Human Stem Cell-Derived Extracellular Vesicles: A Pioneering Path from Biogenesis to Cerebral Ischemic Stroke Therapy
Abstract
1. Introduction
2. EV Biogenesis and Bioengineering
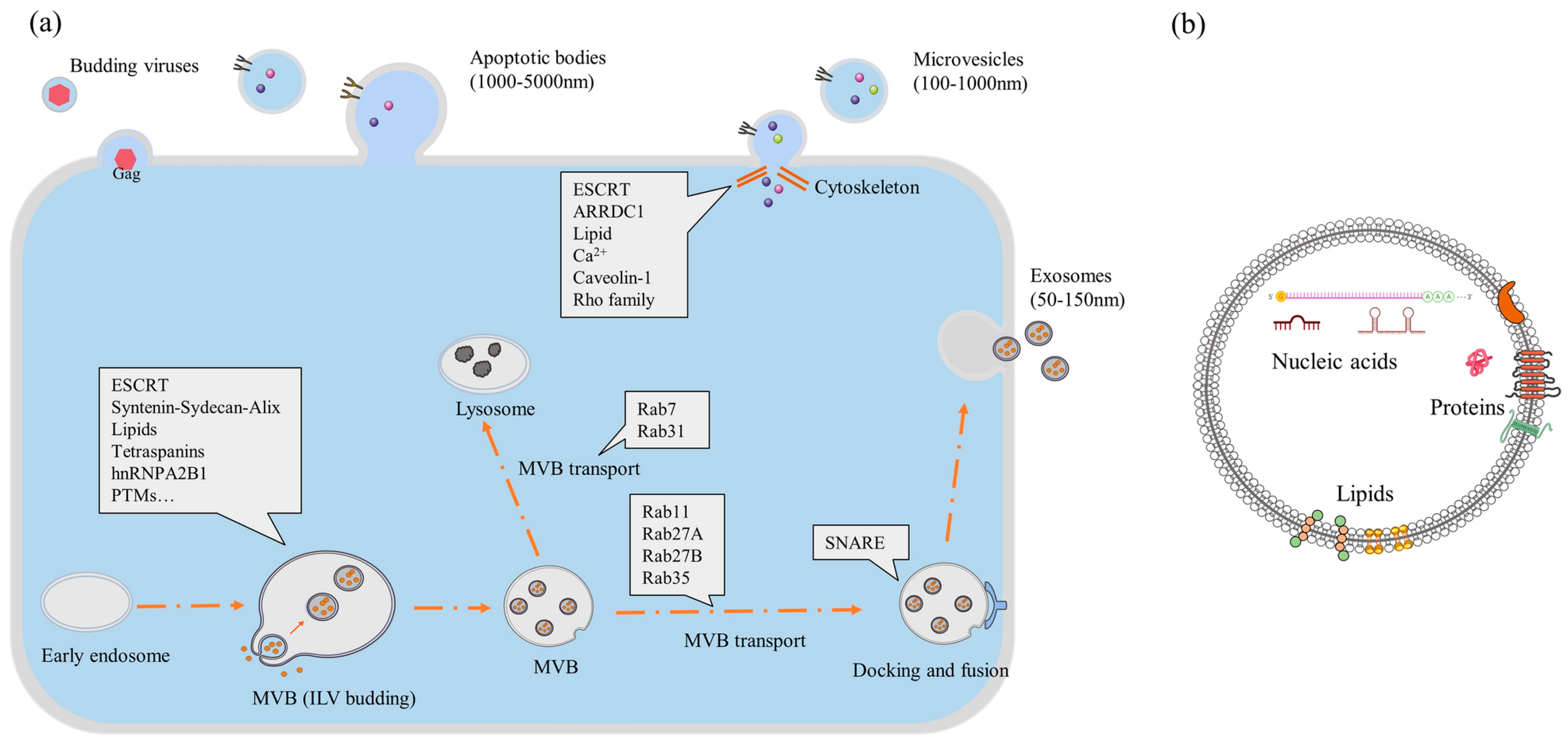
3. EV Separation and Purification
4. EV Classification
5. Nucleic Acid Cargo in EVs Relevant to Ischemia
| miRNA/RNA | Expected Activities | Ref. |
|---|---|---|
| miRNA-134 | Potentially a novel biomarker for diagnosis and prognosis. | [82] |
| miRNA-422a and miRNA-125b-2-3p | Potential biomarker for prognosis of the acuteness of stroke. | [83] |
| miRNA-9 | Biomarker for assessing the extent of injury caused by ischemic injury. | [84] |
| miRNA-21-5p and miRNA-30a-5p | Biomarkers for acute and hyperacute ischemic stroke. | [85] |
| miRNA-PC-5P-12969 | Potential candidate for ischemic stroke peripheral marker but also a drug target for ischemic stroke. | [86] |
| miRNA-125a-5p, miRNA-125b-5p, and miRNA-143-3p | Potential biomarkers for transient and acute ischemic stroke. | [87] |
| CircOGDH expression | Upregulated expression in the penumbra tissue of ischemic mice. | [88] |
| miRNA-126 | Regulators of angiogenesis and endothelial cell function, found in stem cell-derived exosomes. | [89] |
| miRNA-124 | In animal models, promotes neurogenesis. In humans, can be used as a biomarker of ischemic injury. | [84,90] |
| miRNA-21-5p and miRNA-30a-5p | Can be indicative of the recovery phase of ischemia in human patients. | [85] |
| miRNA-223 | Upregulation in blood serum indicates poor recovery of patients. | [91] |
| miRNA-21a | Enhance microenvironment necessary to promote neurogenesis of induced NPC in vitro. | [94] |
6. In Vivo Detection and Tracking of EVs
| Labeling Agent | EV Derived From | Detection Method or Use | Reference |
|---|---|---|---|
| DiR | Bone marrow-derived | Used to observe therapeutic effects of exosomes for ischemic stroke by molecular imaging in mice. | [101] |
| Gadolinium Chelates | Umbilical cord-derived MSC | Used to capture tumor-homing ability of MSC exosome in mice via MRI. | [106] |
| USPION | USPION-labeled Adipose tissue derived MSC | Used to tract exosomes in the muscle of mice via MRI. | [112] |
| SPION | Mouse macrophages | Used to image (MRI) target drug delivery by exosomes for glioma in mouse models. | [113] |
| FTH1 | Viral transfected MSC | Indirect labeling of exosomes detectable in mice via MRI. | [118] |
| Gaussia luciferase–lactadherin | Viral transfected Murine melanoma cells | In vivo detection of exosome in mice via fluorescent imaging | [119] |
| 99Tc-tricarbonyl | Erythrocytes | Used to image the biodistribution of EVs in mice. | [121] |
| 99Tc-HMPAO | Exosome mimicking nanovesicles from mouse macrophage cells | For quantitative measurement of in vivo clearance of exosome mimicking nanovesicles from mice with the help of CT scanning. | [120] |
| 111In | Melanoma cells | Used to image the biodistribution of EVs in mice. | [124] |
7. Preclinical Studies of EV Treatments Applied to Cerebral Ischemia
7.1. Evident Preclinical Studies of EV Treatments—The Motivation for Stem Cell-Derived EVs
| EV Source | Stroke Model, Administration Route, and Observation Period | Outcome | Reference |
|---|---|---|---|
| Rat bone marrow-derived MSCs | Male Wister rat MCAO model, Tail vein injection, 28 days | Reduction in stroke lesion volume and better functional recovery were reported. Long-term neuroprotection, promotion of neurogenesis, and angiogenesis also were observed in histopathology. | [141] |
| Mouse bone marrow-derived MSCs | Male C57BL/6J mice, transient global cerebral ischemia model, intracerebroventricular injection, 7 days | Significant reduction in stroke lesion volume and functional recovery. Lesion site restored partially as well as hippocampal and basal synaptic plasticity improved. | [142] |
| Mouse adipose-derived MSCs | Male C57BL/6J mice, transient global cerebral ischemia model, intracerebroventricular injection, 7 days | Not significant recovery was noticed compared to placebo mice. | [143] |
| Mouse adipose-derived MSCs | Male C57BL/6 mice MCAO model, right femoral vein injection, 14 days | A noticeable reduction in stroke lesion volume was not observed. But better functional recovery and less neural death in the penumbra region was observed in immunohistochemistry. | [144] |
