AMPK Limits MNNG-Induced Parthanatos by Inhibiting BH3-Only Protein Bim
Abstract
1. Introduction
2. Results
2.1. MNNG Induces Parthanatos in a BAX/BAK-Dependent Manner
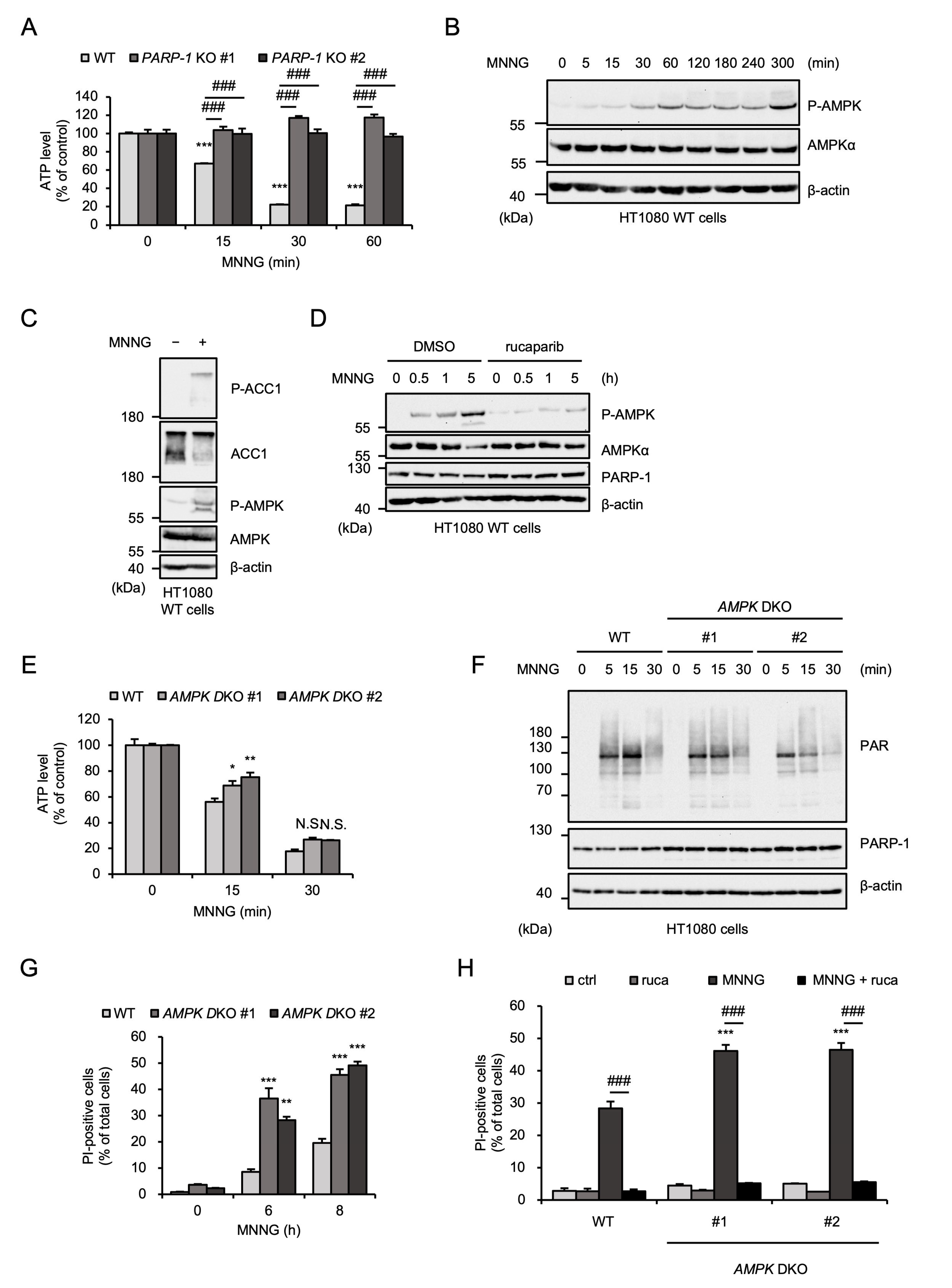
2.2. AMPK Negatively Regulates MNNG-Induced Parthanatos
2.3. AMPK Limits MNNG-Induced Parthanatos Mediated by BAX/BAK-Independent Pathway
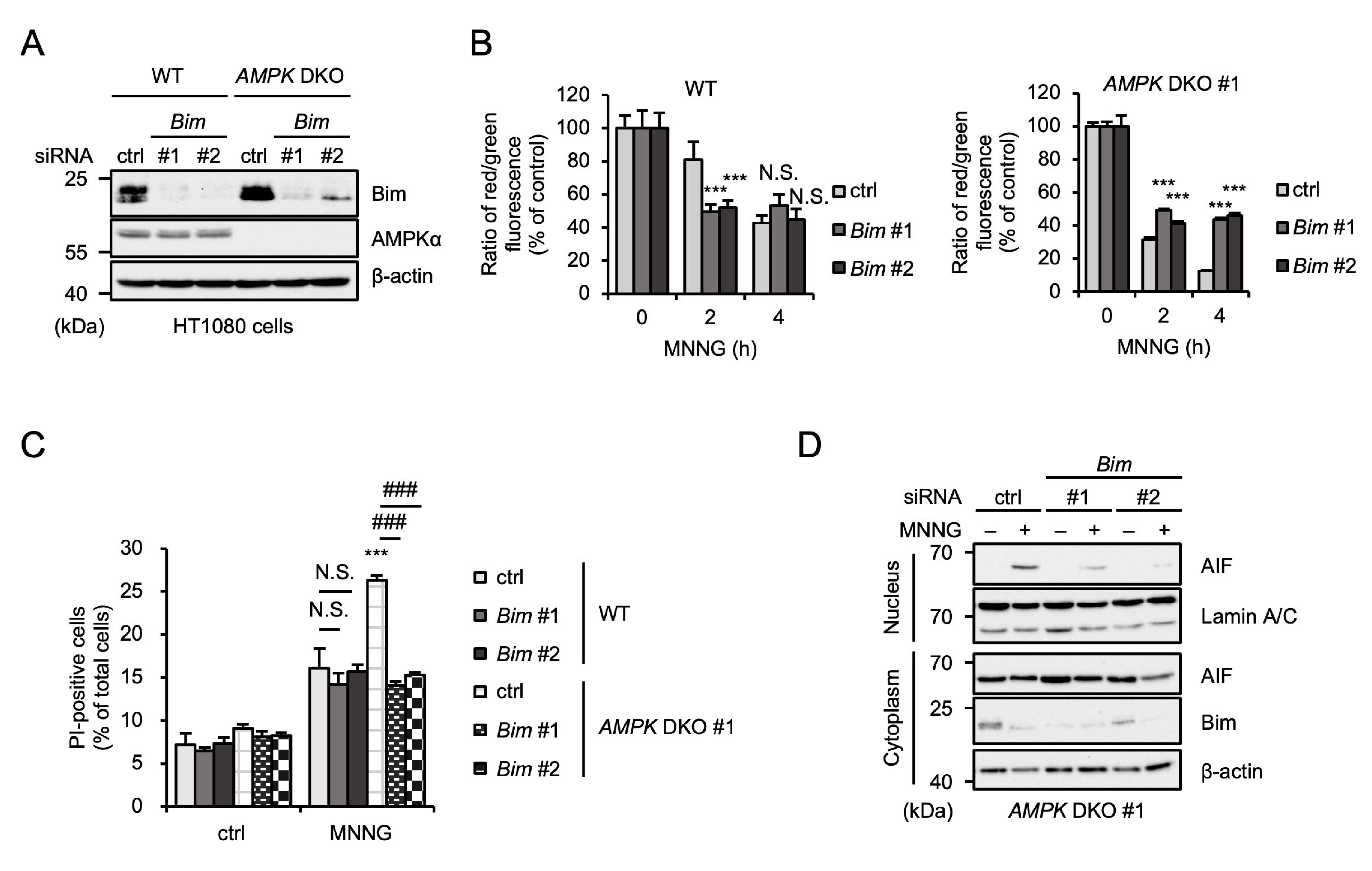
2.4. AMPK Limits MNNG-Induced Upregulation of Pro-Apoptotic Protein Bim
2.5. Bim Is Responsible for the Enhancement of MNNG-Induced Parthanatos in AMPK DKO HT1080 Cells
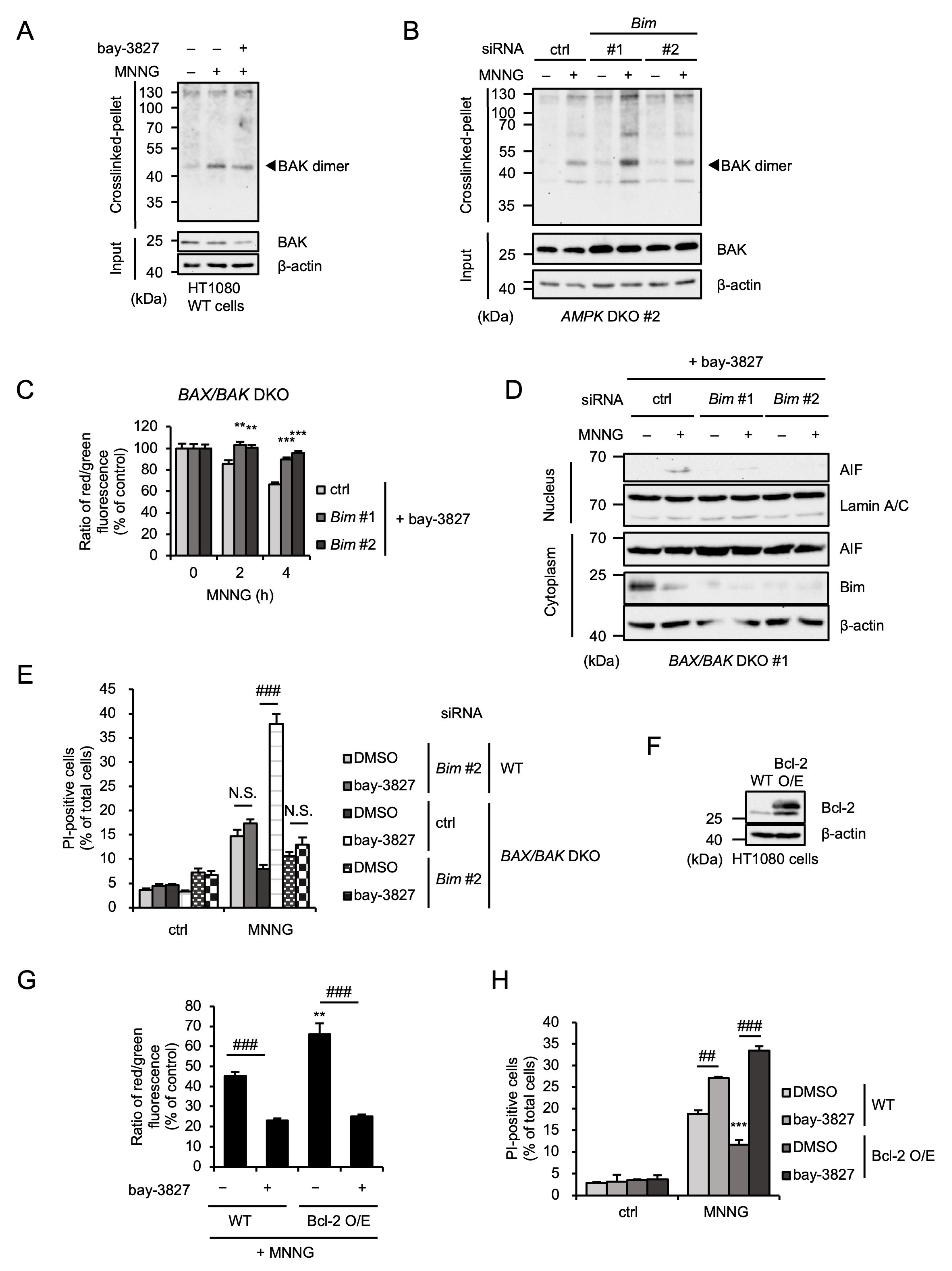
2.6. Bim Mediates MNNG-Induced Parthanatos Independently of BAX/BAK
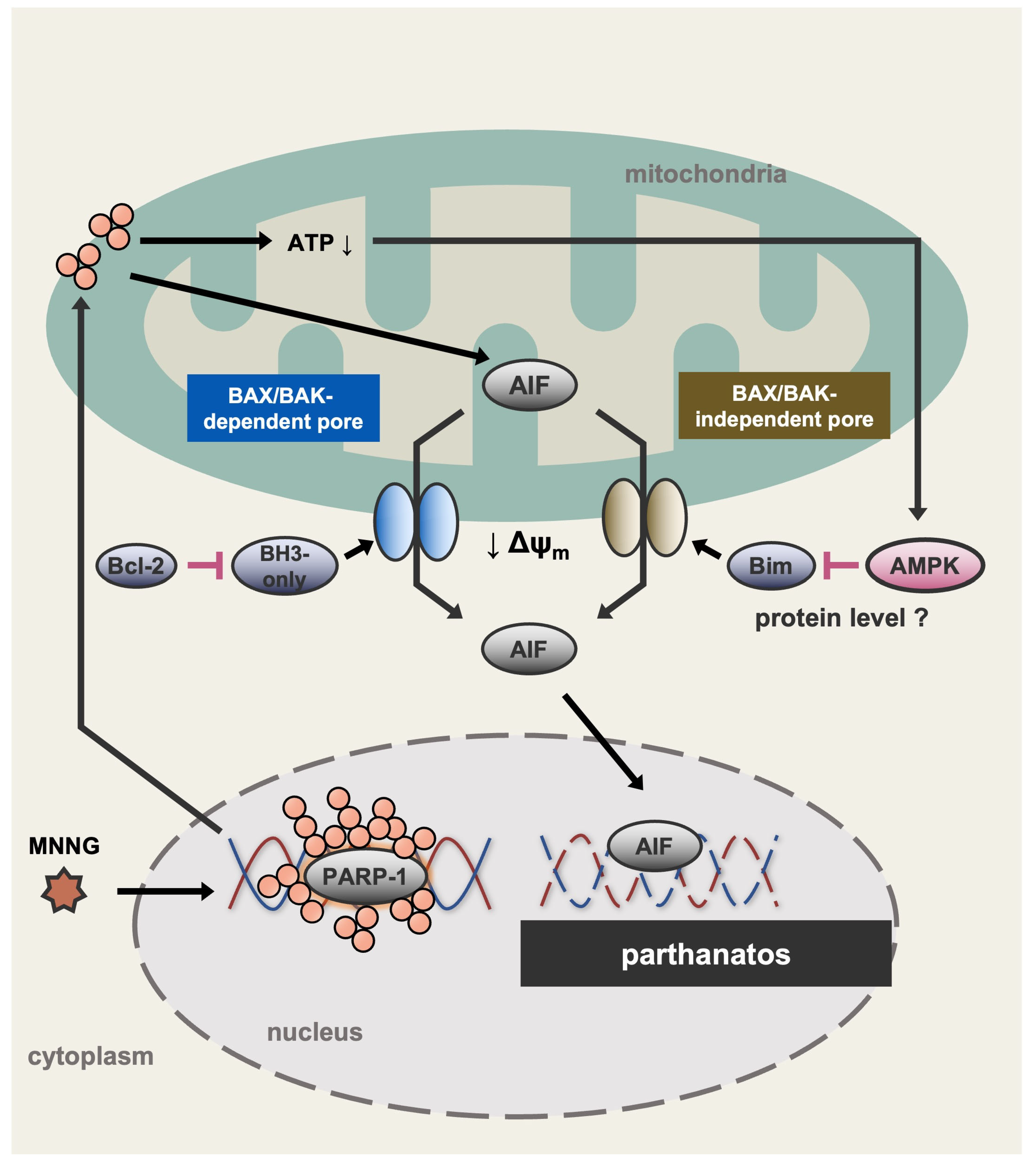
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Generation of Knockout Cell Lines
4.3. Generation of Stable Cell Line
4.4. siRNA Transfection
4.5. Immunoblot
4.6. Nuclear Extraction
4.7. Mitochondrial Membrane Potential Assay
4.8. Dimerization Assay
4.9. Propidium Iodide (PI) Staining and PI/Annexin Staining
4.10. Quantitative Real-Time PCR (qRT-PCR)
4.11. ATP Assay
4.12. Colorimetric Caspase Assay
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Robinson, N.; Ganesan, R.; Hegedus, C.; Kovacs, K.; Kufer, T.A.; Virag, L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019, 26, 101239. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and its associated nucleases in DNA damage response. DNA Repair 2019, 81, 102651. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Burkle, A. Pleiotropic cellular functions of PARP1 in longevity and aging: Genome maintenance meets inflammation. Oxid. Med. Cell Longev. 2012, 2012, 321653. [Google Scholar] [CrossRef]
- Sethi, G.S.; Dharwal, V.; Naura, A.S. Poly(ADP-Ribose)Polymerase-1 in Lung Inflammatory Disorders: A Review. Front. Immunol. 2017, 8, 1172. [Google Scholar] [CrossRef]
- Ditsworth, D.; Zong, W.-X.; Thompson, C.B. Activation of poly (ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J. Biol. Chem. 2007, 282, 17845–17854. [Google Scholar] [CrossRef]
