Soil and Seed: Tumor Microenvironment Nurtures Immunotherapy Resistance and Renewal
Abstract
1. Introduction
2. Mechanisms Underlying Resistance to Tumor Microenvironment-Mediated Immunotherapy
2.1. Tumor Immune Microenvironment
2.1.1. ICIs
2.1.2. CAR-T
2.2. Metabolic Microenvironment
2.2.1. ICIs
2.2.2. CAR-T
2.3. Mechanical Microenvironment
2.3.1. ICIs
2.3.2. CAR-T
2.4. Microbiota Niche
2.5. Others
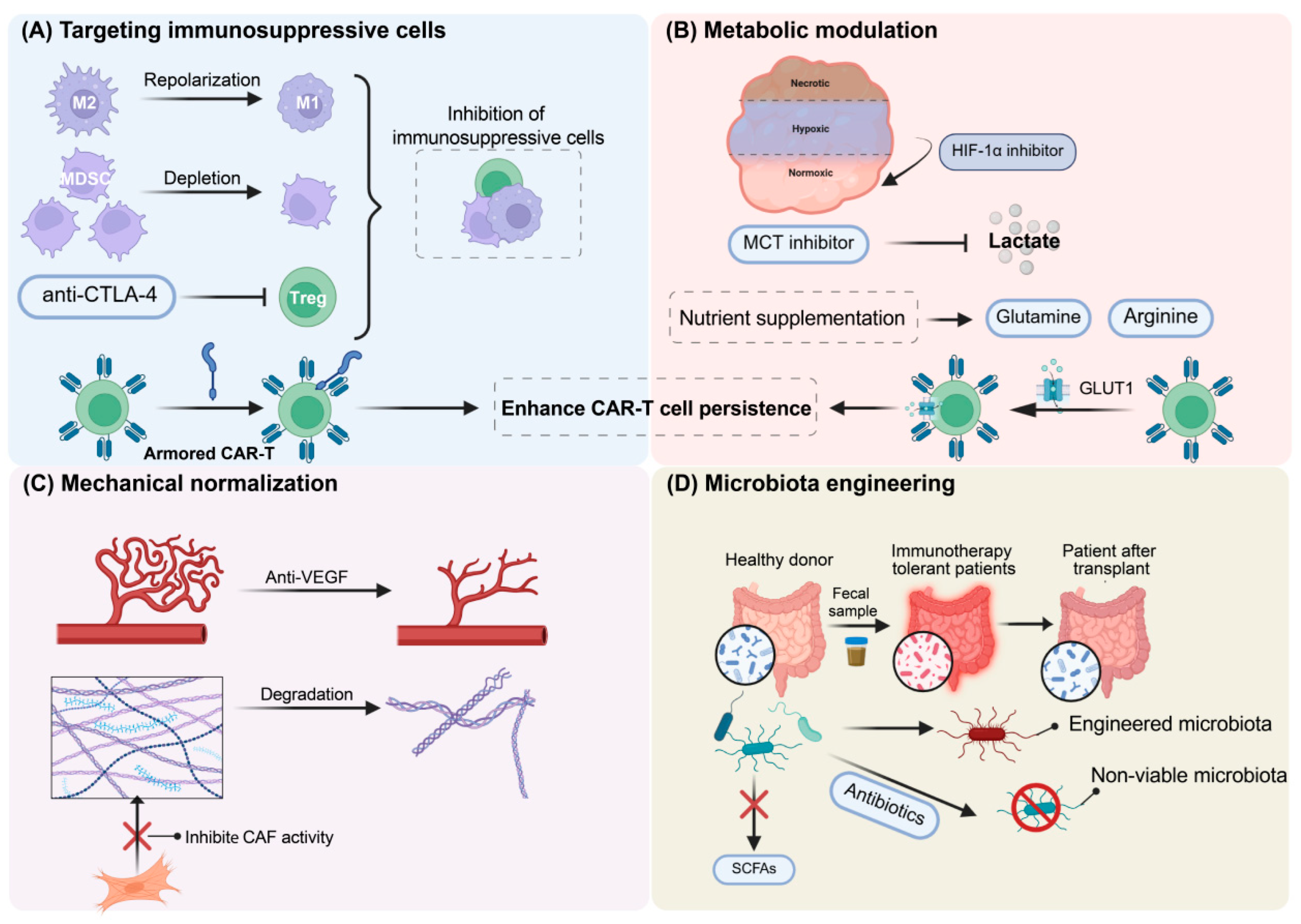
3. Strategies to Overcome Clinical Resistance to Immunotherapy
3.1. Targeting TIME
3.2. Targeting Metabolic Microenvironment
3.3. Targeting Mechanical Microenvironment
3.4. Modulatory Role of the Microbiota Niche
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFs | Cancer-Associated Fibroblasts |
| CAR-T | Chimeric Antigen Receptor T-cell |
| circRNA | Circular RNA |
| CNN | Convolutional Neural Network |
| Col1 | Collagen Type I |
| CRC | Colorectal Cancer |
| CSF1 | Colony Stimulating Factor 1 |
| CTLA-4 | Cytotoxic T Lymphocyte-Associated Protein 4 |
| CTLs | Cytotoxic T Lymphocytes |
| DCs | Dendritic Cells |
| DDR1 | Discoidin Domain Receptor Tyrosine Kinase 1 |
| ECM | Extracellular Matrix |
| FABP | Fatty Acid Binding Protein |
| FMT | Fecal Microbiota Transplantation |
| F. nucleatum | Fusobacterium nucleatum |
| HCC | Hepatocellular Carcinoma |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| ICIs | Immune Checkpoint Inhibitors |
| JAK | Janus Kinase |
| MDSCs | Myeloid-Derived Suppressor Cells |
| NK cells | Natural Killer Cells |
| NNMT | Nicotinamide N-Methyltransferase |
| NSCLC | Non-Small Cell Lung Cancer |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PD-1 | Programmed Cell Death 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PGE2 | Prostaglandin E2 |
| SCFAs | Short-Chain Fatty Acids |
| sEVs | Small Extracellular Vesicles |
| SPP1 | Secreted Phosphoprotein 1 (Osteopontin) |
| STAT | Signal Transducer and Activator of Transcription |
| TAMs | Tumor-Associated Macrophages |
| TECs | Tumor-Associated Endothelial Cells |
| TME | Tumor Microenvironment |
| VEGFA | Vascular Endothelial Growth Factor A |
References
- Quesada, J.R.; Hersh, E.M.; Manning, J.; Reuben, J.; Keating, M.; Schnipper, E.; Itri, L.; Gutterman, J.U. Treatment of Hairy Cell Leukemia with Recombinant Alpha-Interferon. Blood 1986, 68, 493–497. [Google Scholar] [CrossRef]
- Ahmed, S.; Rai, K.R. Interferon in the Treatment of Hairy-Cell Leukemia. Best Pract. Res. Clin. Haematol. 2003, 16, 69–81. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Abaza, A.; Sid Idris, F.; Anis Shaikh, H.; Vahora, I.; Moparthi, K.P.; Al Rushaidi, M.T.; Muddam, M.R.; Obajeun, O.A.; Jaramillo, A.P.; Khan, S. Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) Immunotherapy: A Promising Breakthrough in Cancer Therapeutics. Curēus 2023, 15, e44582. [Google Scholar] [CrossRef]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T Cells: The Promise and Challenges of Cancer Immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Finck, A.V.; Blanchard, T.; Roselle, C.P.; Golinelli, G.; June, C.H. Engineered Cellular Immunotherapies in Cancer and Beyond. Nat. Med. 2022, 28, 678–689. [Google Scholar] [CrossRef]
- Patel, K.K.; Tariveranmoshabad, M.; Kadu, S.; Shobaki, N.; June, C. From Concept to Cure: The Evolution of CAR-T Cell Therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2025, 33, 2123–2140. [Google Scholar] [CrossRef]
- Khan, T.H.; Muhammad, N.; Tarique, M.; Usmani, D.; Naz, H.; Sarode, A. The Role of Cancer-Specific Target Antigens in CAR T Cell Therapy in Hematological Malignancies. Curr. Tissue Microenviron. Rep. 2024, 5, 61–67. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Bell, H.N.; Zou, W. Beyond the Barrier: Unraveling the Mechanisms of Immunotherapy Resistance. Annu. Rev. Immunol. 2024, 42, 521–550. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675. [Google Scholar] [CrossRef]
- Choi, H.; Moon, A. Crosstalk Between Cancer Cells and Endothelial Cells: Implications for Tumor Progression and Intervention. Arch. Pharmacal Res. 2018, 41, 711–724, Erratum in Arch. Pharmacal Res. 2018, 41, 941. [Google Scholar] [CrossRef]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Song, J.; Liu, X.; Liu, S.; Yang, N.; Wang, L.; Liu, Y.; Zhao, Y.; Zhou, W.; et al. Tumor Cell-Targeting and Tumor Microenvironment–Responsive Nanoplatforms for the Multimodal Imaging-Guided Photodynamic/Photothermal/Chemodynamic Treatment of Cervical Cancer. Int. J. Nanomed. 2024, 19, 5837–5858. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The Tumor Microenvironment at a Glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Patel, H.; Nilendu, P.; Jahagirdar, D.; Pal, J.K.; Sharma, N.K. Modulating Secreted Components of Tumor Microenvironment: A Masterstroke in Tumor Therapeutics. Cancer Biol. Ther. 2018, 19, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Aranda, V.; Bardelli, A.; Blanpain, C.; Bock, C.; Borowski, C.; Caldas, C.; Califano, A.; Doherty, M.; Elsner, M.; et al. Toward Understanding and Exploiting Tumor Heterogeneity. Nat. Med. 2015, 21, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor Microbiome—An Integral Part of the Tumor Microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, A.; Yuan, Y.; Zhu, B.; Long, H. Heterogeneity of the Tumor Immune Microenvironment and Its Clinical Relevance. Exp. Hematol. Oncol. 2022, 11, 24. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Li, X.; Singhal, K.; Deng, Q.; Chihara, D.; Russler-Germain, D.; Harkins, R.A.; Henderson, J.; Arita, K.; Kizhakeyil, A.; Sun, R.; et al. Large B Cell Lymphoma Microenvironment Archetype Profiles. Cancer Cell 2025, 43, 1347–1364.e13. [Google Scholar] [CrossRef]
- Li, X.; Lian, J.; Lu, H. The Role of SPP1+TAMs in Cancer: Impact on Patient Prognosis and Future Therapeutic Targets. Int. J. Cancer 2025, 157, 1763–1771. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor Endothelium FasL Establishes a Selective Immune Barrier Promoting Tolerance in Tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Tepper, A.; Falcomatà, C.; Potak, H.T.; Pia, L.; Amabile, A.; Mateus-Tique, J.; Rabinovich, N.; Park, M.D.; LaMarche, N.M.; et al. Ovarian Cancer-Derived IL-4 Promotes Immunotherapy Resistance. Cell 2024, 187, 7492–7510.e22. [Google Scholar] [CrossRef]
