Acalabrutinib May Offer a New Therapeutic Approach for Consolidation and Maintenance of Primary CNS Lymphoma with Expression of MYD88 and CD79B Gene Variants: A Case Report and Literature Review of Primary CNS Lymphoma in the BTKi Era
Abstract
1. Introduction
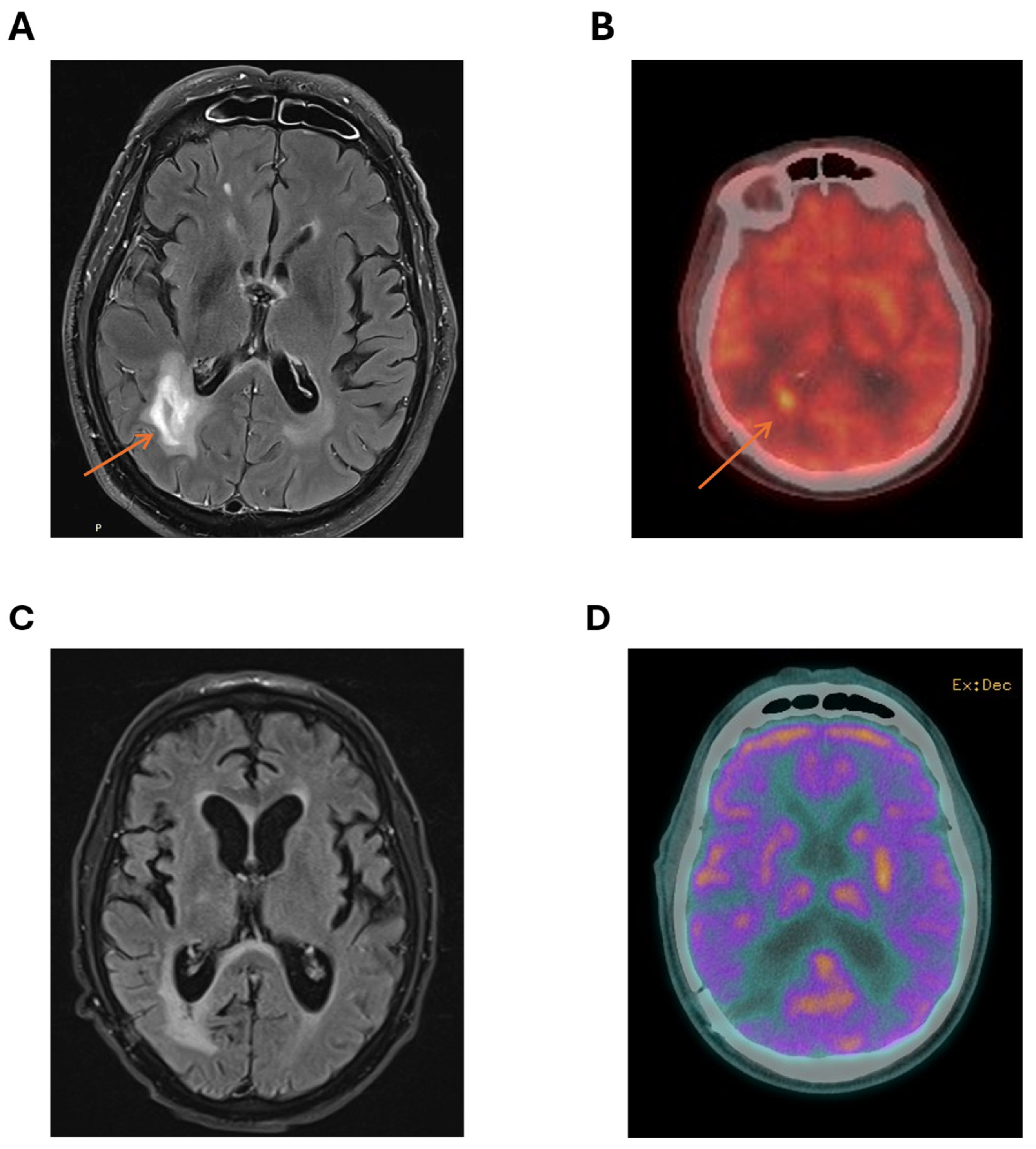
2. Case
3. Outline of Primary CNS Lymphoma
4. Molecular Characterisation
5. Non-Invasive Diagnostics and Monitoring
6. Treatment Evolution
6.1. Induction
6.2. Consolidation
6.3. Relapsed/Refractory Disease
6.4. Treatment in the Elderly and Frail
6.5. Emerging Novel Therapies
6.6. Bruton’s Tyrosine Kinase, B-Cell Activation, and BTK Inhibitors
6.7. Other Therapies
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreri, A.J.; Calimeri, T.; Cwynarski, K.; Dietrich, J.; Grommes, C.; Hoang-Xuan, K.; Hu, L.S.; Illerhaus, G.; Nayak, L.; Ponzoni, M.; et al. Primary central nervous system lymphoma. Nat. Rev. Dis. Primers 2023, 9, 29. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748, Correction in Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Illerhaus, G.; Doorduijn, J.K.; Auer, D.P.; Bromberg, J.E.; Calimeri, T.; Cwynarski, K.; Fox, C.P.; Hoang-Xuan, K.; Malaise, D.; et al. Primary central nervous system lymphomas: EHA–ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. HemaSphere 2024, 8, e89. [Google Scholar] [CrossRef] [PubMed]
- de Koning, M.E.; Hof, J.J.; Jansen, C.; Doorduijn, J.K.; Bromberg, J.E.; van der Meulen, M. Primary central nervous system lymphoma. J. Neurol. 2023, 271, 2906–2913. [Google Scholar] [CrossRef]
- Khwaja, J.; Nayak, L.; Cwynarski, K. Evidence-based management of primary and secondary CNS lymphoma. Semin. Hematol. 2023, 60, 313–321. [Google Scholar] [CrossRef]
- Martinez-Calle, N.; Isbell, L.K.; Cwynarski, K.; Schorb, E. Treatment of elderly patients with primary CNS lymphoma. Ann. Lymphoma 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Schaff, L.R.; Grommes, C. Primary central nervous system lymphoma. Blood 2022, 140, 971–979. [Google Scholar] [CrossRef]
- Mo, S.S.; Cleveland, J.; Rubenstein, J.L. Primary CNS lymphoma: Update on molecular pathogenesis and therapy. Leuk. Lymphoma 2023, 64, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Alderuccio, J.P.; Nayak, L.; Cwynarski, K. How I treat secondary CNS involvement by aggressive lymphomas. Blood 2023, 142, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Lei, H.; Han, X.; Wei, Y.; Wei, X. BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: Mechanisms and clinical studies. J. Hematol. Oncol. 2022, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- de Charette, M.; Houot, R. Hide or defend, the two strategies of lymphoma immune evasion: Potential implications for immunotherapy. Haematologica 2018, 103, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.; Godfrey, J.; Ansell, S.M. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood 2020, 135, 523–533. [Google Scholar] [CrossRef]
- Radke, J.; Ishaque, N.; Koll, R.; Gu, Z.; Schumann, E.; Sieverling, L.; Uhrig, S.; Hübschmann, D.; Toprak, U.H.; López, C.; et al. The genomic and transcriptional landscape of primary central nervous system lymphoma. Nat. Commun. 2022, 13, 2558. [Google Scholar] [CrossRef]