| Human iPSC-derived MSCs | Male C57BL/6J mice MCAO model, intravenous injection, 28 days | Significant reduction in stroke lesion volume and better functional recovery. Promotion of angiogenesis and axonal plasticity were also observed. | [145] |
| Human NSCs | Porcine MCAO model, intravenous injection, 84 days | Significant reduction in stroke lesion volume and edema and improved functional recovery. | [146] |
| Human NSCs | Porcine MCAO model, intravenous injection, 84 days | From MRI, midline shift recovery was considered a recovery metric. It improved in treated animals along with anti-immune response and better functional recovery. | [147] |
| Human MSC and NSC | Murine thromboembolic stroke model, tail vein injection, 24 days | NSC-derived EVs had a more positive outcome than MSC-derived EVs. NSC-derived EV had better anti-immune factors that resulted in reduced lesion size and better functional recovery. | [148] |
7.2. Challenges in Bridging Preclinical Findings to Clinical Application
| Route | Description | Benefits | Drawbacks | Reference |
|---|---|---|---|---|
| Intravenous (IV) | Systemic injection into the bloodstream |
|
| [159,160] |
| Intranasal | Delivery through the nasal cavity |
|
| [164] |
| Intracerebral | Direct injection into the brain parenchyma |
|
| [163] |
| Intrathecal | Injection into cerebrospinal fluid (CSF) via lumbar puncture |
|
| [164] |
| Intra-arterial (IA) | Injection into cerebral arteries (e.g., internal carotid) |
|
| [162] |
| Intraperitoneal | Injection into the abdominal cavity or gastrointestinal (GI) tract |
|
| [159,161] |
| Subcutaneous | Injection into the fat layer under the skin |
|
| [161] |
8. Challenges in the EV Production Process
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| EVs | extracellular vesicles |
| MSCs | mesenchymal stem cells |
| NPCs | neural progenitor cells |
| miRNA | micro-ribonucleic acid |
| mRNA | messenger ribonucleic acid |
| lncRNA | long non-coding ribonucleic acid |
| circRNA | circular ribonucleic acid |
| siRNA | small interfering ribonucleic acid |
| ISEV | International Society for Extracellular Vesicles |
| MVBs | multivesicular bodies |
| BBB | blood–brain barrier |
| ESCRT | the endosomal sorting complex required for transport |
| ASCs | adipose tissue-derived mesenchymal stem cells |
| MRI | magnetic resonance imaging |
| MCAO | middle cerebral artery occlusion |
| iPSC | induced pluripotent stem cells |
| CT | computed tomography |
| PET | positron emission tomography |
| CircOGDH | oxoglutarate dehydrogenase |
| CSF | cerebrospinal Fluid |
| EM | electron microscopy |
| NTA | nanoparticle tracking analysis |
| DLS | dynamic light scattering |
| SPION | superparamagnetic iron oxide nanoparticles |
| USPION | ultra-small superparamagnetic iron oxide nanoparticles |
References
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, e41–e660, Correction in Circulation 2025, 151, e1096. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913, Correction in Circulation 2024, 149, e1164. [Google Scholar] [CrossRef] [PubMed]
- Lansberg, M.G.; Schrooten, M.; Bluhmki, E.; Thijs, V.N.; Saver, J.L. Treatment Time-Specific Number Needed to Treat Estimates for Tissue Plasminogen Activator Therapy in Acute Stroke Based on Shifts Over the Entire Range of the Modified Rankin Scale. Stroke 2009, 40, 2079–2084. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.E.; Savitz, S.; Sitton, C.; Grotta, J.; Chen, P.; Cai, C.; Cutter, G.; Imam, B.; Reddy, S.; et al. Outcomes of Endovascular Thrombectomy vs Medical Management Alone in Patients With Large Ischemic Cores: A Secondary Analysis of the Optimizing Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) Study. JAMA Neurol. 2019, 76, 1147–1156. [Google Scholar] [CrossRef]
- Brazelton, T.R.; Rossi, F.M.V.; Keshet, G.I.; Blau, H.M. From Marrow to Brain: Expression of Neuronal Phenotypes in Adult Mice. Science 2000, 290, 1775–1779. [Google Scholar] [CrossRef]
- Walker, P.A.; Harting, M.T.; Jimenez, F.; Shah, S.K.; Pati, S.; Dash, P.K.; Cox, C.S. Direct Intrathecal Implantation of Mesenchymal Stromal Cells Leads to Enhanced Neuroprotection via an NFκB-Mediated Increase in Interleukin-6 Production. Stem Cells Dev. 2010, 19, 867–876. [Google Scholar] [CrossRef]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V.; Nagaeva, O.; Dehlin, E. Isolation and Characterization of Exosomes from Cultures of Tissue Explants and Cell Lines. Curr. Protoc. Immunol. 2016, 115, 14–42. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Maltsev, N.; Vorobjev, I.A. Microvesicles and intercellular communication in the context of parasitism. Front. Cell. Infect. Microbiol. 2013, 3, 49. [Google Scholar] [CrossRef]