- Noguchi, T.; Sekiguchi, Y.; Kudoh, Y.; Naganuma, R.; Kagi, T.; Nishidate, A.; Maeda, K.; Ishii, C.; Toyama, T.; Hirata, Y.; et al. Gefitinib initiates sterile inflammation by promoting IL-1beta and HMGB1 release via two distinct mechanisms. Cell Death Dis. 2021, 12, 49. [Google Scholar] [CrossRef]
- Kagi, T.; Noguchi, T.; Matsuzawa, A. Mechanisms of gefitinib-induced interstitial pneumonitis: Why and how the TKI perturbs innate immune systems? Oncotarget 2021, 12, 1321–1322. [Google Scholar] [CrossRef]
- David, K.K.; Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Parthanatos, a messenger of death. Front. Biosci. 2009, 14, 1116–1128. [Google Scholar] [CrossRef]
- Wang, Y.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatos. Exp. Neurol. 2009, 218, 193–202. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Umanah, G.K.; Chang, C.; Stevens, D.A.; Karuppagounder, S.S.; Gagne, J.P.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 10209–10214. [Google Scholar] [CrossRef]
- Chiu, L.Y.; Ho, F.M.; Shiah, S.G.; Chang, Y.; Lin, W.W. Oxidative stress initiates DNA damager MNNG-induced poly(ADP-ribose)polymerase-1-dependent parthanatos cell death. Biochem. Pharmacol. 2011, 81, 459–470. [Google Scholar] [CrossRef]
- Noguchi, T.; Suzuki, M.; Mutoh, N.; Hirata, Y.; Tsuchida, M.; Miyagawa, S.; Hwang, G.; Aoki, J.; Matsuzawa, A. Nuclear-accumulated SQSTM1/p62-based ALIS act as microdomains sensing cellular stresses and triggering oxidative stress-induced parthanatos. Cell Death Dis. 2018, 9, 1193. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, S.; Liu, Z.G.; Han, J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem. 2006, 281, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Moubarak, R.S.; Yuste, V.J.; Artus, C.; Bouharrour, A.; Greer, P.A.; Murcia, J.M.D.; Susin, S.A. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell Biol. 2007, 27, 4844–4862. [Google Scholar] [CrossRef] [PubMed]
