Hormonal and Sex-Specific Regulation of Key Players in Fibro-Calcific Aortic Valve Disease
Abstract
1. Introduction
2. Results
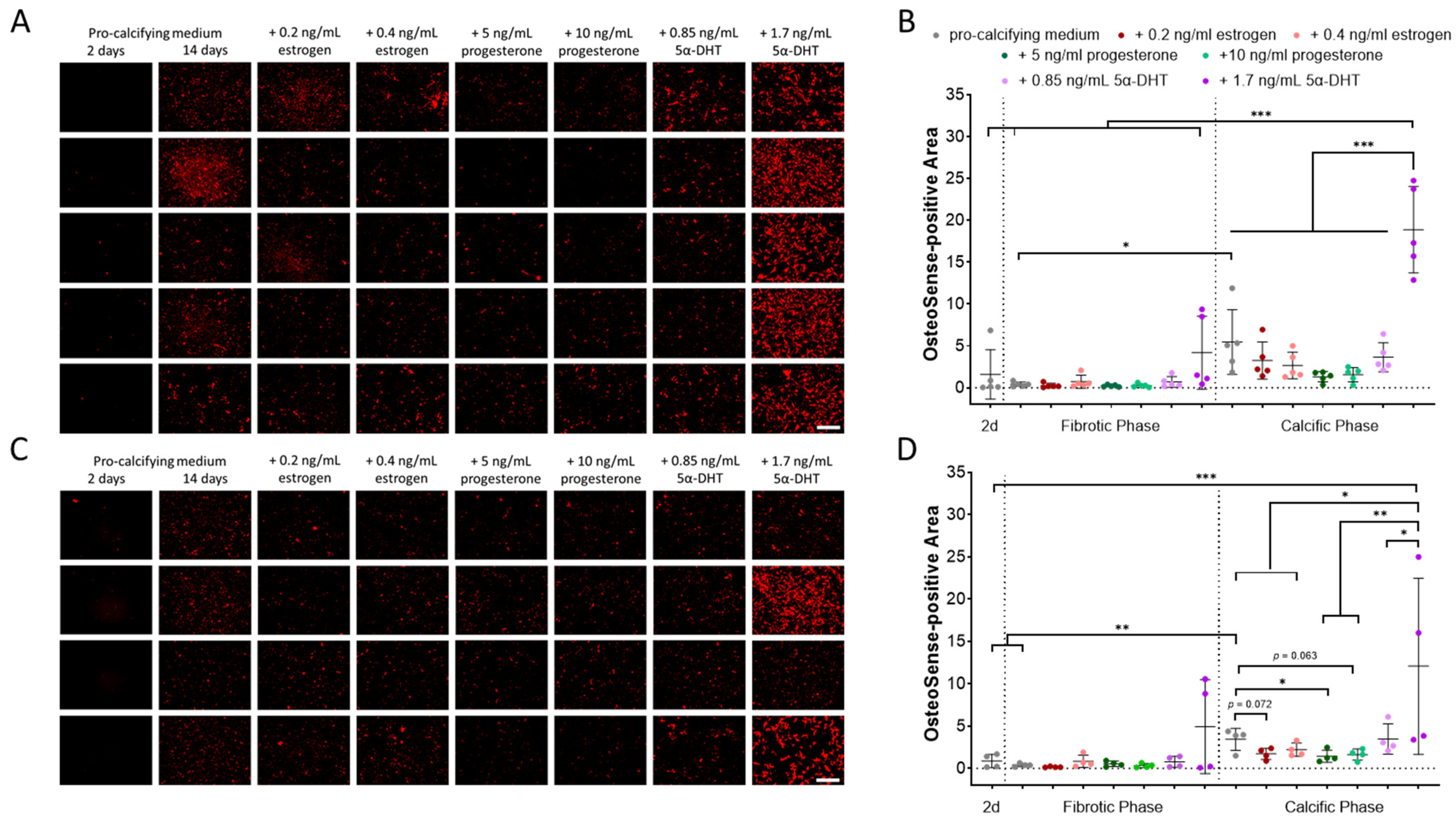
2.1. Impact of Donor Sex and Sex Hormones on In Vitro Calcification
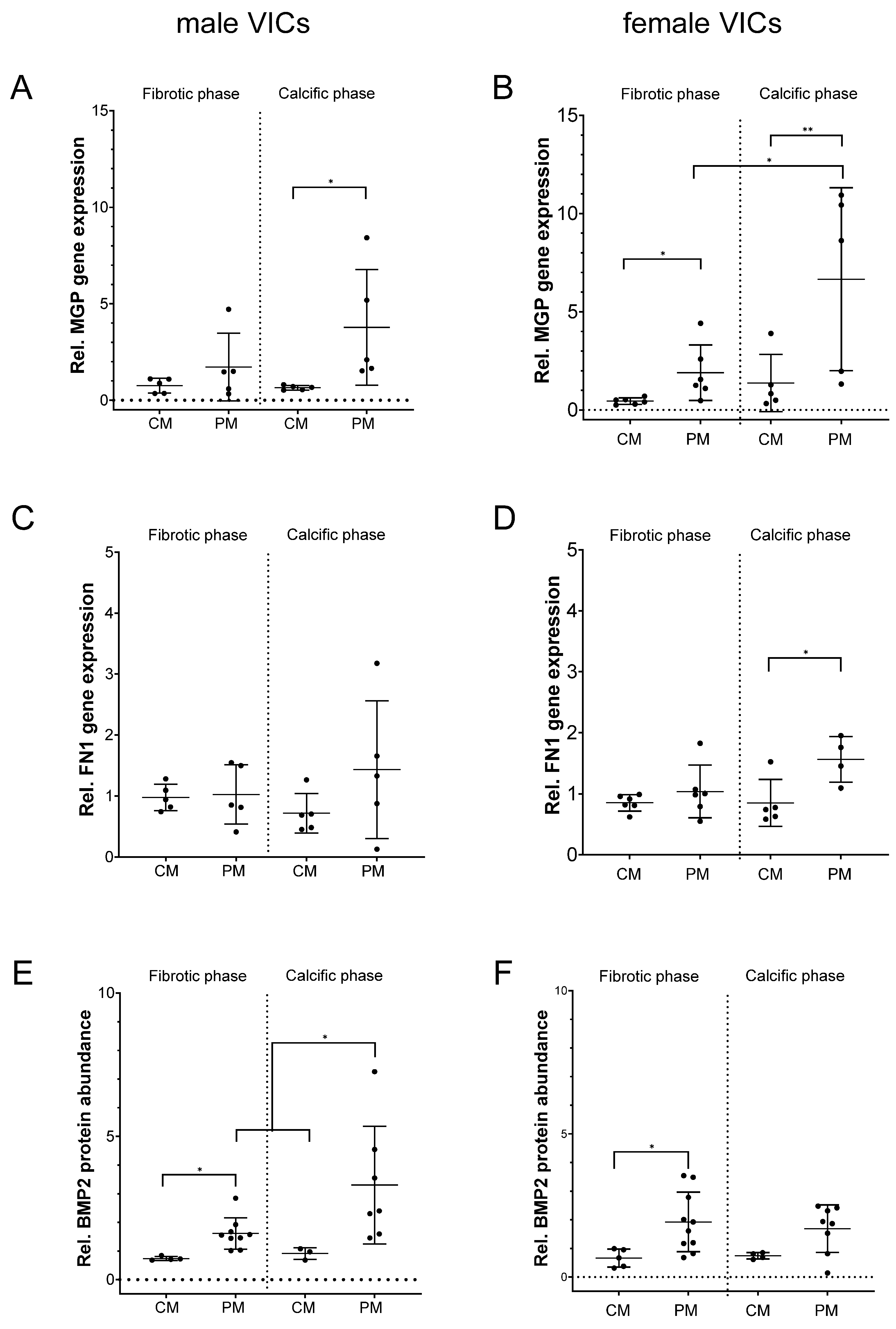
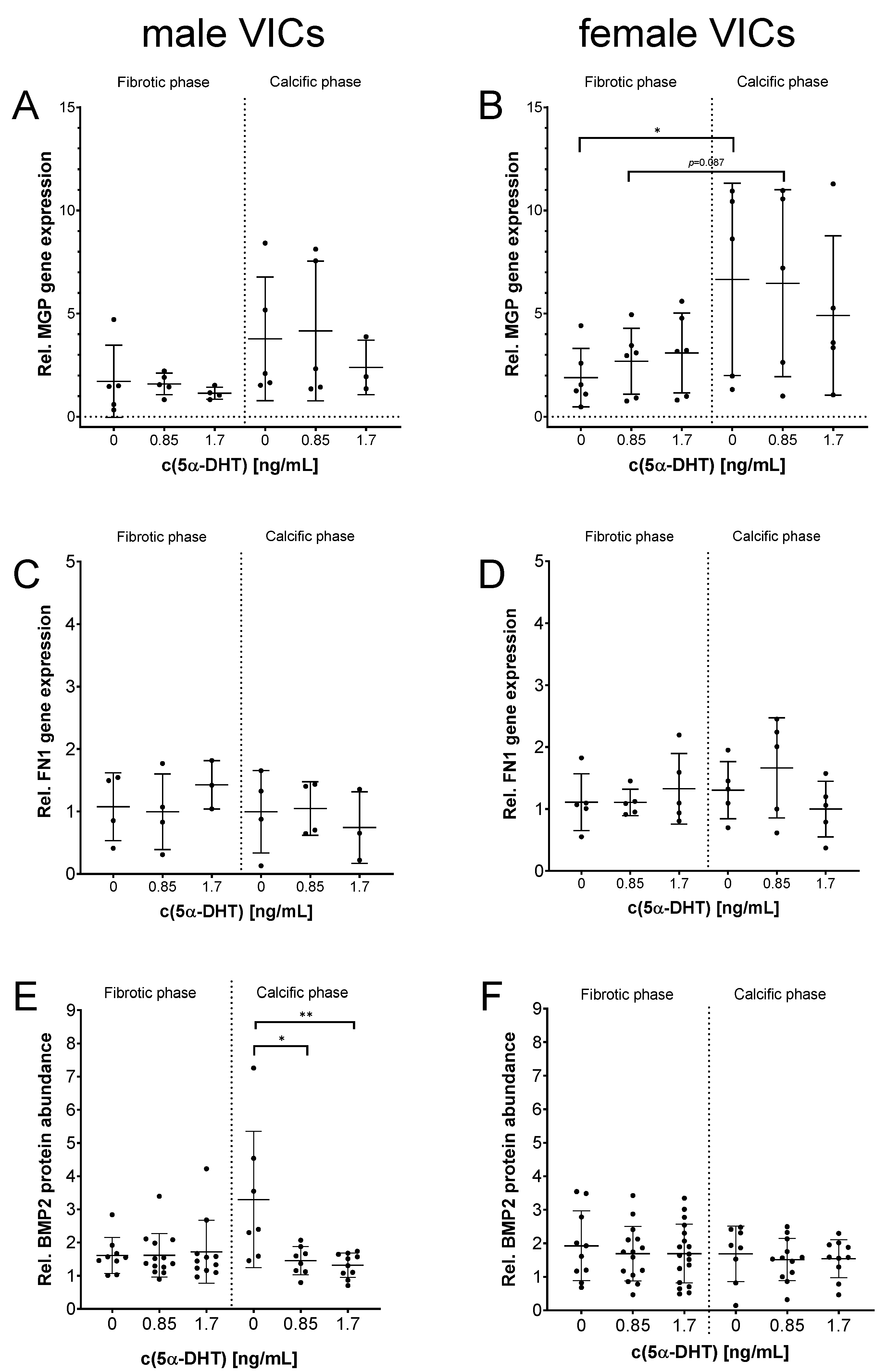
2.2. MGP, FN1 and BMP2 Expression in Relation to Donor Sex and After Exposure to 5α-DHT
3. Discussion
4. Materials and Methods
4.1. Aortic Valves and Isolation and Cultivation of Human Valvular Interstitial Cells
4.2. Near-Infrared Molecular Staining and Quantification of PM-Induced HA Deposition
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Western Blot
4.5. Statistical Analysis
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, J.D.; Chu, Y.; Brooks, R.M.; Richenbacher, W.E.; Peña-Silva, R.; Heistad, D.D. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008, 52, 843–850. [Google Scholar] [CrossRef]
- Mascherbauer, J.; Kammerlander, A.; Nitsche, C.; Bax, J.; Delgado, V.; Evangelista, A.; Laroche, C.; Maggioni, A.P.; Magne, J.; Vahanian, A.; et al. Sex-related differences in severe native valvular heart disease: The ESC-EORP Valvular Heart Disease II survey. Eur. Heart J. 2024, 45, 3818–3833. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Guo, J.; Sun, W.; Ji, Y.; Wang, Y.; Liu, J.; Kong, X. Overexpression of microRNA-93-5p and microRNA-374a-5p Suppresses the Osteogenic Differentiation and Mineralization of Human Aortic Valvular Interstitial Cells Through the BMP2/Smad1/5/RUNX2 Signaling Pathway. J. Cardiovasc. Pharmacol. 2023, 82, 138–147. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Woodward, H.J.; Zhu, D.; Hadoke, P.W.F.; MacRae, V.E. Regulatory Role of Sex Hormones in Cardiovascular Calcification. Int. J. Mol. Sci. 2021, 22, 4620. [Google Scholar] [CrossRef] [PubMed]