- Ling, H.; Guo, Z.; Tan, L.; Cao, Q.; Song, C. Stem cell-derived exosomes: Role in the pathogenesis and treatment of atherosclerosis. Int. J. Biochem. Cell Biol. 2021, 130, 105884. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Horstman, L.L.; Jy, W.; Minagar, A.; Bidot, C.J.; Jimenez, J.J.; Alexander, J.S.; Ahn, Y.S. Cell-Derived Microparticles and Exosomes in Neuroinflammatory Disorders. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 79, pp. 227–268. ISBN 978-0-12-373736-6. [Google Scholar]
- Li, J.-Y.; Li, Q.-Q.; Sheng, R. The role and therapeutic potential of exosomes in ischemic stroke. Neurochem. Int. 2021, 151, 105194. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; O’Loughlin, A.J.; Lakhal, S. Exosomes and the blood–brain barrier: Implications for neurological diseases. Ther. Deliv. 2011, 2, 1095–1099. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mesci, P.; Carromeu, C.; McClatchy, D.R.; Schiapparelli, L.; Yates, J.R.; Muotri, A.R.; Cline, H.T. Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 16086–16094. [Google Scholar] [CrossRef]
- Tan, A.; Rajadas, J.; Seifalian, A.M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv. Drug Deliv. Rev. 2013, 65, 357–367. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Alenquer, M.; Amorim, M. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151. [Google Scholar] [CrossRef]
- Phan, T.K.; Ozkocak, D.C.; Poon, I.K.H. Unleashing the therapeutic potential of apoptotic bodies. Biochem. Soc. Trans. 2020, 48, 2079–2088. [Google Scholar] [CrossRef]
- Roseblade, A.; Luk, F.; Ung, A.; Bebawy, M. Targeting Microparticle Biogenesis: A Novel Approach to the Circumvention of Cancer Multidrug Resistance. Curr. Cancer Drug Targets 2015, 15, 205–214. [Google Scholar] [CrossRef]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 2018, 17, e12734. [Google Scholar] [CrossRef]
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2017, 2, 170–179. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Aryal, S. Surface functionalization strategies of extracellular vesicles. J. Mater. Chem. B 2020, 8, 4552–4569. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 32. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, L.; Zhang, W.; Yang, W.; Feng, Q.; Wang, H. Extracellular signals regulate the biogenesis of extracellular vesicles. Biol. Res. 2022, 55, 35. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Jeske, R.; Chen, X.; Ma, S.; Zeng, E.Z.; Driscoll, T.; Li, Y. Bioreactor expansion reconfigures metabolism and extracellular vesicle biogenesis of human adipose-derived stem cells in vitro. Biochem. Eng. J. 2022, 188, 108711. [Google Scholar] [CrossRef]
- Pinto, D.; Bandeiras, C.; Fuzeta, M.; Rodrigues, C.A.V.; Jung, S.; Hashimura, Y.; Tseng, R.; Milligan, W.; Lee, B.; Ferreira, F.C.; et al. Scalable Manufacturing of Human Mesenchymal Stromal Cells in the Vertical-Wheel Bioreactor System: An Experimental and Economic Approach. Biotechnol. J. 2019, 14, 1800716. [Google Scholar] [CrossRef]
- Borys, B.S.; So, T.; Colter, J.; Dang, T.; Roberts, E.L.; Revay, T.; Larijani, L.; Krawetz, R.; Lewis, I.; Argiropoulos, B.; et al. Optimized serial expansion of human induced pluripotent stem cells using low-density inoculation to generate clinically relevant quantities in vertical-wheel bioreactors. Stem Cells Transl. Med. 2020, 9, 1036–1052. [Google Scholar] [CrossRef]
- Neto, P.M.; Nogueira, D.E.S.; Hashimura, Y.; Jung, S.; Pedras, B.; Berberan-Santos, M.N.; Palmeira, T.; Lee, B.; Cabral, J.M.S.; Geraldes, V.; et al. Characterization of the Aeration and Hydrodynamics in Vertical-WheelTM Bioreactors. Bioengineering 2022, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar] [CrossRef]
- Tanziela, T.; Shaikh, S.; Jiang, H.; Lu, Z.; Wang, X. Efficient encapsulation of biocompatible nanoparticles in exosomes for cancer theranostics. Nano Today 2020, 35, 100964. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef]
