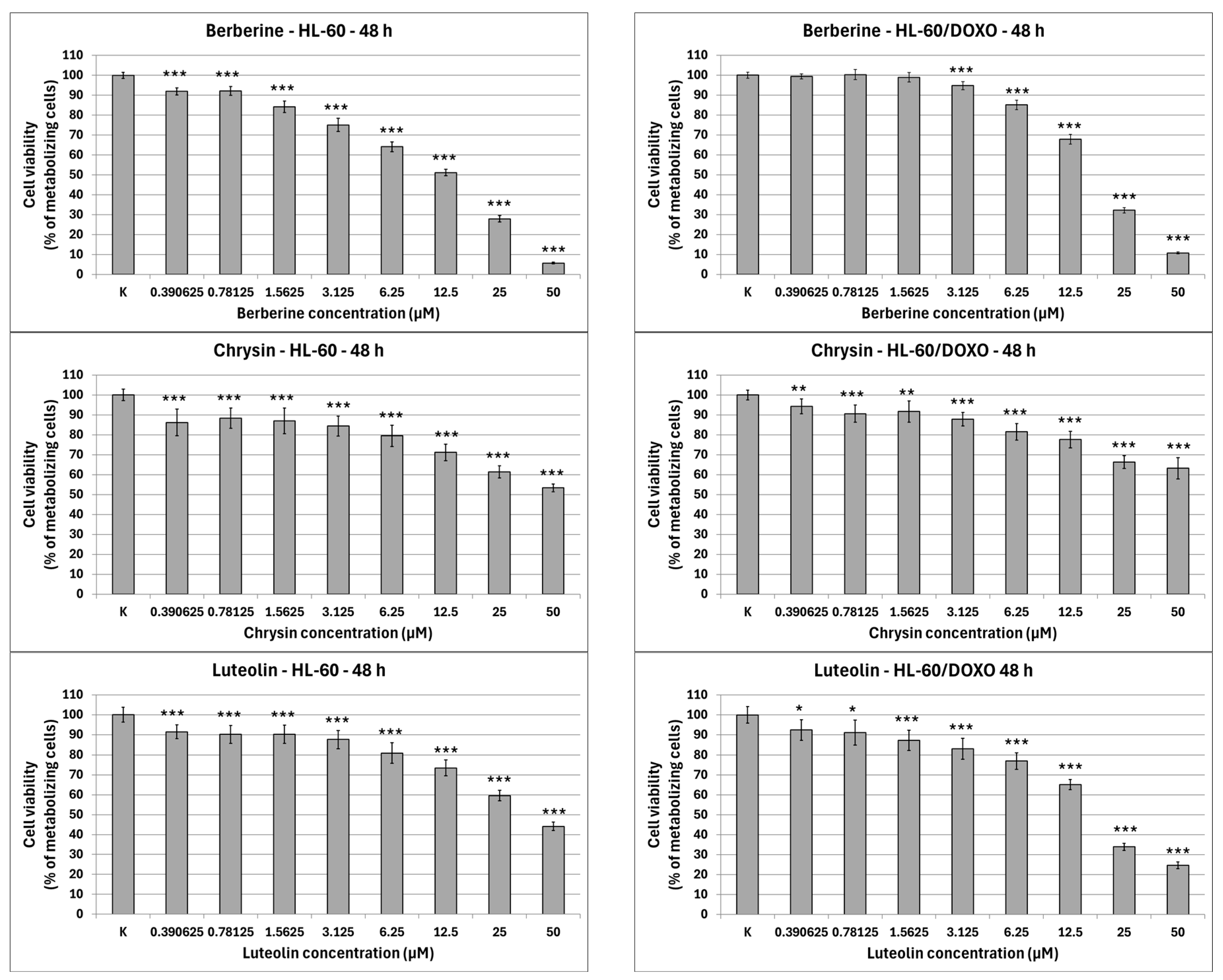
Figure 2). Berberine is highly cytotoxic towards both doxorubicin-sensitive cells, with an IC
50 at 16.66 µM, significantly reducing the percentage of metabolizing cells. At the highest concentration used (50 µM), Berberine reduces the number of living cells to 5.74 ± 0.39% in HL-60 cells (
p < 0.001) and to 10.77 ± 0.49% in HL-60/DOXO cells (
p < 0.001), with an IC
50 at 18.42 µM. Chrysin is moderately cytotoxic towards both HL-60 and HL-60/DOXO cells, reducing the percentage of metabolizing cells at all used concentrations. Chrysin is also the least cytotoxic out of ABCL, with an IC
50 at 90.26 µM and 102.58 µM for HL-60 and HL-60/DOXO, respectively. Luteolin is moderately cytotoxic towards doxorubicin-sensitive HL-60 cells, with an IC
50 at 43.41 µM, significantly reducing the percentage of metabolizing cells at all used concentrations. At the highest concentration used (50 µM), Luteolin reduces the number of living cells to 44.14 ± 2.10% (
p < 0.001). Luteolin exhibits higher cytotoxicity towards doxorubicin-resistant HL-60/DOXO cells, with an IC
50 at 17.81 µM, reducing the percentage of metabolizing cells to 24.69 ± 1.70 at 50 µM (
p < 0.001).
The results of 24 h incubation with ABCL are included in the
Supplementary Materials (Figure S1); overall, the effects of ABCL on both sensitive and resistant cell lines were similar to 48 h incubation, with the key difference being lower cytotoxicity due to shorter exposure time. As with the 48 h incubation, Berberine was found to be the most cytotoxic, while Chrysin was found to be the least cytotoxic (
Figure S1).
2.2. Choice of Incubation Model—48 h Preincubation with ABCL and Doxorubicin 24 h Incubation
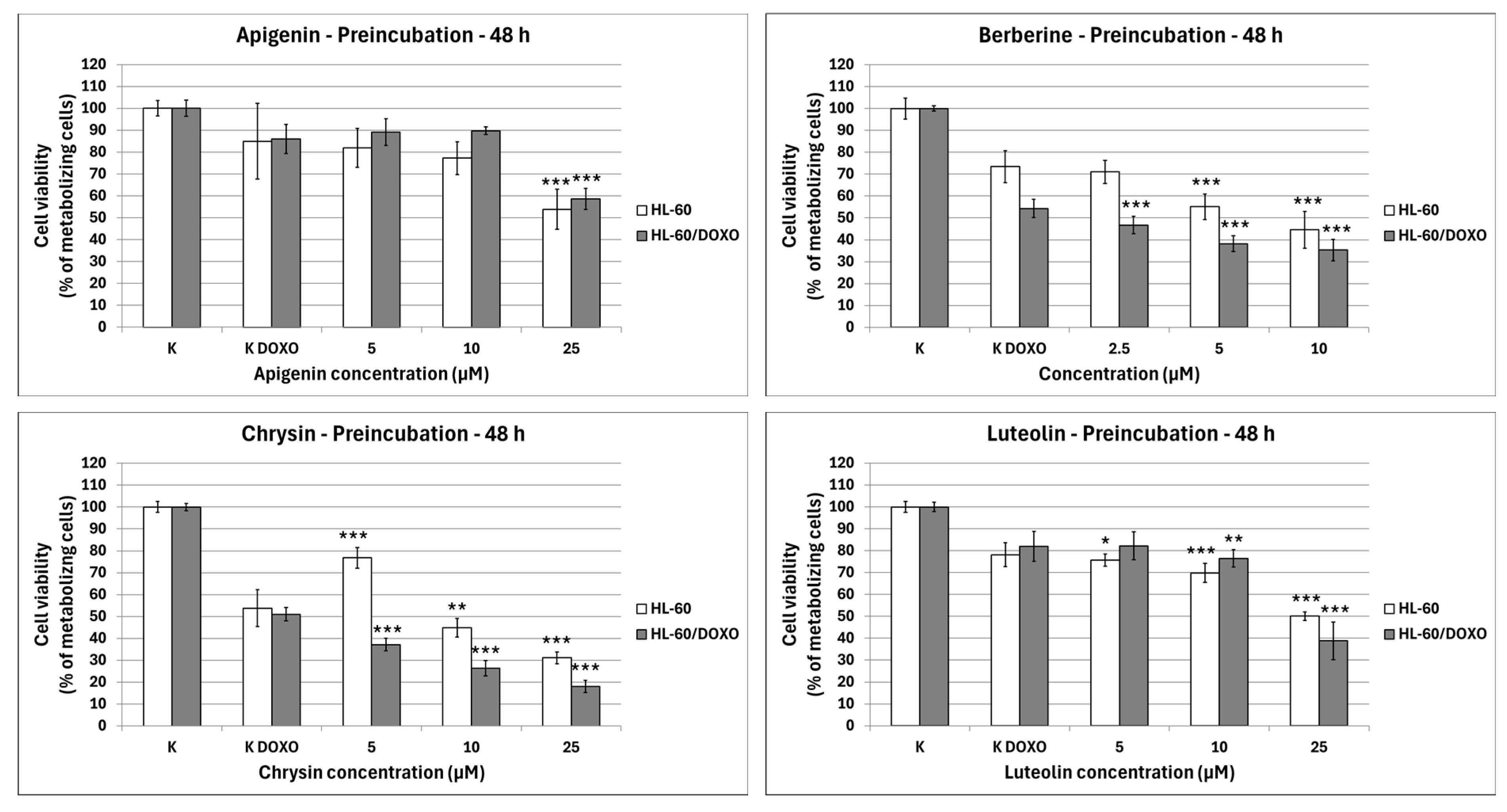
After 48 h preincubation with ABCL and a subsequent 24 h incubation with doxorubicin, Apigenin reduced the percentage of metabolizing cells to 53.89 ± 9.20% at 25 µM concentration (
p < 0.001) compared to 84.97 ± 17.21% in doxorubicin control in doxorubicin-sensitive HL-60 cells, and 58.62 ± 4.86% at 25 µM concentration (
p < 0.001) compared to 86.05 ± 6.64% in doxorubicin control in doxorubicin-resistant HL-60/DOXO cells (
Figure 3). Berberine reduced the percentage of metabolizing cells to 55.14 ± 5.80% at 5 µM (
p < 0.001) and 44.54 ± 8.36% at 10 µM concentration (
p < 0.001) compared to 73.42 ± 7.25% in doxorubicin control in doxorubicin-sensitive cell line, and 46.76 ± 3.90% at 2.5 µM (
p < 0.001), 38.21 ± 3.57% at 5 µM (
p < 0.001) and 35.30 ± 4.90% at 10 µM concentration (
p < 0.001), compared to 54.32 ± 4.12% in doxorubicin control in doxorubicin-resistant cell line. Chrysin increased the percentage of metabolizing cells to 76.78 ± 4.74% at 5 µM concentration (
p < 0.001) but reduced it to 44.91 ± 4.26% at 10 µM (
p < 0.01) and 31.13 ± 2.67% at 25 µM concentration (
p < 0.001) compared to 53.83 ± 8.43% in the doxorubicin control in the doxorubicin-sensitive cell line. In the doxorubicin-resistant cell line, Chrysin reduced the percentage of metabolizing cells to 37.18 ± 2.94% at 5 µM (
p < 0.001), 26.30 ± 3.53% at 10 µM (
p < 0.001), and 17.99 ± 2.77% at 25 µM concentration (
p < 0.001) compared to 50.99 ± 3.05% in the doxorubicin control. Luteolin reduced the percentage of metabolizing cells to 75.74 ± 2.79% at 5 µM (
p < 0.05), 69.80 ± 4.35% at 10 µM (
p < 0.001), and 50.07 ± 1.86% at 25 µM concentration (
p < 0.001) compared to 78.11 ± 5.46% in doxorubicin control in doxorubicin-sensitive cell line, and 76.46 ± 3.96% at 10 µM (
p < 0.01) and 38.82 ± 8.62% at 25 µM concentration (
p < 0.001) compared to 82.01 ± 6.80% in doxorubicin control in doxorubicin-resistant cell line.
Because the 48 h preincubation model with ABCL has been shown to exhibit the highest increase in effectiveness of doxorubicin-induced cytotoxicity when compared to 24 h coincubation as well as 24 h preincubation, this model was chosen for all further experiments (results of other incubation models with ABCL are included in the
Supplementary Materials, Figures S2 and S3). Additionally, centrifugation after incubation with ABCL ensured that cells that died due to ABCL’s cytotoxicity were separated at that point.
Gao et al. have shown that Apigenin sensitizes BEL-7402/ADM cells to doxorubicin after 48 h preincubation followed by 48 h incubation with doxorubicin [
21]. Paramasivan et al. acquired similar results with neferine in A549 and A549/DOXO cell lines, and also observed the increased effectiveness of doxorubicin when combined with neferine, when compared to doxorubicin alone [
22].
2.3. DNA Damage
Apigenin increased the DNA damage percentage in doxorubicin-sensitive HL-60 cells to 14.06 ± 0.92% at 12.5 µM (
p < 0.001) and 7.95 ± 5.90% at 25 µM (
p < 0.001) compared to 5.28 ± 0.50% in control (
Figure 4). In combination with doxorubicin, the DNA damage percentage increased to 20.67 ± 0.78% at 12.5 µM (
p < 0.001) and 12.73 ± 1.47% at 25 µM (
p < 0.05) compared to 9.19 ± 0.72% in the doxorubicin control. Apigenin reduced the DNA damage percentage in doxorubicin-resistant HL-60/DOXO cells to 3.93 ± 0.52% at 25 µM (
p < 0.001) compared to 4.59 ± 0.40% in control. In combination with doxorubicin, the DNA damage percentage increased to 24.91 ± 1.67 at 12.5 µM (
p < 0.001) and 23.64 ± 1.81 at 25 µM (
p < 0.01) compared to 16.09 ± 1.40 in the doxorubicin control.
Berberine increased the DNA damage percentage in doxorubicin-sensitive HL-60 cells to 8.77 ± 0.75% at 10 µM (p < 0.001) compared to 5.28 ± 0.50% in control; in combination with doxorubicin, the DNA damage percentage increased to 13.70 ± 0.83% at 5 µM (p < 0.001) and 12.85 ± 1.05% at 10 µM (p < 0.01) compared to 9.19 ± 0.72% in the doxorubicin control. Berberine decreased the DNA damage percentage in doxorubicin-resistant HL-60/DOXO cells to 1.93 ± 0.26% at 5 µM (p < 0.001) and 0.71 ± 0.14% at 10 µM (p < 0.001) compared to 4.59 ± 0.40% in the control. In combination with doxorubicin, the DNA damage percentage decreased to 11.19 ± 1.00% at 5 µM (p < 0.01) and 11.19 ± 1.18% at 10 µM (p < 0.01) compared to 16.09 ± 1.40% in the doxorubicin control.
Chrysin increased the DNA damage percentage in doxorubicin-sensitive HL-60 cells to 6.98 ± 0.59% at 25 µM (p < 0.05) and 9.72 ± 0.86% at 50 µM (p < 0.001) compared to 5.28 ± 0.50% in control; in combination with doxorubicin, the DNA damage increased to 11.17 ± 0.62% at 25 µM (p < 0.05) compared to 9.19 ± 0.72% in the doxorubicin control. Chrysin decreased the DNA damage percentage to 1.56 ± 0.21% at 25 µM (p < 0.001) and increased the DNA damage percentage to 8.12 ± 0.64% at 50 µM compared to 4.59 ± 0.40% in control in HL-60/DOXO cells. In combination with doxorubicin, the DNA damage percentage increased to 37.71 ± 1.87% at 25 µM (p < 0.001) and 17.40 ± 2.04% at 50 µM (p < 0.05) compared to 16.09 ± 1.40% in the doxorubicin control.
Luteolin increased the DNA damage percentage in doxorubicin-sensitive HL-60 cells to 17.04 ± 1.35% at 12.5 µM (p < 0.001) and 9.40 ± 1.32% at 25 µM (p < 0.05) compared to 5.28 ± 0.50% in control; in combination with doxorubicin, the DNA damage percentage increased to 14.01 ± 0.81% at 12.5 µM (p < 0.001) and 16.50 ± 1.64% at 25 µM (p < 0.001) compared to 9.19 ± 0.72% in the doxorubicin control. Luteolin reduced the DNA damage percentage in doxorubicin-resistant HL-60/DOXO cells to 1.27 ± 0.19% at 12.5 µM (p < 0.001) and 1.16 ± 0.19% at 25 µM (p < 0.001) compared to 4.59 ± 0.40% in the control. In combination with doxorubicin, the DNA damage percentage increased to 22.50 ± 1.52% at 12.5 µM (p < 0.01) compared to 16.09 ± 1.40% in the doxorubicin control.
ABCL has shown the ability to induce DNA damage in doxorubicin-sensitive HL-60 cells; in combination with doxorubicin, it increases its ability to induce DNA damage. In doxorubicin-resistant HL-60/DOXO cells, ABCL has been shown to decrease DNA damage, while at the same time highly increasing the DNA damage-inducing properties of doxorubicin, one exception being Berberine, in which the combination with doxorubicin resulted in lower levels of DNA damage.
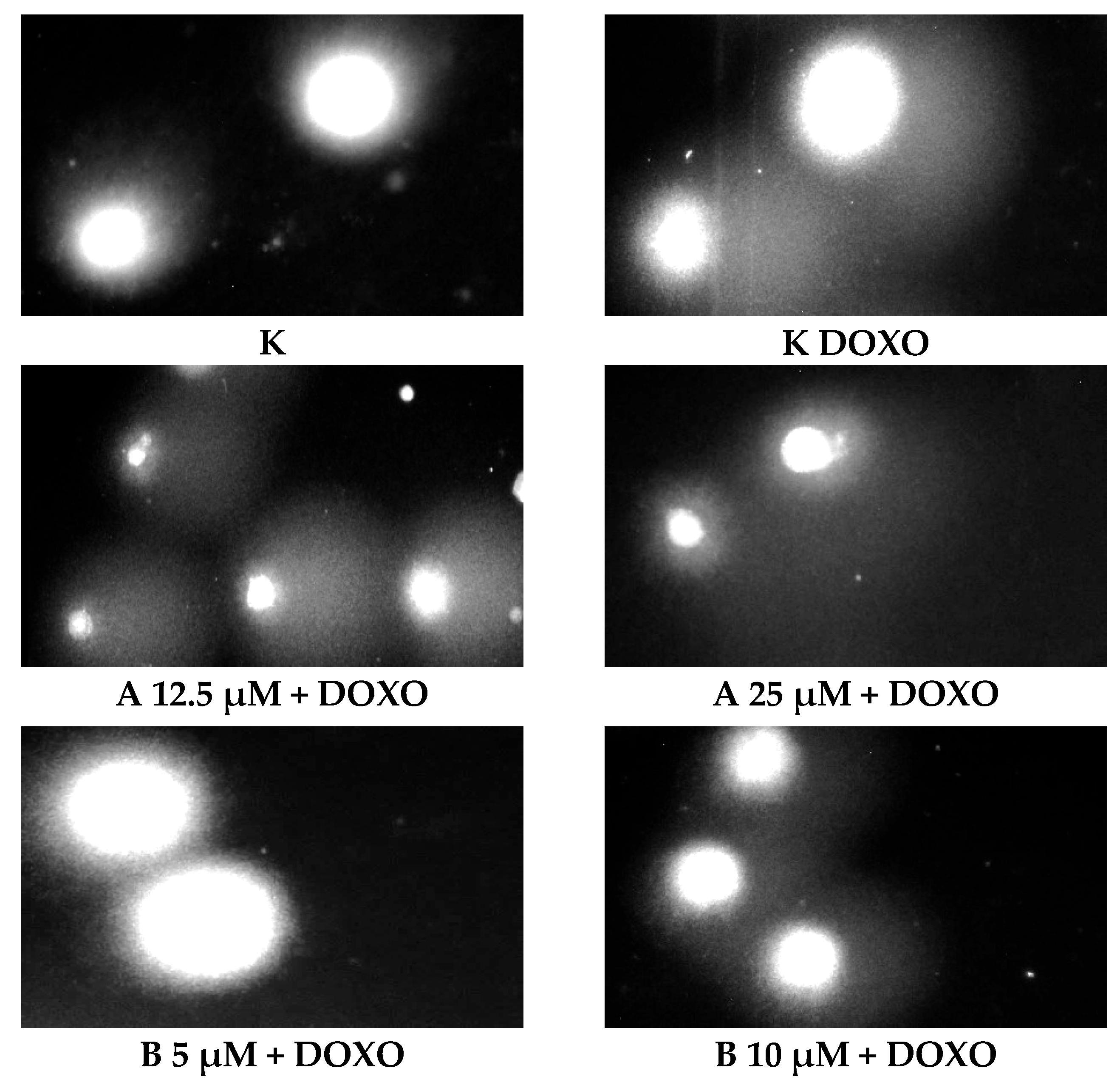
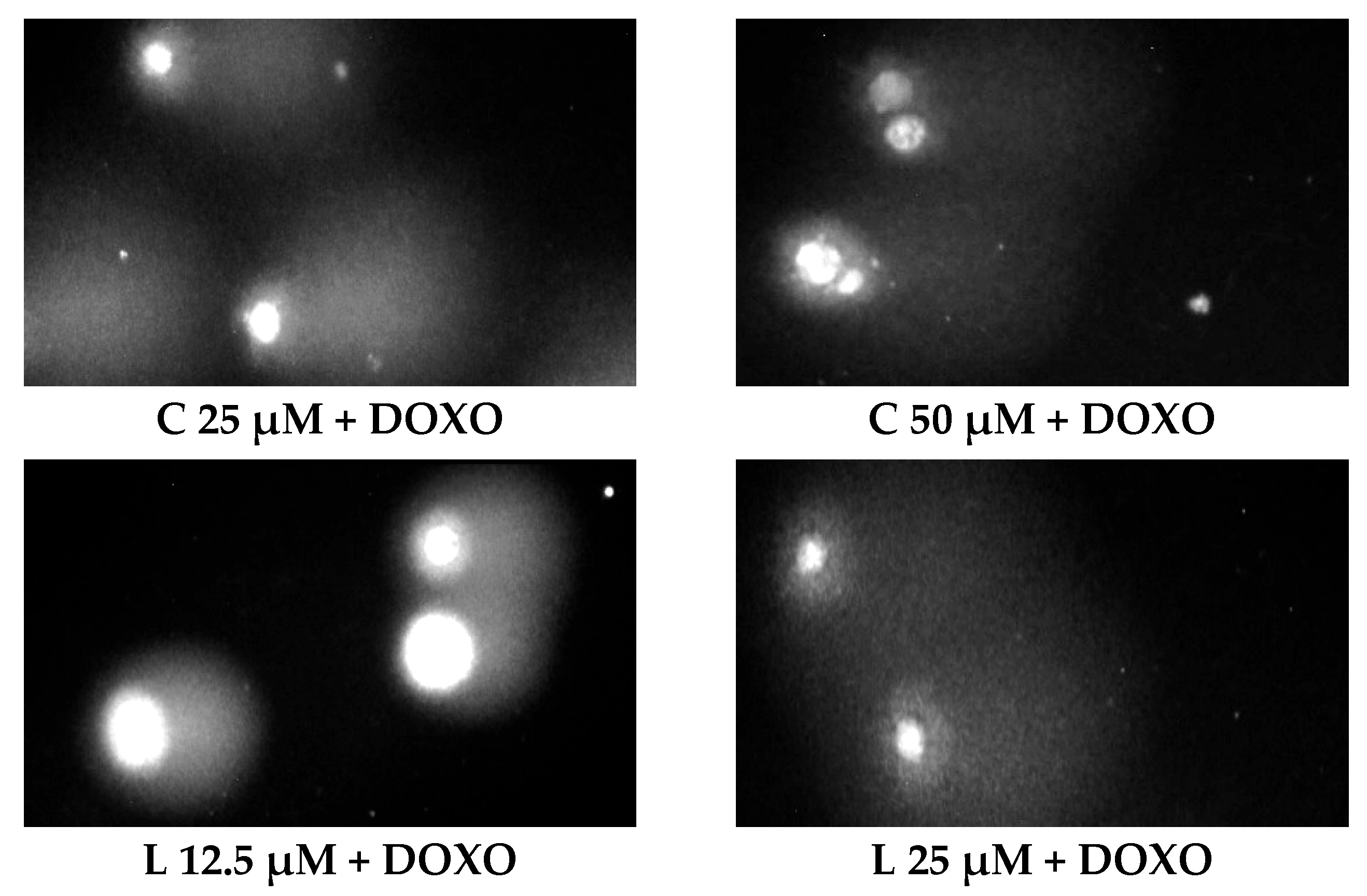
Figure 5 shows representative comet images obtained after 48 h of preincubation of HL-60/DOXO cells with ABCL followed by 24 h of incubation with doxorubicin. The comet images correspond well with the numerical data describing the level of DNA damage presented in
Figure 4. It should also be noted that Chrysin at 50 µM might have induced far greater DNA damage levels than shown in
Figure 4; upon examination of photos of the sample in
Figure 5, it is quite possible that the DNA damage was so high that it rendered accurate measurement impossible. Another noteworthy aspect is that in doxorubicin-sensitive cells, Apigenin has induced lower DNA damage at 25 µM than at 12.5 µM, and similar results were obtained by Arango et al. [
23]; in their study, Apigenin at 50 µM concentration induced higher DNA damage levels after 1 and 3 h of incubation and lower DNA damage levels after 6 and 9 h of incubation. We suspect that this effect might be caused by the cells’ activation of DNA repair pathways caused by prolonged exposure to high concentrations of Apigenin.
2.4. Apoptosis
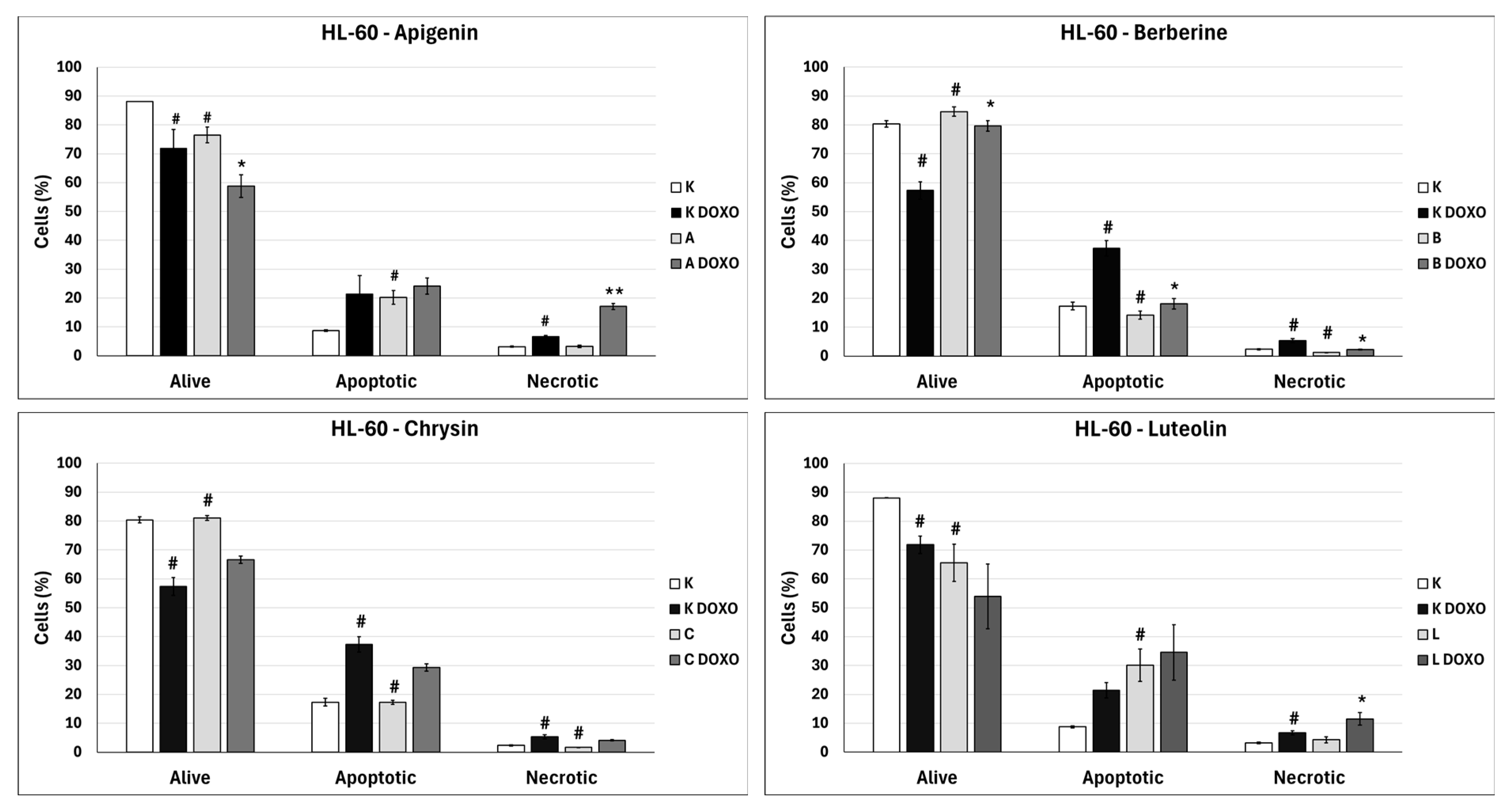
In doxorubicin-sensitive HL-60 cells, incubation with Apigenin increased the percentage of apoptotic cells to 20.21 ± 2.43% (
p < 0.05) compared to 8.74 ± 0.38% in control; in combination with doxorubicin, the percentage of necrotic cells increased to 17.09 ± 1.07% (
p < 0.01) compared to 6.72 ± 2.69% in the doxorubicin control (
Figure 6).
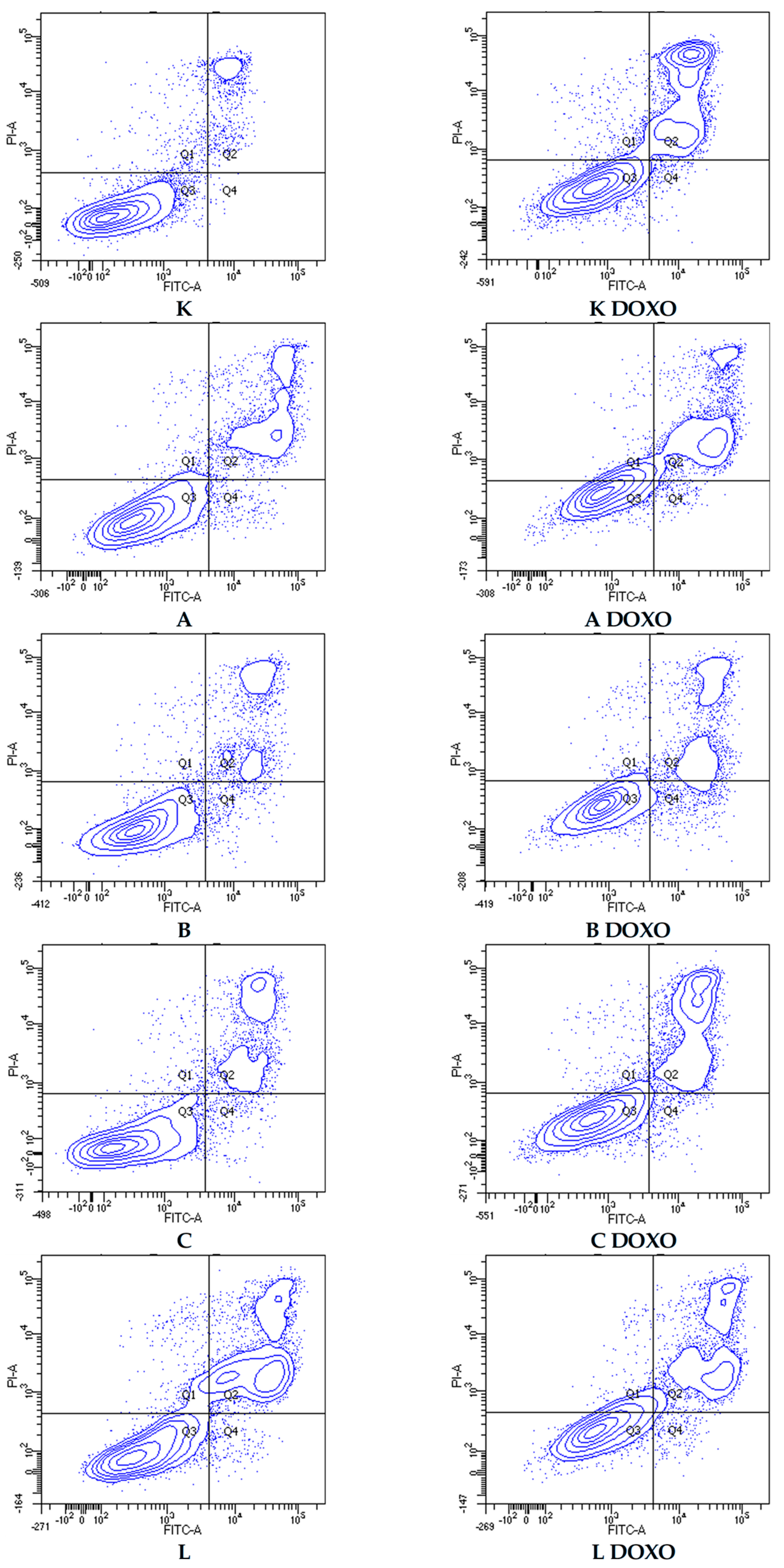
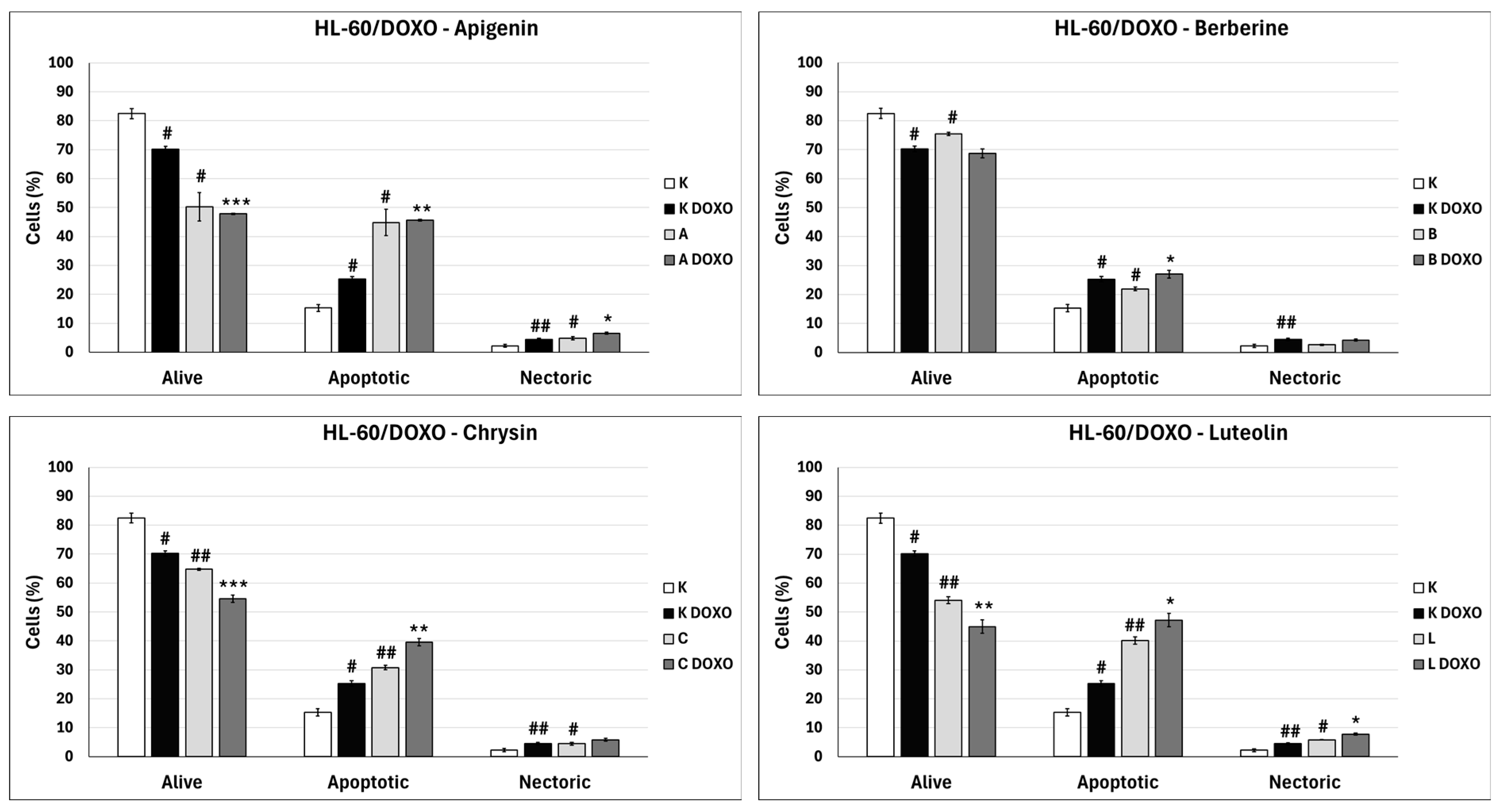
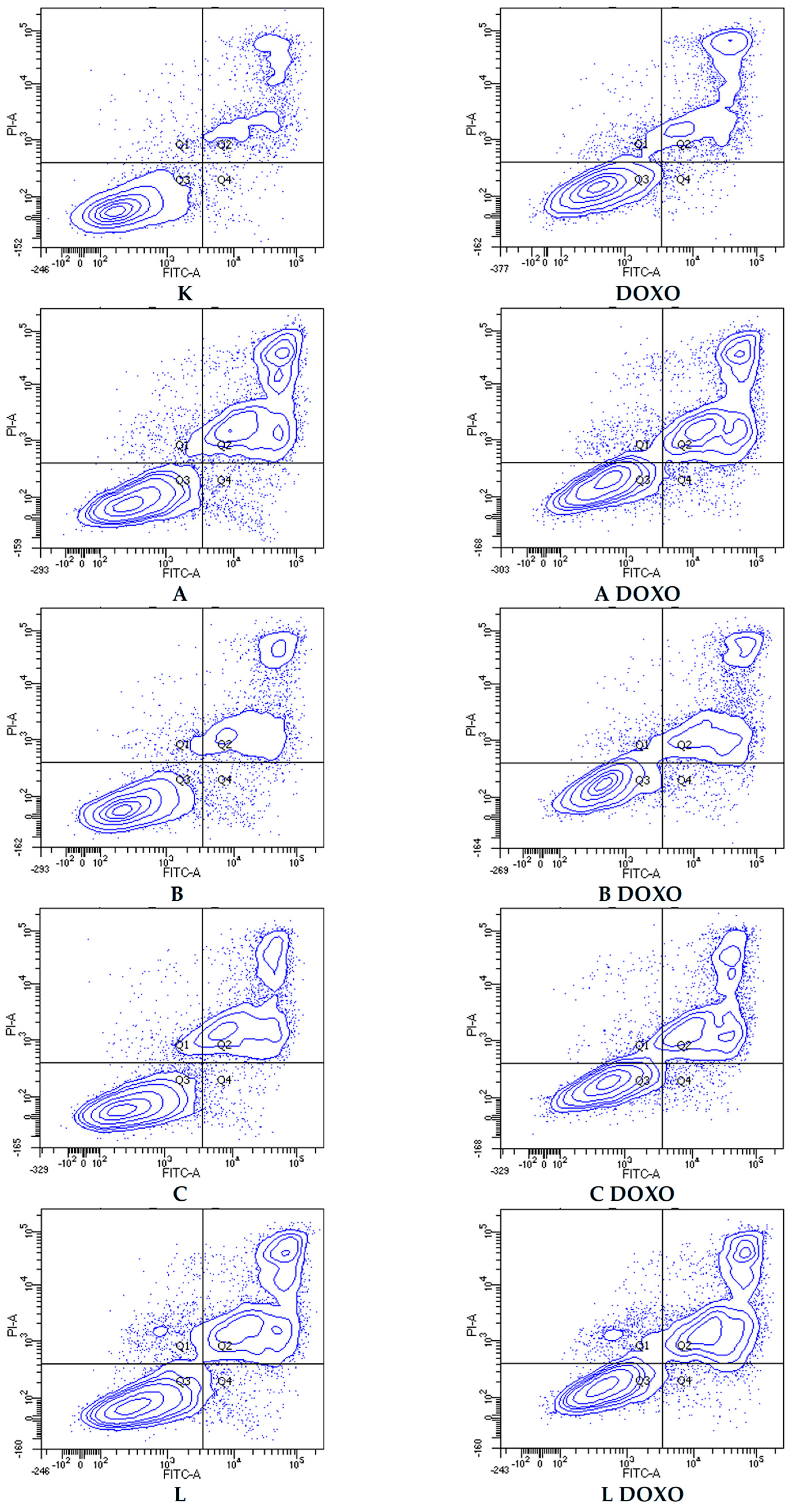
Figure 7 shows representative flow cytometric dot plots of HL-60 cells after 48 h preincubation with ABCL followed by 24 h incubation with doxorubicin in viable (Q3), early apoptotic (Q4), late apoptotic (Q2), and necrotic (Q1) stages. In doxorubicin-resistant HL-60/DOXO cells, incubation with Apigenin increased the percentage of apoptotic cells to 44.86 ± 4.60% (
p < 0.05) compared to 15.30 ± 1.24% in control; in combination with doxorubicin, the percentage of apoptotic cells increased to 45.64 ± 0.26% (
p < 0.01) compared to 25.31 ± 0.88% in the doxorubicin control (
Figure 8).
In doxorubicin-sensitive HL-60 cells after incubation with Berberine in combination with doxorubicin, the percentage of apoptotic cells decreased to 18.10 ± 1.77% (
p < 0.05) compared to 37.32 ± 2.62% in the doxorubicin control (
Figure 6). In doxorubicin-resistant HL-60/DOXO cells, incubation with Berberine increased the percentage of apoptotic cells to 21.94 ± 0.63% (
p < 0.05) compared to 15.30 ± 1.24% in the control; in combination with doxorubicin, the percentage of apoptotic cells increased to 27.01 ± 0.72% compared to 25.31 ± 0.88% (
p < 0.05) in the doxorubicin control (
Figure 8).
In doxorubicin-sensitive HL-60 cells after incubation with Chrysin in combination with doxorubicin, no statistically significant differences were observed when compared to the doxorubicin control (
p > 0.05) (
Figure 6). In doxorubicin-resistant HL-60/DOXO cells, incubation with Chrysin increased the percentage of apoptotic cells to 30.79 ± 0.72% (
p < 0.01) compared to 15.30 ± 1.24% in the control; in combination with doxorubicin, the percentage of apoptotic cells increased to 39.59 ± 1.30% (
p < 0.01) compared to 25.31 ± 0.88% in the doxorubicin control (
Figure 8).
In doxorubicin-sensitive HL-60 cells, incubation with Luteolin increased the percentage of apoptotic cells to 30.11 ± 5.56% (
p < 0.05) compared to 8.74 ± 0.38% in control; in combination with doxorubicin, no statistically significant differences were observed when compared to the doxorubicin control (
Figure 6). In doxorubicin-resistant HL-60/DOXO cells, incubation with Luteolin increased the percentage of apoptotic cells 40.12 ± 1.24% (
p < 0.01) compared to 15.30 ± 1.24% in the control. In combination with doxorubicin, the percentage of apoptotic cells increased to 47.21 ± 2.36% (
p < 0.05) compared to 25.31 ± 0.88% in the doxorubicin control (
Figure 8).
In doxorubicin-sensitive HL-60 cells, ABCL increases the percentage of apoptotic cells; however, in combination with doxorubicin, no increase in apoptotic cell percentage is observed; furthermore, Berberine has caused a significant drop in apoptotic cells in combination with doxorubicin. Interestingly, Apigenin in combination with doxorubicin has been shown to greatly increase the percentage of necrotic cells (
p < 0.01) (
Figure 6).
In doxorubicin-resistant HL-60/DOXO cells, ABCL increases the percentage of apoptotic cells. A combination of ABCL with doxorubicin also results in an increased percentage of apoptotic cells. It should be noted, however, that in cases of Apigenin and Berberine, this effect may be attributed to the compounds themselves (
Figure 8).
Figure 9 shows representative flow cytometric dot plots of HL-60/DOXO cells after 48 h preincubation with ABCL, followed by 24 h incubation with doxorubicin in viable (Q3), early apoptotic (Q4), late apoptotic (Q2), and necrotic (Q1) stages.
Paramasivan et al. showed that combined treatment of A549 and A549/DOXO cells with doxorubicin and neferine for 48 h increased the percentage of phosphatidyl serine externalization and mitochondrial membrane potential, pointing to increased induction of apoptosis when compared to doxorubicin alone [
22]. Juszczak et al. have shown that 24 h preincubation with retinoic acid and subsequent 24 h incubation with doxorubicin increased the amount of apoptotic HL-60/DOXO cells, compared to doxorubicin alone [
16].
2.5. Oxidative Stress Evaluation with H2DCFDA
ABCL exhibits the ability to increase oxidative stress in both doxorubicin-sensitive HL-60 cells (
Figure 10) as well as resistant HL-60/DOXO cells (
Figure 11). Oxidative stress-inducing properties of doxorubicin are basically nonexistent in doxorubicin control (K DOXO) (evaluation of doxorubicin’s ability to induce oxidative stress in HL-60/DOXO cells is included in the
Supplementary Materials, Figure S4). However, in doxorubicin-resistant cells, ABCL does in fact seem to affect this property of doxorubicin.
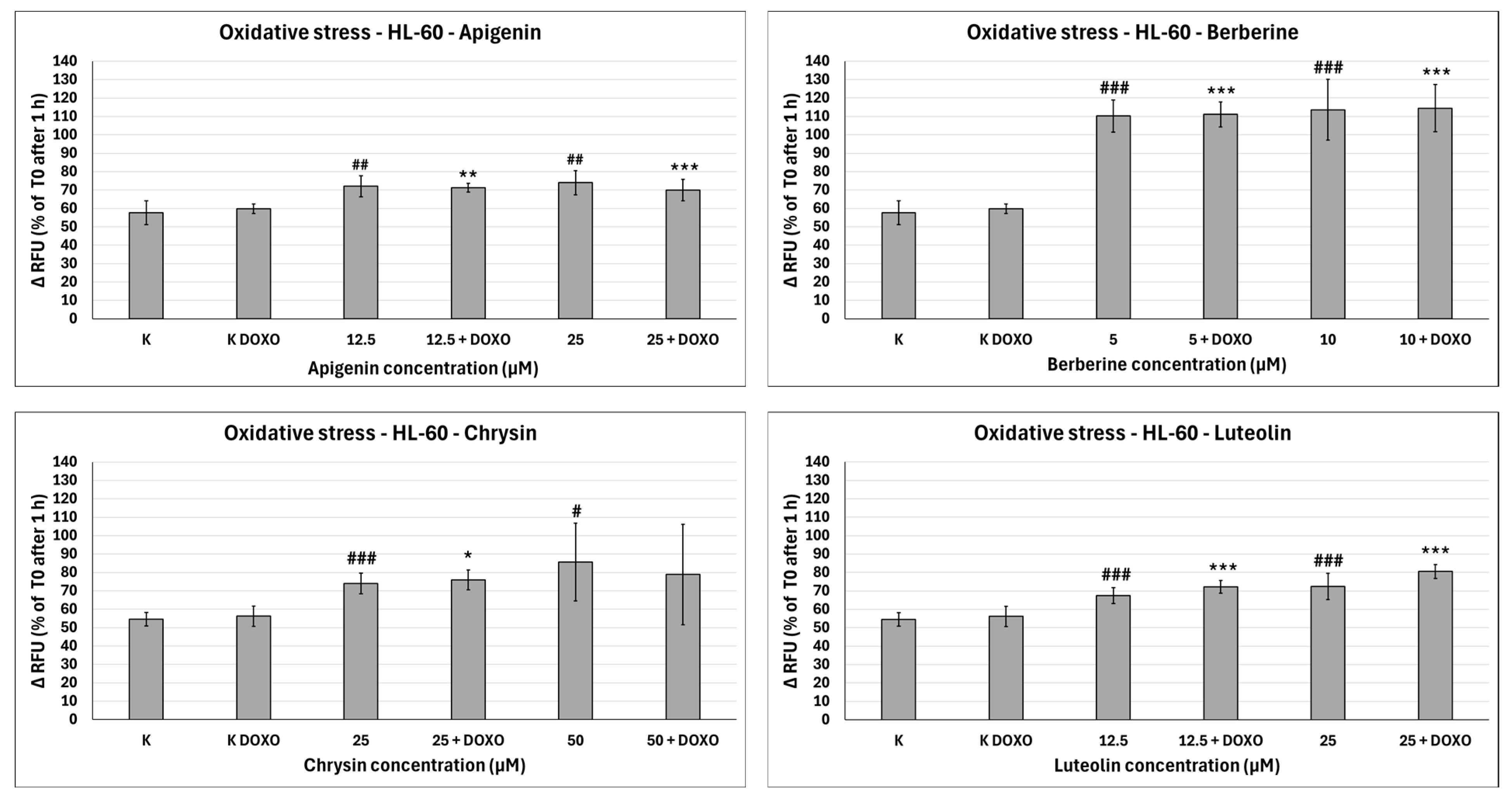
In doxorubicin-sensitive HL-60 cells, Apigenin increased relative fluorescence units to 72.10 ± 5.68 at 12.5 µM (
p < 0.01) and 74.01 ± 6.59 at 25 µM (
p < 0.01), compared to 57.68 ± 6.46 in control; samples with doxorubicin yielded similar results (
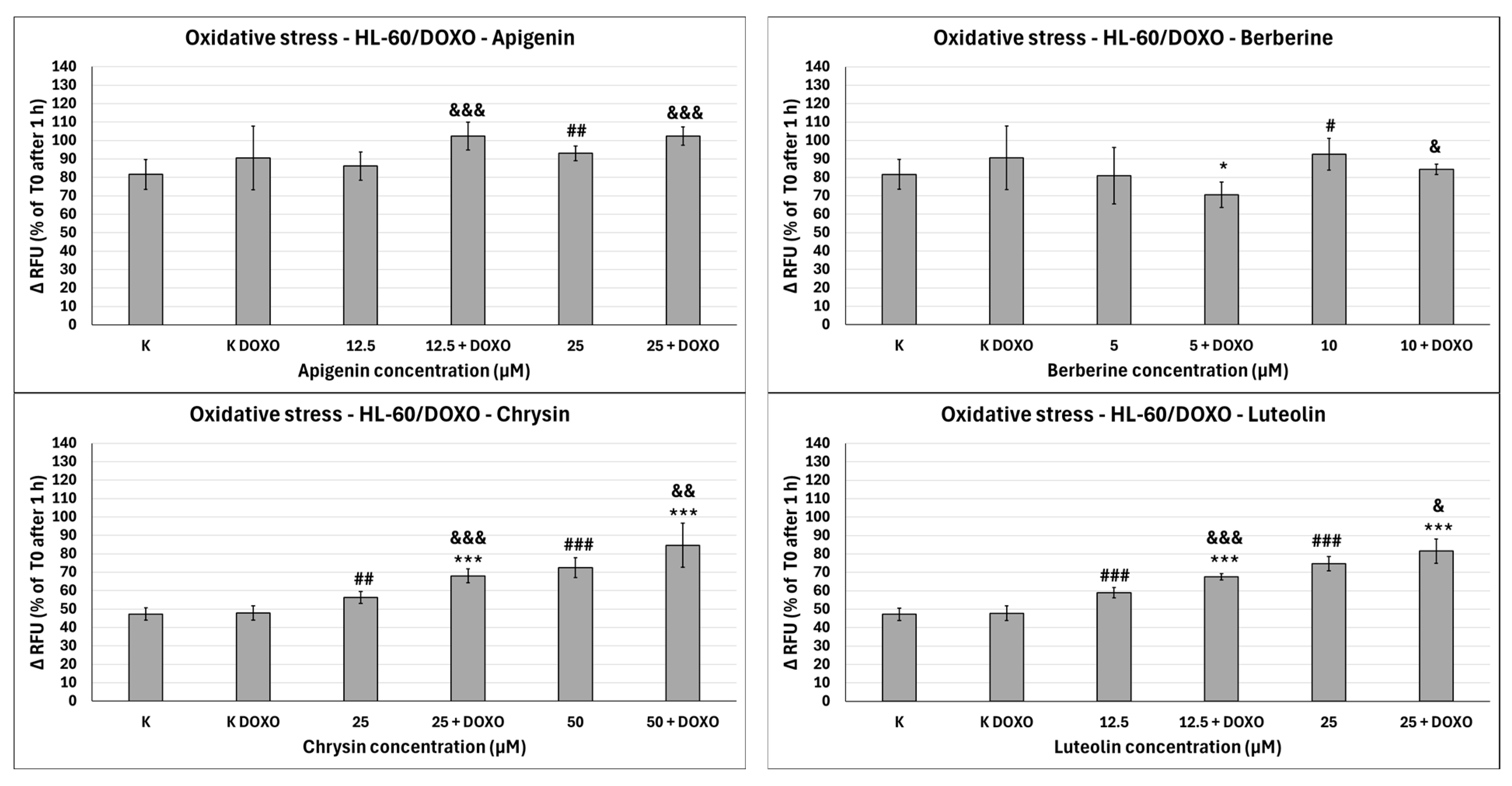
Figure 10). In doxorubicin-resistant HL-60/DOXO cells, Apigenin increased relative fluorescence units to 93.04 ± 4.09 at 25 µM (
p < 0.01) compared to 81.61 ± 8.16 in control (
Figure 11).
In doxorubicin-sensitive HL-60 cells, Berberine increased relative fluorescence units to 110.19 ± 8.66 at 5 µM (
p < 0.001) and 113.61 ± 16.57 at 10 µM (
p < 0.001), compared to 57.68 ± 6.46 in control; samples with doxorubicin yielded similar results (
Figure 10). In doxorubicin-resistant HL-60/DOXO cells, Berberine increased relative fluorescence units to 92.57 ± 8.65 at 10 µM (
p < 0.05) compared to 57.68 ± 6.46 in the control. A total of 5 µM Berberine combined with doxorubicin lowered relative fluorescence units to 70.50 ± 6.89 (
p < 0.05), compared to 90.55 ± 17.24 in the doxorubicin control (
Figure 11).
In doxorubicin-sensitive HL-60 cells, Chrysin increased relative fluorescence units to 74.01 ± 5.58 at 25 µM (
p < 0.001) and 85.54 ± 21.14 at 50 µM (
p < 0.05), compared to 54.56 ± 3.72 in control; samples with doxorubicin yielded similar results (
Figure 10). In doxorubicin-resistant HL-60/DOXO cells, Chrysin increased relative fluorescence units to 56.29 ± 3.31 at 25 µM (
p < 0.01) and 72.44 ± 5.33 at 50 µM (
p < 0.001), compared to 47.27 ± 3.37 in control (
Figure 9). In combination with doxorubicin, Chrysin increased relative fluorescence units to 67.99 ± 3.73 at 25 µM (
p < 0.001) and 84.59 ± 11.98 at 50 µM (
p < 0.001), compared to 47.83 ± 3.94 in the doxorubicin control (
Figure 11).
In doxorubicin-sensitive HL-60 cells, Luteolin increased relative fluorescence units to 67.41 ± 4.29 at 12.5 µM (
p < 0.001) and 72.50 ± 7.14 at 25 µM (
p < 0.001), compared to 54.56 ± 3.72 in control; samples with doxorubicin yielded similar results (
Figure 10). In doxorubicin-resistant HL-60/DOXO cells, Luteolin increased relative fluorescence units to 58.97 ± 2.85 at 12.5 µM (
p < 0.001) and 74.70 ± 3.88 at 25 µM (
p < 0.001), compared to 47.27 ± 3.37 in the control. In combination with doxorubicin, Luteolin increased relative fluorescence units to 67.59 ± 1.74 at 12.5 µM (
p < 0.001) and 81.57 ± 6.58 at 25 µM (
p < 0.001), compared to 47.83 ± 3.94 in the doxorubicin control (
Figure 11).
In HL-60/DOXO cells, Apigenin at 12.5 µM combined with doxorubicin increased the amount of relative fluorescence units to 102.38 ± 7.52 (
p < 0.001) compared to 86.15 ± 7.72 in Apigenin alone, and at 25 µM to 102.33 ± 4.97 (
p < 0.001) compared to 93.04 ± 4.09 in Apigenin alone (
Figure 11). Berberine at 10 µM in combination with doxorubicin lowered relative fluorescence units to 84.28 ± 2.79 (
p < 0.05) compared to 92.57 ± 8.65 in Berberine alone. Chrysin at 25 µM combined with doxorubicin increased the amount of relative fluorescence units to 67.99 ± 3.73 (
p < 0.001) compared to 56.29 ± 3.31 in Chrysin alone, and at 50 µM concentration to 84.59 ± 11.98 (
p < 0.01) compared to 72.44 ± 5.33 in Chrysin alone. Luteolin at 12.5 µM combined with doxorubicin increased the amount of relative fluorescence units to 67.59 ± 1.74 (
p < 0.001) compared to 58.97 ± 2.85 in Luteolin alone and at 25 µM to 81.57 ± 6.58 (
p < 0.05) compared to 74.70 ± 3.88 in Luteolin alone (
Figure 11).
Overall, ABCL does not seem to affect doxorubicin’s ability to induce oxidative stress in doxorubicin-sensitive HL-60 cells; they themselves, however, caused a slight increase in endogenous ROS, with one exception being Berberine, which doubled the amount of ROS measured in this cell line (
Figure 10). In doxorubicin-resistant HL-60/DOXO cells, Apigenin caused a rise in ROS only at a 25 µM concentration; at the same time, it enhanced the oxidative stress-inducing properties of doxorubicin at both 12.5 and 25 µM concentrations (
Figure 9). Interestingly, Berberine at 5 µM concentration, when combined with doxorubicin, caused even lower levels of ROS than in the doxorubicin control (
p < 0.05), which even further hints at its code of conduct being NRF2 activation. Chrysin and Luteolin cause an increase in ROS levels when combined with doxorubicin (
p < 0.001) (
Figure 9).
Another noteworthy thing is that ABCLs are all anti-oxidants; therefore, it is fair to assume that the observed results are the effect of two antagonistic properties of these compounds. On one hand, they act as NRF2 inhibitors, hindering cells’ anti-oxidant responses, and on the other, they themselves act as anti-oxidants. Having all that in mind, it is fair to claim that Apigenin, Chrysin, and Luteolin do hinder cells’ anti-oxidant responses. Similar results with the use of Luteolin were obtained by Yang et al., but in their study, it also increased ROS generation in HT29 cells [
24].
Apigenin, Chrysin, and Luteolin exhibited the ability to boost both cytotoxic and DNA-damaging effects of doxorubicin in both doxorubicin-sensitive and doxorubicin-resistant cell lines. Interestingly, while they did not increase in apoptosis levels after doxorubicin treatment in doxorubicin-sensitive cells, they increased in doxorubicin-resistant cells, which may point to the inhibition of NRF2 in this cell line as an underlying mechanism. Their effects on doxorubicin-resistant cells’ DNA damage levels are also interesting; on their own, they generally reduced DNA damage; however, in combination with doxorubicin, their genotoxic properties were greatly increased. Berberine, on the other hand, seems to act more as an NRF2 activator, rather than an inhibitor. While it reduced the percentage of metabolizing cells in combination with doxorubicin in the resazurin assay, after examination of the comet assay and apoptosis assay results, it is much more likely that these deviations are a result of Berberine’s high cytotoxicity towards cancer cells. Apigenin, Chrysin, and Luteolin hinder HL-60 cells’ anti-oxidant responses, and in combination with doxorubicin, they have been shown to significantly enhance its ability to induce oxidative stress, as on its own doxorubicin caused little to no difference. Berberine, when combined with doxorubicin, caused the doxorubicin-resistant cells to strengthen their anti-oxidant response, which even further proves that, in HL-60 cells, Berberine causes NRF2 activation.
Other studies have found that Luteolin inhibits the NRF2 pathway in vivo using C57BL/6 mice, where it greatly reduced the expression of NRF2-controlled genes like
NQO1,
AKR1C,
HO-1, and
GSTm1 [
25]. On top of that, it was shown that Luteolin alone, as well as in combination with cisplatin, in xenograft mice inoculated with human non-small lung cancer A549 cells, greatly reduced the tumor growth; furthermore, analysis of tumors after Luteolin treatment also revealed lowered expression of NRF2-controlled proteins. Lowered GSH levels were also noted after Luteolin treatment [
25]. Another in vivo study, on the other hand, mentions luteolin acting as an NRF2 activator in mice liver, promoting its nuclear translocation, which led to increased expression of
HO-1 and
NQO1 genes [
26]. In another study, it was found that Luteolin sensitized two oxaliplatin-resistant colorectal cancer cell lines, namely HCT116-OX and SW620-OX, both of which have shown lowered protein levels of NRF2 and NQO1 in vitro after Luteolin treatment. Further studies on xenograft mice have shown that treatment with Luteolin caused lowered expression of NQO1, HO-1, and GSTα1/2, as well as lowered levels of GSH. Luteolin also induced higher sensitivity to doxorubicin, cisplatin, and oxaliplatin in those two cell lines [
27]. In yet another study, Luteolin treatment reduced expression of NRF2, HO-1, Cripto-1, and Sirt3 protein levels in MDA-MB-231 breast cancer cells [
28]. Luteolin was also shown to reduce mRNA expression of
HO-1,
NQO1,
GCLC,
MRP2, and
AKR1C1 in an MCF7 breast cancer cell line through increasing KEAP1-independent NRF2 mRNA degradation, as well as reducing GSH levels in A549 cells and sensitizing them to anti-cancer drugs like bleomycin, oxaliplatin, and doxorubicin [
29]. Chrysin was able to sensitize BEL-7402/ADM and MCF/ADM cells to doxorubicin. In BEL-7402/ADM, a decrease in mRNA levels of NRF2 was observed, as well as of its target genes
HO-1,
AK1B10, and
MRP5 [
30]. Berberine was successfully used in surmounting lapatinib resistance in BT-474
LapR and AU-565
LapR breast cancer cells. It has caused lowered viability of the cells and elevated apoptosis induction, as well as increased induction of ROS when combined with lapatinib. This was proven to be the result of NRF2 inhibition through KEAP1-independent ubiquitination of the NRF2 protein [
31].