- Voisine, M.; Hervault, M.; Shen, M.; Boilard, A.-J.; Filion, B.; Rosa, M.; Bossé, Y.; Mathieu, P.; Côté, N.; Clavel, M.-A. Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve. J. Am. Heart Assoc. 2020, 9, e015610. [Google Scholar] [CrossRef] [PubMed]
- Böttner, J.; Werner, S.; Feistner, L.; Fischer-Schaepmann, T.; Neussl, K.; Borger, M.A.; Thiele, H.; Büttner, P.; Schlotter, F. High resolution monitoring of valvular interstitial cell driven pathomechanisms in procalcific environment using label-free impedance spectroscopy. Front. Cardiovasc. Med. 2023, 10, 1155371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Di Chen Tsao, C.; Deng, H.-W.; Zhao, M. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 2013, 52, 145–156. [Google Scholar] [CrossRef]
- Kruithof, B.P.T.; Xu, J.; Fritz, D.T.; Cabral, C.S.; Gaussin, V.; Rogers, M.B. An in vivo map of bone morphogenetic protein 2 post-transcriptional repression in the heart. Genesis 2011, 49, 841–850. [Google Scholar] [CrossRef]
- O’Young, J.; Liao, Y.; Xiao, Y.; Jalkanen, J.; Lajoie, G.; Karttunen, M.; Goldberg, H.A.; Hunter, G.K. Matrix Gla protein inhibits ectopic calcification by a direct interaction with hydroxyapatite crystals. J. Am. Chem. Soc. 2011, 133, 18406–18412. [Google Scholar] [CrossRef]
- Yang, P.; Troncone, L.; Augur, Z.M.; Kim, S.S.J.; McNeil, M.E.; Yu, P.B. The role of bone morphogenetic protein signaling in vascular calcification. Bone 2020, 141, 115542. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Shanahan, C.M. Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein. Nephrology 2006, 11, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.M.; Ma, W.; Mosher, D.F. Dynamic structure of plasma fibronectin. Crit. Rev. Biochem. Mol. Biol. 2015, 51, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Fayet, C.; Bendeck, M.P.; Gotlieb, A.I. Cardiac valve interstitial cells secrete fibronectin and form fibrillar adhesions in response to injury. Cardiovasc. Pathol. 2007, 16, 203–211. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Zhou, J.; Liang, Q.; He, F.; Li, F.; Chen, J.; Zhang, F.; Han, C.; Liu, J.; et al. Fibronectin 1 activates WNT/β-catenin signaling to induce osteogenic differentiation via integrin β1 interaction. Lab. Investig. 2020, 100, 1494–1502. [Google Scholar] [CrossRef]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef]
- Kloner, R.A.; Carson, C.; Dobs, A.; Kopecky, S.; Mohler, E.R. Testosterone and Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 67, 545–557. [Google Scholar] [CrossRef]
- Lai, J.; Ge, Y.; Shao, Y.; Xuan, T.; Xia, S.; Li, M. Low serum testosterone level was associated with extensive coronary artery calcification in elderly male patients with stable coronary artery disease. Coron. Artery Dis. 2015, 26, 437–441. [Google Scholar] [CrossRef]
- Zhang, B.; Miller, V.M.; Miller, J.D. Influences of Sex and Estrogen in Arterial and Valvular Calcification. Front. Endocrinol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Chung, C.-C.; Hsu, R.-C.; Kao, Y.-H.; Liou, J.-P.; Lu, Y.-Y.; Chen, Y.-J. Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-β and angiotensin II signaling. Int. J. Cardiol. 2014, 176, 386–393. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Akiyoshi, T.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Eto, M.; Ouchi, Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: Protective role of eNOS and SIRT1. PLoS ONE 2012, 7, e29598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Dou, Y.; Zhao, J.; Jiang, T.; Qiao, Z.; Qiao, J. Testosterone and estradiol modulate TNF-alpha-induced expression of adhesion molecules in endothelial cells. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B. Is atherosclerotic cardiovascular disease an endocrinological disorder? The estrogen-androgen paradox. J. Clin. Endocrinol. Metab. 2005, 90, 2708–2711. [Google Scholar] [CrossRef] [PubMed]