- Cabon, L.; Galan-Malo, P.; Bouharrour, A.; Delavallee, L.; Brunelle-Navas, M.N.; Lorenzo, H.K.; Gross, A.; Susin, S.A. BID regulates AIF-mediated caspase-independent necroptosis by promoting BAX activation. Cell Death Differ. 2012, 19, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yang, H.; Cao, C.; Song, X.; Wallin, B.; Kivlin, R.; Lu, S.; Hu, G.; Di, W.; Wan, Y. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol. Rep. 2008, 20, 1553–1559. [Google Scholar] [PubMed]
- Chen, Z.; Shen, X.; Shen, F.; Zhong, W.; Wu, H.; Liu, S.; Lai, J. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70. Mol. Cell Biochem. 2013, 377, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.; Mok, S.W.; Chan, W.K.; Xu, S.W.; Wu, A.G.; Yao, X.J.; Wang, J.R.; Liu, L.; Wong, V.K. Hernandezine, a novel AMPK activator induces autophagic cell death in drug-resistant cancers. Oncotarget 2016, 7, 8090–8104. [Google Scholar] [CrossRef]
- Shimada, T.; Yabuki, Y.; Noguchi, T.; Tsuchida, M.; Komatsu, R.; Hamano, S.; Yamada, M.; Ezaki, Y.; Hirata, Y.; Matsuzawa, A. The Distinct Roles of LKB1 and AMPK in p53-Dependent Apoptosis Induced by Cisplatin. Int. J. Mol. Sci. 2022, 23, 10064. [Google Scholar] [CrossRef]
- Cheratta, A.R.; Thayyullathil, F.; Hawley, S.A.; Ross, F.A.; Atrih, A.; Lamont, D.J.; Pallichankandy, S.; Subburayan, K.; Alakkal, A.; Rezgui, R.; et al. Caspase cleavage and nuclear retention of the energy sensor AMPK-alpha1 during apoptosis. Cell Rep. 2022, 39, 110761. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, D.; Trefts, E.; Lv, M.; Inuzuka, H.; Song, G.; Liu, M.; Lu, J.; Liu, J.; Chu, C.; et al. Metabolic orchestration of cell death by AMPK-mediated phosphorylation of RIPK1. Science 2023, 380, 1372–1380. [Google Scholar] [CrossRef]
- Tiainen, M.; Ylikorkala, A.; Mäkelä, T.P. Growth suppression by Lkb1 is mediated by a G cell cycle arrest. Proc. Natl. Acad. Sci. USA 1999, 96, 9248–9251. [Google Scholar] [CrossRef]
