Circulating and Urinary CCL20 in Human Kidney Disease
Abstract
1. Introduction
2. Results
2.1. Study Population
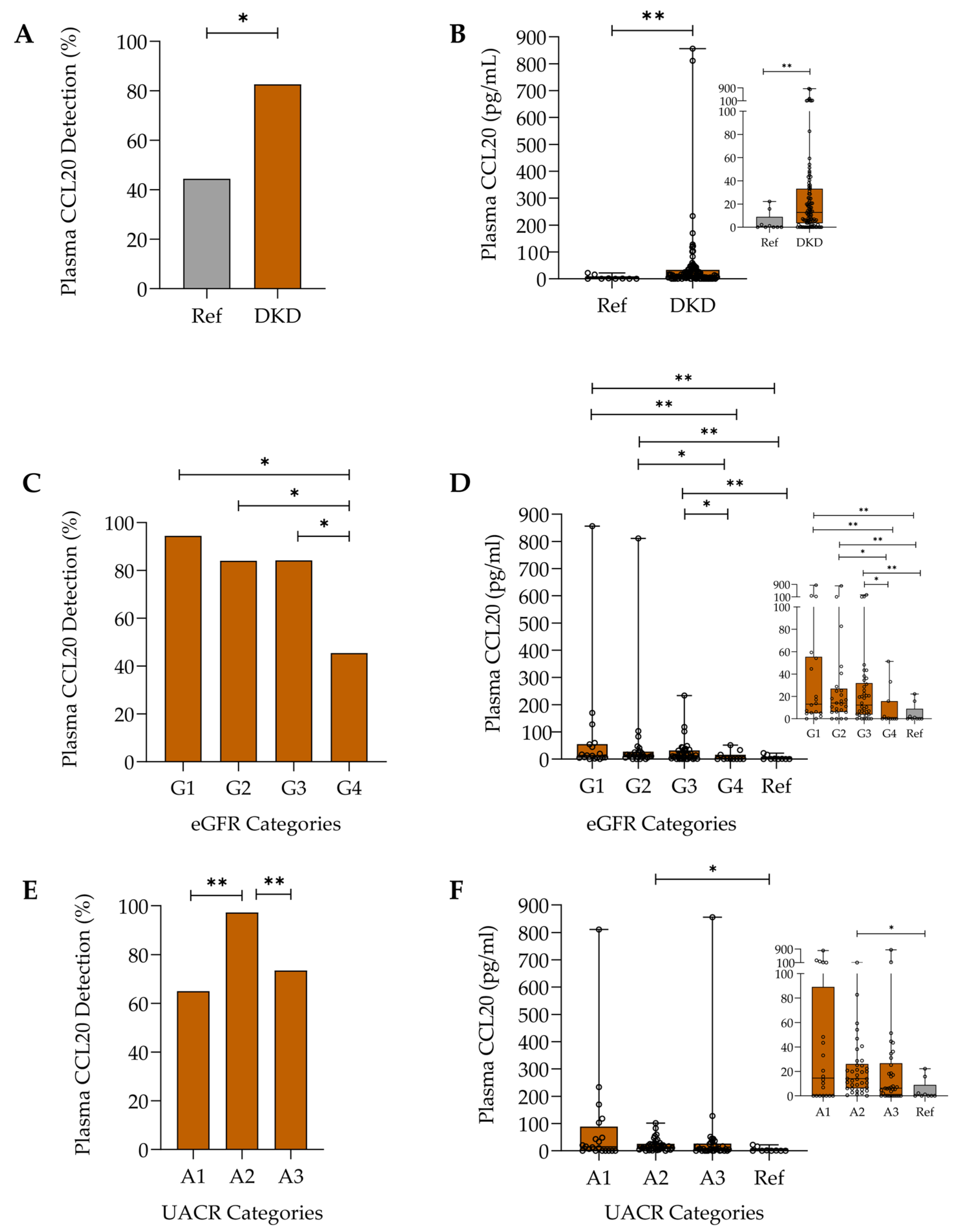
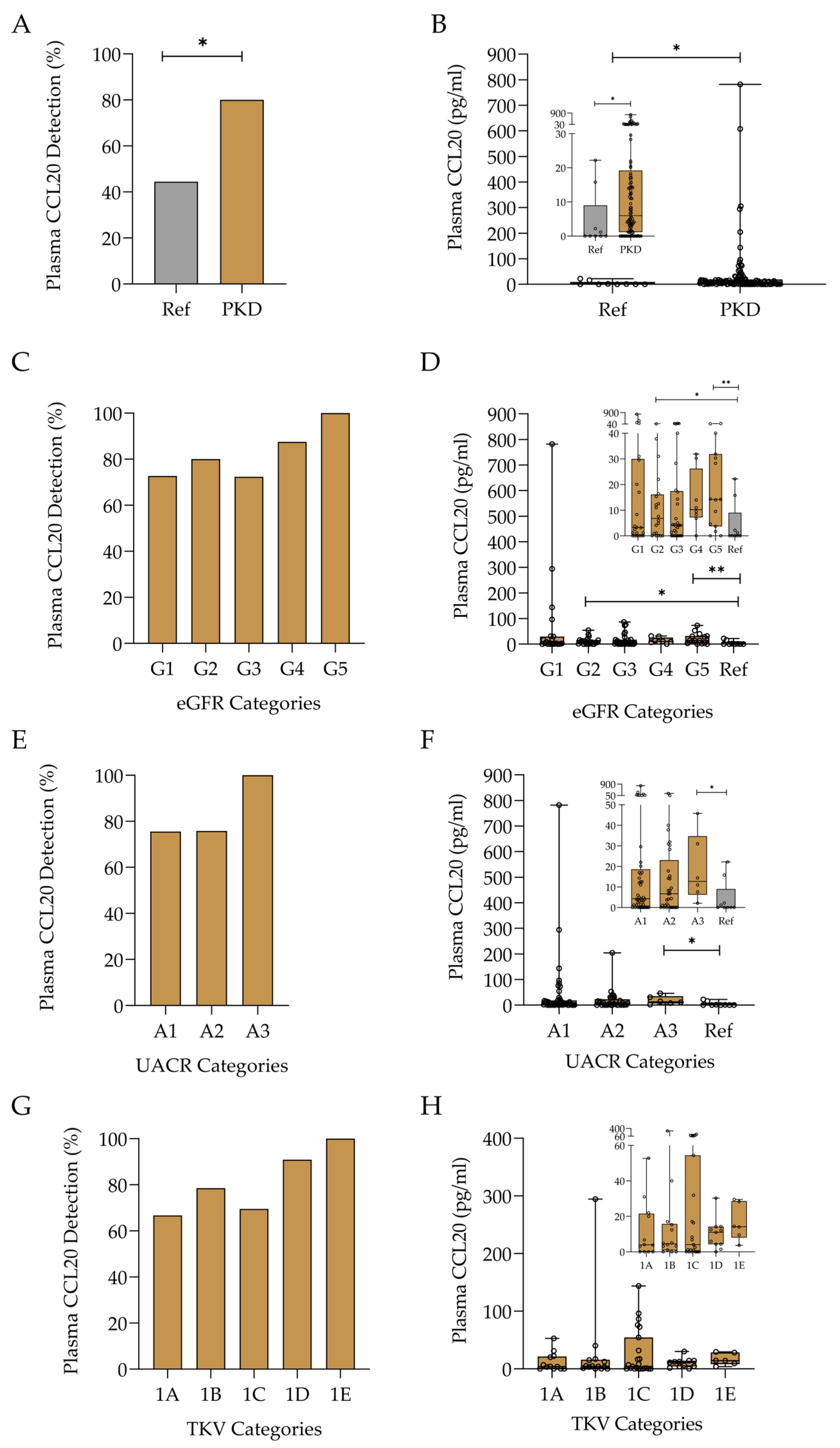
2.2. Plasma CCL20 in Diabetic Kidney Disease
2.2.1. Assessment of CCL20 Levels in Diabetic Kidney Disease and Relationship with Disease Categories
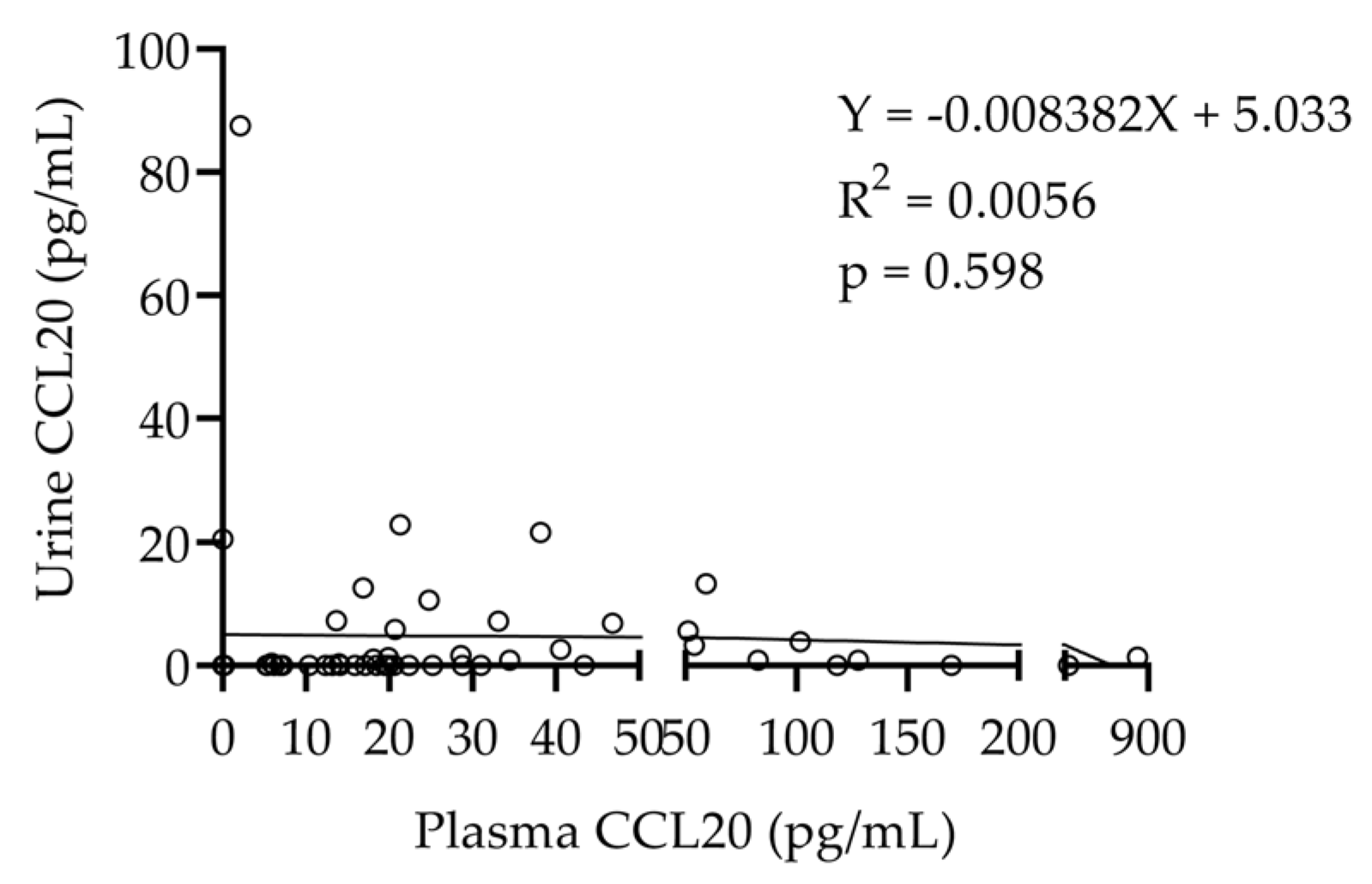
2.2.2. Relationship Between Plasma and Urinary CCL20 Levels
2.3. Plasma CCL20 in Autosomal Dominant Polycystic Kidney Disease
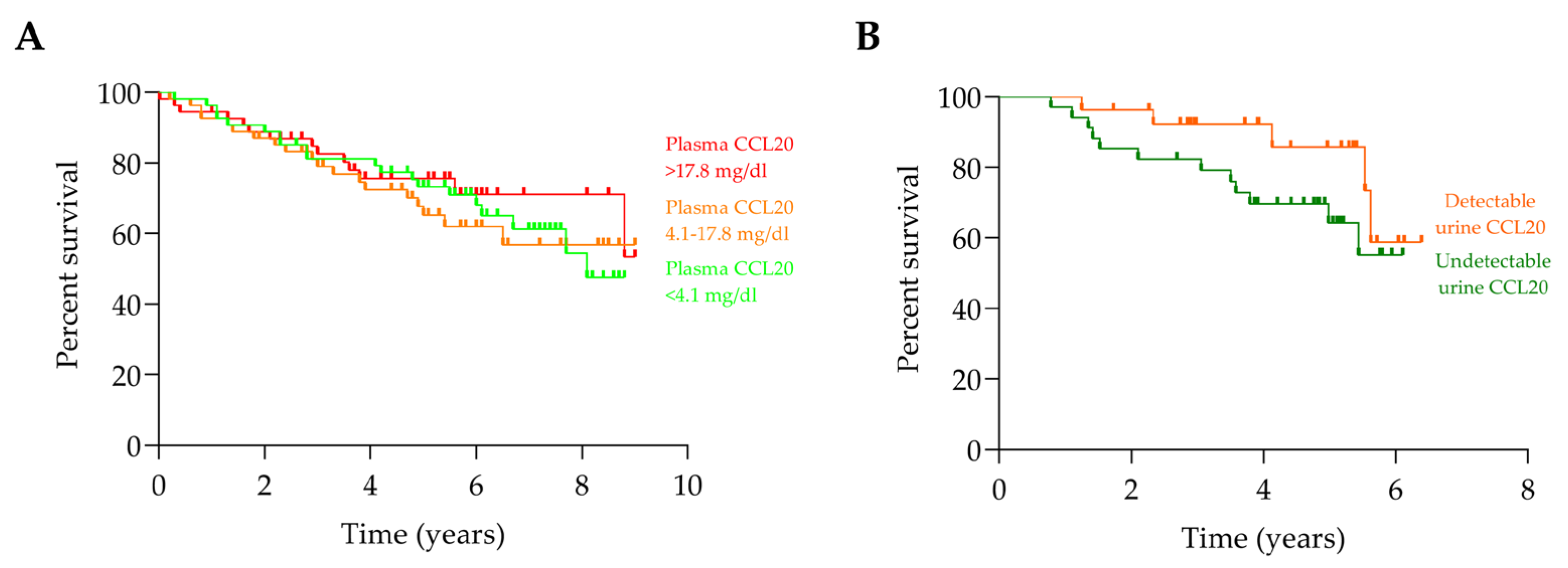
2.4. CCL20 and Progression of Chronic Kidney Disease to Kidney Failure
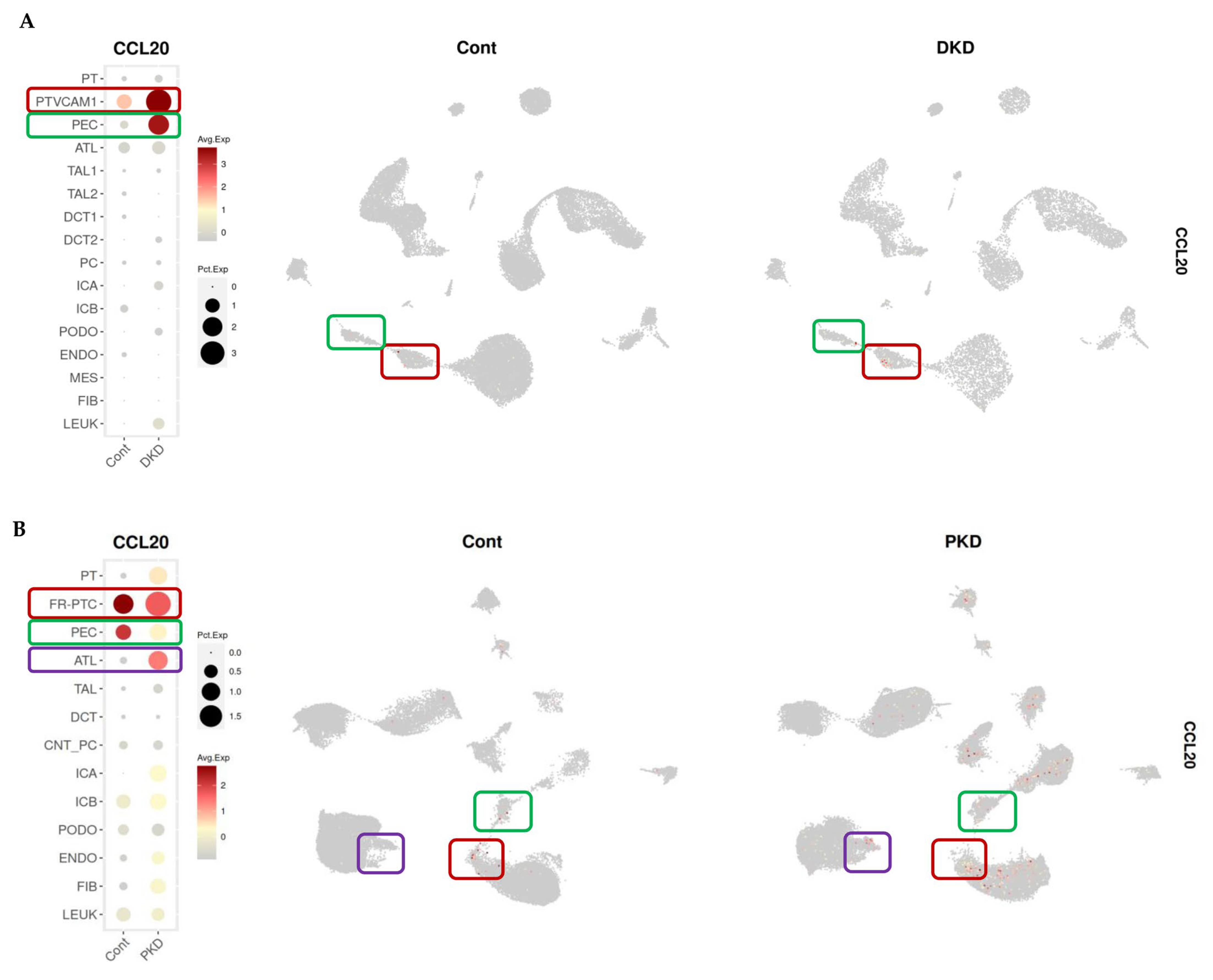
2.5. Identification of Renal CCL20 Sources in Kidney Transcriptomic Databases
3. Discussion
4. Materials and Methods
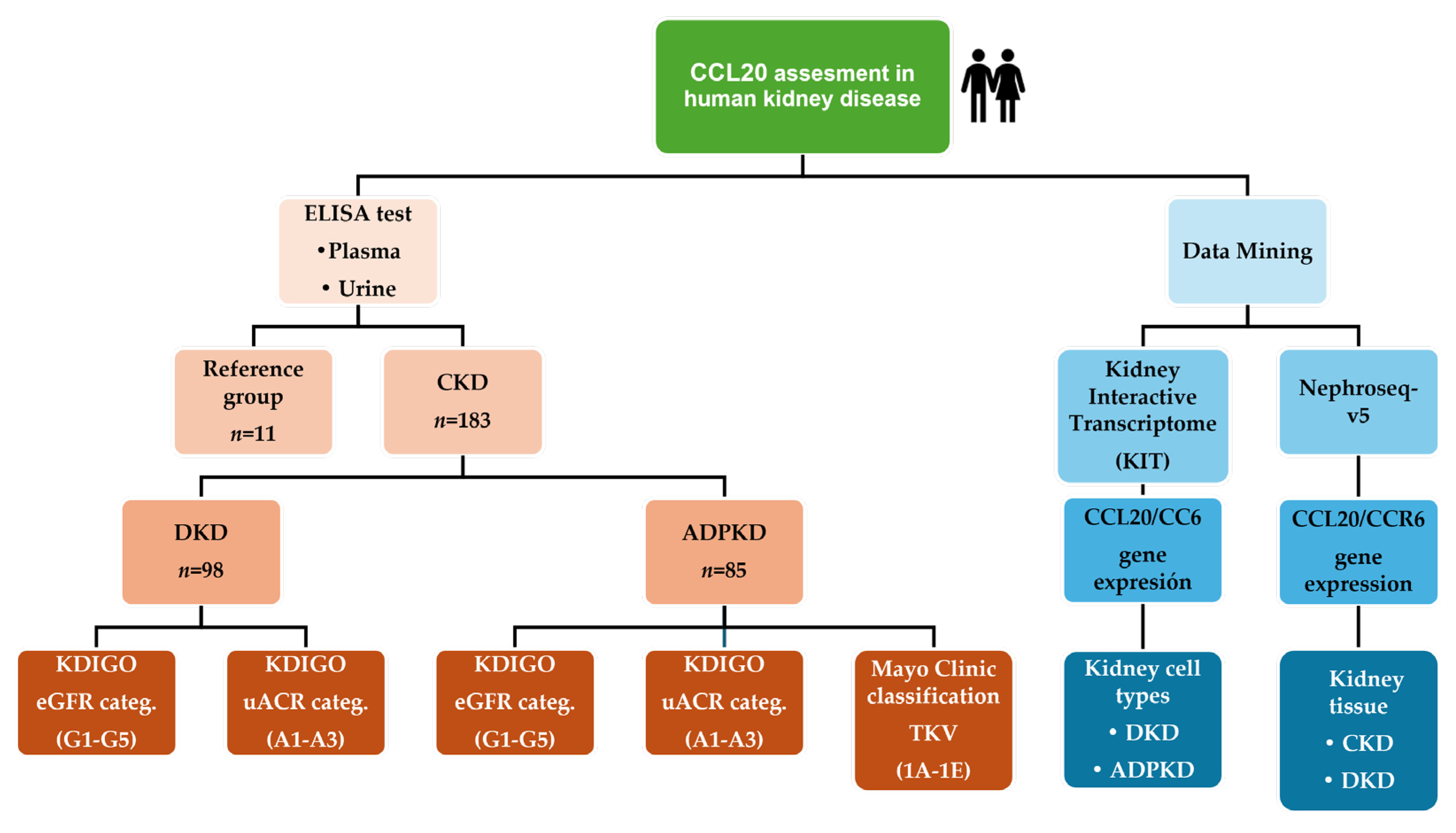
4.1. Study Design and Study Population
4.2. CCL20 Assay
4.3. Data Mining
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| CKM | Cardiovascular–metabolic–kidney spectrum |
| CAKUT | Congenital anomalies of the kidney and urinary tract |
| ADPKD | Autosomal dominant polycystic kidney disease |
| CCL20 | CC motif chemokine ligand 20 |
| CCR6 | CC chemokine receptor type 6 |
| Th17 cells | T helper 17 cells |
| Treg cells | Regulatory T cells |
| AKI | Acute kidney injury |
| DKD | Diabetic kidney disease |
| IQR | Interquartile range |
| eGFR | Estimated glomerular filtration rate |
| UACR | Urine albumin-to-creatinine ratio |
| KRT | Kidney replacement therapy |
| TKV | Total kidney volume |
| Ref | Reference group |
| ELISA | Enzyme-linked immunosorbent assay |
| ns | Not significant |
| HR | Hazard ratio |
| PTVCAM | Proximal tubular cells expressing VCAM |
| PEC | Parietal epithelial cells |
| FR-PTC | Failed-repair proximal tubular cells |
| ATL | Ascending thin limb |
| KIT | Kidney Interactive Transcriptomic |
| snRNA-seq | Single-nucleus RNA sequencing |
| UAMPs | Uniform Manifold Approximation and Projection |
| Cont | Control |
| PT | Proximal tubule |
| TAL1 | Thick ascending limb 1 |
| TAL2 | Thick ascending limb 2 |
| DCT1 | Distal convoluted tubule 1 |
| DCT2 | Distal convoluted tubule 2 |
| PC | Principal cells |
| ICA | Intercalated cells A |
| ICB | Intercalated cells B |
| PODO | Podocytes |
| ENDO | Endothelial cells |
| MES | Mesangial cells |
| FIB | Fibroblasts |
| LEUK | Leukocytes |
| TAL | Thick ascending limb |
| DCT | Distal convoluted tubule |
| CNT_PC | Connecting tubule/Principal cells |
| SGLT2I | Sodium-glucose cotransporter-2-inhibitor |
| Glom | Glomerular |
| TubInt | Tubulointerstitial |
| GLP1Ras | Glucagon-like peptide-1 receptor agonist |
| NF-κB1 | Nuclear Factor kappa-light-chain-enhancer of activated B cells 1 |
| RGC-32 | Response gene to complement-32 |
| α7nAChRs | α7 nicotinic acetylcholine receptors |
| SDH | Succinate Dehydrogenase |
| ACKRs | Atypical chemokine receptors |
| miRNAs | MicroRNAs |
| MRAs | Mineralocorticoid receptor antagonists |
| ADA | American Diabetes Association |
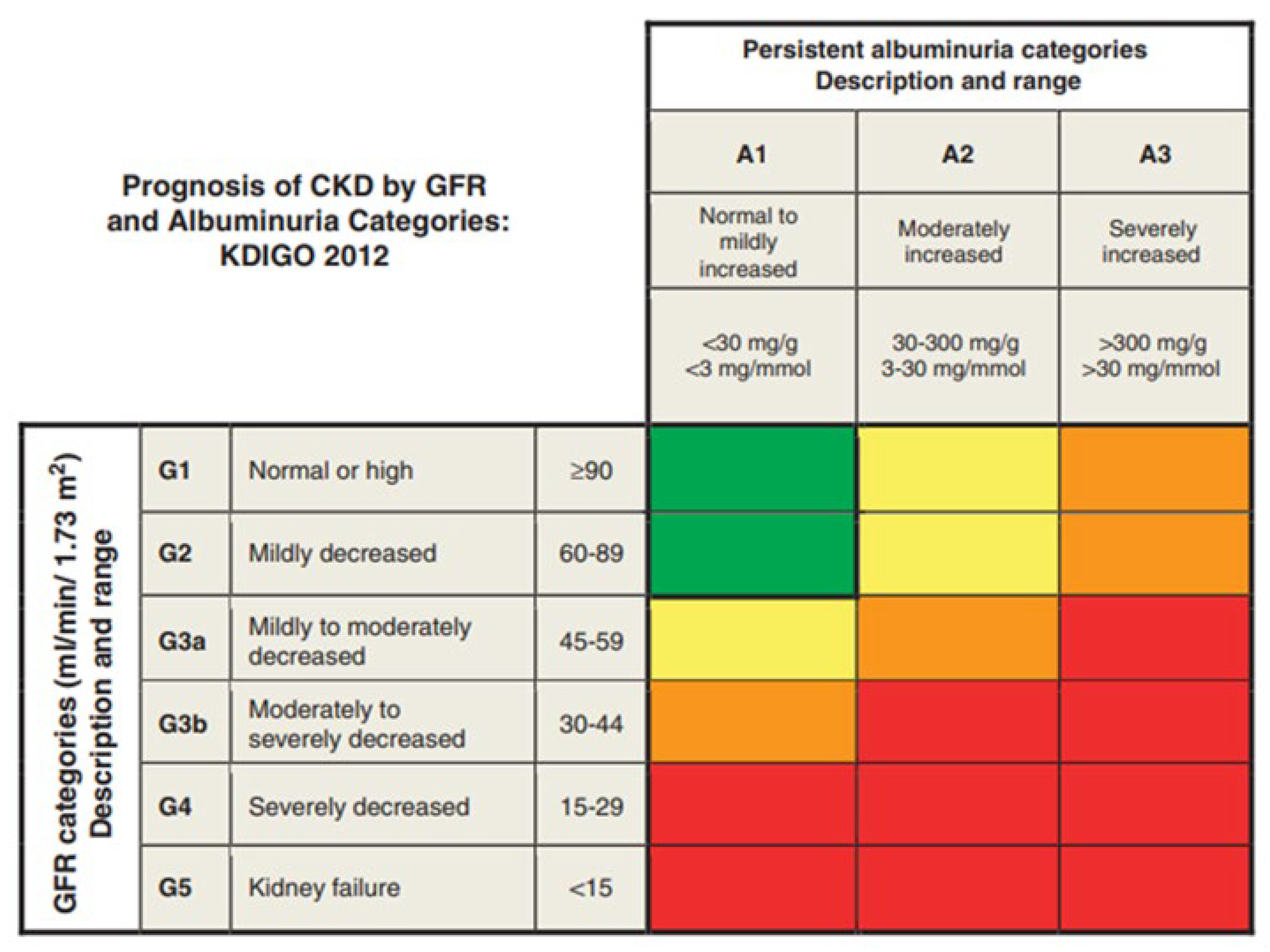
| KDIGO | Kidney Disease: Improving Global Outcomes |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| LOD | Limit of detection |
Appendix A. Supplementary Baseline Characteristics
Appendix A.1. Detailed Characteristics and Outcomes of Patients with Diabetic Kidney Disease
| n (%) | Age (Years), Median (IQR) | Male, n (%) | eGFR (mL/min/1.73 m2), Median (IQR) | UACR (mg/g), Median (IQR) | KRT, n (%) | Death, n (%) | Follow-Up (Years), Median (IQR) | ||
|---|---|---|---|---|---|---|---|---|---|
| Patients | 98 (100) | 69.1 (60.4–76.5) | 75 (76.5) | 57.6 (70.7–83.2) | 159.1 (36.9–437.7) | 5 (5.1) | 21 (21.4) | 4.9 (2.9–5.5) | |
| eGFR categories | G1 | 18 (18.4) | 57.8 (51.7–68.2) | 16 (88.9) | 95.4 (92.23–98.08) | 311.1 (63.4–517.0) | 0 (0) | 2 (11.1) | 4.9 (3.0–5.7) |
| G2 | 26 (26.5) | 67.1 (58.2–71.6) | 19 (73.1) | 75.7 (69.4–83.2) | 154.8 (45.3–313.2) | 0 (0) | 5 (19.2) | 4.2 (2.6–5.4) | |
| G3 | 42 (42.9) | 71.6 (64.7–7.6) | 32 (76.2) | 45.6 (39.9–55.0) | 77.3 (24.6–413.2) | 1 (2.4) | 9 (21.4) | 5.0 (3.6–5.4) | |
| G4 | 11 (11.2) | 76.4 (63.5–84.1) | 7 (63.6) | 25.9 (22.2–28.6) | 312.4 (7.4–823.0) | 4 (36.4) | 4 (36.4) | 4.8 (1.9–5.9) | |
| G5 | 1 (1.0) | 61.7 | 1 (100) | 10.7 | 430.9 | 0(0) | 1 (100) | 4.1 | |
| UACR categories | A1 | 22 (22.5) | 74.6 (64.8–8.1) | 16 (72.7) | 44.7 (32.3–64.4) | 12.7 (6.1–16.85) | 0 (0) | 7 (31.8) | 5.0 (2.2–5.6) |
| A2 | 39 (39.8) | 66.5 (59.9–73.2) | 30 (76.9) | 72.1 (46.8–83.9) | 78.1 (55.5–181.8) | 1 (2.6) | 1 (2.6) | 5.0 (3.3–5.6) | |
| A3 | 37 (37.8) | 68 (60.3–77.4) | 29 (78.4) | 57.0 (35.7–90.0) | 554.7 (371.9–974.2) | 4 (10.8) | 13 (35.1) | 4.2 (2.8–5.4) |
Appendix A.2. Detailed Characteristics and Outcomes of Patients with Autosomal Dominant Polycystic Kidney Disease
| n (%) | Age (Years), Median (IQR) | Male, n (%) | eGFR (mL/min/1.73 m2), Median (IQR) | UACR (mg/g), Median (IQR) | KRT, n (%) | Death, n (%) | Follow-Up (Years), Median (IQR) | ||
|---|---|---|---|---|---|---|---|---|---|
| Patients | 85 (100) | 54.8 (43.2–67.6) | 39 (45.9) | 59.3 (37.5–95.6) | 25.4 (7.1–77.3) | 10 (11.8) | 28 (32.9) | 7.1 (2.9–8.4) | |
| eGFR categories | G1 | 22 (25.9) | 36.6 (32.0–44.5) | 8 (36.4) | 104.3 (98.6–115.4) | 8.3 (3.7–19.1) | 0 (0) | 6 (27.3) | 7.4 (2.2–8.3) |
| G2 | 20 (23.5) | 58.9 (49.9–73.2) | 9 (45) | 70.3 (64.6–82.2) | 20.3 (6.9–62.0) | 0 (0) | 4 (20) | 7.9 (6.4–8.6) | |
| G3 | 29 (34.1) | 65.1 (54.6–73.9) | 13 (44.8) | 44.0 (37.5–57.5) | 27.5 (7.1–94.3) | 1 (3.5) | 14 (48.3) | 7.2 (4.7–8.5) | |
| G4 | 8 (9.4) | 57.3 (44.8–68.0) | 6 (75) | 25.0 (22.3–26.8) | 80.3 (51.4–163.6) | 5 (62.5) | 3 (37.5) | 2.9 (1.5–3.1) | |
| G5 | 6 (7.1) | 50.6 (43.0–69.5) | 3 (50) | 11.0 (8.3–12.3) | 193.2 (68.1–269.5) | 4 (66.7) | 1 (16.7) | 1.5 (0.3–3.9) | |
| UACR categories | A1 | 45 (52.9) | 51.9 (37.4–63.8) | 19 (42.2) | 81.2 (58.0–103.1) | 7.5 (3.3–14.7) | 0 (0) | 9 (20) | 7.7 (6.1–8.5) |
| A2 | 33 (38.8) | 60.4 (45.9–72.0) | 13 (39.4) | 38.0 (25.0–69.1) | 69.0 (51.3–139.5) | 8 (24.2) | 14 (42.4) | 4.7 (2.6–8.0) | |
| A3 | 6 (7.1) | 63.2 (55.4–77.4) | 6 (100) | 43.5 (23.3–64.1) | 713.5 (320.0–958.3) | 1 (10) | 5 (90) | 1.8 (0.3–4.4) | |
| TKV categories | 1A | 12 (14.1) | 56.3 (38.6–66.7) | 0 (0) | 74.3 (42.0–105.3) | 6.0 (2.2–58.6) | 1 (8.3) | 3 (25) | 6.5 (2.3–7.5) |
| 1B | 14 (16.5) | 66.1 (49.0–73.9) | 8 (57.1) | 77.8 (42.0–102.3) | 12.1 (6.2–39.2) | 0 (0) | 5 (35.7) | 7.4 (4.9–8.4) | |
| 1C | 23 (27.1) | 55.2 (43.7–66.4) | 13 (56.5) | 59.3 (33.0–88.4) | 21,3 (6.3–57.9) | 2 (8.7) | 5 (21.7) | 7.6 (3.0–8.5) | |
| 1D | 11 (12.9) | 53.4 (41.1–57.5) | 4 (36.4) | 52.0 (27.0–83.1) | 10.0 (6.4–69.0) | 2 (18.2) | 2 (18.2) | 7.6 (3.11–8.3) | |
| 1E | 6 (7.1) | 43.7 (38.3–48.0) | 5 (90) | 27.5 (19.5–49.3) | 108.2 (37.5–199.6) | 4 (66.7) | 2 (33.3) | 2.5 (1.5–3.9) |
Appendix B. Extended Information on the Study Design and Study Population Section
References
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635, Erratum in Circulation 2024, 149, 1023. [Google Scholar] [CrossRef]
- Ortiz, A.; Kramer, A.; Ariceta, G.; Rodríguez Arévalo, O.L.; Gjerstad, A.C.; Santiuste, C.; Trujillo-Alemán, S.; Ferraro, P.M.; Methven, S.; Santamaría, R.; et al. Inherited kidney disease and CAKUT are common causes of kidney failure requiring kidney replacement therapy: An ERA Registry study. Nephrol. Dial. Transplant. 2025, 40, 1020–1031. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) ADPKD Work Group. KDIGO 2025 Clinical Practice Guideline for the Evaluation, Management, and Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Kidney Int. 2025, 107, S1–S239. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Li, X.; Ao, X.; Zhong, Y.; Tang, R.; Peng, W.; Zhang, Y.; Liu, J. Hemolytic Streptococcus May Exacerbate Kidney Damage in IgA Nephropathy through CCL20 Response to the Effect of Th17 Cells. PLoS ONE 2014, 9, e108723. [Google Scholar] [CrossRef]
- Welsh-Bacic, D.; Lindenmeyer, M.; Cohen, C.D.; Draganovici, D.; Mandelbaum, J.; Edenhofer, I.; Ziegler, U.; Regele, H.; Wüthrich, R.P.; Segerer, S. Expression of the chemokine receptor CCR6 in human renal inflammation. Nephrol. Dial. Transplant. 2011, 26, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Armas-González, E.; Domínguez-Luis, M.J.; Díaz-Martín, A.; Arce-Franco, M.T.; Castro-Hernández, J.; Danelon, G.; Hernández-Hernández, V.; Bustabad-Reyes, S.; Cantabrana, A.; Uguccioni, M.; et al. Role of CXCL13 and CCL20 in the recruitment of B cells to inflammatory foci in chronic arthritis. Arthritis Res. Ther. 2018, 20, 114. [Google Scholar] [CrossRef]
- Woltman, A.M.; de Fijter, J.W.; van der Kooij, S.W.; Jie, K.E.; Massacrier, C.; Caux, C.; Daha, M.R.; Van Kooten, C. MIP-3alpha/CCL20 in renal transplantation and its possible involvement as dendritic cell chemoattractant in allograft rejection. Am. J. Trasplant. 2005, 5, 2114–2125. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Overcoming barriers to implementing new guideline-directed therapies for chronic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 532–540. [Google Scholar] [CrossRef]
- Comerford, I.; Bunting, M.; Fenix, K.; Haylock-Jacobs, S.; Litchfield, W.; Harata-Lee, Y.; Turvey, M.; Brazzatti, J.; Gregor, C.; Nguyen, P.; et al. An immune paradox: How can the same chemokine axis regulate both immune tolerance and activation? BioEssays 2010, 32, 1067–1076. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, X.; Shen, L.; Qiao, Q.; Li, Y.; Sun, J.; Zhang, J. CCL20 secreted from IgA1-stimulated human mesangial cells recruits inflammatory Th17 cells in IgA nephropathy. PLoS ONE 2017, 12, e0178352. [Google Scholar] [CrossRef]
- Chan, A.J.; Alikhan, M.A.; Odobasic, D.; Gan, P.Y.; Khouri, M.B.; Steinmetz, O.M.; Mansell, A.S.; Kitching, A.R.; Holdsworth, S.R.; Summers, S.A. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am. J. Pathol. 2014, 184, 1411–1418. [Google Scholar] [CrossRef]
- Turner, J.E.; Paust, H.J.; Steinmetz, O.M.; Peters, A.; Riedel, J.H.; Erhardt, A.; Wegscheid, C.; Velden, J.; Fehr, S.; Mittrücker, H.W.; et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 974–985. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, C.; Morgado-Pascual, J.L.; Cannata-Ortiz, P.; Ramos-Barron, M.A.; Gómez-Alamillo, C.; Arias, M.; Mezzano, S.; Egido, J.; Ruiz-Ortega, M.; Ortiz, A.; et al. CCL20 blockade increases the severity of nephrotoxic folic acid-induced acute kidney injury. J. Pathol. 2018, 246, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.D.; Yu, M.Y.; Kim, K.H.; Lee, S.; Park, E.; Kang, S.; Lim, D.H.; Lee, Y.; Song, J.; Kown, S.; et al. Role of the CCL20/CCR6 axis in tubular epithelial cell injury: Kidney-specific translational insights from acute kidney injury to chronic kidney disease. FASEB J. 2024, 38, e23407. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Karlstad, M.D.; Regal, K.M.; Collier, J.J. CCL20 is elevated during obesity and differentially regulated by NF-κB subunits in pancreatic β-cells. Biochim. Biophys. Acta 2015, 1849, 637–652. [Google Scholar] [CrossRef]
- Lee, S.M.; Yang, H.; Tartar, D.M.; Liu, C.; Song, J.; Ma, X.; Wang, C.; Serreze, D.V.; Zaghouani, H. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia 2011, 54, 1136–1146. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, J.; Zhao, Q.; Jiang, Y.; Liu, Y.N.; Liu, W.J. From diabetic nephropathy to end-stage renal disease: The effect of chemokines on the immune system. J. Diabetes Res. 2023, 2023, 3931043. [Google Scholar] [CrossRef]
- Humphreys Lab. Kidney Interactive Transcriptomics (KIT) Platform: Visualization of Single-Cell/Nucleus RNA-seq Data from Human and Mouse Kidneys. 2025. Available online: https://humphreyslab.com/SingleCell/ (accessed on 29 August 2025).
- Wilson, P.C.; Muto, Y.; Wu, H.; Karihaloo, A.; Waikar, S.S.; Humphreys, B.D. Multimodal single cell sequencing implicates chromatin accessibility and genetic background in diabetic kidney disease progression. Nat. Commun. 2022, 13, 5253. [Google Scholar] [CrossRef]
- Muto, Y.; Dixon, E.E.; Yoshimura, Y.; Wu, H.; Omachi, K.; Ledru, N.; Wilson, P.C.; King, A.J.; Eric Olson, N.; Gunawan, M.G.; et al. Defining cellular complexity in human autosomal dominant polycystic kidney disease by multimodal single cell analysis. Nat. Commun. 2022, 13, 6497. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Levinsohn, J.; Klötzer, K.A.; Dumoulin, B.; Ma, Z.; Frederick, J.; Dhillon, P.; Balzer, M.S.; Shrestha, R.; Liu, H.; et al. Single-cell multi-omic and spatial profiling of human kidneys implicates the fibrotic microenvironment in kidney disease progression. Nat. Genet. 2024, 56, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Nephroseq Research Edition, Developed by the University of Michigan. Nephroseq Web Platform. 2025. Available online: https://www.nephroseq.org (accessed on 29 August 2025).
- Nakagawa, S.; Nishihara, K.; Miyata, H.; Shinke, H.; Tomita, E.; Kajiwara, M.; Matsubara, T.; Iehara, N.; Igarashi, Y.; Yamada, H.; et al. Molecular Markers of Tubulointerstitial Fibrosis and Tubular Cell Damage in Patients with Chronic Kidney Disease. PLoS ONE 2015, 10, e0136994. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Park, A.S.D.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome analysis of human diabetic kidney disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef]
- Ju, W.; Greene, C.S.; Eichinger, F.; Nair, V.; Hodgin, J.B.; Bitzer, M.; Lee, Y.S.; Zhu, Q.; Kehata, M.; Li, M.; et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. 2013, 23, 1862–1873. [Google Scholar] [CrossRef]
- Schmid, H.; Boucherot, A.; Yasuda, Y.; Henger, A.; Brunner, B.; Eichinger, F.; Nitsche, A.; Kiss, E.; Bleich, M.; Gröne, H.J.; et al. Modular activation of nuclear factor-κB transcriptional programs in human diabetic nephropathy. Diabetes 2006, 55, 2993–3003. [Google Scholar] [CrossRef]
- Palomares, S.M.; Ferrara, G.; Sguanci, M.; Gazineo, D.; Godino, L.; Palmisano, A.; Paderno, A.; Vrenna, G.; Faraglia, E.; Petrelli, F.; et al. The impact of artificial intelligence technologies on nutritional care in patients with chronic kidney disease: A systematic review. J. Ren. Nutr. 2025; in press. [Google Scholar] [CrossRef]
- Gaweda, A.E.; Lederer, E.D.; Brier, M.E. Artificial Intelligence-Guided Precision Treatment of Chronic Kidney Disease–Mineral Bone Disorder. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1305–1315. [Google Scholar] [CrossRef]
- Ortiz, A.; Yanagita, M.; Yokoi, H.; Torra, R. Evolving Strategies for Early Diagnosis, Proactive Prevention and Treatment of CKD. Nephrol. Dial. Transplant. 2025, gfaf151. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Bozkurt, B. Pre-Heart Failure, Heart Stress, and Subclinical Heart Failure: Bridging Heart Health and Heart Failure. JACC Heart Fail. 2024, 12, 1115–1118. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 3. Prevention or Delay of Diabetes and Associated Comorbidities: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Arreola Guerra, J.M.; Chan, J.C.N.; Jha, V.; Kramer, H.; Nicholas, S.B.; Pavkov, M.E.; Wanner, C.; Wong, L.P.; Cheung, M.; et al. Preventing Chronic Kidney Disease and Maintaining Kidney Health: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2025, 108, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Wanner, C.; Luyckx, V.; Fontana, M.; Gallego, D.; Vanholder, R.; Torra, R.; Ortiz, A. A Call for Urgent Action on Chronic Kidney Disease across Europe. Lancet Reg. Health Eur. 2025, 54, 101347. [Google Scholar] [CrossRef] [PubMed]
- Abasheva, D.; Ortiz, A.; Fernandez-Fernandez, B. GLP-1 Receptor Agonists in Patients with Chronic Kidney Disease and Either Overweight or Obesity. Clin. Kidney J. 2024, 17 (Suppl. 2), 19–35. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Ortiz, A.; Lucia, A.; Miranda, B.; Alvarez-Llamas, G.; Barderas, M.G.; Volpe, M.; Ruiz-Hurtado, G.; Pitt, B. Prevention of Cardiorenal Damage: Importance of Albuminuria. Eur. Heart J. 2023, 44, 1112–1123. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Ruiz-Hurtado, G.; Miranda, B.; Ortiz, A. Use of Chronic Kidney Disease Blind Spot to Prevent Cardiorenal Outcomes. Eur. Heart J. 2022, 43, 257–260. [Google Scholar] [CrossRef]
- Latosinska, A.; Mina, I.K.; Nguyen, T.M.N.; Golovko, I.; Keller, F.; Mayer, G.; Rossing, P.; Staessen, J.A.; Delles, C.; Beige, J.; et al. In Silico Prediction of Optimal Multifactorial Intervention in Chronic Kidney Disease. J. Transl. Med. 2025, 23, 943. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, B.; Hasegawa, T.; Saruta, Y.; Li Guan, Y.; Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Sanchez-Rodriguez, J.; Navarro-Gonzalez, J.F.; Ortiz, A. Plasma IL-22 Predicts Progression of Early Diabetic Kidney Disease. Am. J. Nephrol. 2025; submitted. [Google Scholar] [CrossRef]
- Astley, M.E.; Chesnaye, N.C.; Gambaro, G.; Ortiz, A.; Hallan, S.; Carrero, J.J.; Ebert, N.; Eriksen, B.O.; Faucon, A.L.; Ferraro, P.M.; et al. Prevalence of Reduced eGFR in European Adults Using KDIGO and Age-Adapted eGFR Thresholds. Nephrol. Dial. Transplant. 2025; submitted. [Google Scholar] [CrossRef]
- Astley, M.E.; Chesnaye, N.C.; Hallan, S.; Gambaro, G.; Ortiz, A.; Carrero, J.J.; Ebert, N.; Eriksen, B.O.; Faucon, A.L.; Ferraro, P.M.; et al. Age- and Sex-Specific Reference Values of Estimated Glomerular Filtration Rate for European Adults. Kidney Int. 2025, 107, 1076–1087. [Google Scholar] [CrossRef]
- Rojas-Rivera, J.E.; Hasegawa, T.; Fernandez-Juarez, G.; Praga, M.; Saruta, Y.; Fernandez-Fernandez, B.; Ortiz, A. Prognostic and Therapeutic Monitoring Value of Plasma and Urinary Cytokine Profile in Primary Membranous Nephropathy: The STARMEN Trial Cohort. Clin. Kidney J. 2024, 17, sfae239. [Google Scholar] [CrossRef]
- Wu, P.H.; Glerup, R.I.; Svensson, M.H.S.; Eriksson, N.; Christensen, J.H.; Laval, P.D.; Soveri, I.; Westerlund, M.; Linde, T.; Ljunggren, Ö.; et al. Novel Biomarkers Detected by Proteomics Predict Death and Cardiovascular Events in Hemodialysis Patients. Biomedicines 2022, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Feldreich, T.; Nowak, C.; Carlsson, A.C.; Östgren, C.J.; Nyström, F.H.; Sundström, J.; Carrero-Roig, J.J.; Leppert, J.; Hedberg, P.; Giedraitis, V.; et al. The Association between Plasma Proteomics and Incident Cardiovascular Disease Identifies MMP-12 as a Promising Cardiovascular Risk Marker in Patients with Chronic Kidney Disease. Atherosclerosis 2020, 307, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 Axis in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 5186. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated Cell Death Pathways in Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef]
- Fernández-Fernandez, B.; Sarafidis, P.; Soler, M.J.; Ortiz, A. EMPA-KIDNEY: Expanding the Range of Kidney Protection by SGLT2 Inhibitors. Clin. Kidney J. 2023, 16, 1187–1198. [Google Scholar] [CrossRef]
- EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Agrawal, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; et al. Long-Term Effects of Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2025, 392, 777–787. [Google Scholar] [CrossRef]
- Sobbe, I.V.; Krieg, N.; Dennhardt, S.; Coldewey, S.M. Involvement of NF-κB1 and the Non-Canonical NF-κB Signaling Pathway in the Pathogenesis of Acute Kidney Injury in Shiga-toxin-2-Induced Hemolytic-Uremic Syndrome in Mice. Shock 2021, 56, 573–581. [Google Scholar] [CrossRef]
- Hu, X.; Feng, J.; Deng, S.; Tang, J.; Liao, Z.; Luo, L.; Luo, L.; Meng, T.; Gong, G.; Li, X. Anaphylatoxins Enhance Th9 Cell Recruitment via the CCL20-CCR6 Axis in IgA Nephropathy. J. Nephrol. 2020, 33, 1027–1036. [Google Scholar] [CrossRef]
- Tatomir, A.; Vlaicu, S.; Nguyen, V.; Luzina, I.G.; Atamas, S.P.; Drachenberg, C.; Papadimitriou, J.; Badea, T.C.; Rus, H.G.; Rus, V. RGC-32 Mediates Proinflammatory and Profibrotic Pathways in Immune-Mediated Kidney Disease. Clin. Immunol. 2024, 265, 110279. [Google Scholar] [CrossRef]
- Yang, A.; Wu, C.H.; Matsuo, S.; Umene, R.; Nakamura, Y.; Inoue, T. Activation of the α7nAChR by GTS-21 Mitigates Septic Tubular Cell Injury and Modulates Macrophage Infiltration. Int. Immunopharmacol. 2024, 138, 112555. [Google Scholar] [CrossRef]
- Carlström, M.; Rannier Ribeiro Antonino Carvalho, L.; Guimaraes, D.; Boeder, A.; Schiffer, T.A. Dimethyl Malonate Preserves Renal and Mitochondrial Functions Following Ischemia-Reperfusion via Inhibition of Succinate Dehydrogenase. Redox Biol. 2024, 69, 102984. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, P.; Ning, L.; Huang, X.; Sun, H.; Wang, Y.; Zhao, Y.; Zeng, C.; Huang, D.; Gao, H.; et al. Inhibition of Mitochondrial Succinate Dehydrogenase with Dimethyl Malonate Promotes M2 Macrophage Polarization by Enhancing STAT6 Activation. Inflammation 2025, 48, 2516–2530. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Z.; Liu, X.; Wang, L.; Zhou, Y.; Huang, J.; Liu, Z.; Lin, D.; Liu, L. GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro. Metabolites 2023, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Melgrati, S.; Radice, E.; Ameti, R.; Hub, E.; Thelen, S.; Pelczar, P.; Jarrossay, D.; Rot, A.; Thelen, M. Atlas of the Anatomical Localization of Atypical Chemokine Receptors in Healthy Mice. PLoS Biol. 2023, 21, e3002111. [Google Scholar] [CrossRef]
- Han, L.; Zou, Y.; Yu, C. Targeting CC Chemokine Ligand (CCL) 20 by miR-143-5p Alleviates Lead Poisoning-Induced Renal Fibrosis by Regulating Interstitial Fibroblasts Excessive Proliferation and Dysfunction. Bioengineered 2022, 13, 11156–11168. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- KDIGO CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2013-Lipids-Guideline-English.pdf (accessed on 26 October 2025).
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015, 26, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Elewa, U.; Fernández-Fernández, B.; Mahillo-Fernández, I.; Martin-Cleary, C.; Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. PCSK9 in diabetic kidney disease. Eur. J. Clin. Investig. 2016, 46, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Elewa, U.; Sanchez-Niño, M.D.; Mahillo-Fernández, I.; Martin-Cleary, C.; Belen Sanz, A.; Perez-Gomez, M.V.; Fernandez-Fernandez, B.; Ortiz, A. Circulating CXCL16 in diabetic kidney disease. Kidney Blood Press. Res. 2016, 41, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, B.; Mahillo, I.; Sánchez-Rodríguez, J.; Carriazo, S.; Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. Gender, albuminuria and chronic kidney disease progression in treated diabetic kidney disease. J. Clin. Med. 2020, 9, 1611. [Google Scholar] [CrossRef]
- Ars, E.; Bernis, C.; Fraga, G.; Martínez, V.; Martins, J.; Ortiz, A.; Rodríguez-Pérez, J.C.; Sans, L.; Torra, R.; Spanish Working Group on Inherited Kidney Disease. Spanish guidelines for the management of autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2014, 29 (Suppl. 4), iv95–iv105. [Google Scholar] [CrossRef]
- Ars, E.; Bernis, C.; Fraga, G.; Furlano, M.; Martínez, V.; Martins, J.; Ortiz, A.; Pérez-Gómez, M.V.; Rodríguez-Pérez, J.C.; Sans, L.; et al. Consensus document on autosomal dominant polycystic kidney disease from the Spanish Working Group on Inherited Kidney Diseases. Nefrología 2022, 42, 367–389. [Google Scholar] [CrossRef]
- Wu, H.; Malone, A.F.; Donnelly, E.L.; Kirita, Y.; Uchimura, K.; Ramakrishnan, S.M.; Gaut, J.P.; Humphreys, B.D. Single-Cell Transcriptomics of a Human Kidney Allograft Biopsy Specimen Defines a Diverse Inflammatory Response. J. Am. Soc. Nephrol. 2018, 29, 2069–2080. [Google Scholar] [CrossRef]


| Age (Years) | Male, n (%) | eGFR (mL/min/1.73 m2) | UACR (mg/g) | TKV (mL) | KRT at Baseline n (%) | KRT, n (%) | Death, n (%) | Follow-Up Time (Years) | |
|---|---|---|---|---|---|---|---|---|---|
| DKD n = 98 | 69.1 (60.4–76.4) | 75 (76.5) | 57.6 (70.7–83.2) | 159.1 (36.9–437.7) | NA | 0(0) | 5 (5.1) | 21 (21.4) | 4.9 (2.9–5.5) |
| ADPKD n = 85 | 54.75 (43.2–67.6) | 39 (45.9) | 59.26 (37.5–95.6) | 25.35 (7.1–77.3) | 1526 (667.8–2586) | 10 (11.8) | 10 (11.8) | 28 (32.9) | 7.1 (2.9–8.4) |
| Gene | Dataset | Disease | n | Fold Change | p-Value | References |
|---|---|---|---|---|---|---|
| CCL20 | Nakagawa CKD Kidney | CKD vs. Normal Kidney (Discovery set) | 53 | 3.9 | 1.27 × 10−7 | 25 |
| Nakagawa CKD Kidney | CKD vs. Normal Kidney (Validation set) | 8 | 12.4 | 0.001 | 25 | |
| Woroniecka DKD TubInt | DKD vs. Healthy Living Donor | 22 | 2.1 | 0.006 | 26 | |
| Ju CKD Glom | DKD vs. Healthy Living Donor | 33 | 2.1 | 0.008 | 27 | |
| Schmid DKD TubInt | Nephrotic vs. Subnephrotic (DKD) | 11 | 1.6 | 0.01 | 28 | |
| CCR6 | Nakagawa CKD Kidney | CKD vs. Normal Kidney (Discovery set) | 53 | 2.2 | 0.001 | 25 |
| Nakagawa CKD Kidney | CKD vs. Normal Kidney (Validation set) | 8 | 5.1 | 0.012 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Cazallas, N.; García-Ayuso, D.; Fernández-Fernández, B.; Rodríguez-Osorio, L.; Sanchez-Rodriguez, J.; Pérez-Gómez, M.V.; Ortiz, A.; Ramos, A.M. Circulating and Urinary CCL20 in Human Kidney Disease. Int. J. Mol. Sci. 2025, 26, 10563. https://doi.org/10.3390/ijms262110563
Molina-Cazallas N, García-Ayuso D, Fernández-Fernández B, Rodríguez-Osorio L, Sanchez-Rodriguez J, Pérez-Gómez MV, Ortiz A, Ramos AM. Circulating and Urinary CCL20 in Human Kidney Disease. International Journal of Molecular Sciences. 2025; 26(21):10563. https://doi.org/10.3390/ijms262110563
Chicago/Turabian StyleMolina-Cazallas, Noelia, Diego García-Ayuso, Beatriz Fernández-Fernández, Laura Rodríguez-Osorio, Jinny Sanchez-Rodriguez, María Vanessa Pérez-Gómez, Alberto Ortiz, and Adrián M. Ramos. 2025. "Circulating and Urinary CCL20 in Human Kidney Disease" International Journal of Molecular Sciences 26, no. 21: 10563. https://doi.org/10.3390/ijms262110563
APA StyleMolina-Cazallas, N., García-Ayuso, D., Fernández-Fernández, B., Rodríguez-Osorio, L., Sanchez-Rodriguez, J., Pérez-Gómez, M. V., Ortiz, A., & Ramos, A. M. (2025). Circulating and Urinary CCL20 in Human Kidney Disease. International Journal of Molecular Sciences, 26(21), 10563. https://doi.org/10.3390/ijms262110563