- Ranjan, K.; Rajendran, B.K.; Deen, I.U.; Costantini, A.; de Rodas, M.L.; Desai, S.S.; Scallo, F.; Gianino, N.; Ferrone, S.; Schalper, K.A. IL-4 Mediated TAP2 Downregulation Is a Dominant and Reversible Mechanism of Immune Evasion and Immunotherapy Resistance in Non-Small Cell Lung Cancer. Mol. Cancer 2025, 24, 80. [Google Scholar] [CrossRef]
- Ravi, V.M.; Neidert, N.; Will, P.; Joseph, K.; Maier, J.P.; Kückelhaus, J.; Vollmer, L.; Goeldner, J.M.; Behringer, S.P.; Scherer, F.; et al. T-Cell Dysfunction in the Glioblastoma Microenvironment Is Mediated by Myeloid Cells Releasing Interleukin-10. Nat. Commun. 2022, 13, 925. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, Y.-Q.; Gao, M.; Zhao, Y.; Franco, F.; Wenes, M.; Siddiqui, I.; Bevilacqua, A.; Wang, H.; Yang, H.; et al. Metabolic Reprogramming of Terminally Exhausted CD8+ T Cells by IL-10 Enhances Anti-Tumor Immunity. Nat. Immunol. 2021, 22, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Bai, Z.; Zhou, X.; Zhao, Y.; Xie, Y.-Q.; Huang, X.; Liu, Y.; Enbar, T.; Li, R.; Wang, Y.; et al. The Type 2 Cytokine Fc-IL-4 Revitalizes Exhausted CD8+ T Cells Against Cancer. Nature 2024, 634, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, C.T.; Dougan, S.K. Cytokines in Cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Cai, Z.; Wang, B.; Zeng, C.; Xi, Y.; Hu, S.; Qu, R.; Yuan, Z.; Yue, J.; Tian, Y.; et al. TGFβ Antagonizes IFNγ-Mediated Adaptive Immune Evasion via Activation of the AKT-Smad3-SHP1 Axis in Lung Adenocarcinoma. Cancer Res. 2023, 83, 2262–2277. [Google Scholar] [CrossRef]
- Lamorte, S.; Quevedo, R.; Jin, R.; Neufeld, L.; Liu, Z.Q.; Ciudad, M.T.; Lukhele, S.; Bruce, J.; Mishra, S.; Zhang, X.; et al. Lymph Node Macrophages Drive Immune Tolerance and Resistance to Cancer Therapy by Induction of the Immune-Regulatory Cytokine IL-33. Cancer Cell 2025, 43, 955–969.e10. [Google Scholar] [CrossRef]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.-I.; Cheng, P.; Cho, H.-I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α Regulates Function and Differentiation of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Lasser, S.A.; Ozbay Kurt, F.G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells in Cancer and Cancer Therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, S.; Zhao, X.; Xu, W.; Ju, F.; Gu, M.; Weng, J.; Du, Y. AKAP12 Positive Fibroblast Determines Immunosuppressive Contexture and Immunotherapy Response in Patients with TNBC by Promoting Macrophage M2 Polarization. J. Immunother. Cancer 2024, 12, e009877. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; To, K.K.W.; Zhu, S.; Wang, F.; Fu, L. Tumor-Associated Macrophages Remodel the Suppressive Tumor Immune Microenvironment and Targeted Therapy for Immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 145. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.U.; Subramanian, M.; Lee, D.H.; Wang, X.; Krchma, K.; Wu, J.; Naismith, T.; Halabi, C.M.; Kim, J.Y.; Pulous, F.E.; et al. Dual Role of Endothelial Myct1 in Tumor Angiogenesis and Tumor Immunity. Sci. Transl. Med. 2021, 13, abb6731. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, Y.; Mei, J.; Liu, Y.; Zhou, G. Extracellular Matrix Dynamics in Tumor Immunoregulation: From Tumor Microenvironment to Immunotherapy. J. Hematol. Oncol. 2025, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Herzog, B.H.; Baer, J.M.; Borcherding, N.; Kingston, N.L.; Belle, J.I.; Knolhoff, B.L.; Hogg, G.D.; Ahmad, F.; Kang, L.-I.; Petrone, J.; et al. Tumor-Associated Fibrosis Impairs Immune Surveillance and Response to Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Sci. Transl. Med. 2023, 15, eadh8005. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Li, G.; Zhu, B.; Wu, F.; Zhou, J.; Chen, X.; Qin, B.; Gao, Y.; Wang, F.; et al. PLAUR+ Neutrophils Drive Anti-PD-1 Therapy Resistance in Patients with Hepatocellular Carcinoma by Shaping an Immunosuppressive Microenvironment. Adv. Sci. 2025, 12, e07167. [Google Scholar] [CrossRef]
- Meng, Y.; Ye, F.; Nie, P.; Zhao, Q.; An, L.; Wang, W.; Qu, S.; Shen, Z.; Cao, Z.; Zhang, X.; et al. Immunosuppressive CD10+ALPL+ Neutrophils Promote Resistance to Anti-PD-1 Therapy in HCC by Mediating Irreversible Exhaustion of T Cells. J. Hepatol. 2023, 79, 1435–1449. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Huang, J.; Nie, R.-C.; Wu, Q.-N.; Zuo, Z.; Yuan, S.; Yu, K.; Liang, C.-C.; Pan, Y.-Q.; et al. CAF-Macrophage Crosstalk in Tumour Microenvironments Governs the Response to Immune Checkpoint Blockade in Gastric Cancer Peritoneal Metastases. Gut 2024, 74, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, T.; Yu, H.; Cao, Y.; Zhang, R.; Shao, R.; Liu, B.; Chen, L.; Xu, K.; Chen, W.; et al. Mechanisms Underlying Resistance to CAR-T Cell Therapy and Strategies for Enhancement. Cytokine Growth Factor Rev. 2025, 83, 66–76. [Google Scholar] [CrossRef]
- Stahl, D.; Gödel, P.; Balke-Want, H.; Gholamipoorfard, R.; Segbers, P.; Tetenborg, L.; Koker, M.; Dörr, J.; Gregor, L.; Bachurski, D.; et al. CSF1R+ Myeloid-Monocytic Cells Drive CAR-T Cell Resistance in Aggressive B Cell Lymphoma. Cancer Cell 2025, 43, 1476–1494.e10. [Google Scholar] [CrossRef]
- Ruella, M.; Korell, F.; Porazzi, P.; Maus, M.V. Mechanisms of Resistance to Chimeric Antigen Receptor-T Cells in Haematological Malignancies. Nat. Rev. Drug Discov. 2023, 22, 976–995. [Google Scholar] [CrossRef]
- Bailey, S.R.; Vatsa, S.; Larson, R.C.; Bouffard, A.A.; Scarfò, I.; Kann, M.C.; Berger, T.R.; Leick, M.B.; Wehrli, M.; Schmidts, A.; et al. Blockade or Deletion of IFNγ Reduces Macrophage Activation Without Compromising CAR T-Cell Function in Hematologic Malignancies. Blood Cancer Discov. 2022, 3, 136–153. [Google Scholar] [CrossRef]
- Larson, R.C.; Kann, M.C.; Bailey, S.R.; Haradhvala, N.J.; Llopis, P.M.; Bouffard, A.A.; Scarfó, I.; Leick, M.B.; Grauwet, K.; Berger, T.R.; et al. CAR T Cell Killing Requires the IFNγR Pathway in Solid but Not Liquid Tumours. Nature 2022, 604, 563–570. [Google Scholar] [CrossRef]
- Jung, I.-Y.; Bartoszek, R.L.; Rech, A.J.; Collins, S.M.; Ooi, S.-K.; Williams, E.F.; Hopkins, C.R.; Narayan, V.; Haas, N.B.; Frey, N.V.; et al. Type I Interferon Signaling via the EGR2 Transcriptional Regulator Potentiates CAR T Cell-Intrinsic Dysfunction. Cancer Discov. 2023, 13, 1636–1655. [Google Scholar] [CrossRef] [PubMed]
- Elia, I.; Haigis, M.C. Metabolites and the Tumour Microenvironment: From Cellular Mechanisms to Systemic Metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Hu, F.; Hu, C.; Sun, Y.; Yang, K.; Yang, S. Metabolic Reprogramming of Immune Cells in the Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 12223. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, X.; Yu, W.; Liang, X.; Du, X.; Liu, Z.; Long, J.; Zhao, G.; Liu, H. Lactic Acid in Macrophage Polarization: The Significant Role in Inflammation and Cancer. Int. Rev. Immunol. 2021, 41, 4–18. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Hong, B.-J.; Lee, C.-J.; Kim, Y.-E.; Bok, S.; Oh, J.-M.; Gwak, S.-H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, X.; Shi, J.; Huang, J.; Wang, S.; Li, X.; Lin, H.; Zhao, D.; Ye, M.; Zhang, S.; et al. Elevated Protein Lactylation Promotes Immunosuppressive Microenvironment and Therapeutic Resistance in Pancreatic Ductal Adenocarcinoma. J. Clin. Investig. 2025, 135, jci187024. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Hu, Z.; Xiong, F.; Yang, Y.; Peng, C.; Wang, D.; Li, X. Lipid Metabolism Reprogramming in Tumor-Associated Macrophages and Implications for Therapy. Lipids Health Dis. 2023, 22, 45. [Google Scholar] [CrossRef]
- Xu, S.; Chaudhary, O.; Rodríguez-Morales, P.; Sun, X.; Chen, D.; Zappasodi, R.; Xu, Z.; Pinto, A.F.M.; Williams, A.; Schulze, I.; et al. Uptake of Oxidized Lipids by the Scavenger Receptor CD36 Promotes Lipid Peroxidation and Dysfunction in CD8+ T Cells in Tumors. Immunity 2021, 54, 1561–1577.e7. [Google Scholar] [CrossRef]
- Huang, C.-X.; Lao, X.-M.; Wang, X.-Y.; Ren, Y.-Z.; Lu, Y.-T.; Shi, W.; Wang, Y.-Z.; Wu, C.-Y.; Xu, L.; Chen, M.-S.; et al. Pericancerous Cross-Presentation to Cytotoxic T Lymphocytes Impairs Immunotherapeutic Efficacy in Hepatocellular Carcinoma. Cancer Cell 2024, 42, 2082–2097.e10. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Wang, Y.; Liu, M.; Zhang, Y.; Feng, T.; Xiao, C.; Song, H.; Miao, R.; Xu, L.; et al. Lactylated Apolipoprotein C-II Induces Immunotherapy Resistance by Promoting Extracellular Lipolysis. Adv. Sci. 2024, 11, e2406333. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Cassmann, T.J.; Bhagwate, A.V.; Hitosugi, T.; Ip, W.K.E. Lactic Acid Induces Transcriptional Repression of Macrophage Inflammatory Response via Histone Acetylation. Cell Rep. 2024, 43, 113746. [Google Scholar] [CrossRef]
- Noe, J.T.; Rendon, B.E.; Geller, A.E.; Conroy, L.R.; Morrissey, S.M.; Young, L.E.A.; Bruntz, R.C.; Kim, E.J.; Wise-Mitchell, A.; Barbosa de Souza Rizzo, M.; et al. Lactate Supports a Metabolic-Epigenetic Link in Macrophage Polarization. Sci. Adv. 2021, 7, eabi8602. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yan, P.; Wang, D.; Bai, L.; Liang, H.; Zhu, X.; Zhu, H.; Ding, C.; Wei, H.; Wang, Y. Targeting Lactylation Reinforces NK Cell Cytotoxicity Within the Tumor Microenvironment. Nat. Immunol. 2025, 26, 1099–1112. [Google Scholar] [CrossRef]
- Lv, H.; Lv, G.; Chen, C.; Zong, Q.; Jiang, G.; Ye, D.; Cui, X.; He, Y.; Xiang, W.; Han, Q.; et al. NAD+ Metabolism Maintains Inducible PD-L1 Expression to Drive Tumor Immune Evasion. Cell Metab. 2021, 33, 110–127.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, C.; Wang, T.; Deng, H. Complex Roles of Nicotinamide N-Methyltransferase in Cancer Progression. Cell Death Dis. 2022, 13, 267. [Google Scholar] [CrossRef]
- Ma, X.; Bi, E.; Lu, Y.; Su, P.; Huang, C.; Liu, L.; Wang, Q.; Yang, M.; Kalady, M.F.; Qian, J.; et al. Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019, 30, 143–156.e5. [Google Scholar] [CrossRef]
- Bell, H.N.; Huber, A.K.; Singhal, R.; Korimerla, N.; Rebernick, R.J.; Kumar, R.; El-Derany, M.O.; Sajjakulnukit, P.; Das, N.K.; Kerk, S.A.; et al. Microenvironmental Ammonia Enhances T Cell Exhaustion in Colorectal Cancer. Cell Metab. 2023, 35, 134–149.e6. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Deng, Y.; Ye, C.; Peng, H. HIF-1α Signaling: Essential Roles in Tumorigenesis and Implications in Targeted Therapies. Genes Dis. 2024, 11, 234–251. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 Is a Novel Direct Target of HIF-1α, and Its Blockade under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Xun, Z.; Zhou, H.; Shen, M.; Liu, Y.; Sun, C.; Du, Y.; Jiang, Z.; Yang, L.; Zhang, Q.; Lin, C.; et al. Identification of Hypoxia-ALCAMhigh Macrophage- Exhausted T Cell Axis in Tumor Microenvironment Remodeling for Immunotherapy Resistance. Adv. Sci. 2024, 11, e2309885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lao, M.; Sun, K.; Yang, H.; He, L.; Liu, X.; Liu, L.; Zhang, S.; Guo, C.; Wang, S.; et al. Sphingolipid Synthesis in Tumor-Associated Macrophages Confers Immunotherapy Resistance in Hepatocellular Carcinoma. Sci. Adv. 2025, 11, eadv0558. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, B.; Zhao, W.; Guo, Y.; Wan, Y.; Wu, Z.; Su, S.; Gu, J.; Hu, X.; Feng, W.; et al. FABP5+ Lipid-Loaded Macrophages Process Tumour-Derived Unsaturated Fatty Acid Signal to Suppress T-Cell Antitumour Immunity. J. Hepatol. 2025, 82, 676–689. [Google Scholar] [CrossRef]
- Sun, J.; Esplugues, E.; Bort, A.; Cardelo, M.P.; Ruz-Maldonado, I.; Fernández-Tussy, P.; Wong, C.; Wang, H.; Ojima, I.; Kaczocha, M.; et al. Fatty Acid Binding Protein 5 Suppression Attenuates Obesity-Induced Hepatocellular Carcinoma by Promoting Ferroptosis and Intratumoral Immune Rewiring. Nat. Metab. 2024, 6, 741–763. [Google Scholar] [CrossRef]
- Xu, S.; Peng, X.; Wang, Z.; Le, C.; Wu, X.; Zeng, Z.; Zeng, S.; Zhang, C.; Qiu, M.; Zou, X.; et al. FABP7-Mediated Lipid-Laden Macrophages Drive the Formation of Pre-Metastatic Niche and Liver Metastasis. Int. J. Biol. Sci. 2025, 21, 4388–4409. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino Acid Metabolism in Immune Cells: Essential Regulators of the Effector Functions, and Promising Opportunities to Enhance Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef]
- Guo, C.; You, Z.; Shi, H.; Sun, Y.; Du, X.; Palacios, G.; Guy, C.; Yuan, S.; Chapman, N.M.; Lim, S.A.; et al. SLC38A2 and Glutamine Signalling in cDC1s Dictate Anti-Tumour Immunity. Nature 2023, 620, 200–208. [Google Scholar] [CrossRef]
- Yu, T.; Van der Jeught, K.; Zhu, H.; Zhou, Z.; Sharma, S.; Liu, S.; Eyvani, H.; So, K.M.; Singh, N.; Wang, J.; et al. Inhibition of Glutamate-to-Glutathione Flux Promotes Tumor Antigen Presentation in Colorectal Cancer Cells. Adv. Sci. 2025, 12, e2310308. [Google Scholar] [CrossRef]
- Du, L.; Xing, Z.; Tao, B.; Li, T.; Yang, D.; Li, W.; Zheng, Y.; Kuang, C.; Yang, Q. Both IDO1 and TDO Contribute to the Malignancy of Gliomas via the Kyn-AhR-AQP4 Signaling Pathway. Signal Transduct. Target. Ther. 2020, 5, 10, Erratum in Signal Transduct. Target. Ther. 2021, 6, 385. [Google Scholar] [CrossRef] [PubMed]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.-H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR Restricts a Treg-Macrophage Suppressive Axis Induced by L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(Reg) and T(H)17 Cell Differentiation by the Aryl Hydrocarbon Receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction Between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and Lactylation in Cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef]
- Lin, H.; Tison, K.; Du, Y.; Kirchhoff, P.; Kim, C.; Wang, W.; Yang, H.; Pitter, M.; Yu, J.; Liao, P.; et al. Itaconate Transporter SLC13A3 Impairs Tumor Immunity via Endowing Ferroptosis Resistance. Cancer Cell 2024, 42, 2032–2044.e6. [Google Scholar] [CrossRef]
- Fan, Y.; Dan, W.; Wang, Y.; Ma, Z.; Jian, Y.; Liu, T.; Li, M.; Wang, Z.; Wei, Y.; Liu, B.; et al. Itaconate Transporter SLC13A3 Confers Immunotherapy Resistance via Alkylation-Mediated Stabilization of PD-L1. Cell Metab. 2025, 37, 514–526.e5. [Google Scholar] [CrossRef]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-Associated Fibroblasts in the Single-Cell Era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef]
- Yang, M.; Wang, B.; Hou, W.; Zeng, H.; He, W.; Zhang, X.-K.; Yan, D.; Yu, H.; Huang, L.; Pei, L.; et al. NAD+ Metabolism Enzyme NNMT in Cancer-Associated Fibroblasts Drives Tumor Progression and Resistance to Immunotherapy by Modulating Macrophages in Urothelial Bladder Cancer. J. Immunother. Cancer 2024, 12, e009281. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Ugolini, A.; Yu, X.; Scirocchi, F.; Scocozza, D.; Peixoto, B.; Pace, A.; D’Angelo, L.; Liu, J.K.C.; Etame, A.B.; et al. Glucose-Driven Histone Lactylation Promotes the Immunosuppressive Activity of Monocyte-Derived Macrophages in Glioblastoma. Immunity 2024, 57, 1105–1123.e8. [Google Scholar] [CrossRef] [PubMed]
- Lamplugh, Z.L. Microenvironmental Regulation of Solid Tumour Resistance to CAR T Cell Therapy. Nat. Rev. Immunol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why Do Cancers Have High Aerobic Glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Herman, C.E.; Maciver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. J. Immunol. 2008, 180, 4476–4486. [Google Scholar] [CrossRef]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-Programmed Nutrient Partitioning in the Tumour Microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef]
- Shen, L.; Xiao, Y.; Zhang, C.; Li, S.; Teng, X.; Cui, L.; Liu, T.; Wu, N.; Lu, Z. Metabolic Reprogramming by Ex Vivo Glutamine Inhibition Endows CAR-T Cells with Less-Differentiated Phenotype and Persistent Antitumor Activity. Cancer Lett. 2022, 538, 215710. [Google Scholar] [CrossRef]
- Zou, W.; Han, Z.; Wang, Z.; Liu, Q. Targeting Glutamine Metabolism as a Potential Target for Cancer Treatment. J. Exp. Clin. Cancer Res. 2025, 44, 180. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α) and Cancer: Mechanisms of Tumor Hypoxia and Therapeutic Targeting. Cureus 2024, 16, e70700. [Google Scholar] [CrossRef]
- Zhang, D.; Li, A.M.; Hu, G.; Huang, M.; Yang, F.; Zhang, L.; Wellen, K.E.; Xu, X.; Conn, C.S.; Zou, W.; et al. PHGDH-Mediated Endothelial Metabolism Drives Glioblastoma Resistance to Chimeric Antigen Receptor T Cell Immunotherapy. Cell Metab. 2023, 35, 517–534.e8. [Google Scholar] [CrossRef]
- Yan, Z.-X.; Dong, Y.; Qiao, N.; Zhang, Y.-L.; Wu, W.; Zhu, Y.; Wang, L.; Cheng, S.; Xu, P.-P.; Zhou, Z.-S.; et al. Cholesterol Efflux from C1QB-Expressing Macrophages Is Associated with Resistance to Chimeric Antigen Receptor T Cell Therapy in Primary Refractory Diffuse Large B Cell Lymphoma. Nat. Commun. 2024, 15, 5183. [Google Scholar] [CrossRef]
- Ren, T.; Sun, L.; Zheng, Y.; Jiang, Y.; Guo, Y.; Ma, J. Mechanical Forces and Immune Cells in the Tumor Microenvironment: From Regulation Mechanisms to Therapeutic Strategies. Int. J. Surg. 2025, 111, 5420–5434. [Google Scholar] [CrossRef] [PubMed]
- Flies, D.B.; Langermann, S.; Jensen, C.; Karsdal, M.A.; Willumsen, N. Regulation of Tumor Immunity and Immunotherapy by the Tumor Collagen Extracellular Matrix. Front. Immunol. 2023, 14, 1199513. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bartleson, J.M.; Butenko, S.; Alonso, V.; Liu, W.F.; Winer, D.A.; Butte, M.J. Tuning Immunity Through Tissue Mechanotransduction. Nat. Rev. Immunol. 2022, 23, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Regan, K.; Najera, J.; Grinstaff, M.W.; Datta, M.; Nia, H.T. The Peritumor Microenvironment: Physics and Immunity. Trends Cancer 2023, 9, 609–623. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay Between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef]
- Muliaditan, T.; Caron, J.; Okesola, M.; Opzoomer, J.W.; Kosti, P.; Georgouli, M.; Gordon, P.; Lall, S.; Kuzeva, D.M.; Pedro, L.; et al. Macrophages Are Exploited from an Innate Wound Healing Response to Facilitate Cancer Metastasis. Nat. Commun. 2018, 9, 2951. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.-C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix Architecture Defines the Preferential Localization and Migration of T Cells into the Stroma of Human Lung Tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor Stiffening Reversion Through Collagen Crosslinking Inhibition Improves T Cell Migration and Anti-PD-1 Treatment. Elife 2021, 10, e58688. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Naba, A. Mechanisms of Assembly and Remodelling of the Extracellular Matrix. Nat. Rev. Mol. Cell Biol. 2024, 25, 865–885. [Google Scholar] [CrossRef]
- Tharp, K.M.; Kersten, K.; Maller, O.; Timblin, G.A.; Stashko, C.; Canale, F.P.; Menjivar, R.E.; Hayward, M.-K.; Berestjuk, I.; ten Hoeve, J.; et al. Tumor-Associated Macrophages Restrict CD8+ T Cell Function Through Collagen Deposition and Metabolic Reprogramming of the Breast Cancer Microenvironment. Nat. Cancer 2024, 5, 1045–1062. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Hou, Y.; Lin, Y.; Zhao, H.; Shi, Y.; Chen, K.; Nian, C.; Tang, J.; Pan, L.; et al. Osr2 Functions as a Biomechanical Checkpoint to Aggravate CD8+ T Cell Exhaustion in Tumor. Cell 2024, 187, 3409–3426.e24. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Sun, W.; Yang, Y.; Wen, D.; Lin, F.; Wang, D.; Li, K.; Zhang, N.; Liang, J.; Xiong, C.; et al. PIEZO1 Mechanically Regulates the Antitumour Cytotoxicity of T Lymphocytes. Nat. Biomed. Eng. 2024, 8, 1162–1176. [Google Scholar] [CrossRef]
- Bonner, M.; Askew, D.; Sathish Kumar, V.; Tomchuck, S.L.; Eid, S.; Abiff, M.; Myers, J.T.; Nguyen, P.; Garyu, J.W.A.; Miller, T.E.; et al. Piezo1 Deletion Enhances Cross-Priming of CD8+ T Cells by Tumor-Infiltrating CD11b+ Dendritic Cells. J. Immunother. Cancer 2025, 13, e011815. [Google Scholar] [CrossRef]
- Targeting the PIEZO1 Pathway Boosts T Cell Antitumour Cytotoxicity. Nat. Biomed. Eng. 2024, 8, 1071–1072. [CrossRef] [PubMed]
- Micek, H.M.; Yang, N.; Dutta, M.; Rosenstock, L.; Ma, Y.; Hielsberg, C.; McCord, M.; Notbohm, J.; McGregor, S.; Kreeger, P.K. The Role of Piezo1 Mechanotransduction in High-Grade Serous Ovarian Cancer: Insights from an in Vitro Model of Collective Detachment. Sci. Adv. 2024, 10, eadl4463. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Wang, M.; Wang, Y.; Qian, J.; Xing, X.; Wang, Z.; You, Y.; Guo, K.; Chen, J.; et al. Activation of Piezo1 Contributes to Matrix Stiffness-Induced Angiogenesis in Hepatocellular Carcinoma. Cancer Commun. 2022, 42, 1162–1184. [Google Scholar] [CrossRef] [PubMed]
- Mpekris, F.; Panagi, M.; Charalambous, A.; Voutouri, C.; Stylianopoulos, T. Modulating Cancer Mechanopathology to Restore Vascular Function and Enhance Immunotherapy. Cell Rep. Med. 2024, 5, 101626. [Google Scholar] [CrossRef]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Zuo, X.; Li, G.; Wang, J.; Liu, J.; Wang, X.; Wang, S.; Zhang, W.; Zhang, K.; et al. CXCL12+ Tumor-Associated Endothelial Cells Promote Immune Resistance in Hepatocellular Carcinoma. J. Hepatol. 2025, 82, 634–648. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, D.; Hu, J.; Chen, Y.; Sun, L.; Yu, H.; Xiong, Y.; Huang, Z.; Xia, H.; Zhu, X.; et al. Multi-Omic Profiling Highlights Factors Associated with Resistance to Immuno-Chemotherapy in Non-Small-Cell Lung Cancer. Nat. Genet. 2025, 57, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Z.; Zaretsky, J.M.; Hu-Lieskovan, S.; Kim, D.W.; Algazi, A.; Johnson, D.B.; Liniker, E.; Kong, B.; Munhoz, R.; Rapisuwon, S.; et al. High Response Rate to PD-1 Blockade in Desmoplastic Melanomas. Nature 2018, 553, 347–350. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The Extracellular Matrix and the Immune System: A Mutually Dependent Relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef]
- Mei, J.; Yang, K.; Zhang, X.; Luo, Z.; Tian, M.; Fan, H.; Chu, J.; Zhang, Y.; Ding, J.; Xu, J.; et al. Intratumoral Collagen Deposition Supports Angiogenesis Suggesting Anti-Angiogenic Therapy in Armored and Cold Tumors. Adv. Sci. 2025, 12, e2409147. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Fu, R.; Trinh, B.; Sun, N.; Liu, J.; Kumar, A.; Baglieri, J.; Siruno, J.; Le, M.; et al. Collagenolysis-Dependent DDR1 Signalling Dictates Pancreatic Cancer Outcome. Nature 2022, 610, 366–372, Erratum in Nature 2023, 615, E24. [Google Scholar] [CrossRef]
- Shen, X.-T.; Xie, S.-Z.; Zheng, X.; Zou, T.-T.; Hu, B.-Y.; Xu, J.; Liu, L.; Xu, Y.-F.; Wang, X.-F.; Wang, H.; et al. Cirrhotic-Extracellular Matrix Attenuates aPD-1 Treatment Response by Initiating Immunosuppressive Neutrophil Extracellular Traps Formation in Hepatocellular Carcinoma. Exp. Hematol. Oncol. 2024, 13, 20. [Google Scholar] [CrossRef]
- Xie, W.; Yu, X.; Yang, Q.; Ke, N.; Wang, P.; Kong, H.; Wu, X.; Ma, P.; Chen, L.; Yang, J.; et al. Data from the Immunomechanical Checkpoint PYK2 Governs Monocyte-to-Macrophage Differentiation in Pancreatic Cancer; American Association for Cancer Research: Philadelphia, PA, USA, 2025. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Yang, Z.; Zhang, X.; Xu, Z.; Yang, L.; Yang, X.; Zhou, W.; Liu, W. Matrix Stiffness-Dependent PD-L2 Deficiency Improves SMYD3/xCT-Mediated Ferroptosis and the Efficacy of Anti-PD-1 in HCC. J. Adv. Res. 2025, 73, 265–282. [Google Scholar] [CrossRef]
- Young, R.M.; Engel, N.W.; Uslu, U.; Wellhausen, N.; June, C.H. Next-Generation CAR t-Cell Therapies. Cancer Discov. 2022, 12, 1625–1633. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, L.; Ferrara, N. Biology and Therapeutic Targeting of Vascular Endothelial Growth Factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef] [PubMed]
- Lanitis, E.; Irving, M.; Coukos, G. Tumour-Associated Vasculature in T Cell Homing and Immunity: Opportunities for Cancer Therapy. Nat. Rev. Immunol. 2025, 25, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Bouzin, C.; Brouet, A.; De Vriese, J.; DeWever, J.; Feron, O. Effects of Vascular Endothelial Growth Factor on the Lymphocyte-Endothelium Interactions: Identification of Caveolin-1 and Nitric Oxide as Control Points of Endothelial Cell Anergy. J. Immunol. 2007, 178, 1505–1511. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Damen, C.A.; Martinotti, S.; Blijham, G.H.; Groenewegen, G. Endothelial Intercellular Adhesion Molecule-1 Expression Is Suppressed in Human Malignancies: The Role of Angiogenic Factors. Cancer Res. 1996, 56, 1111–1117. [Google Scholar] [PubMed]
- Mavuluri, J.; Dhungana, Y.; Jones, L.L.; Bhatara, S.; Shi, H.; Yang, X.; Lim, S.-E.; Reyes, N.; Chi, H.; Yu, J.; et al. GPR65 Inactivation in Tumor Cells Drives Antigen-Independent CAR T-Cell Resistance via Macrophage Remodeling. Cancer Discov. 2025, 15, 1018–1036. [Google Scholar] [CrossRef]
- Kabir, A.U.; Subramanian, M.; Kwon, Y.; Choi, K. Linking Tumour Angiogenesis and Tumour Immunity. Nat. Rev. Immunol. 2025. [Google Scholar] [CrossRef]
- Uslu, U.; June, C.H. Beyond the Blood: Expanding CAR T Cell Therapy to Solid Tumors. Nat. Biotechnol. 2025, 43, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the Gut and Tumor Microbiota in Cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral Microbiota: Roles in Cancer Initiation, Development and Therapeutic Efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, K.; Wang, L.; Liu, N.-N.; Mao, W. Unraveling the Fungi–Cancer Connection. Research 2025, 8, 931. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Y.; Ren, H.; Liu, Y.; Wang, Q.; Ran, M.; Zhou, W.; Tian, L.; Zheng, X.; Qiao, C.; et al. The Tumor Microbiome in Cancer Progression: Mechanisms and Therapeutic Potential. Mol. Cancer 2025, 24, 195. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Thi-Thu Ngo, H.; Son, J.; Hong, Y.; Min, J.-J. Exploiting Bacteria for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 569–589. [Google Scholar] [CrossRef]