- Osako, M.K.; Nakagami, H.; Koibuchi, N.; Shimizu, H.; Nakagami, F.; Koriyama, H.; Shimamura, M.; Miyake, T.; Rakugi, H.; Morishita, R. Estrogen inhibits vascular calcification via vascular RANKL system: Common mechanism of osteoporosis and vascular calcification. Circ. Res. 2010, 107, 466–475. [Google Scholar] [CrossRef]
- Harper, E.; Forde, H.; Davenport, C.; Rochfort, K.D.; Smith, D.; Cummins, P.M. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vascul. Pharmacol. 2016, 82, 30–40. [Google Scholar] [CrossRef]
- Sahoo, S.; Meijles, D.N.; Pagano, P.J. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317–335. [Google Scholar] [CrossRef]
- Zapata, E.; Ventura, J.L.; La Cruz Kde Rodriguez, E.; Damián, P.; Massó, F.; Montaño, L.F.; López-Marure, R. Dehydroepiandrosterone inhibits the proliferation of human umbilical vein endothelial cells by enhancing the expression of p53 and p21, restricting the phosphorylation of retinoblastoma protein, and is androgen- and estrogen-receptor independent. FEBS J. 2005, 272, 1343–1353. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Shao, Z.M.; Chen, J.C.; Fontana, J.A. Differential regulation of matrix Gla protein (MGP) gene expression by retinoic acid and estrogen in human breast carcinoma cells. Mol. Cell. Endocrinol. 1993, 92, 153–160. [Google Scholar] [CrossRef]
- Castardo-de-Paula, J.C.; Campos, B.H.; de Amorim, E.D.T.; da Silva, R.V.; de Farias, C.C.; Higachi, L.; Pinge-Filho, P.; Barbosa, D.S.; Martins-Pinge, M.C. Cardiovascular risk and the effect of nitric oxide synthase inhibition in female rats: The role of estrogen. Exp. Gerontol. 2017, 97, 38–48. [Google Scholar] [CrossRef]
- Nevzati, E.; Shafighi, M.; Bakhtian, K.D.; Treiber, H.; Fandino, J.; Fathi, A.R. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir. Suppl. 2015, 120, 141–145. [Google Scholar]