- Xu, R.; Bai, Y.; Min, S.; Xu, X.; Tang, T.; Ju, S. In vivo Monitoring and Assessment of Exogenous Mesenchymal Stem Cell-Derived Exosomes in Mice with Ischemic Stroke by Molecular Imaging. Int. J. Nanomed. 2020, 15, 9011–9023. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Kuroda, S. Engineering of Extracellular Vesicles for Small Molecule-Regulated Cargo Loading and Cytoplasmic Delivery of Bioactive Proteins. Mol. Pharm. 2022, 19, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Bui, S.; Dancourt, J.; Lavieu, G. Virus-Free Method to Control and Enhance Extracellular Vesicle Cargo Loading and Delivery. ACS Appl. Bio Mater. 2023, 6, 1081–1091. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Taha, H.B. Plasma versus serum for extracellular vesicle (EV) isolation: A duel for reproducibility and accuracy for CNS-originating EVs biomarker analysis. J. Neurosci. Res. 2023, 101, 1677–1686. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Wasmuth, E.V.; Januszyk, K.; Lima, C.D. Structure of an Rrp6–RNA exosome complex bound to poly(A) RNA. Nature 2014, 511, 435–439. [Google Scholar] [CrossRef]
- Yakubovich, E.I.; Polischouk, A.G.; Evtushenko, V.I. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2022, 16, 115–126. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Ji, X.; Tan, Z.; Lubman, D.M. Column-based Technology for CD9-HPLC Immunoaffinity Isolation of Serum Extracellular Vesicles. J. Proteome Res. 2021, 20, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Brett, S.I.; Lucien, F.; Guo, C.; Williams, K.C.; Kim, Y.; Durfee, P.N.; Brinker, C.J.; Chin, J.I.; Yang, J.; Leong, H.S. Immunoaffinity based methods are superior to kits for purification of prostate derived extracellular vesicles from plasma samples. Prostate 2017, 77, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Skog, J.; Hsu, C.-H.; Lessard, R.T.; Balaj, L.; Wurdinger, T.; Carter, B.S.; Breakefield, X.O.; Toner, M.; Irimia, D. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 2010, 10, 505–511. [Google Scholar] [CrossRef]
- Yuan, X.; Rosenberg, J.T.; Liu, Y.; Grant, S.C.; Ma, T. Aggregation of human mesenchymal stem cells enhances survival and efficacy in stroke treatment. Cytotherapy 2019, 21, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef] [PubMed]
- Saury, C.; Lardenois, A.; Schleder, C.; Leroux, I.; Lieubeau, B.; David, L.; Charrier, M.; Guével, L.; Viau, S.; Delorme, B.; et al. Human serum and platelet lysate are appropriate xeno-free alternatives for clinical-grade production of human MuStem cell batches. Stem Cell Res. Ther. 2018, 9, 128. [Google Scholar] [CrossRef]
- Østergaard, O.; Nielsen, C.T.; Iversen, L.V.; Jacobsen, S.; Tanassi, J.T.; Heegaard, N.H.H. Quantitative Proteome Profiling of Normal Human Circulating Microparticles. J. Proteome Res. 2012, 11, 2154–2163. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X.; et al. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018, 46, D106–D112. [Google Scholar] [CrossRef]
- Schorey, J.S.; Bhatnagar, S. Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Palviainen, M.; Saari, H.; Kärkkäinen, O.; Pekkinen, J.; Auriola, S.; Yliperttula, M.; Puhka, M.; Hanhineva, K.; Siljander, P.R.-M. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J. Extracell. Vesicles 2019, 8, 1596669. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Yeo, R.W.Y.; Choo, A.B.H.; Reiner, A.T.; Su, Y.; Shen, Y.; Fu, Z.; Alexander, L.; Sze, S.K.; et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Coumans, F.A.W.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; Van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhu, N.; Zhang, Y.; Yu, Y.; Kang, K.; Yi, Q.; Wu, Y. Hedgehog-inspired immunomagnetic beads for high-efficient capture and release of exosomes. J. Mater. Chem. B 2022, 10, 4059–4069, Correction in J. Mater. Chem. B 2025, 13, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Curley, N.; Levy, D.; Do, M.A.; Brown, A.; Stickney, Z.; Marriott, G.; Lu, B. Sequential deletion of CD63 identifies topologically distinct scaffolds for surface engineering of exosomes in living human cells. Nanoscale 2020, 12, 12014–12026. [Google Scholar] [CrossRef]
- Rasihashemi, S.Z.; Rezazadeh Gavgani, E.; Majidazar, R.; Seraji, P.; Oladghaffari, M.; Kazemi, T.; Lotfinejad, P. Tumor-derived exosomal PD-L1 in progression of cancer and immunotherapy. J. Cell. Physiol. 2022, 237, 1648–1660. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Nolte-’t Hoen, E.N.M.; Buermans, H.P.J.; Waasdorp, M.; Stoorvogel, W.; Wauben, M.H.M.; ’T Hoen, P.A.C. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012, 40, 9272–9285. [Google Scholar] [CrossRef]
- Németh, A.; Orgovan, N.; Sódar, B.W.; Osteikoetxea, X.; Pálóczi, K.; Szabó-Taylor, K.É.; Vukman, K.V.; Kittel, Á.; Turiák, L.; Wiener, Z.; et al. Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci. Rep. 2017, 7, 8202. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Chen, B.; Huang, S.; Zeng, C.; Wu, H.; Chen, C.; Long, F. Increased serum exosomal miR-134 expression in the acute ischemic stroke patients. BMC Neurol. 2018, 18, 198. [Google Scholar] [CrossRef]
- Li, D.-B.; Liu, J.-L.; Wang, W.; Li, R.-Y.; Yu, D.-J.; Lan, X.-Y.; Li, J.-P. Plasma Exosomal miR-422a and miR-125b-2-3p Serve as Biomarkers for Ischemic Stroke. Curr. Neurovasc. Res. 2018, 14, 330–337. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.-B.; Li, R.-Y.; Zhou, X.; Yu, D.-J.; Lan, X.-Y.; Li, J.-P.; Liu, J.-L. Diagnosis of Hyperacute and Acute Ischaemic Stroke: The Potential Utility of Exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc. Dis. 2018, 45, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Alamri, F.F.; Al Shoyaib, A.; Karamyan, V.T.; Reddy, P.H. Novel miRNA PC-5P-12969 in Ischemic Stroke. Mol. Neurobiol. 2019, 56, 6976–6985. [Google Scholar] [CrossRef] [PubMed]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Böck, J.; Northoff, B.H.; Klein, M.; et al. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zang, J.; Zhang, T.; Li, Y.; Tan, Z.; Ma, D.; Zhang, T.; Wang, S.; Zhang, Y.; et al. CircOGDH Is a Penumbra Biomarker and Therapeutic Target in Acute Ischemic Stroke. Circ. Res. 2022, 130, 907–924, Correction in Circ. Res. 2022, 130, e24. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, X.; He, Z. Association of miR-122, miR-124 miR-126 and miR-143 gene polymorphisms with ischemic stroke in the northern Chinese Han population. Int. J. Neurosci. 2019, 129, 916–922. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Pastrana, E.; Tavazoie, M.; Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009, 12, 399–408. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.-Y. Increased Circulating Exosomal miRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Zhang, M.; Sun, J.; Li, W.; Jiang, R.; Han, R.; Wang, Y.; Tian, Y.; Kang, X.; et al. miRNA-223 targets the GPAM gene and regulates the differentiation of intramuscular adipocytes. Gene 2019, 685, 106–113. [Google Scholar] [CrossRef]
- Bao, M.-H.; Szeto, V.; Yang, B.B.; Zhu, S.; Sun, H.-S.; Feng, Z.-P. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, C.; Huang, Y.; Wang, Y.; Xia, X.; Zheng, J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F.; Odintsova, E. Tetraspanins as Regulators of Protein Trafficking. Traffic 2007, 8, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Munich, S.; Sobo-Vujanovic, A.; Buchser, W.J.; Beer-Stolz, D.; Vujanovic, N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. OncoImmunology 2012, 1, 1074–1083. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Betzer, O.; Barnoy, E.; Sadan, T.; Elbaz, I.; Braverman, C.; Liu, Z.; Popovtzer, R. Advances in imaging strategies for in vivo tracking of exosomes. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1594. [Google Scholar] [CrossRef]
- Hoffman, R.M. The Advantages of Using Fluorescent Proteins for In Vivo Imaging. Curr. Protoc. Essent. Lab. Tech. 2017, 15, 9.6.1–9.6.24. [Google Scholar] [CrossRef]
- Dabrowska, S.; Del Fattore, A.; Karnas, E.; Frontczak-Baniewicz, M.; Kozlowska, H.; Muraca, M.; Janowski, M.; Lukomska, B. Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int. J. Nanomed. 2018, 13, 1653–1664. [Google Scholar] [CrossRef]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Exosome labeling by lipophilic dye PKH26 results in significant increase in vesicle size. Biochemistry 2019. preprint. [Google Scholar] [CrossRef]
- Shimomura, T.; Seino, R.; Umezaki, K.; Shimoda, A.; Ezoe, T.; Ishiyama, M.; Akiyoshi, K. New Lipophilic Fluorescent Dyes for Labeling Extracellular Vesicles: Characterization and Monitoring of Cellular Uptake. Bioconjug. Chem. 2021, 32, 680–684. [Google Scholar] [CrossRef]
- Bower, D.V.; Richter, J.K.; Von Tengg-Kobligk, H.; Heverhagen, J.T.; Runge, V.M. Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent. Investig. Radiol. 2019, 54, 453–463. [Google Scholar] [CrossRef]
- Runge, V.M. Safety of the Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging, Focusing in Part on Their Accumulation in the Brain and Especially the Dentate Nucleus. Investig. Radiol. 2016, 51, 273–279. [Google Scholar] [CrossRef]