- Liu, C.; Fu, L.; Wang, Y.; Yang, W. Influence of the Gut Microbiota on Immune Cell Interactions and Cancer Treatment. J. Transl. Med. 2024, 22, 939. [Google Scholar] [CrossRef]
- Datorre, J.G.; dos Reis, M.B.; Sorroche, B.P.; Teixeira, G.R.; Hatano, S.S.; de Carvalho, A.C.; Gama, R.R.; Rebolho Batista Arantes, L.M.; Reis, R.M. Intratumoral Fusobacterium nucleatum Is Associated with Better Cancer-Specific Survival in Head and Neck Cancer Patients. J. Oral Microbiol. 2025, 17, 2487644. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial Bile Acid Metabolites Modulate Gut RORγ+ Regulatory T Cell Homeostasis. Nature 2019, 577, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Fidelle, M.; Rauber, C.; Alves Costa Silva, C.; Tian, A.-L.; Lahmar, I.; de La Varende, A.-L.M.; Zhao, L.; Thelemaque, C.; Lebhar, I.; Messaoudene, M.; et al. A Microbiota-Modulated Checkpoint Directs Immunosuppressive Intestinal T Cells into Cancers. Science 2023, 380, eabo2296. [Google Scholar] [CrossRef]
- Rah, B.; Mohamed, M.W.; Janeeh, A.S.; Kurabi, T.M.; Salam, J.S.A.; Hamad, M. Fatty Allies: How Short-Chain Fatty Acids Turn the Tumor Microenvironment Against Cancer. Cancer Immunol. Connect 2025, 1, 1. [Google Scholar] [CrossRef]
- Liao, Q.; Zhou, X.; Wu, L.; Yang, Y.; Zhu, X.; Liao, H.; Zhang, Y.; Lian, W.; Zhang, F.; Wang, H.; et al. Gut Microbial Metabolite 4-Hydroxybenzeneacetic Acid Drives Colorectal Cancer Progression via Accumulation of Immunosuppressive PMN-MDSCs. J. Clin. Investig. 2025, 135, e181243. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wu, R.; Mao, M.; Ji, Y.; Wang, X.; Dou, S.; Yan, M.; Chen, W. Fusobacterium nucleatum-Derived Outer Membrane Vesicles Promote Immunotherapy Resistance via Changes in Tryptophan Metabolism in Tumour-Associated Macrophages. J. Extracell. Vesicles 2025, 14, e70070. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Guo, S.; Li, Y.; Lu, Y.; Liu, L.; Chen, S.; An, J.; Zhang, G. Fusobacterium nucleatum-Driven CX3CR1+ PD-L1+ Phagocytes Route to Tumor Tissues and Reshape Tumor Microenvironment. Gut Microbes 2025, 17, 2442037. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Miao, Y.; Wang, D.; Xu, D.; Liu, R.; Liu, X.; Zhang, Y.; Zhang, X.; Qin, H. Engineered Tumor-Symbiotic Bacterial Membrane Nanovesicles Enable Precise Immuno-Chemotherapy of Colorectal Cancer. J. Control. Release 2025, 388, 114291. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum Facilitates Anti-PD-1 Therapy in Microsatellite Stable Colorectal Cancer. Cancer Cell 2024, 42, 1729–1746.e8, Erratum in Cancer Cell 2025, 43, 564–574. [Google Scholar] [CrossRef]
- Battaglia, T.W.; Mimpen, I.L.; Traets, J.J.H.; van Hoeck, A.; Zeverijn, L.J.; Geurts, B.S.; de Wit, G.F.; Noë, M.; Hofland, I.; Vos, J.L.; et al. A Pan-Cancer Analysis of the Microbiome in Metastatic Cancer. Cell 2024, 187, 2324–2335.e19. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast Cancer Colonization by Fusobacterium nucleatum Accelerates Tumor Growth and Metastatic Progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-Resident Intracellular Microbiota Promotes Metastatic Colonization in Breast Cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef]
- Johnson, J.L.; Jones, M.B.; Cobb, B.A. Polysaccharide a from the Capsule of Bacteroides Fragilis Induces Clonal CD4+ T Cell Expansion. J. Biol. Chem. 2015, 290, 5007–5014. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.; Lenardon, M.; Traven, A. Bacteria-Derived Short-Chain Fatty Acids as Potential Regulators of Fungal Commensalism and Pathogenesis. Trends Microbiol. 2024, 32, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Seelbinder, B.; Lohinai, Z.; Vazquez-Uribe, R.; Brunke, S.; Chen, X.; Mirhakkak, M.; Lopez-Escalera, S.; Dome, B.; Megyesfalvi, Z.; Berta, J.; et al. Candida Expansion in the Gut of Lung Cancer Patients Associates with an Ecological Signature That Supports Growth Under Dysbiotic Conditions. Nat. Commun. 2023, 14, 2673. [Google Scholar] [CrossRef]
- Liu, N.-N.; Yi, C.-X.; Wei, L.-Q.; Zhou, J.-A.; Jiang, T.; Hu, C.-C.; Wang, L.; Wang, Y.-Y.; Zou, Y.; Zhao, Y.-K.; et al. The Intratumor Mycobiome Promotes Lung Cancer Progression via Myeloid-Derived Suppressor Cells. Cancer Cell 2023, 41, 1927–1944.e9, Erratum in Cancer Cell 2024, 42, 318–322. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Li, Z.-Q.; Wang, Y.; Luo, Y.-L.; Guo, W.-P.; Meng, N.; Bu, G.-L.; Zhang, L.-L.; Li, S.-X.; Kong, X.-W.; et al. Extracellular Vesicles Derived EBV Tegument Protein BRRF2 Suppresses cGAS Phase Separation to Promote Anti-Viral Innate Immune Evasion. Nat. Commun. 2025, 16, 9015. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Sanders, P.D.; Guram, K.; Kim, S.S.; Paolini, F.; Venuti, A.; Cohen, E.E.W.; Gutkind, J.S.; Califano, J.A.; Sharabi, A.B. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res. 2020, 80, 732–746. [Google Scholar] [CrossRef]
- Roy, S.; Miyauchi, S.; Jones, R.N.; Kim, S.S.; Venuti, A.; Boparai, A.S.; Mell, L.K.; Guo, T.; Califano, J.A.; Sharabi, A.B. HPV E5 Mediates Resistance to Chemotherapy and Radiation by Promoting a Stem Cell-like Phenotype in Head and Neck Squamous Cell Carcinoma. Cancer Res. 2025, 85, 3875–3893. [Google Scholar] [CrossRef]
- Gao, Y.; You, M.; Fu, J.; Tian, M.; Zhong, X.; Du, C.; Hong, Z.; Zhu, Z.; Liu, J.; Markowitz, G.J.; et al. Intratumoral Stem-like CCR4+ Regulatory T Cells Orchestrate the Immunosuppressive Microenvironment in HCC Associated with Hepatitis B. J. Hepatol. 2022, 76, 148–159. [Google Scholar] [CrossRef]