- Yamada, Y.; Tsuchida, M.; Noguchi, T.; Yokosawa, T.; Mitsuya, M.; Shimada, T.; Oikawa, D.; Hirata, Y.; Tokunaga, F.; Schneider, P.; et al. Truncated LKB1 nonenzymatically enhances Fas-induced apoptosis by acting as a surrogate of Smac. Cell Death Discov. 2025, 11, 285. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef]
- Chhipa, R.R.; Fan, Q.; Anderson, J.; Muraleedharan, R.; Huang, Y.; Ciraolo, G.; Chen, X.; Waclaw, R.; Chow, L.M.; Khuchua, Z.; et al. AMP kinase promotes glioblastoma bioenergetics and tumour growth. Nat. Cell Biol. 2018, 20, 823–835, Erratum in: Nat Cell Biol. 2018, 20, 328. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Gross, A.; Katz, S.G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 2017, 24, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Sauter, G.; Moch, H.; Jordan, P.; Blochlinger, A.; Gasser, T.C.; Mihatsch, M.J. Prognostic significance of Bcl-2 in clinically localized prostate cancer. Am. J. Pathol. 1996, 148, 1557–1565. [Google Scholar] [PubMed]
- Martin, B.; Paesmans, M.; Berghmans, T.; Branle, F.; Ghisdal, L.; Mascaux, C.; Meert, A.P.; Steels, E.; Vallot, F.; Verdebout, J.M.; et al. Role of Bcl-2 as a prognostic factor for survival in lung cancer: A systematic review of the literature with meta-analysis. Brit. J. Cancer 2003, 89, 55–64. [Google Scholar] [CrossRef]
- Augsburger, D.; Nelson, P.J.; Kalinski, T.; Udelnow, A.; Knosel, T.; Hofstetter, M.; Qin, J.W.; Wang, Y.; Gupta, A.S.; Bonifatius, S.; et al. Current diagnostics and treatment of fibrosarcoma -perspectives for future therapeutic targets and strategies. Oncotarget 2017, 8, 104638–104653. [Google Scholar] [CrossRef]
- Mahdavi, N.; Derakhshan, S.; Etemadi, M. Fibrosarcoma of the maxilla with maxillary sinus invasion: A case report and review of the literature. J. Med. Case Rep. 2025, 19, 254. [Google Scholar] [CrossRef]
- Keil, C.; Grobe, T.; Oei, S.L. MNNG-induced cell death is controlled by interactions between PARP-1, poly(ADP-ribose) glycohydrolase, and XRCC1. J. Biol. Chem. 2006, 281, 34394–34405. [Google Scholar] [CrossRef]
- Tentori, L.; Orlando, L.; Lacal, P.M.; Benincasa, E.; Faraoni, I.; Bonmassar, E.; D’Atri, S.; Graziani, G. Inhibition of O6-alkylguanine DNA-alkyltransferase or poly(ADP-ribose) polymerase increases susceptibility of leukemic cells to apoptosis induced by temozolomide. Mol. Pharmacol. 1997, 52, 249–258. [Google Scholar] [CrossRef]
- Yamada, Y.; Noguchi, T.; Suzuki, M.; Yamada, M.; Hirata, Y.; Matsuzawa, A. Reactive sulfur species disaggregate the SQSTM1/p62-based aggresome-like induced structures via the HSP70 induction and prevent parthanatos. J. Biol. Chem. 2023, 299, 104710. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Goellner, E.M.; Yu, Z.; Gagne, J.P.; Barbi de Moura, M.; Feinstein, T.; Wheeler, D.; Redpath, P.; Li, J.; Romero, G.; et al. ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD+ depletion. Cell Rep. 2014, 8, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Chen, Y.; Alano, C.C.; Swanson, R.A. Tricarboxylic acid cycle substrates prevent PARP-mediated death of neurons and astrocytes. J. Cereb. Blood Flow. Metab. 2002, 22, 774–779. [Google Scholar] [CrossRef]
- Nechushtan, A.; Smith, C.L.; Lamensdorf, I.; Yoon, S.H.; Youle, R.J. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 2001, 153, 1265–1276. [Google Scholar] [CrossRef]
- Luciano, F.; Jacquel, A.; Colosetti, P.; Herrant, M.; Cagnol, S.; Pages, G.; Auberger, P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 2003, 22, 6785–6793. [Google Scholar] [CrossRef]
- Moustafa-Kamal, M.; Gamache, I.; Lu, Y.; Li, S.; Teodoro, J.G. BimEL is phosphorylated at mitosis by Aurora A and targeted for degradation by βTrCP1. Cell Death Differ. 2013, 20, 1393–1403. [Google Scholar] [CrossRef]
- Strasser, A.; Cory, S.; Adams, J.M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011, 30, 3667–3683. [Google Scholar] [CrossRef]
- Dong, L.; Vaux, D.L. Glucocorticoids can induce BIM to trigger apoptosis in the absence of BAX and BAK1. Cell Death Dis. 2020, 11, 442. [Google Scholar] [CrossRef]
- Llambi, F.; Wang, Y.M.; Victor, B.; Yang, M.; Schneider, D.M.; Gingras, S.; Parsons, M.J.; Zheng, J.H.; Brown, S.A.; Pelletier, S.; et al. BOK Is a Non-canonical BCL-2 Family Effector of Apoptosis Regulated by ER-Associated Degradation. Cell 2016, 165, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells die with increased cytosolic ATP during apoptosis: A bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.B.; Halmos, B.; Kumar, A.; Schumer, S.T.; Huberman, M.S.; Boggon, T.J.; Tenen, D.G.; Kobayashi, S. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007, 4, 1669–1679; discussion 1680. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.C.; Corcoran, R.B.; Ebi, H.; Sequist, L.V.; Waltman, B.A.; Chung, E.; Incio, J.; Digumarthy, S.R.; Pollack, S.F.; Song, Y.; et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011, 1, 352–365. [Google Scholar] [CrossRef]
- Sofi, S.; Mehraj, U.; Jan, N.; Almilaibary, A.; Ahmad, I.; Ahmad, F.; Ahmad Mir, M. Clinicopathological Significance and Expression Pattern of Bcl2 in Breast Cancer: A Comprehensive in silico and in vitro Study. Saudi J. Biol. Sci. 2024, 31, 103916. [Google Scholar] [CrossRef]
- Placzek, W.J.; Wei, J.; Kitada, S.; Zhai, D.; Reed, J.C.; Pellecchia, M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010, 1, e40. [Google Scholar] [CrossRef]
- Maru, B.; Messikommer, A.; Huang, L.; Seipel, K.; Kovecses, O.; Valk, P.J.M.; Theocharides, A.P.A.; Mercier, F.E.; Pabst, T.; McKeague, M.; et al. PARP-1 improves leukemia outcomes by inducing parthanatos during chemotherapy. Cell Rep. Med. 2023, 4, 101191. [Google Scholar] [CrossRef]