- Hernández-Verdin, I.; Morales-Martínez, A.; Hoang-Xuan, K.; Alentorn, A. Primary central nervous system lymphoma: Advances in its pathogenesis, molecular markers and targeted therapies. Curr. Opin. Neurol. 2022, 35, 779–786. [Google Scholar] [CrossRef]
- Hernández-Verdin, I.; Kirasic, E.; Wienand, K.; Mokhtari, K.; Eimer, S.; Loiseau, H.; Rousseau, A.; Paillassa, J.; Ahle, G.; Lerintiu, F.; et al. Molecular and clinical diversity in primary central nervous system lymphoma. Ann. Oncol. 2023, 34, 186–199. [Google Scholar] [CrossRef]
- Hai, L.; Friedel, D.; Hinz, F.; Hoffmann, D.C.; Doubrovinskaia, S.; Rohdjess, H.; Weidenauer, K.; Denisova, E.; Scheffler, G.T.; Kessler, T.; et al. Distinct epigenetic and transcriptional profiles of Epstein-Barr virus-positive and negative primary CNS lymphomas. Neuro-Oncology 2024, 27, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating tumour DNA—Looking beyond the blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, Y.; Ding, T.; Zhang, X.; Chen, B.; Guan, M. Effectiveness of digital PCR for MYD88L265P detection in vitreous fluid for primary central nervous system lymphoma diagnosis. Exp. Ther. Med. 2020, 20, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Them, L.; Alentorn, A.; Ahle, G.; Soussain, C.; Mathon, B.; Tavernier, M.L.; Houillier, C.; Hoang-Xuan, K. CSF biomarkers in primary CNS lymphoma. Rev. Neurol. 2023, 179, 141–149. [Google Scholar] [CrossRef]
- Aastha, A.; Wilding, H.; Mikolajewicz, N.; Khan, S.; Ignatchenko, V.; De Macedo Filho, L.J.; Bhanja, D.; Remite-Berthet, G.; Heebner, M.; Glantz, M.; et al. Cerebrospinal fluid protein biomarkers are associated with response to multiagent intraventricular chemotherapy in patients with CNS lymphoma. Neuro-Oncol. Adv. 2025, 7, vdaf046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, D.; Yin, J.; Zhang, L.; Zhang, X.; Wang, W.; Zhang, M.; Zhou, D.; Zhang, W. Changes of CSF IL-10 levels display better performance in predicting disease relapse than conventional MRI in PCNSL. BMC Cancer 2020, in press. [Google Scholar]
- Landry, A.P.; Zuccato, J.A.; Patil, V.; Voisin, M.; Wang, J.Z.; Ellenbogen, Y.; Gui, C.; Ajisebutu, A.; Nassiri, F.; Zadeh, G. Integration of cerebrospinal fluid methylome and proteome can obviate the need for biopsy in central nervous system lymphoma. Cancer Res. 2024, 84 (Suppl. S6), 1022. [Google Scholar] [CrossRef]
- Watanabe, J.; Natsumeda, M.; Okada, M.; Kobayashi, D.; Kanemaru, Y.; Tsukamoto, Y.; Oishi, M.; Kakita, A.; Fujii, Y. High detection rate of MYD88 mutations in cerebrospinal fluid from patients with CNS lymphomas. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fontanilles, M.; Marguet, F.; Bohers, É.; Viailly, P.J.; Dubois, S.; Bertrand, P.; Camus, V.; Mareschal, S.; Ruminy, P.; Maingonnat, C.; et al. Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget 2017, 8, 48157–48168. [Google Scholar] [CrossRef] [PubMed]
- Bobillo, S.; Crespo, M.; Escudero, L.; Mayor, R.; Raheja, P.; Carpio, C.; Rubio-Perez, C.; Tazon-Vega, B.; Palacio, C.; Carabia, J.; et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica 2021, 106, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gocke, C.D. Circulating Tumor DNA in Lymphoma, in Precision Molecular Pathology of Aggressive B-Cell Lymphomas; Crane, G.M., Loghavi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 395–426. [Google Scholar]