- Al Subeh, Z.Y.; Pierre, H.C.; Pearce, C.J.; Grinstaff, M.W.; Colby, A.H.; Liu, K.; Oberlies, N.H. Verticillin A-Loaded Surgical Buttresses Prevent Local Pancreatic Cancer Recurrence in a Murine Model. Mol. Pharm. 2025, 22, 1220–1229. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome–Mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Wu, Q.-W.; Han, Y.; Xiang, S.-J.; Wang, Y.-N.; Wu, W.-W.; Chen, Y.-X.; Feng, Z.-Q.; Wang, Y.-Y.; Xu, Z.-G.; et al. Alistipes Finegoldii Augments the Efficacy of Immunotherapy Against Solid Tumors. Cancer Cell 2025, 43, 1714–1730.e12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.-W.; Chan, L.-C.; Wei, Y.; Hsu, J.-M.; Xia, W.; Cha, J.-H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 Harbors Active Defense Function to Suppress T Cell Killing of Breast Cancer Cells and Promote Tumor Growth. Cell Res. 2018, 28, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Rubenich, D.S.; Domagalski, J.L.; Gentil, G.F.S.; Eichberger, J.; Fiedler, M.; Weber, F.; Federlin, M.; Poeck, H.; Reichert, T.E.; Ettl, T.; et al. The Immunomodulatory Ballet of Tumour-Derived Extracellular Vesicles and Neutrophils Orchestrating the Dynamic CD73/PD-L1 Pathway in Cancer. J. Extracell. Vesicles 2024, 13, e12480. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.; Zhang, Y.; Tao, Y.; Zhao, L.; Abusalamah, H.; Huffman, C.; Harbison, R.A.; Puram, S.V.; Wang, Y.; et al. Tumor Extracellular Vesicle-Derived PD-L1 Promotes T Cell Senescence Through Lipid Metabolism Reprogramming. Sci. Transl. Med. 2025, 17, eadm7269. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-Derived circCCAR1 Promotes CD8 + T-Cell Dysfunction and Anti-PD1 Resistance in Hepatocellular Carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef]
- Can, C.; Yang, X.; Jia, H.; Wu, H.; Guo, X.; Wei, Y.; Jia, Z.; Liu, W.; Zhang, A.; He, N.; et al. Exosomal Circ_0006896 Promotes AML Progression via Interaction with HDAC1 and Restriction of Antitumor Immunity. Mol. Cancer 2025, 24, 4. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Zhang, M.; Li, Y.; Tang, H.; Wang, Y.; Song, C.; Song, B.; Tan, B. Circ_0001947 Encapsulated by Small Extracellular Vesicles Promotes Gastric Cancer Progression and Anti-PD-1 Resistance by Modulating CD8+ T Cell Exhaustion. J. Nanobiotechnol. 2024, 22, 563. [Google Scholar] [CrossRef]
- Lan, T.; Gao, F.; Cai, Y.; Lv, Y.; Zhu, J.; Liu, H.; Xie, S.; Wan, H.; He, H.; Xie, K.; et al. The Protein circPETH-147aa Regulates Metabolic Reprogramming in Hepatocellular Carcinoma Cells to Remodel Immunosuppressive Microenvironment. Nat. Commun. 2025, 16, 333. [Google Scholar] [CrossRef]
- Zhong, W.; Qin, Z.; Yu, Z.; Yang, J.; Yan, D.; Engel, N.W.; Sheppard, N.C.; Fan, Y.; Radhakrishnan, R.; Xu, X.; et al. Overcoming Extracellular Vesicle-Mediated Fratricide Improves CAR T Cell Treatment against Solid Tumors. Nat. Cancer 2025, 6, 1157–1172. [Google Scholar] [CrossRef]
- Zak, J.; Pratumchai, I.; Marro, B.S.; Marquardt, K.L.; Zavareh, R.B.; Lairson, L.L.; Oldstone, M.B.A.; Varner, J.A.; Hegerova, L.; Cao, Q.; et al. JAK Inhibition Enhances Checkpoint Blockade Immunotherapy in Patients with Hodgkin Lymphoma. Science 2024, 384, eade8520. [Google Scholar] [CrossRef]
- Liu, X.; Hogg, G.D.; Zuo, C.; Borcherding, N.C.; Baer, J.M.; Lander, V.E.; Kang, L.-I.; Knolhoff, B.L.; Ahmad, F.; Osterhout, R.E.; et al. Context-Dependent Activation of STING-Interferon Signaling by CD11b Agonists Enhances Anti-Tumor Immunity. Cancer Cell 2023, 41, 1073–1090.e12. [Google Scholar] [CrossRef]
- Liu, T.; Sun, T.; Chen, X.; Wu, J.; Sun, X.; Liu, X.; Yan, H.; Fu, Q.; Fan, Z.; Wang, X.; et al. Targeting ARPC1B Overcomes Immune Checkpoint Inhibitor Resistance in Glioblastoma by Reversing Protumorigenic Macrophage Polarization. Cancer Res. 2025, 85, 1236–1252. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Long, X. Targeting Polymorphonuclear Myeloid-Derived Suppressor Cells in the Immunosuppressive Tumor Microenvironment for Cancer Immunotherapy. Medcomm 2024, 5, e602. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Ma, Q.; Du, M.; Wang, H.; Zhu, Y.; Deng, L.; Gao, W.; Wang, C.; Liu, Y.; et al. OX40L-Armed Oncolytic Virus Boosts t-Cell Response and Remodels Tumor Microenvironment for Pancreatic Cancer Treatment. Theranostics 2023, 13, 4016–4029. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Zhou, J.; Xiao, Z.; Liu, J.; Deng, S.; Hong, X.; Huang, W.; Cai, M.; Guo, Y.; et al. T Cell-Mediated Targeted Delivery of Tadalafil Regulates Immunosuppression and Polyamine Metabolism to Overcome Immune Checkpoint Blockade Resistance in Hepatocellular Carcinoma. J. Immunother. Cancer 2023, 11, e006493. [Google Scholar] [CrossRef]
- Dai, Y.; Knisely, A.; Yano, M.; Dang, M.; Hinchcliff, E.M.; Lee, S.; Welp, A.; Chelvanambi, M.; Lastrapes, M.; Liu, H.; et al. PPP2R1A Mutations Portend Improved Survival After Cancer Immunotherapy. Nature 2025, 644, 537–546, Erratum in Nature 2025, 645, E10. [Google Scholar] [CrossRef]
- Bao, Y.; Cruz, G.; Zhang, Y.; Qiao, Y.; Mannan, R.; Hu, J.; Yang, F.; Gondal, M.; Shahine, M.; Kang, S.; et al. The UBA1–STUB1 Axis Mediates Cancer Immune Escape and Resistance to Checkpoint Blockade. Cancer Discov. 2024, 15, 363–381. [Google Scholar] [CrossRef]
- Yamada-Hunter, S.A.; Theruvath, J.; McIntosh, B.J.; Freitas, K.A.; Lin, F.; Radosevich, M.T.; Leruste, A.; Dhingra, S.; Martinez-Velez, N.; Xu, P.; et al. Engineered CD47 Protects T Cells for Enhanced Antitumour Immunity. Nature 2024, 630, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Rodriguez, J.L.; Yin, Y.; Logun, M.T.; Zhang, L.; Yu, S.; Hicks, K.A.; Zhang, J.V.; Zhang, L.; Xie, C.; et al. Armored Bicistronic CAR T Cells with Dominant-Negative TGF-β Receptor II to Overcome Resistance in Glioblastoma. Mol. Ther. 2024, 32, 3522–3538. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Takei, H.N.; Escobar, G.; Kann, M.C.; Bouffard, A.A.; Kienka, T.; Supper, V.M.; Armstrong, A.; Salas-Benito, D.; Phillips, M.K.; et al. IFN-γ–Resistant CD28 CAR T Cells Demonstrate Increased Survival, Efficacy, and Durability in Multiple Murine Tumor Models. Sci. Transl. Med. 2025, 17, eadp8166. [Google Scholar] [CrossRef]
- Qiao, T.; Xiong, Y.; Feng, Y.; Guo, W.; Zhou, Y.; Zhao, J.; Jiang, T.; Shi, C.; Han, Y. Inhibition of LDH-a by Oxamate Enhances the Efficacy of Anti-PD-1 Treatment in an NSCLC Humanized Mouse Model. Front. Oncol. 2021, 11, 632364. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Du, X.; Li, J.; Qin, F.X.-F. Evolving Roles of CD38 Metabolism in Solid Tumour Microenvironment. Br. J. Cancer 2022, 128, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Cai, Y.; Chen, D.; Jiang, G.; Xu, Y.; Chen, R.; Wang, F.; Wang, X.; Zheng, M.; Zhao, X.; et al. Statin Shapes Inflamed Tumor Microenvironment and Enhances Immune Checkpoint Blockade in Non–Small Cell Lung Cancer. JCI Insight 2022, 7, e161940. [Google Scholar] [CrossRef]