- Wang, X.Z.; Liang, S.P.; Chen, X.; Wang, Z.C.; Li, C.; Feng, C.S.; Lu, S.; He, C.; Wang, Y.B.; Chi, G.F.; et al. TAX1BP1 contributes to deoxypodophyllotoxin-induced glioma cell parthanatos via inducing nuclear translocation of AIF by activation of mitochondrial respiratory chain complex I. Acta Pharmacol. Sin. 2023, 44, 1906–1919. [Google Scholar] [CrossRef]
- Li, B.; Luo, C.; Chowdhury, S.; Gao, Z.H.; Liu, J.L. Parp1 deficient mice are protected from streptozotocin-induced diabetes but not caerulein-induced pancreatitis, independent of the induction of Reg family genes. Regul. Pept. 2013, 186, 83–91. [Google Scholar] [CrossRef]
- Noguchi, T.; Sekiguchi, Y.; Shimada, T.; Suzuki, W.; Yokosawa, T.; Itoh, T.; Yamada, M.; Suzuki, M.; Kurokawa, R.; Hirata, Y.; et al. LLPS of SQSTM1/p62 and NBR1 as outcomes of lysosomal stress response limits cancer cell metastasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2311282120. [Google Scholar] [CrossRef]
- Otani, K.; Komatsu, R.; Noguchi, T.; Suzuki, W.; Hirata, Y.; Matsuzawa, A. The Selective 3-MST Inhibitor I3MT-3 Works as a Potent Caspase-1 Inhibitor. Int. J. Mol. Sci. 2025, 26, 2237. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Kudoh, Y.; Noguchi, T.; Kagi, T.; Suzuki, M.; Tsuchida, M.; Komatsu, H.; Takahashi, M.; Hirata, Y.; Matsuzawa, A. The E3 Ubiquitin-Protein Ligase RNF4 Promotes TNF-alpha-Induced Cell Death Triggered by RIPK1. Int. J. Mol. Sci. 2021, 22, 5796. [Google Scholar] [CrossRef]
- Kagi, T.; Naganuma, R.; Inoue, A.; Noguchi, T.; Hamano, S.; Sekiguchi, Y.; Hwang, G.W.; Hirata, Y.; Matsuzawa, A. The polypeptide antibiotic polymyxin B acts as a pro-inflammatory irritant by preferentially targeting macrophages. J. Antibiot. 2022, 75, 29–39. [Google Scholar] [CrossRef]
- Yokosawa, T.; Miyagawa, S.; Suzuki, W.; Nada, Y.; Hirata, Y.; Noguchi, T.; Matsuzawa, A. The E3 Ubiquitin Protein Ligase LINCR Amplifies the TLR-Mediated Signals through Direct Degradation of MKP1. Cells 2024, 13, 687. [Google Scholar] [CrossRef]