- Kambhampati, S.; Zain, J. Circulating Tumor DNA in Lymphoma. Curr. Hematol. Malig. Rep. 2022, 17, 298–305. [Google Scholar]
- Watanabe, J.; Natsumeda, M.; Kanemaru, Y.U.; Okada, M.; Oishi, M.; Kakita, A.; Fujii, Y. Comparison of circulating tumor DNA between body fluids in patients with primary central nervous system lymphoma. Leuk. Lymphoma 2019, 60, 3587–3589. [Google Scholar] [CrossRef] [PubMed]
- Heger, J.M.; Mattlener, J.; Gödel, P.; Balke-Want, H.; Sieg, N.; Kutsch, N.; Becker, K.; Weiss, J.; Reinhardt, H.C.; Hallek, M.; et al. Noninvasive, dynamic risk profiling of primary central nervous system lymphoma by peripheral blood ctdna-sequencing. Blood 2022, 140 (Suppl. S1), 3537–3538. [Google Scholar] [CrossRef]
- Mutter, J.A.; Alig, S.; Lauer, E.M.; Esfahani, M.S.; Mitschke, J.; Kurtz, D.M.; Kühn, J.; Bleul, S.; Olsen, M.; Liu, C.L.; et al. Profiling of circulating tumor DNA for noninvasive disease detection, risk stratification, and MRD monitoring in patients with CNS lymphoma. Blood 2021, 138 (Suppl. S1), 6. [Google Scholar] [CrossRef]
- Rimelen, V.; Ahle, G.; Pencreach, E.; Zinniger, N.; Debliquis, A.; Zalmaï, L.; Harzallah, I.; Hurstel, R.; Alamome, I.; Lamy, F.; et al. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol. Commun. 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Sasaki, N.; Nakano, Y.; Matushita, Y.; Omura, T.; Shimizu, S.; Saito, K.; Kobayashi, K.; Narita, Y.; Kondo, A.; et al. Liquid biopsy of cerebrospinal fluid for MYD88 L265P mutation is useful for diagnosis of central nervous system lymphoma. Cancer Sci. 2021, 112, 4702–4710. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, J.; Cwynarski, K. Management of primary and secondary CNS lymphoma. Hematol. Oncol. 2023, 41 (Suppl. S1), 25–35. [Google Scholar] [CrossRef]
- Hiraga, S.; Arita, N.; Ohnishi, T.; Kohmura, E.; Yamamoto, K.; Oku, Y.; Taki, T.; Sato, M.; Aozasa, K.; Yoshimine, T. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J. Neurosurg. 1999, 91, 221–230. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Reni, M.; Foppoli, M.; Martelli, M.; Pangalis, G.A.; Frezzato, M.; Cabras, M.G.; Fabbri, A.; Corazzelli, G.; Ilariucci, F.; et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009, 374, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Ollila, T.A.; Taher, R.; Moku, P.; Olszewski, A.J. Immunochemotherapy or chemotherapy alone in primary central nervous system lymphoma: A National Cancer Database analysis. Blood Adv. 2023, 7, 5470–5479. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; La Rosée, P.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; Celico, C.; Falautano, M.; Nonis, A.; La Rosée, P.; Binder, M.; et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia 2022, 36, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Schorb, E.; Fox, C.P.; Kasenda, B.; Linton, K.; Martinez-Calle, N.; Calimeri, T.; Ninkovic, S.; Eyre, T.A.; Cummin, T.; Smith, J.; et al. Induction therapy with the MATRix regimen in patients with newly diagnosed primary diffuse large B-cell lymphoma of the central nervous system–an international study of feasibility and efficacy in routine clinical practice. Br. J. Haematol. 2020, 189, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Batara, J.M.; Apor, A.D.; Mojica, C.V.; Mondia, M.W. Use of rituximab, temozolomide, and radiation in recurrent and refractory primary central nervous system lymphoma in the Philippines: A retrospective analysis. Neuro-Oncol. Adv. 2022, 4, vdac105. [Google Scholar] [CrossRef] [PubMed]
- Enting, R.H.; Demopoulos, A.; DeAngelis, L.M.; Abrey, L.E. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004, 63, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calle, N.; Isbell, L.K.; Cwynarski, K.; Schorb, E. Advances in treatment of elderly primary central nervous system lymphoma. Br. J. Haematol. 2022, 196, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Dureau, S.; Taillandier, L.; Houot, R.; Chinot, O.; Moluçon-Chabrot, C.; Schmitt, A.; Gressin, R.; Choquet, S.; Damaj, G.; et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients age 60 years and younger: Long-term results of the randomized phase II PRECIS study. J. Clin. Oncol. 2022, 40, 3692–3698. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosée, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017, 4, e510–e523. [Google Scholar] [PubMed]
- Martinez-Calle, N.; Poynton, E.; Alchawaf, A.; Kassam, S.; Horan, M.; Rafferty, M.; Kelsey, P.; Scott, G.; Culligan, D.J.; Buckley, H.; et al. Outcomes of older patients with primary central nervous system lymphoma treated in routine clinical practice in the UK: Methotrexate dose intensity correlates with response and survival. Br. J. Haematol. 2020, 190, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, K.; Kasenda, B.; Schorb, E.; Hau, P.; Bloehdorn, J.; Möhle, R.; Loew, S.; Binder, M.; Atta, J.; Keller, U.; et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017, 31, 846–852. [Google Scholar] [CrossRef]
- Schorb, E.; Isbell, L.K.; Kerkhoff, A.; Mathas, S.; Braulke, F.; Egerer, G.; Röth, A.; Schliffke, S.; Borchmann, P.; Brunnberg, U.; et al. High-dose chemotherapy and autologous haematopoietic stem-cell transplantation in older, fit patients with primary diffuse large B-cell CNS lymphoma (MARTA): A single-arm, phase 2 trial. Lancet Haematol. 2024, 11, e196–e205. [Google Scholar] [CrossRef]
- Satterthwaite1; AB and Witte1, Owen N, The role of Bruton’s tyrosine kinase in B-cell development and function: A genetic perspective. Immunol. Rev. 2000, 175, 120–127. [CrossRef]
- Neys, S.F.; Hendriks, R.W.; Corneth, O.B. Targeting Bruton’s tyrosine kinase in inflammatory and autoimmune pathologies. Front. Cell Dev. Biol. 2021, 9, 668131. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Tanasi, I.; Quaglia, F.M.; Ferrarini, I.; Fraenza, C.; Krampera, M. Oncogenic mutations of MYD88 and CD79B in diffuse large B-cell lymphoma and implications for clinical practice. Cancers 2020, 12, 2913. [Google Scholar] [CrossRef] [PubMed]
- de Groen, R.A.; Schrader, A.M.; Kersten, M.J.; Pals, S.T.; Vermaat, J.S. MYD88 in the driver’s seat of B-cell lymphomagenesis: From molecular mechanisms to clinical implications. Haematologica 2019, 104, 2337. [Google Scholar] [CrossRef]
- Flümann, R.; Hansen, J.; Meinel, J.; Pfeiffer, P.; Goldfarb Wittkopf, H.; Lütz, A.; Wirtz, J.; Möllmann, M.; Zhou, T.; Tabatabai, A.; et al. An inducible Cd79b mutation confers ibrutinib sensitivity in mouse models of Myd88-driven diffuse large B-cell lymphoma. Blood Adv. 2024, 8, 1063–1074. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518, Correction in N. Engl. J. Med. 2016, 374, 998. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Dunleavy, K.; Roschewski, M.; Widemann, B.C.; Butman, J.A.; Schmitz, R.; Yang, Y.; Cole, D.E.; Melani, C.; Higham, C.S.; et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 2017, 31, 833–843.e5. [Google Scholar] [CrossRef]
- Schaff, L.; Nayak, L.; Grommes, C. Bruton’s tyrosine kinase (BTK) inhibitors for the treatment of primary central nervous system lymphoma (PCNSL): Current progress and latest advances. Leuk. Lymphoma 2024, 65, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Goldwirt, L.; Beccaria, K.; Ple, A.; Sauvageon, H.; Mourah, S. Ibrutinib brain distribution: A preclinical study. Cancer Chemother. Pharmacol. 2018, 81, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Goldwirt, L.; Amorim, S.; Brice, P.; Brière, J.; de Kerviler, E.; Mourah, S.; Sauvageon, H.; Thieblemont, C. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood 2015, 126, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kong, H.; Li, C.; Dong, X.; Wu, Y.; Zhuang, Y.; Han, S.; Lei, T.; Yang, H. Bruton’s tyrosine kinase inhibitors in primary central nervous system lymphoma-evaluation of anti-tumor efficacy and brain distribution. Transl. Cancer Res. 2021, 10, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Zhuang, Z.; Wang, W.; Wei, C.; Zhao, D.; Zhou, D.; Zhang, W. Preliminary Evaluation of Zanubrutinib-Containing Regimens in DLBCL and the Cerebrospinal Fluid Distribution of Zanubrutinib: A 13-Case Series. Front. Oncol. 2021, 11, 760405. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kwak, M.; Kang, J.; Cesaire, M.; Tang, K.; Robey, R.W.; Frye, W.J.; Karim, B.; Butcher, D.; Lizak, M.J.; et al. Ibrutinib disrupts blood-tumor barrier integrity and prolongs survival in rodent glioma model. Acta Neuropathol. Commun. 2024, 12, 56. [Google Scholar] [CrossRef]
- Grommes, C.; Pastore, A.; Palaskas, N.; Tang, S.S.; Campos, C.; Schartz, D.; Codega, P.; Nichol, D.; Clark, O.; Hsieh, W.Y.; et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017, 7, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Nandakumar, S.; Schaff, L.R.; Gavrilovic, I.; Kaley, T.J.; Nolan, C.P.; Stone, J.; Thomas, A.A.; Tang, S.S.; Wolfe, J.; et al. A Phase II study assessing Long-Term Response to Ibrutinib Monotherapy in recurrent or refractory CNS Lymphoma. Clin. Cancer Res. 2024, 30, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Soussain, C.; Choquet, S.; Blonski, M.; Leclercq, D.; Houillier, C.; Rezai, K.; Bijou, F.; Houot, R.; Boyle, E.; Gressin, R.; et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur. J. Cancer 2019, 117, 121–130. [Google Scholar]
- Soussain, C.; Malaise, D.; Choquet, S.; Ghesquières, H.; Houillier, C. Long-lasting CRs after ibrutinib monotherapy for relapse or refractory primary CNS lymphoma (PCNSL) and primary vitreoretinal lymphoma (PVRL): Long-term results of the iLOC study by the Lymphoma Study Association (LYSA) and the French Oculo-Cerebral Lymphoma (LOC) Network (clinical trial number: NCT02542514). Eur. J. Cancer 2023, 189, 112909. [Google Scholar] [PubMed]
- Yonezawa, H.; Narita, Y.; Nagane, M.; Mishima, K.; Terui, Y.; Arakawa, Y.; Asai, K.; Fukuhara, N.; Sugiyama, K.; Shinojima, N.; et al. Three-year follow-up analysis of phase 1/2 study on tirabrutinib in patients with relapsed or refractory primary central nervous system lymphoma. Neuro-Oncol. Adv. 2024, 6, vdae037. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Nagane, M.; Mishima, K.; Terui, Y.; Arakawa, Y.; Yonezawa, H.; Asai, K.; Fukuhara, N.; Sugiyama, K.; Shinojima, N.; et al. Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro-Oncology 2021, 23, 122–133. [Google Scholar] [CrossRef]
- Nayak, L.; Grommes, C.; Kallam, A.; Peereboom, D.M.; Ambady, P.; Mendez, J.S.; Aregawi, D.G.; Sumrall, A.L.; Omuro, A.M.; Iwamoto, F.; et al. Tirabrutinib for the treatment of relapsed or refractory primary central nervous system lymphoma: Efficacy and safety from the phase II PROSPECT study. J. Clin. Oncol. 2025, 43 (Suppl. S16), 2019. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, J.; Lv, C.; Xu, J. Successful Management of a Patient with Refractory Primary Central Nervous System Lymphoma by Zanubrutinib. Onco Targets Ther. 2021, 14, 3367–3372. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, F.L.; Pan, L.; Teng, Y.; Wei, X.J.; Guo, H.G.; Jiang, X.M.; Huang, L.; Liu, S.C.; Liang, Z.L.; et al. Clinical outcomes of newly diagnosed primary central nervous system lymphoma treated with zanubrutinib-based combination therapy. World J. Clin. Oncol. 2023, 14, 606–619. [Google Scholar] [CrossRef]
- Fox, C.P.; Wang, J.; McIlroy, G.; Khwaja, J.; Jackson, A.; Boucher, R.; Maycock, S.; Yates, F.; Homer, E.; Cummin, T.; et al. The Prizm+ Phase II Platform Study for Relapsed and Refractory Primary Cns Lymphoma: First Results from Cohort 1 Zanubrutinib Monotherapy. Hematol. Oncol. 2025, 43, e87_70093. [Google Scholar] [CrossRef]
- Nizamuddin, I.A.; Epperla, N.; Malacek, M.K.; Kahl, B.S.; Wan, F.; Fischer, A.; Moreno, C.; King, E.R.; Krivenko, A.; Bartlett, N.L.; et al. Phase I Results of Acalabrutinib in Combination with Durvalumab in Primary Central Nervous System Lymphoma: Safety, Efficacy, and Central Nervous System Penetration. Blood 2024, 144, 985. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, T.; Zuo, X.; Ke, X.; Hu, K. Central nervous system posttransplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation successfully treated with combination therapy of acalabrutinib and immunochemotherapy: A case report and literature review. EJHaem 2024, 6, e1078. [Google Scholar] [CrossRef]
- Barrett, A.; Eyre, T.A.; Bhuva, S.; Aljurf, M.; Fakih, R.E.; Ashshi, M.A.; Alshaibani, A. Complete response of mantle cell lymphoma with central nervous system involvement at diagnosis with acalabrutinib–Case report. EJHaem 2023, 5, 238–241. [Google Scholar] [CrossRef]
- Yohannan, B.; Sridhar, A.; Nguyen, N.; Rios, A. Durable remission with Bruton’s tyrosine kinase inhibitor therapy in a patient with leptomeningeal disease secondary to relapsed mantle cell lymphoma. BMJ Case Rep. 2022, 15, e249631. [Google Scholar] [CrossRef]
- Bai, S.J.; He, J.X.; Zheng, Y.J.; Geng, Y.; Gao, Y.N.; Zhang, C.X.; Wang, Y.R.; Qin, L.Y.; Wang, W.J.; Yang, L.H. Clinical characteristics and prognosis of patients with newly diagnosed primary central nervous system lymphoma: A multicentre retrospective analysis. Ann. Hematol. 2024, 103, 4649–4660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, J.; Chen, H.; Zhou, D.; Ji, C. Efficacy and Safety of BTKis in Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 860. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Sun, X.; Wu, Y.; Cui, Q.; Chen, Y.; Liu, Y. Efficacy and Safety of Ibrutinib in Central Nervous System Lymphoma: A PRISMA-Compliant Single-Arm Meta-Analysis. Front. Oncol. 2021, 11, 707285. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, J. Bruton’s tyrosine kinase inhibitors in the treatment of primary central nervous system lymphoma: A mini-review. Front. Oncol. 2022, 12, 1034668. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Jiang, H.; Han, Y.; Li, C.; Zhang, L.; Zhang, Y.; Chai, Y.; Zeng, P.; Yue, L.; Wu, C. Frequent gene mutations and their possible roles in the pathogenesis, treatment and prognosis of primary central nervous system lymphoma. World Neurosurg. 2023, 170, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Severson, E.A.; Haberberger, J.; Hemmerich, A.; Huang, R.S.; Edgerly, C.; Schiavone, K.; Najafian, A.; Hiemenz, M.; Lechpammer, M.; Vergilio, J.A.; et al. Genomic profiling reveals differences in primary central nervous system lymphoma and large B-cell lymphoma, with subtyping suggesting sensitivity to BTK inhibition. Oncology 2023, 28, e26–e35. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Y.; Qian, X.; Ding, T.; Yuan, Y.; Li, Y.; Wu, H.; Chen, T. The outcome of ibrutinib-based regimens in relapsed/refractory central nervous system lymphoma and the potential impact of genomic variants. Adv. Clin. Exp. Med. 2023, 32, 855–863. [Google Scholar] [CrossRef]
- Karschnia, P.; Blobner, J.; Teske, N.; Schöberl, F.; Fitzinger, E.; Dreyling, M.; Tonn, J.C.; Thon, N.; Subklewe, M.; von Baumgarten, L. CAR T-cells for CNS lymphoma: Driving into new terrain? Cancers 2021, 13, 2503. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.R.; Dorris, C.S.; Makambi, K.H.; Luo, Y.; Munshi, P.N.; Donato, M.; Rowley, S.; Saad, A.; Goy, A.; Dunleavy, K.; et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: A meta-analysis of 128 patients. Blood Adv. 2023, 7, 32–39. [Google Scholar] [CrossRef]
- Mahdi, J.; Dietrich, J.; Straathof, K.; Roddie, C.; Scott, B.J.; Davidson, T.B.; Prolo, L.M.; Batchelor, T.T.; Campen, C.J.; Davis, K.L.; et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 2023, 29, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Ricciotti, I.; Tosoni, A.; Di Nunno, V.; Bartolini, S.; Ranieri, L.; Franceschi, E. CAR-T cells neurotoxicity from consolidated practice in hematological malignancies to fledgling experience in CNS tumors: Fill the gap. Front. Oncol. 2023, 13, 1206983. [Google Scholar] [CrossRef]
- Kaulen, L.D.; Martinez-Lage, M.; Abramson, J.S.; Karschnia, P.; Doubrovinskaia, S.; Shankar, G.; Choi, B.; Ramundo, C.M.; Ehret, F.; Barnes, J.A.; et al. Clinical presentation, management and outcome of TIAN in CNS lymphoma treated with CD19-CAR T-cell Therapy. Blood 2025, 146, 1902–1913. [Google Scholar] [CrossRef]
- Graham, C.E.; Velasco, R.; Tomas, A.A.; Stewart, O.P.; Dachy, G.; Del Bufalo, F.; Doglio, M.; Henter, J.I.; Ortí, G.; Peric, Z.; et al. Non-ICANS neurological complications after CAR T-cell therapies: Recommendations from the EBMT Practice Harmonisation and Guidelines Committee. Lancet Oncol. 2025, 26, e203–e213. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J.K.; Gao, L.; Shouse, G.; Song, J.Y.; Pak, S.; Lee, B.; Chen, B.T.; Kallam, A.; Baird, J.H.; Marcucci, G.; et al. Glofitamab stimulates immune cell infiltration of CNS tumors and induces clinical responses in secondary CNS lymphoma. Blood 2024, 144, 457–461. [Google Scholar] [CrossRef]
- Furuse, M.; Nonoguchi, N.; Omura, N.; Shirahata, M.; Iwasaki, K.; Inui, T.; Kuroiwa, T.; Kuwabara, H.; Miyatake, S.I. Immunotherapy of nivolumab with dendritic cell vaccination is effective against intractable recurrent primary central nervous system lymphoma: A case report. Neurol. Med.-Chir. 2017, 57, 191–197. [Google Scholar] [CrossRef]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood J. Am. Soc. Hematol. 2017, 129, 3071–3073. [Google Scholar] [CrossRef] [PubMed]
- Graber, J.J.; Plato, B.; Mawad, R.; Moore, D.J. Pembrolizumab immunotherapy for relapsed CNS Lymphoma. Leuk. Lymphoma 2020, 61, 1766–1768. [Google Scholar] [CrossRef] [PubMed]
- Ambady, P.; Szidonya, L.; Firkins, J.; James, J.; Johansson, K.; White, T.; Jezierski, C.; Doolittle, N.D.; Neuwelt, E.A. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk. Lymphoma 2019, 60, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, A.N.; Volkov, N.P.; Shmidt, D.I.; Polushin, A.Y.; Kondakova, E.; Lepik, K.V.; Zalaylov, Y.R.; Popova, M.O.; Kulagin, A.D.; Afanasyev, B.V.; et al. Nivolumab in primary CNS lymphoma and primary testicular lymphoma with CNS involvement: Single center experience. Blood 2020, 136, 4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allison, E.; Campbell, A.; Watson, A.-M.; Beaton, B. Acalabrutinib May Offer a New Therapeutic Approach for Consolidation and Maintenance of Primary CNS Lymphoma with Expression of MYD88 and CD79B Gene Variants: A Case Report and Literature Review of Primary CNS Lymphoma in the BTKi Era. Int. J. Mol. Sci. 2025, 26, 10521. https://doi.org/10.3390/ijms262110521
Allison E, Campbell A, Watson A-M, Beaton B. Acalabrutinib May Offer a New Therapeutic Approach for Consolidation and Maintenance of Primary CNS Lymphoma with Expression of MYD88 and CD79B Gene Variants: A Case Report and Literature Review of Primary CNS Lymphoma in the BTKi Era. International Journal of Molecular Sciences. 2025; 26(21):10521. https://doi.org/10.3390/ijms262110521
Chicago/Turabian StyleAllison, Eleanor, Ashlea Campbell, Anne-Marie Watson, and Brendan Beaton. 2025. "Acalabrutinib May Offer a New Therapeutic Approach for Consolidation and Maintenance of Primary CNS Lymphoma with Expression of MYD88 and CD79B Gene Variants: A Case Report and Literature Review of Primary CNS Lymphoma in the BTKi Era" International Journal of Molecular Sciences 26, no. 21: 10521. https://doi.org/10.3390/ijms262110521
APA StyleAllison, E., Campbell, A., Watson, A.-M., & Beaton, B. (2025). Acalabrutinib May Offer a New Therapeutic Approach for Consolidation and Maintenance of Primary CNS Lymphoma with Expression of MYD88 and CD79B Gene Variants: A Case Report and Literature Review of Primary CNS Lymphoma in the BTKi Era. International Journal of Molecular Sciences, 26(21), 10521. https://doi.org/10.3390/ijms262110521