- Song, C.; Zhang, X.; Cao, Z.; Wei, Z.; Zhou, M.; Wang, Y.; Han, S.; Cai, Y.; Han, W. Regulating Tumor Cholesterol Microenvironment to Enhance Photoimmunotherapy in Oral Squamous Cell Carcinoma. Chem. Eng. J. 2023, 462, 142160. [Google Scholar] [CrossRef]
- Zhao, H.; Niu, M.; Guo, Y.; Li, Q.; Wang, Y.; Jiang, Q.; Song, Q.; Zhang, Y.; Wang, L. A Lipid Starvation Strategy-Synergized Neutrophil Activation for Postoperative Melanoma Immunotherapy. J. Control. Release 2025, 380, 860–874. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, L.; Jiang, S.; Yang, H.; Guo, J.; Jiang, L.; Li, T.; Zhang, H.; Bai, Y.; Lou, Y.; et al. Exhaustion-Associated Cholesterol Deficiency Dampens the Cytotoxic Arm of Antitumor Immunity. Cancer Cell 2023, 41, 1276–1293.e11. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Klysz, D.D.; Chen, Y.; Malipatlolla, M.; Lone, J.; Fowler, C.; Stuani, L.; May, A.; Bashti, M.; Xu, P.; et al. GLUT1 Overexpression in CAR-T Cells Induces Metabolic Reprogramming and Enhances Potency. Nat. Commun. 2024, 15, 8658. [Google Scholar] [CrossRef]
- Chan, J.D.; Scheffler, C.M.; Munoz, I.; Sek, K.; Lee, J.N.; Huang, Y.-K.; Yap, K.M.; Saw, N.Y.L.; Li, J.; Chen, A.X.Y.; et al. FOXO1 Enhances CAR T Cell Stemness, Metabolic Fitness and Efficacy. Nature 2024, 629, 201–210. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Andreatta, M.; Feng, B.; Xie, Y.-Q.; Wenes, M.; Wang, Y.; Gao, M.; Hu, X.; Romero, P.; et al. IL-10-Expressing CAR T Cells Resist Dysfunction and Mediate Durable Clearance of Solid Tumors and Metastases. Nat. Biotechnol. 2024, 42, 1693–1704. [Google Scholar] [CrossRef]
- Zhao, S.; Peralta, R.M.; Avina-Ochoa, N.; Delgoffe, G.M.; Kaech, S.M. Metabolic Regulation of T Cells in the Tumor Microenvironment by Nutrient Availability and Diet. Semin. Immunol. 2021, 52, 101485. [Google Scholar] [CrossRef]
- Peng, J.; Yin, X.; Yun, W.; Meng, X.; Huang, Z. Radiotherapy-Induced Tumor Physical Microenvironment Remodeling to Overcome Immunotherapy Resistance. Cancer Lett. 2023, 559, 216108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy Combined with Immunotherapy: The Dawn of Cancer Treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Hao, H.; Sun, S.; Fu, Y.; Wen, S.; Wen, Y.; Yi, Y.; Peng, Z.; Fang, Y.; Tang, J.; Wang, T.; et al. Magnesium Peroxide-Based Biomimetic Nanoigniter Degrades Extracellular Matrix to Awake T Cell-Mediated Cancer Immunotherapy. Biomaterials 2025, 317, 123043. [Google Scholar] [CrossRef]
- Zhang, X.; Lao, M.; Yang, H.; Sun, K.; Dong, Y.; He, L.; Jiang, X.; Wu, H.; Jiang, Y.; Li, M.; et al. Targeting Cancer-Associated Fibroblast Autophagy Renders Pancreatic Cancer Eradicable with Immunochemotherapy by Inhibiting Adaptive Immune Resistance. Autophagy 2024, 20, 1314–1334. [Google Scholar] [CrossRef]
- Panagi, M.; Mpekris, F.; Voutouri, C.; Hadjigeorgiou, A.G.; Symeonidou, C.; Porfyriou, E.; Michael, C.; Stylianou, A.; Martin, J.D.; Cabral, H.; et al. Data from Stabilizing Tumor-Resident Mast Cells Restores t-Cell Infiltration and Sensitizes Sarcomas to PD-L1 Inhibition; American Association for Cancer Research: Philadelphia, PA, USA, 2024. [Google Scholar] [CrossRef]
- Ash, S.L.; Orha, R.; Mole, H.; Dinesh-Kumar, M.; Lee, S.P.; Turrell, F.K.; Isacke, C.M. Targeting the Activated Microenvironment with Endosialin (CD248)-Directed CAR-T Cells Ablates Perivascular Cells to Impair Tumor Growth and Metastasis. J. Immunother. Cancer 2024, 12, e008608. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.-Y.; Do, E.-J.; Bae, D.-J.; Kim, S.; Kweon, M.-N.; Song, J.S.; et al. Fecal Microbiota Transplantation Improves Anti-PD-1 Inhibitor Efficacy in Unresectable or Metastatic Solid Cancers Refractory to Anti-PD-1 Inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Hadi, D.K.; Baines, K.J.; Jabbarizadeh, B.; Miller, W.H.; Jamal, R.; Ernst, S.; Logan, D.; Belanger, K.; Esfahani, K.; Elkrief, A.; et al. Improved Survival in Advanced Melanoma Patients Treated with Fecal Microbiota Transplantation Using Healthy Donor Stool in Combination with Anti-PD1: Final Results of the MIMic Phase 1 Trial. J. Immunother. Cancer 2025, 13, e012659. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut Microbiota-Derived Short-Chain Fatty Acids and Colorectal Cancer: Ready for Clinical Translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef]
- Preet, R.; Islam, M.A.; Shim, J.; Rajendran, G.; Mitra, A.; Vishwakarma, V.; Kutz, C.; Choudhury, S.; Pathak, H.; Dai, Q.; et al. Gut Commensal Bifidobacterium-Derived Extracellular Vesicles Modulate the Therapeutic Effects of Anti-PD-1 in Lung Cancer. Nat. Commun. 2025, 16, 3500. [Google Scholar] [CrossRef]
- Marcos-Kovandzic, L.; Avagliano, M.; Ben Khelil, M.; Srikanthan, J.; Abdallah, R.; Petrocelli, V.; Rengassamy, J.; Alfaro, A.; Bied, M.; Fidelle, M.; et al. Gut Microbiota Modulation Through Akkermansia spp. Supplementation Increases CAR-T Cell Potency. Cancer Discov. 2025, 15, 1905–1926. [Google Scholar] [CrossRef]
- Ye, L.; Zhou, C.; Peng, H.; Wang, J.; Liu, Z.; Yang, Q. Multi-Level Feature Interaction Image Super-Resolution Network Based on Convolutional Nonlinear Spiking Neural Model. Neural Netw. 2024, 177, 106366. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Feng, Q.; Xia, Y.; Liao, L.; Xie, S. Soil and Seed: Tumor Microenvironment Nurtures Immunotherapy Resistance and Renewal. Int. J. Mol. Sci. 2025, 26, 10547. https://doi.org/10.3390/ijms262110547
Li Y, Feng Q, Xia Y, Liao L, Xie S. Soil and Seed: Tumor Microenvironment Nurtures Immunotherapy Resistance and Renewal. International Journal of Molecular Sciences. 2025; 26(21):10547. https://doi.org/10.3390/ijms262110547
Chicago/Turabian StyleLi, Yiya, Qiushi Feng, Yangyang Xia, Lingzi Liao, and Shang Xie. 2025. "Soil and Seed: Tumor Microenvironment Nurtures Immunotherapy Resistance and Renewal" International Journal of Molecular Sciences 26, no. 21: 10547. https://doi.org/10.3390/ijms262110547
APA StyleLi, Y., Feng, Q., Xia, Y., Liao, L., & Xie, S. (2025). Soil and Seed: Tumor Microenvironment Nurtures Immunotherapy Resistance and Renewal. International Journal of Molecular Sciences, 26(21), 10547. https://doi.org/10.3390/ijms262110547