- Buhaescu, I.; Izzedine, H. Gadolinium-induced nephrotoxicity: Gadolinium-induced nephrotoxicity. Int. J. Clin. Pract. 2008, 62, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Abello, J.; Nguyen, T.D.T.; Marasini, R.; Aryal, S.; Weiss, M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 2019, 9, 2325–2345. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Aschner, M. Manganese toxicity in the central nervous system: The glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med. 2013, 273, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Hobson, N.J.; Weng, X.; Siow, B.; Veiga, C.; Ashford, M.; Thanh, N.T.; Schätzlein, A.G.; Uchegbu, I.F. Clustering superparamagnetic iron oxide nanoparticles produces organ-targeted high-contrast magnetic resonance images. Nanomedicine 2019, 14, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall superparamagnetic iron oxide nanoparticles: A next generation contrast agent for magnetic resonance imaging. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1740. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: A biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef]
- Marzola, P.; Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Mariotti, R. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: A new method to obtain labeled exosomes. Int. J. Nanomed. 2016, 11, 2481–2490. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Chen, X.; Du, D.; Shi, J.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale 2020, 12, 173–188. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, Y.; Zhao, R.; Wei, X.; Xin, X. Visualization of exosomes from mesenchymal stem cells in vivo by magnetic resonance imaging. Magn. Reson. Imaging 2020, 68, 75–82. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Choi, H.; Jang, S.C.; Yoo, M.Y.; Park, J.Y.; Choi, N.E.; Oh, H.J.; Ha, S.; Lee, Y.-S.; Jeong, J.M.; et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using 99mTc-HMPAO. Sci. Rep. 2015, 5, 15636. [Google Scholar] [CrossRef]
- Varga, Z.; Gyurkó, I.; Pálóczi, K.; Buzás, E.I.; Horváth, I.; Hegedűs, N.; Máthé, D.; Szigeti, K. Radiolabeling of Extracellular Vesicles with 99m Tc for Quantitative In Vivo Imaging Studies. Cancer Biother. Radiopharm. 2016, 31, 168–173. [Google Scholar] [CrossRef]
- Faruqu, F.N.; Wang, J.T.-W.; Xu, L.; McNickle, L.; Chong, E.M.-Y.; Walters, A.; Gurney, M.; Clayton, A.; Smyth, L.A.; Hider, R.; et al. Membrane Radiolabelling of Exosomes for Comparative Biodistribution Analysis in Immunocompetent and Immunodeficient Mice—A Novel and Universal Approach. Theranostics 2019, 9, 1666–1682. [Google Scholar] [CrossRef]
- Popovtzer, R.; Betzer, O.; Perets, N.; Barnoy, E.; Offen, D. Labeling and tracking exosomes within the brain using gold nanoparticles. In Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XV; Cartwright, A.N., Nicolau, D.V., Fixler, D., Eds.; SPIE: San Francisco, CA, USA, 2018; p. 44. [Google Scholar]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, S.; Villa, A.; Crescenti, D.; Crippa, E.; Brunialti, E.; Shojaei-Ghahrizjani, F.; Rizzi, N.; Rebecchi, M.; Dei Cas, M.; Del Sole, A.; et al. Increased Sensitivity of Computed Tomography Scan for Neoplastic Tissues Using the Extracellular Vesicle Formulation of the Contrast Agent Iohexol. Pharmaceutics 2022, 14, 2766. [Google Scholar] [CrossRef]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.-M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef]
- Lafourcade, C.; Ramírez, J.P.; Luarte, A.; Fernández, A.; Wyneken, U. MIRNAS in Astrocyte-Derived Exosomes as Possible Mediators of Neuronal Plasticity: Supplementary Issue: Brain Plasticity and Repair. J. Exp. Neurosci. 2016, 10 (Suppl. S1), JEN.S39916. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Peruzzotti-Jametti, L.; Giebel, B.; Pluchino, S. Extracellular vesicles set the stage for brain plasticity and recovery by multimodal signalling. Brain 2023, 147, 372–389. [Google Scholar] [CrossRef]
- Nistor-Cseppentö, D.C.; Jurcău, M.C.; Jurcău, A.; Andronie-Cioară, F.L.; Marcu, F. Stem Cell- and Cell-Based Therapies for Ischemic Stroke. Bioengineering 2022, 9, 717. [Google Scholar] [CrossRef]
- Tamama, K.; Kawasaki, H.; Wells, A. Epidermal Growth Factor (EGF) Treatment on Multipotential Stromal Cells (MSCs). Possible Enhancement of Therapeutic Potential of MSC. J. Biomed. Biotechnol. 2010, 2010, 795385. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, T.-H.; Li, H.-D.; Li, G.-Z.; Liang, J.; Wang, P. Exosomes from bone marrow mesenchymal stem cells are a potential treatment for ischemic stroke. Neural Regen. Res. 2023, 18, 2246. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells: hMSC. Cytom. A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef]
- Khabbal, J.; Kerkelä, E.; Mitkari, B.; Raki, M.; Nystedt, J.; Mikkonen, V.; Bergström, K.; Laitinen, S.; Korhonen, M.; Jolkkonen, J. Differential Clearance of Rat and Human Bone Marrow-Derived Mesenchymal Stem Cells from the Brain after Intra-arterial Infusion in Rats. Cell Transplant. 2015, 24, 819–828. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chang, S.; Xu, R.; Chen, L.; Song, X.; Wu, J.; Qian, J.; Zou, Y.; Ma, J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res. Ther. 2020, 11, 224. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic Culture Conditions as a Solution for Mesenchymal Stem Cell Based Regenerative Therapy. Sci. World J. 2013, 2013, 632972. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Deng, M.; Xiao, H.; Zhang, H.; Peng, H.; Yuan, H.; Xu, Y.; Zhang, G.; Hu, Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles Ameliorates Hippocampal Synaptic Impairment after Transient Global Ischemia. Front. Cell. Neurosci. 2017, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zheng, X.; Zhang, L.; Ai, X.; Venkataramani, V.; Kilic, E.; Hermann, D.M.; Majid, A.; Bähr, M.; Doeppner, T.R. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J. Extracell. Vesicles 2020, 10, e12024. [Google Scholar] [CrossRef]
- Lu, G.; Su, X.; Wang, L.; Leung, C.-K.; Zhou, J.; Xiong, Z.; Wang, W.; Liu, H.; Chan, W.-Y. Neuroprotective Effects of Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cell Extracellular Vesicles in Ischemic Stroke Models. Biomedicines 2023, 11, 2550. [Google Scholar] [CrossRef]
- Wang, A.Y.L. Human Induced Pluripotent Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Various Diseases. Int. J. Mol. Sci. 2021, 22, 1769. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.L.; Kaiser, E.E.; Jurgielewicz, B.J.; Spellicy, S.; Scoville, S.L.; Thompson, T.A.; Swetenburg, R.L.; Hess, D.C.; West, F.D.; Stice, S.L. Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke 2018, 49, 1248–1256. [Google Scholar] [CrossRef]
- Spellicy, S.E.; Kaiser, E.E.; Bowler, M.M.; Jurgielewicz, B.J.; Webb, R.L.; West, F.D.; Stice, S.L. Neural Stem Cell Extracellular Vesicles Disrupt Midline Shift Predictive Outcomes in Porcine Ischemic Stroke Model. Transl. Stroke Res. 2020, 11, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef]
- Othman, F.A.; Tan, S.C. Preconditioning Strategies to Enhance Neural Stem Cell-Based Therapy for Ischemic Stroke. Brain Sci. 2020, 10, 893. [Google Scholar] [CrossRef]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Xin, H.; Wang, F.; Li, Y.; Lu, Q.-E.; Cheung, W.L.; Zhang, Y.; Zhang, Z.G.; Chopp, M. Secondary Release of Exosomes from Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery after Stroke in Rats Treated with Exosomes Harvested from MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transplant. 2017, 26, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Kuniyan, M.S.; Jawed, A.M.; Chen, L. Engineered Extracellular Vesicles for Drug Delivery in Therapy of Stroke. Pharmaceutics 2023, 15, 2173. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- El-Andaloussi, S.; Lee, Y.; Lakhal-Littleton, S.; Li, J.; Seow, Y.; Gardiner, C.; Alvarez-Erviti, L.; Sargent, I.L.; Wood, M.J.A. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012, 7, 2112–2126. [Google Scholar] [CrossRef]
- Rennert, R.C.; Wali, A.R.; Steinberg, J.A.; Santiago-Dieppa, D.R.; Olson, S.E.; Pannell, J.S.; Khalessi, A.A. Epidemiology, Natural History, and Clinical Presentation of Large Vessel Ischemic Stroke. Neurosurgery 2019, 85, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Kassem-Moussa, H.; Graffagnino, C. Nonocclusion and Spontaneous Recanalization Rates in Acute Ischemic Stroke: A Review of Cerebral Angiography Studies. Arch. Neurol. 2002, 59, 1870. [Google Scholar] [CrossRef]
- Cook, D.J.; Tymianski, M. Nonhuman Primate Models of Stroke for Translational Neuroprotection Research. Neurotherapeutics 2012, 9, 371–379. [Google Scholar] [CrossRef]
- Macrae, I. Preclinical stroke research—Advantages and disadvantages of the most common rodent models of focal ischaemia: Animal models of focal cerebral ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Mazzaferro, V.; Ciana, P. Systemic Administration and Targeted Delivery of Immunogenic Oncolytic Adenovirus Encapsulated in Extracellular Vesicles for Cancer Therapies. Viruses 2018, 10, 558. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Yang, B.; Migliati, E.; Parsha, K.; Schaar, K.; Xi, X.; Aronowski, J.; Savitz, S.I. Intra-Arterial Delivery Is Not Superior to Intravenous Delivery of Autologous Bone Marrow Mononuclear Cells in Acute Ischemic Stroke. Stroke 2013, 44, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.; Kang, M.; Hisey, C.L.; Chamley, L.W. Studying exogenous extracellular vesicle biodistribution by in vivo fluorescence microscopy. Dis. Model. Mech. 2023, 16, dmm050074. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Zuccolotto, G.; Malvicini, R.; De Lazzari, G.; Penna, A.; Franco, C.; Caicci, F.; Magarotto, F.; Quarta, S.; Pozzobon, M.; et al. Biodistribution of Intratracheal, Intranasal, and Intravenous Injections of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles in a Mouse Model for Drug Delivery Studies. Pharmaceutics 2023, 15, 548. [Google Scholar] [CrossRef]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation. Cancer Res. 2010, 70, 1668–1678, Correction in Cancer Res. 2025, 85, 3177. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.N.; Chaudhuri, A.; Kumar, D.; Singh, S.; Agrawal, A.K. Impact of the Drug Loading Method on the Drug Distribution and Biological Efficacy of Exosomes. AAPS PharmSciTech 2023, 24, 166. [Google Scholar] [CrossRef]
- Colja, S.; Jovčevska, I.; Šamec, N.; Romih, R.; Zottel, A. Sonication is a suitable method for loading nanobody into glioblastoma small extracellular vesicles. Heliyon 2023, 9, e15674. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Encabo-Berzosa, M.D.M.; Beltrán-Visiedo, M.; Fernández-Messina, L.; Sebastián, V.; Sánchez-Madrid, F.; Arruebo, M.; Santamaría, J.; Martín-Duque, P. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: Leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale 2019, 11, 18825–18836. [Google Scholar] [CrossRef]
- Podolak, I.; Grabowska, K.; Sobolewska, D.; Wróbel-Biedrawa, D.; Makowska-Wąs, J.; Galanty, A. Saponins as cytotoxic agents: An update (2010–2021). Part II—Triterpene saponins. Phytochem. Rev. 2023, 22, 113–167. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, H.; Zhang, M.; He, Y.; Li, X.; Xu, Y.; Liu, X. Hypoxia Induced Changes of Exosome Cargo and Subsequent Biological Effects. Front. Immunol. 2022, 13, 824188. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.; Lee, M. Hypoxia-specific anti-RAGE exosomes for nose-to-brain delivery of anti-miR-181a oligonucleotide in an ischemic stroke model. Nanoscale 2021, 13, 14166–14178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Mazure, N.M.; Pouysségur, J. Hypoxia-induced autophagy: Cell death or cell survival? Curr. Opin. Cell Biol. 2010, 22, 177–180. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.E.S.; Rodrigues, C.A.V.; Carvalho, M.S.; Miranda, C.C.; Hashimura, Y.; Jung, S.; Lee, B.; Cabral, J.M.S. Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-WheelTM bioreactors. J. Biol. Eng. 2019, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Doron, G.; Klontzas, M.E.; Mantalaris, A.; Guldberg, R.E.; Temenoff, J.S. Multiomics characterization of mesenchymal stromal cells cultured in monolayer and as aggregates. Biotechnol. Bioeng. 2020, 117, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, A.; Driscoll, T.; Grant, S.C.; Li, Y. Human Stem Cell-Derived Extracellular Vesicles: A Pioneering Path from Biogenesis to Cerebral Ischemic Stroke Therapy. Int. J. Mol. Sci. 2025, 26, 10550. https://doi.org/10.3390/ijms262110550
Roy A, Driscoll T, Grant SC, Li Y. Human Stem Cell-Derived Extracellular Vesicles: A Pioneering Path from Biogenesis to Cerebral Ischemic Stroke Therapy. International Journal of Molecular Sciences. 2025; 26(21):10550. https://doi.org/10.3390/ijms262110550
Chicago/Turabian StyleRoy, Anamika, Tristan Driscoll, Samuel C. Grant, and Yan Li. 2025. "Human Stem Cell-Derived Extracellular Vesicles: A Pioneering Path from Biogenesis to Cerebral Ischemic Stroke Therapy" International Journal of Molecular Sciences 26, no. 21: 10550. https://doi.org/10.3390/ijms262110550
APA StyleRoy, A., Driscoll, T., Grant, S. C., & Li, Y. (2025). Human Stem Cell-Derived Extracellular Vesicles: A Pioneering Path from Biogenesis to Cerebral Ischemic Stroke Therapy. International Journal of Molecular Sciences, 26(21), 10550. https://doi.org/10.3390/ijms262110550