- Richards, J.; El-Hamamsy, I.; Chen, S.; Sarang, Z.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H.; Butcher, J.T. Side-specific endothelial-dependent regulation of aortic valve calcification: Interplay of hemodynamics and nitric oxide signaling. Am. J. Pathol. 2013, 182, 1922–1931. [Google Scholar] [CrossRef]
- Pagana, K.D. Mosby’s® Diagnostic and Laboratory Test Reference, 17th ed.; Mosby: Philadelphia, PA, USA, 2025; Available online: https://ebookcentral.proquest.com/lib/kxp/detail.action?docID=31479954 (accessed on 22 September 2025).
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C. Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease. Circ. Res. 2023, 132, 993–1012. [Google Scholar] [CrossRef] [PubMed]
- Rattazzi, M.; Bertacco, E.; Del Vecchio, A.; Puato, M.; Faggin, E.; Pauletto, P. Aortic valve calcification in chronic kidney disease. Nephrol. Dial. Transplant. 2013, 28, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zhao, X.; Jin, X.; Zhang, Z.; Chen, L.; Zhu, Y.; Yuan, W. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell. Physiol. Biochem. 2014, 34, 2049–2060. [Google Scholar] [CrossRef]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef]
- Masjedi, S.; Lei, Y.; Patel, J.; Ferdous, Z. Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells. Heart Vessels 2017, 32, 217–228. [Google Scholar] [CrossRef]
- Simard, L.; Côté, N.; Dagenais, F.; Mathieu, P.; Couture, C.; Trahan, S.; Bossé, Y.; Mohammadi, S.; Pagé, S.; Joubert, P.; et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ. Res. 2017, 120, 681–691. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Xiang, L.-X.; Shao, J.-Z. Bone morphogenetic protein. Biochem. Biophys. Res. Commun. 2007, 362, 550–553. [Google Scholar] [CrossRef]
- Jung, J.-J.; Ahmad, A.A.; Rajendran, S.; Wei, L.; Zhang, J.; Toczek, J.; Nie, L.; Kukreja, G.; Salarian, M.; Gona, K.; et al. Differential BMP Signaling Mediates the Interplay Between Genetics and Leaflet Numbers in Aortic Valve Calcification. JACC Basic. Transl. Sci. 2022, 7, 333–345. [Google Scholar] [CrossRef]
- Boström, K.; