Key Signals Produced by Gut Microbiota Associated with Metabolic Syndrome, Cancer, Cardiovascular Diseases, and Brain Functions
Abstract
1. Introduction
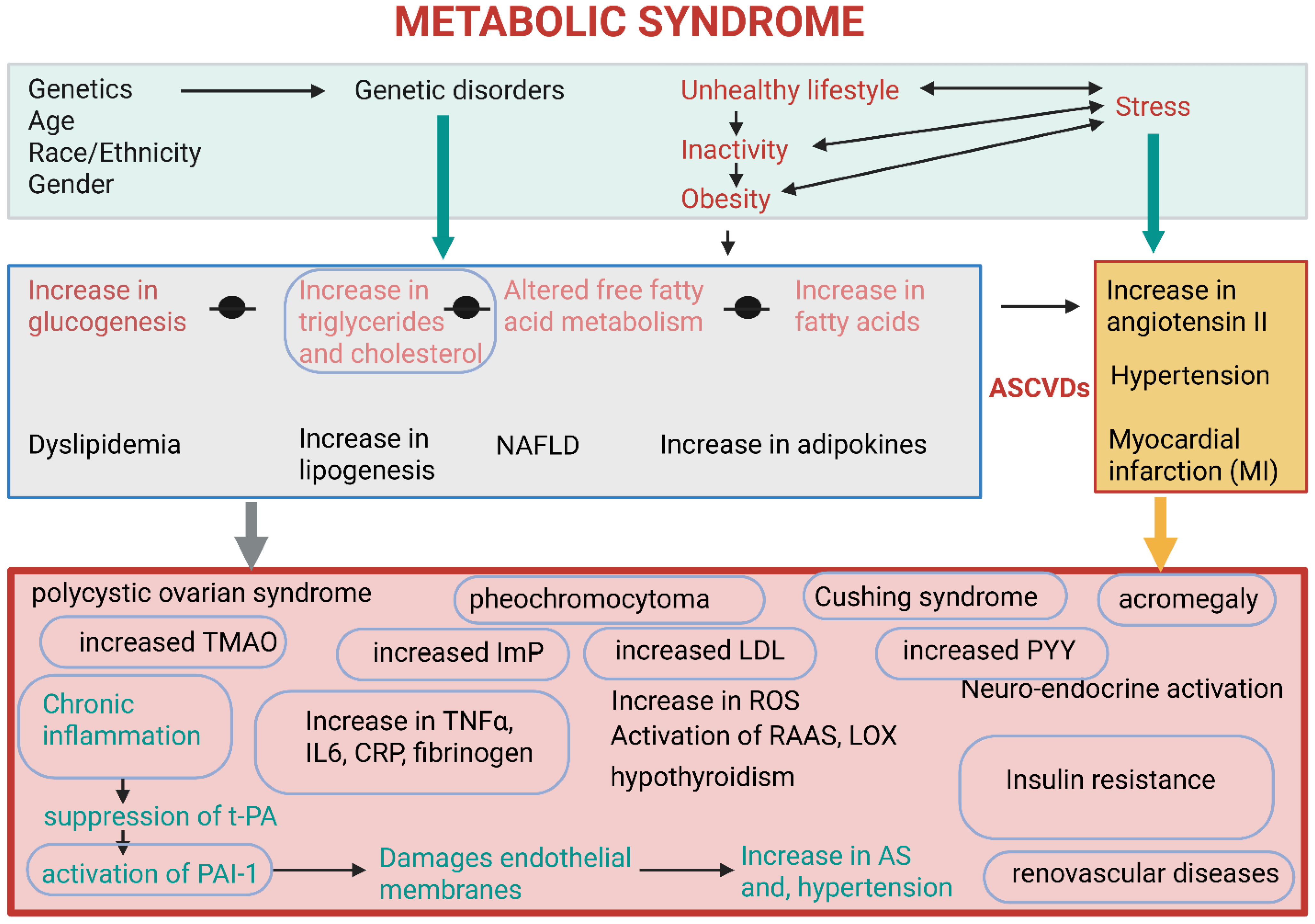
2. Metabolic Syndrome
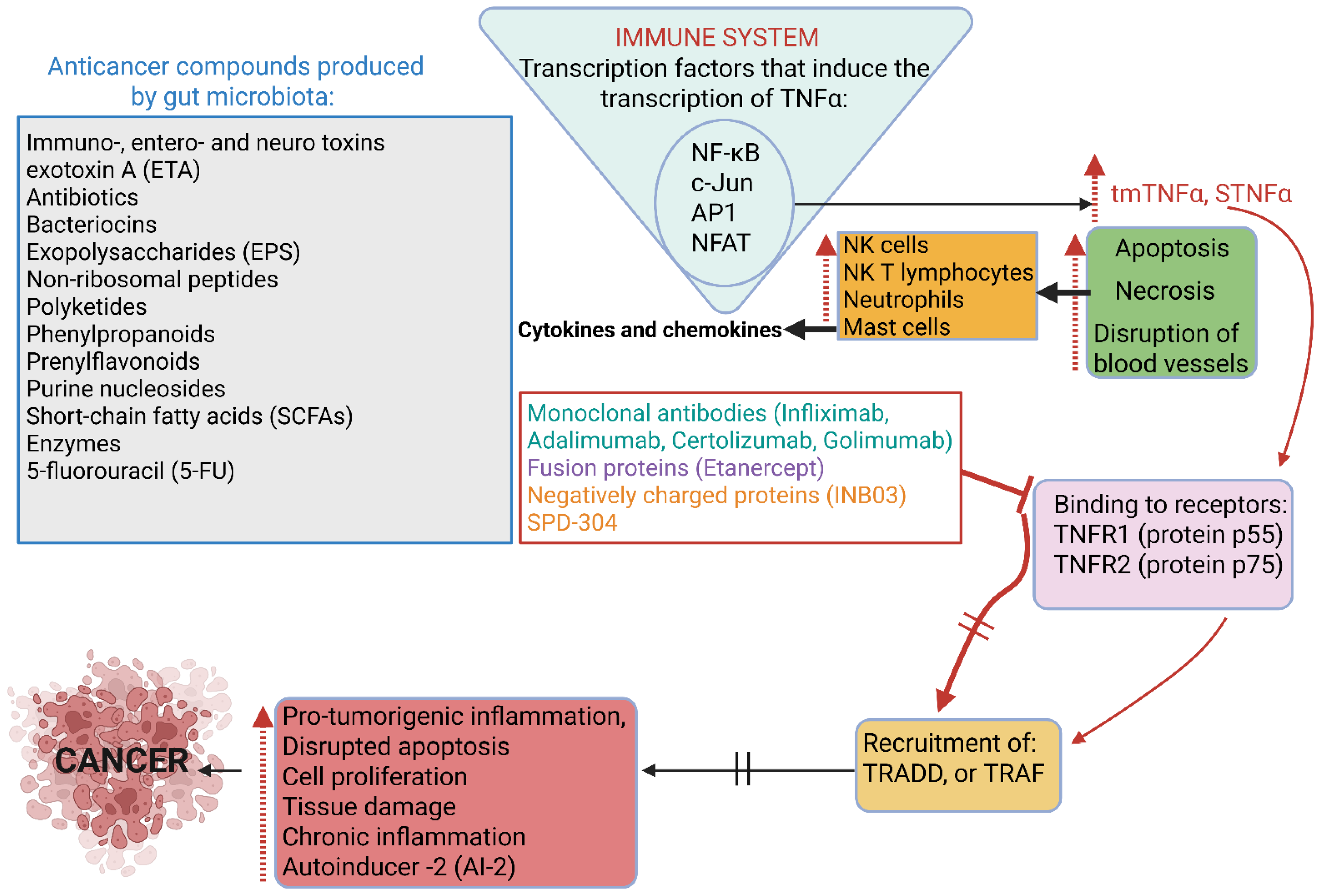
3. Cancer
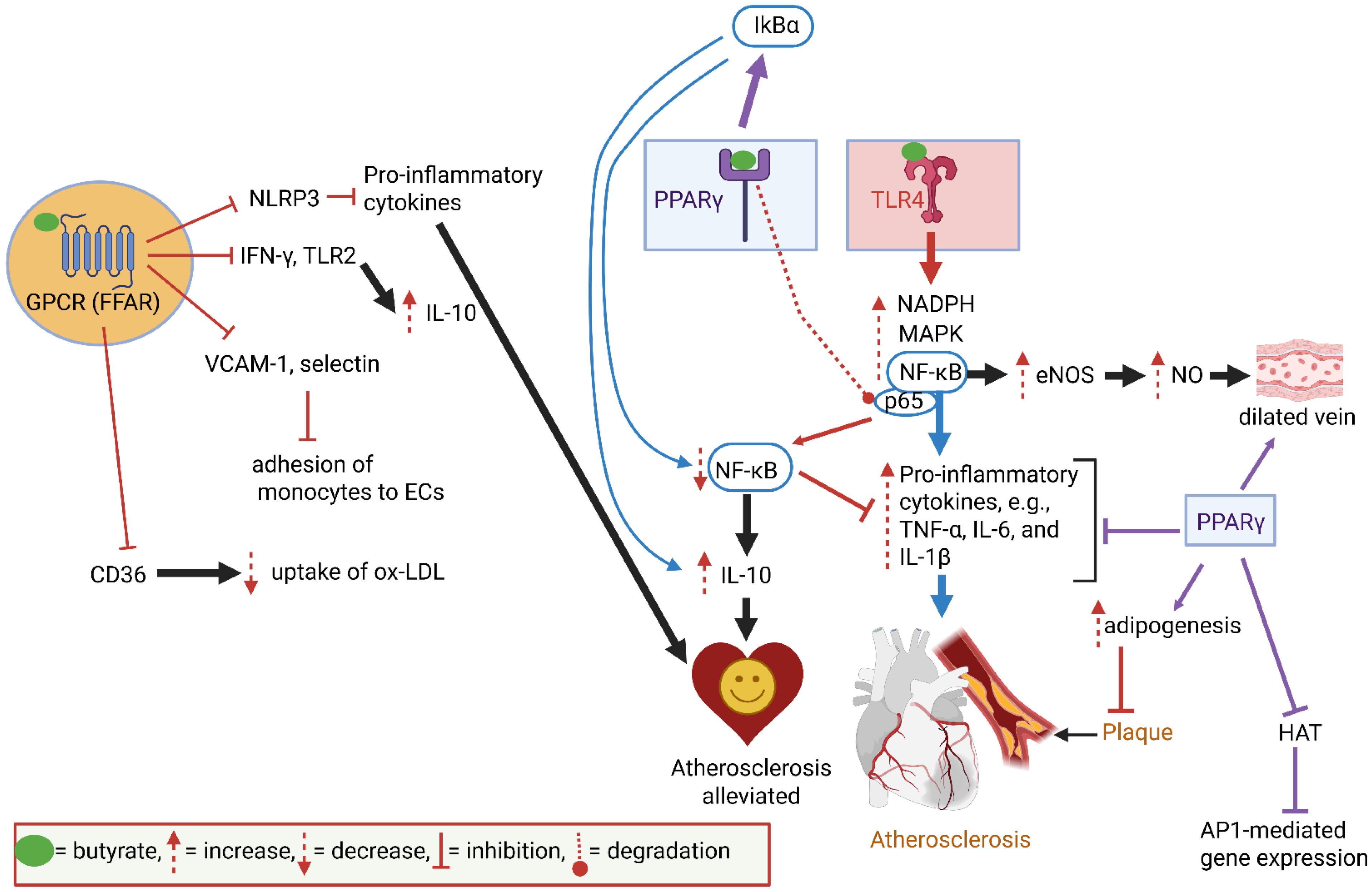
4. Cardiovascular Functions
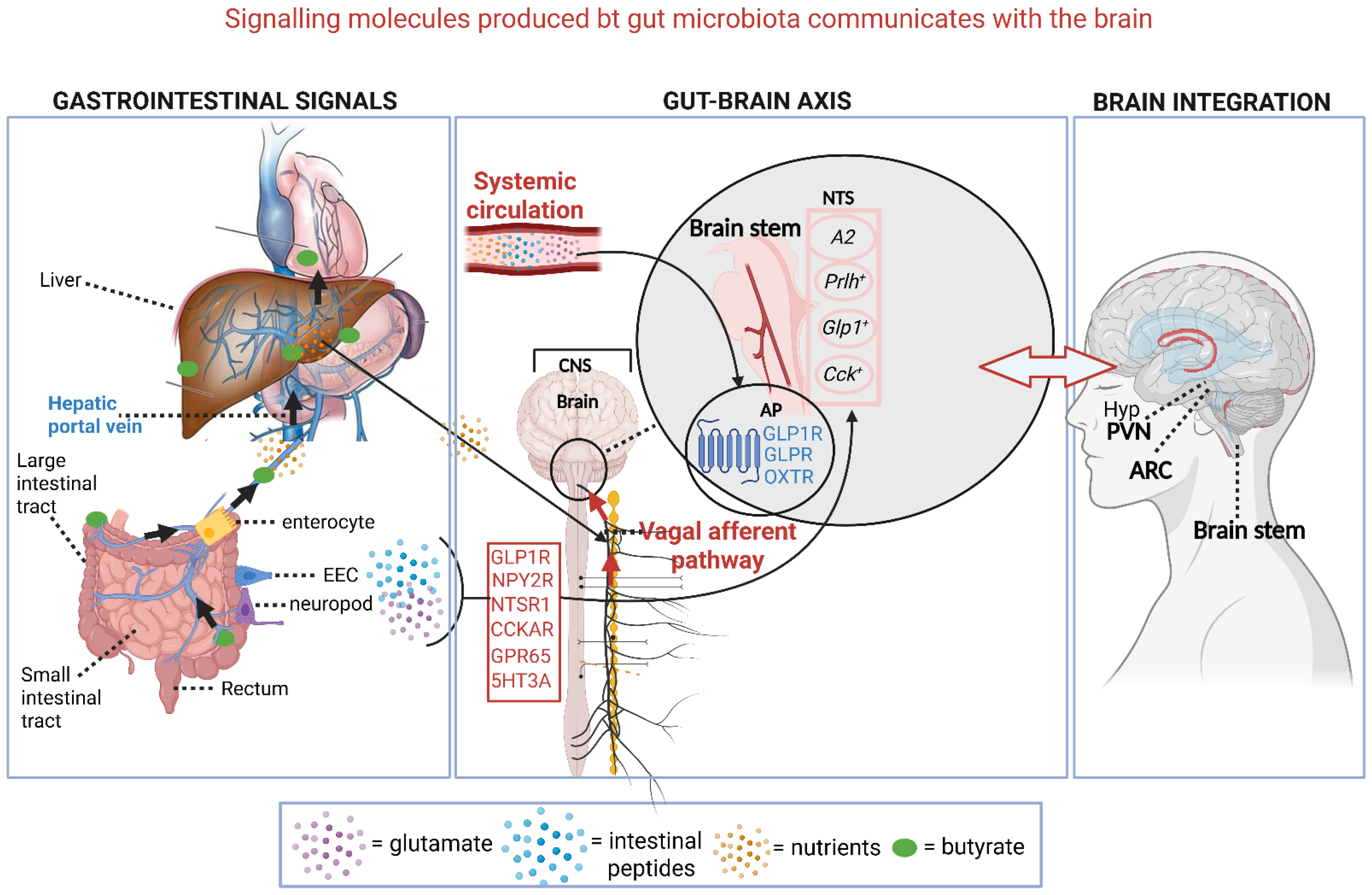
5. Brain Functions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dicks, L.M.T. How important are fatty acids in human health and can they be used in treating diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Andino, R.; Kirkegaard, K.; Macadam, A.; Racaniello, V.R.; Rosenfeld, A.B. The Picornaviridae Family: Knowledge Gaps, Animal Models, Countermeasures, and Prototype Pathogens. J. Infect. Dis. 2023, 228, S427–S445. [Google Scholar] [CrossRef]
- Pawłuszkiewicz, K.; Ryglowski, P.J.; Idzik, N.; Błaszczyszyn, K.; Kucharczyk, E.; Gaweł-Dąbrowska, D.; Siczek, M.; Widelski, J.; Paluch, E. Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination. Pathogens 2025, 14, 480. [Google Scholar] [CrossRef]
- Carlson, K.B.; Dilley, A.; O’Grady, T.; Johnson, J.A.; Lopman, B.; Viscidi, E. A narrative review of norovirus epidemiology, biology, and challenges to vaccine development. npj Vaccines 2024, 9, 94. [Google Scholar] [CrossRef]
- Pargin, E.; Roach, M.J.; Skye, A.; Papudeshi, B.; Inglis, L.K.; Mallawaarachchi, V.; Grigson, S.R.; Harker, C.; Edwards, R.A.; Giles, S.K. The human gut virome: Composition, colonization, interactions, and impacts on human health. Front. Microbiol. 2023, 14, 963173. [Google Scholar] [CrossRef]
- Pedroza Matute, S.; Iyavoo, S. Exploring the gut microbiota: Lifestyle choices, disease associations, and personal genomics. Front. Nutr. 2023, 10, 1225120. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Endo, A. Are fructophilic lactic acid bacteria (FLAB) beneficial to humans? Benef. Microbes 2022, 13, 3–11. [Google Scholar] [CrossRef]
- Garud, N.R.; Good, B.H.; Hallatschek, O.; Pollard, K.S. Evolutionary dynamics of bacteria in the gut microbiome within and across hosts. PLoS Biol. 2019, 17, e3000102. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Cardiovascular disease may be triggered by gut microbiota, microbial metabolites, gut wall reactions, and inflammation. Int. J. Mol. Sci. 2024, 25, 10634. [Google Scholar] [CrossRef]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How does quorum sensing of intestinal bacteria affect our health and mental status? Microorganisms 2022, 10, 1969. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Butyrate produced by gut microbiota regulates atherosclerosis: A narrative review of the latest findings. Int. J. Mol. Sci. 2025, 26, 6744. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Hurn, D.; Hermanus, D. Gut bacteria and neuropsychiatric disorders. Microorganisms 2021, 9, 2583. [Google Scholar] [CrossRef] [PubMed]
- Landini, L.; Souza Monteiro de Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 role in inflammatory disorders: What is known so far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef]
- Samad, A.; Sura, L.; Benedikt, J.; Ettrich, R.; Minofar, B.; Teisinger, J.; Vlachova, V. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem. J. 2011, 433, 197–204. [Google Scholar] [CrossRef]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.; Lu, H.Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021, 29, 179–196. [Google Scholar] [CrossRef]
- Yao, K.; Dou, B.; Zhang, Y.; Chen, Z.; Li, Y.; Fan, Z.; Ma, Y.; Du, S.; Wang, J.; Xu, Z.; et al. Inflammation-the role of TRPA1 channel. Front Physiol. 2023, 14, 1093925. [Google Scholar] [CrossRef]
- Waclawiková, B.; Codutti, A.; Alim, K.; El Aidy, S. Gut microbiota-motility interregulation: Insights from in vivo, ex vivo and in silico studies. Gut Microbes 2022, 14, 1997296. [Google Scholar] [CrossRef]
- Flint, A.; Sun, Y.Q.; Stintzi, A. Cj1386 is an ankyrin-containing protein involved in heme trafficking to catalase in Campylobacter jejuni. J. Bacteriol. 2012, 194, 334–345. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Crowe, M.S.; Mahavadi, S.; Murthy, K.S.; Grider, J.R. Branched short-chain fatty acid isovaleric acid causes colonic smooth muscle relaxation via cAMP/PKA pathway. Dig. Dis. Sci. 2019, 64, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-chain fatty acid receptors and blood pressure regulation: Council on hypertension mid-career award for research excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef]
- Ezzine, C.; Loison, L.; Montbrion, N.; Bôle-Feysot, C.; Déchelotte, P.; Coëffier, M.; Ribet, D. Fatty acids produced by the gut microbiota dampen host inflammatory responses by modulating intestinal SUMOylation. Gut Microbes 2022, 14, 2108280. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Alcoceba, M.; García-Álvarez, M.; Medina, A.; Maldonado, R.; González-Calle, V.; Chillón, M.C.; Sarasquete, M.E.; González, M.; García-Sanz, R.; Jiménez, C. MYD88 mutations: Transforming the landscape of IgM monoclonal gammopathies. Int. J. Mol. Sci. 2022, 23, 5570. [Google Scholar] [CrossRef]
- Lin, T.L.; Shu, C.C.; Chen, Y.M.; Lu, J.J.; Wu, T.S.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Like cures like: Pharmacological activity of anti-Inflammatory lipopolysaccharides from gut microbiome. Front. Pharmacol. 2020, 11, 554. [Google Scholar] [CrossRef]
- Mandl, M.; Depping, R. Hypoxia-inducible aryl hydrocarbon receptor nuclear translocator (ARNT) (HIF-1β): Is it a rare exception? Mol. Med. 2014, 20, 215–220. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Coleman, R.A.; Keating, D.J.; Martin, A.M. Gut microbiome regulation of gut hormone secretion. Endocrinology 2025, 166, bqaf004. [Google Scholar] [CrossRef]
- Collins, L.; Costello, R.A. Glucagon-like peptide-1 receptor agonists. [Updated 2024 Feb 29]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551568/ (accessed on 25 August 2025).
- Zong, Y.; Deng, K.; Chong, W.P. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front. Immunol. 2024, 15, 1387975. [Google Scholar] [CrossRef]
- Alex, K.D.; Pehek, E.A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007, 113, 296–320. [Google Scholar] [CrossRef]
- Cho, H.; Lim, J. The emerging role of gut hormones. Mol. Cells 2024, 47, 100126. [Google Scholar] [CrossRef]
- Lis, J.; Fichna, J.; Tarasiuk-Zawadzka, A. The role of free fatty acid receptors activation in pancreatic disorders. Mol. Asp. Med. 2025, 104, 101386. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Tremaroli, V. Therapeutic potential of butyrate for treatment of type 2 diabetes. Front. Endocrinol. 2021, 12, 761834. [Google Scholar] [CrossRef] [PubMed]
- Nema, P.; Asati, V.; Kendya, P.; Gupta, T.; Agarwal, S.; Kori, S.; Kashaw, V.; Iyer, A.K.; Kashaw, S.K. Structural Insight on GPR119 agonist as potential therapy for type II diabetes: A comprehensive review. Mini Revs. Med. Chem. 2023, 23, 2008–2040. [Google Scholar] [CrossRef]
- Han, Y.E.; Kang, C.W.; Oh, J.H.; Park, S.H.; Ku, C.R.; Cho, Y.H.; Lee, M.K.; Lee, E.J. Olfactory receptor OR51E1 mediates GLP-1 secretion in human and rodent enteroendocrine L cells. J. Endocr. Soc. 2018, 2, 1251–1258. [Google Scholar] [CrossRef]
- Jovancevic, N.; Dendorfer, A.; Matzkies, M.; Kovarova, M.; Heckmann, J.C.; Osterloh, M.; Boehm, M.; Weber, L.; Nguemo, F.; Semmler, J.; et al. Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res. Cardiol. 2017, 112, 13. [Google Scholar] [CrossRef] [PubMed]
- Dobrin, J.S.; Lebeche, D. Diabetic cardiomyopathy: Signaling defects and therapeutic approaches. Expert Rev. Cardiovasc. Ther. 2010, 8, 373–391. [Google Scholar] [CrossRef]
- Høgild, M.L.; Gudiksen, A.; Pilegaard, H.; Stødkilde-Jørgensen, H.; Pedersen, S.B.; Moller, N.; Jørgensen, J.O.L.; Jessen, N. Redundancy in regulation of lipid accumulation in skeletal muscle during prolonged fasting in obese men. Physiol. Rep. 2019, 7, e14285. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zhou, X.; Li, Z.; Hideki, N. Diacylglycerol kinases and its role in lipid metabolism and related diseases. Int. J. Mol. Sci. 2024, 25, 13207. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile acid physiology. Ann. Hepatol 2017, 16, s4–s14. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.D.; Könner, A.C.; Brüning, J.C. Sensing the fuels: Glucose and lipid signaling in the CNS controlling energy homeostasis. Cell. Mol. Life Sci. 2010, 67, 3255–3273. [Google Scholar] [CrossRef]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and abnormal glucose and lipid metabolism. Front. Endocrinol. 2019, 10, 703. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.W.; Huang, X.; Song, B.L.; Wang, Y.; Wang, Y. Regulation of glucose and lipid metabolism in health and disease. Sci. China Life Sci. 2019, 62, 1420–1458. [Google Scholar] [CrossRef]
- Sudo, N. Biogenic amines: Signals between commensal microbiota and gut physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Franceschetti, L.; Gianò, M.; Favero, G. A Focus on enterochromaffin cells among the enteroendocrine cells: Localization, morphology, and role. Int. J. Mol. Sci. 2022, 23, 3758. [Google Scholar] [CrossRef]
- Lukiw, W.J. Gastrointestinal (GI) tract microbiome-derived neurotoxins—Potent neuro-inflammatory signals from the GI tract via the systemic circulation into the brain. Front. Cell. Infect. Microbiol. 2020, 10, 22. [Google Scholar] [CrossRef]
- Alvarenga, L.; Kemp, J.A.; Baptista, B.G.; Ribeiro, M.; Lima, L.S.; Mafra, D. Production of toxins by the gut microbiota: The role of dietary protein. Curr. Nutr. Rep. 2024, 13, 340–350. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Vermeulen, W. Do bacteria provide an alternative to cancer treatment and what role does lactic acid bacteria play? Microorganisms 2022, 10, 1733. [Google Scholar] [CrossRef]
- Campbell, M.; Jialal, I. Physiology, Endocrine Hormones. [Updated 2022 Sep 26]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538498/ (accessed on 25 August 2025).
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern concepts in cardiovascular disease: Inflamm-Aging. Front. Cell. Dev. Biol. 2022, 10, 882211. [Google Scholar] [CrossRef]
- Kennedy-Wood, K.; Ng, C.A.S.; Alaiyed, S.; Foley, P.L.; Conant, K. Increased MMP-9 levels with strain-dependent stress resilience and tunnel handling in mice. Behav. Brain Res. 2021, 408, 113288. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Why are internal mammary (thoracic) arteries less prone to developing atherosclerosis compared to coronary arteries? Do gut microbiota play a role? A narrative review. Int. J. Mol. Sci. 2025, 26, 9052. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Are an aging gut and a decrease in butyrate production the reasons for atherosclerosis? Int. J. Mol. Sci. 2025, 26, 8276. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic syndrome. [Updated 2024 Mar 7]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL. USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459248/ (accessed on 25 August 2025).
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The role of adipokines in health and disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Singh, B.; Goyal, A.; Patel, B.C. C-reactive protein: Clinical relevance and interpretation. [Updated 2025 May 3]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 25 August 2025).
- He, L.; Fan, C.; Li, G. The relationship between serum C-reactive protein and senile hypertension. BMC Cardiovasc. Disord. 2022, 22, 500. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Wu, S.; Zheng, H.M.; Li, P.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.X.; et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Cӑtoi, A.F.; Pârvu, A.E.; Andreicuț, A.D.; Mironiuc, A.; Crӑciun, A.; Cӑtoi, C.; Pop, I.D. Metabolically healthy versus unhealthy morbidly obese: Chronic inflammation, nitro-oxidative stress, and insulin resistance. Nutrients 2018, 10, 1199. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. A Narrative review on plasminogen activator inhibitor-1 and its (patho)physiological role: To target or not to target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef]
- Jilani, T.N.; Siddiqui, A.H. Tissue plasminogen activator. [Updated 2023 Feb 20]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507917/ (accessed on 20 August 2025).
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin resistance. [Updated 2023 Aug 17]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507839/ (accessed on 15 August 2025).
- Semo, D.; Reinecke, H.; Godfrey, R. Gut microbiome regulates inflammation and insulin resistance: A novel therapeutic target to improve insulin sensitivity. Sig. Transduct. Target Ther. 2024, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Fernandes, J.-M.; Gonçalves, R.; Pinheiro, A.C.; Duarte, M.S.; Alves, M.A.; Maximo, G.J.; Vicente, A.A. Evaluating the in vitro digestion of lipids rich in medium-chain fatty acids (MCFAs) using dynamic and static protocols. Food Chem. 2023, 406, 135080. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the fine-tuning of colonic homeostasis: Implication for inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Yu, W.; Sun, S.; Yan, Y.; Zhou, H.; Liu, Z.; Fu, Q. The role of short-chain fatty acid in metabolic syndrome and its complications: Focusing on immunity and inflammation. Front. Immunol. 2025, 16, 1519925, Erratum in Front. Immunol. 2025, 16, 1580492. [Google Scholar]
- Ilyés, T.; Silaghi, C.N.; Crăciun, A.M. Diet-related changes of short-chain fatty acids in blood and feces in obesity and metabolic syndrome. Biology 2022, 11, 1556. [Google Scholar] [CrossRef]
- Henderson, G.C. Plasma free fatty acid concentration as a modifiable risk factor for metabolic disease. Nutrients 2021, 13, 2590. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, Y. Metabolic syndrome and PCOS: Pathogenesis and the role of metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef]
- Klag, K.A.; Round, J.L. Microbiota-immune interactions regulate metabolic disease. J. Immunol. 2021, 207, 1719–1724. [Google Scholar] [CrossRef]
- Burcelin, R. Gut microbiota and immune crosstalk in metabolic disease. Mol. Metabol. 2016, 5, 771–781. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Nozu, T.; Okumura, T. Pathophysiological commonality between irritable bowel syndrome and metabolic syndrome: Role of corticotropin-releasing factor-Toll-like receptor 4-proinflammatory cytokine signaling. J. Neurogastroenterol. Motil. 2022, 28, 173–184. [Google Scholar] [CrossRef]
- Javadekar, N.S.; Oka, G.A.; Joshi, A.S.; Vaste, P.; Tamane, S.; Lawate, P.S. Prevalence of irritable bowel syndrome and metabolic syndrome among young adults in an annual health check-up setting. JGH Open 2021, 5, 1148–1153. [Google Scholar] [CrossRef]
- Khoubai, F.Z.; Grosset, C.F. DUSP9, a dual-specificity phosphatase with a key role in cell biology and human diseases. Int. J. Mol. Sci. 2021, 22, 11538. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Sig. Transduct. Target Ther. 2022, 7, 298, Erratum in Sig. Transduct. Target Ther. 2022, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. Am. Cancer Soc. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Assembly, Cancer Prevention and Control in the Context of an Integrated Approach. Available online: https://apps.who.int/iris/handle/10665/275676 (accessed on 19 August 2025).
- American Cancer Society, Cancer Facts and Figures. 2018. Available online: https://doi.org/10.1182/blood-2015-12-687814 (accessed on 19 August 2025).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus. Proc. R. Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Ben-Baruch, A. Tumor necrosis factor α: Taking a personalized road in cancer therapy. Front. Immunol. 2022, 13, 903679. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Mandal, S.M. Bacteria and bacterial anticancer agents as a promising alternative for cancer therapeutics. Biochimie 2020, 177, 164–189. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.S. Unfolding transmembrane TNFα dynamics in cancer therapeutics. Cytokine 2021, 137, 155303. [Google Scholar] [CrossRef]
- Karpinski, T.; Adamczak, A. Anticancer activity of bacterial proteins and peptides. Pharmaceutics 2018, 10, 54. [Google Scholar] [CrossRef]
- Tsioumpekou, M.; Krijgsman, D.; Leusen, J.H.W.; Olofsen, P.A. The role of cytokines in neutrophil development, tissue homing, function and plasticity in health and disease. Cells 2023, 12, 1981. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor necrosis factor α blockade: An opportunity to tackle breast cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Gamboa, M.; Kitamura, N.; Miura, K.; Noda, S.; Kaminuma, O. Evolutionary mechanisms underlying the diversification of nuclear factor of activated T cells across vertebrates. Sci. Rep. 2023, 13, 6468. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninezhad, F.; Leone, P.; Alemohammad, H.; Najafzadeh, B.; Nourbakhsh, N.S.; Prete, M.; Malerba, E.; Saeedi, H.; Tabrizi, N.J.; Racanelli, V.; et al. Tumor necrosis factor-α in systemic lupus erythematosus: Structure, function and therapeutic implications. Int. J. Mol. Med. 2022, 49, 43. [Google Scholar] [CrossRef]
- Cai, R.; Hao, Y.; Liu, Y.Y.; Huang, L.; Yao, Y.; Zhou, M.S. Tumor necrosis factor alpha deficiency improves endothelial function and cardiovascular injury in deoxycorticosterone acetate/salt-hypertensive mice. BioMed Res. Int. 2020, 2020, 3921074. [Google Scholar] [CrossRef]
- O’Connell, J.; Porter, J.; Kroeplien, B.; Norman, T.; Rapecki, S.; Davis, R.; McMillan, D.; Arakaki, T.; Burgin, A.; Fox Iii, D.; et al. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat. Commun. 2019, 10, 5795. [Google Scholar] [CrossRef]
- McMillan, D.; Martinez-Fleites, C.; Porter, J.; Fox, D.R., 3rd; Mori, P.; Ceska, T.; Carrington, B.; Lawson, A.; Bourne, T.; O’Connell , J. Structural insights into the disruption of TNF-TNFR1 signalling by small molecules stabilising a distorted TNF. Nat. Commun. 2021, 12, 582. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Cuomo, C.; Mignini, I.; Gasbarrini, A.; Papa, A. Focus on anti-tumour necrosis factor (TNF)-α-related autoimmune diseases. Int. J. Mol. Sci. 2023, 24, 8187. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, A.; Radhakrishnan, S. Diphtheria. [Updated 2024 Feb 26]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560911/ (accessed on 15 August 2025).
- Parveen, S.; Bishai, W.R.; Murphy, J.R. Corynebacterium diphtheriae: Diphtheria toxin, the tox operon, and its regulation by Fe2+ activation of apo-DtxR. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Yagi, K.; Kondoh, M. Roles of the first-generation claudin binder, Clostridium perfringens enterotoxin, in the diagnosis and claudin-targeted treatment of epithelium-derived cancers. Pflug. Arch. Eur. J. Physiol. 2017, 469, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; McClane, B.; Uzal, F. Mechanisms of action and cell death associated with Clostridium perfringens toxins. Toxins 2018, 10, 212. [Google Scholar] [CrossRef]
- Shim, M.K.; Na, J.; Cho, I.K.; Jang, E.H.; Park, J.; Lee, S.; Kim, J.-H. Targeting of claudin-4 by Clostridium perfringens enterotoxin- conjugated polysialic acid nanoparticles for pancreatic cancer therapy. J. Control. Release 2021, 331, 434–442. [Google Scholar] [CrossRef]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From tight junctions to biological systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- James, N.G.; Malik, S.; Sanstrum, B.J.; Rheaume, C. Characterization of clostridium botulinum neurotoxin serotype A (BoNT/A) and fibroblast growth factor receptor interactions using novel receptor dimerization assay. Sci. Rep. 2021, 11, 7832. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Seu, M.Y.; Dorafshar, A.H.; Shafikhani, S.H. Pseudomonas aeruginosa cytotoxins: Mechanisms of cytotoxicity and impact on inflammatory responses. Cells 2023, 12, 195. [Google Scholar] [CrossRef]
- Morgan, R.N.; Saleh, S.E.; Farrag, H.A.; Aboshanab, K.M. New insights on Pseudomonas aeruginosa exotoxin A-based immunotoxins in targeted cancer therapeutic delivery. Ther. Deliv. 2023, 14, 31–60. [Google Scholar] [CrossRef]
- Li, Q.; Peng, W.; Wu, J.; Wang, X.; Ren, Y.; Li, H.; Peng, Y.; Tang, X.; Fu, X. Autoinducer-2 of gut microbiota, a potential novel marker for human colorectal cancer, is associated with the activation of TNFSF9 signaling in macrophages. Oncoimmunology 2019, 8, e1626192. [Google Scholar] [CrossRef]
- Schroeder, J.-H.; Howard, J.K.; Lord, G.M. Transcription factor-driven regulation of ILC1 and ILC3. Trends Immunol. 2022, 43, 564–579. [Google Scholar] [CrossRef]
- Zia, S.; Tehreem, K.; Batool, S.; Ishfaq, M.; Mirza, S.B.; Khan, S.; Almashjary, M.N.; Hazzazi, M.S.; Qanash, H.; Shaikh, A.; et al. Epithelial Cell Adhesion Molecule (EpCAM) Expression Can Be Modulated via NFκB. BioMedicines 2022, 10, 2985. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Peng, L.; Kuhn, C.; Chelariu-Raicu, A.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Expression of the carbohydrate Lewis antigen, sialyl Lewis A, sialyl Lewis X, Lewis X, and Lewis Y in the placental villi of patients with unexplained miscarriages. Front. Immunol. 2023, 12, 679424. [Google Scholar] [CrossRef]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the cells of origin of squamous cell carcinomas. Nat. Rev. Cancer. 2018, 18, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, Y.; Gao, H.; Hu, X. Mechanisms of angiogenesis in tumour. Front. Oncol. 2024, 14, 1359069. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Lenz, H.J. What roles do colon stem cells and gap junctions play in the left and right location of origin of colorectal cancers? J. Cell Commun. Signal. 2017, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Chavan, S.S. Nerve Stimulation for Treatment of Diseases and Disorders. International Patent PCT/US2016/018573, 19 February 2016. [Google Scholar]
- Ashaq, M.S.; Zhang, S.; Xu, M.; Li, Y.; Zhao, B. The regulatory role of CD36 in hematopoiesis beyond fatty acid uptake. Life Sci. 2024, 339, 122–442. [Google Scholar] [CrossRef]
- Pražienková, V.; Popelová, A.; Kuneš, J.; Maletínská, L. Prolactin-releasing peptide: Physiological and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 5297. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Q.; He, X.; Zhang, Y.; You, C.; Wu, C.; Li, J.; Xu, H.E.; Zhao, L.H. Molecular mechanism of prolactin-releasing peptide recognition and signaling via its G protein-coupled receptor. Cell Discov. 2024, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, F.; Bech, E.M.; Pedersen, S.L.; Thorbek, D.D.; Leurs, U.; Rudkjaer, L.C.B.; Fosgerau, K.; Hansen, H.H.; Vrang, N.; Jelsing, J.; et al. Lipidated PrRP31 metabolites are long acting dual GPR10 and NPFF2 receptor agonists with potent body weight lowering effect. Sci. Rep. 2022, 12, 1696. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef]
- Yamashita, T. The role of gut microbiota in cardiovascular diseases and their potential as novel therapeutic targets. J. Cardiol. 2025, 86, 141–147. [Google Scholar] [CrossRef]
- Lansbury, E.L.; Vana, V.; Lund, M.L.; Ludwig, M.Q.; Mamedova, E.; Gautron, L.; Arnold, M.; Egerod, K.L.; Kuhre, R.E.; Holst, J.J.; et al. Neurons co-expressing GLP-1, CCK, and PYY receptors particularly in right nodose ganglion and innervating entire GI tract in mice. Int. J. Mol. Sci. 2025, 26, 2053. [Google Scholar] [CrossRef]
- Gruber, T.; Lechner, F.; Krieger, J.-P.; García-Cáceres, C. Neuroendocrine gut–brain signaling in obesity. Trends Endocrinol. Metabol. 2025, 36, 42–54. [Google Scholar] [CrossRef]
- Aklan, I.; Sayar Atasoy, N.; Yavuz, Y.; Ates, T.; Coban, I.; Koksalar, F.; Filiz, G.; Topcu, I.C.; Oncul, M.; Dilsiz, P.; et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab. 2020, 31, 313–326.e5. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, M.; Wang, L.; Zhang, L.; Xu, D.; Cao, P.; Wang, F.; Herzog, H.; Song, S.; Zhan, C. A vagal-NTS neural pathway that stimulates feeding. Curr. Biol. 2020, 30, 3986–3998.e552. [Google Scholar] [CrossRef] [PubMed]
- Christ-Crain, M.; Ball, S. The neurohypophysis: Endocrinology of vasopressin and oxytocin. [Updated 2022 Nov 9]. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279157/ (accessed on 15 August 2025).
- Jewett, B.E.; Sharma, S. Physiology, GABA; StatPearls: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/30020683/ (accessed on 14 October 2021).
- Venegas, P.D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277, Erratum in Front. Immunol. 2019, 10, 1486. [Google Scholar] [PubMed]

| Abnormality | Key Signals and Gut Microbiota a | Physiological Effects | References |
|---|---|---|---|
| Metabolic Syndrome | Short-chain fatty acids (SCFAs). Faecalibacterium, Eubacterium, Bacteroidetes, Phascolarctobacterium, Veillonella, Lachnospiraceae, Ruminococcaceae, Bifidobacterium, Lactobacillus, Blautia, Coprococcus, Roseburia, Clostridium | Improve insulin sensitivity, regulate lipid metabolism, inhibit histone deacetylases (HDACs), reduce cholesterol synthesis, serve as an energy source for colonocytes, anti-inflammatory, improve gut peristalsis, promote neurogenesis and synaptic plasticity, have pro- and anticancer effects (depending on concentration) | [1,14,25,60,81,82] |
| Bile acids (BAs), e.g., deoxycholic acid (DCA) and taurine-conjugated BAs. Clostridium, Bacteroides, and Eubacterium | Metabolized by gut microbiota into secondary BAs that impact lipid and glucose metabolism. DCA is associated with inflammation and colorectal carcinogenesis by activating specific signaling pathways, such as mitogen-activated protein kinase (MAPK). Dysregulation of BAs can contribute to neurological dysfunction | [1,14,45,48] | |
| Tryptophan (Trp) metabolites, e.g., indole-3-propionic acid (IPA), and indole. Lactobacillus, Bifidobacterium, Clostridium, Ruminococcus | Trp is a precursor to serotonin (5-HT). Dysregulation of Trp metabolism is linked to anxiety and depression. IPA and indoles activate aryl hydrocarbon receptors (AhRs), which regulate metabolism and reduce inflammation. Lower levels of these metabolites are linked to insulin resistance. Indoles suppress cancer progression by activating AhR | [1,21,22,30,50] | |
| Trimethylamine N-oxide (TMAO). Generated from trimethylamine (TMA) by the liver. TMA is produced by gut bacteria metabolizing choline and carnitine. Enterobacteriaceae, Lactobacillus, Clostridium. | High levels of TMAO are associated with insulin resistance and type 2 diabetes, atherosclerosis (AS), endothelial dysfunction, heart failure, certain cancers, and neuroinflammation | [78,127] | |
| Lipopolysaccharides (LPSs). Mainly Gram-negative bacteria, e.g., Escherichia coli, Bacteroides, Akkermansia, Proteobacteria | Causes increased intestinal permeability (leaky gut), triggers chronic inflammation, and insulin resistance. Promote inflammation-related carcinogenesis by activating inflammatory pathways, such as nuclear factor kappa B (NF-κB), and induce DNA damage. Chronic systemic inflammation impairs endothelial wall function and promotes AS. Causes neuroinflammation when the blood–brain barrier is crossed | [27,28,80,81,82] | |
| Gamma-amino butyric acid (GABA). Bifidobacterium, Lactobacillus, Bacteroides, Akkermansia | Primary inhibitory neurotransmitter in the central nervous system (CNS), affecting mood and anxiety. Modulates intestinal motility | [10,15,16] | |
| Serotonin (5-HT). Enterococcus, Streptococcus, Escherichia | Enterochromaffin cells produce most of the body’s 5-HT, but gut bacteria can produce it directly or stimulate its production. Regulates gut motility and is a key signaling molecule in the gut–brain axis (GBA) | [19,27] | |
| Dopamine. Enterococcus, Eubacterium, Blautia, Bacillus, Staphylococcus, Escherichia, Serratia | A neurotransmitter, crucial for motor control, motivation, pleasure, reward, and cognitive functions such as learning, memory, and attention | [49] | |
| Gut hormones, e.g., glucagon-like peptide-1 (GLP-1), peptide tyrosine-tyrosine (peptide YY), and cholecystokinin (CCK). Produced by intestinal epithelial cells (IECs) | Suppresses appetite and decreases food intake by signaling satiety from the gut to the brain | [11,35] | |
| Ankyrin. Not produced by gut microbiota | Initiates inflammatory responses by releasing neuropeptides that stimulate immune cells. Plays a role in tissue repair and intestinal motility | [19,22] | |
| Toxins, e.g., fragilysin (BFT), uremic toxin, diphtheria toxin, enterotoxin, botulinum toxin, exotoxin A. Bacteroides fragilis, Clostridium diphtheriae, Clostridium perfringens, Clostridium botulinum, Clostridium butyrricum, Clostridium barati, Clostridium argentinensis, Pseudomonas aeruginosa | Damage tight junction proteins, increase gut permeability, and cause inflammation | [13,53,54] | |
| Cancer: | Secondary BAs, e.g., deoxycholic acid (DCA) and lithocholic acid (LCA). Clostridium, Bacteroides, Eubacterium, Lactobacillus, Bifidobacterium | Elevated levels linked to colon cancer | [1,14,45,48] |
| Bacterial toxins. See microbiota listed under “Toxins” | Interact with host cells, alter physiological functions, and promote tumor growth | [91,92] | |
| Cardiovascular Diseases (CVDs): | Trimethylamine N-oxide (TMAO). Enterobacteriaceae, Lactobacillus, Clostridium | Produced from choline and carnitine, linked to vascular inflammation and endothelial damage | [78,127] |
| BAs. Clostridium, Bacteroides, and Eubacterium | Affect cardiac muscle function, and influence lipid metabolism and plaque formation | [1,14,45,48] | |
| Inflammatory mediators. Escherichia coli, Bacteroides, Akkermansia, Proteobacteria | Lipopolysaccharides (LPSs) trigger inflammation | [27,28,80,81,82] | |
| Olfactory receptor OR51E1 (Olfr588). Produced by IECs | Influences blood pressure, vascular reactivity, and arterial stiffness | [25] | |
| Transient receptor potential ankyrin A1 (Trpa1). Not produced by gut microbiota | Increases intestinal motility and the transfer of Ca2+ | [20,21,22] | |
| Oleoylethanolamide (OEA), palmitoylethanolamide (PEA), lysophosphatidylcholine (LPC). Not produced by gut microbiota | Regulate the release of glucagon-like peptide-1 (GLP-1) and peptide tyrosine-tyrosine (PYY), which control food intake. The activation of the brainstem nucleus tractus solitarius (NTS) by GLP-1 influences mood, cognition, and gastrointestinal motility | [1,24,30] | |
| Isovaleric acid. Bacteroides, Clostridium Senescence-associated secretory phenotype (SASP), e.g., matrix metalloproteinase 9 (MMP-9). Not produced by gut microbiota | An intermediate in the synthesis of cholesterol and fatty acids. Interacts with olfactory receptor OR51E1 (equivalent to olfactory receptor 558, Olfr588, in mice). Regulates blood pressure, vascular reactivity, and arterial stiffness | [23,25] | |
| Senescence-associated secretory phenotype (SASP), e.g., matrix metalloproteinase 9 (MMP-9). Not produced by gut microbiota | Degrades the extracellular matrix, resulting in chronic inflammation | [10,56,57,58,59] | |
| Brain Functions: | 5-HT. Enterococcus, Streptococcus, Escherichia | The majority of 5-HT is produced in the intestine. Gut microbiota influences the levels produced. Impact on mood | [30,31,32,33,49,50,128] |
| GABA. Bifidobacterium, Lactobacillus, Bacteroides, Akkermansia | Produced by some microorganisms. A neurotransmitter that affects emotions | [10,15,16,133] | |
| Inflammatory molecules. Escherichia coli, Bacteroides, Akkermansia, Proteobacteria | LPS triggers cytokine production (e.g., TNF-α, IL-6), which affects brain function. Associated with anxiety, depression, and memory | [1,14,28,33] | |
| Polysaccharide A (PSA). Bacillus fragilis | May protect against CNS inflammation | [28,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, L.M.T. Key Signals Produced by Gut Microbiota Associated with Metabolic Syndrome, Cancer, Cardiovascular Diseases, and Brain Functions. Int. J. Mol. Sci. 2025, 26, 10539. https://doi.org/10.3390/ijms262110539
Dicks LMT. Key Signals Produced by Gut Microbiota Associated with Metabolic Syndrome, Cancer, Cardiovascular Diseases, and Brain Functions. International Journal of Molecular Sciences. 2025; 26(21):10539. https://doi.org/10.3390/ijms262110539
Chicago/Turabian StyleDicks, Leon M. T. 2025. "Key Signals Produced by Gut Microbiota Associated with Metabolic Syndrome, Cancer, Cardiovascular Diseases, and Brain Functions" International Journal of Molecular Sciences 26, no. 21: 10539. https://doi.org/10.3390/ijms262110539
APA StyleDicks, L. M. T. (2025). Key Signals Produced by Gut Microbiota Associated with Metabolic Syndrome, Cancer, Cardiovascular Diseases, and Brain Functions. International Journal of Molecular Sciences, 26(21), 10539. https://doi.org/10.3390/ijms262110539