- Yamada, Y.; Ito, R.; Noguchi, T.; Hamano, S.; Otani, K.; Komatsu, T.; Hirata, Y.; Matsuzawa, A. The degree of caspase-3 aggregation determines the selectivity of arsenic-induced cell death. J. Toxicol. Sci. 2025, 50, 351–359. [Google Scholar] [CrossRef]
- Kagi, T.; Tan, M.; Suzuki, W.; Otani, K.; Suzuki, S.; Hirata, Y.; Noguchi, T.; Matsuzawa, A. Benzalkonium chloride initiates proinflammatory responses via NLRP3 inflammasome activation. J. Toxicol. Sci. 2025, 50, 11–21. [Google Scholar] [CrossRef]
- Hamano, S.; Noguchi, T.; Asai, Y.; Ito, R.; Komatsu, R.; Sato, T.; Inoue, A.; Maruyama, T.; Kudo, T.A.; Hirata, Y.; et al. Aggregability of the SQSTM1/p62-based aggresome-like induced structures determines the sensitivity to parthanatos. Cell Death Discov. 2024, 10, 74. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamano, S.; Maruyama, T.; Suzuki, M.; Mitsuya, M.; Yokosawa, T.; Hirata, Y.; Matsuzawa, A.; Noguchi, T. AMPK Limits MNNG-Induced Parthanatos by Inhibiting BH3-Only Protein Bim. Int. J. Mol. Sci. 2025, 26, 10519. https://doi.org/10.3390/ijms262110519
Hamano S, Maruyama T, Suzuki M, Mitsuya M, Yokosawa T, Hirata Y, Matsuzawa A, Noguchi T. AMPK Limits MNNG-Induced Parthanatos by Inhibiting BH3-Only Protein Bim. International Journal of Molecular Sciences. 2025; 26(21):10519. https://doi.org/10.3390/ijms262110519
Chicago/Turabian StyleHamano, Shuhei, Tomoe Maruyama, Midori Suzuki, Maki Mitsuya, Takumi Yokosawa, Yusuke Hirata, Atsushi Matsuzawa, and Takuya Noguchi. 2025. "AMPK Limits MNNG-Induced Parthanatos by Inhibiting BH3-Only Protein Bim" International Journal of Molecular Sciences 26, no. 21: 10519. https://doi.org/10.3390/ijms262110519
APA StyleHamano, S., Maruyama, T., Suzuki, M., Mitsuya, M., Yokosawa, T., Hirata, Y., Matsuzawa, A., & Noguchi, T. (2025). AMPK Limits MNNG-Induced Parthanatos by Inhibiting BH3-Only Protein Bim. International Journal of Molecular Sciences, 26(21), 10519. https://doi.org/10.3390/ijms262110519







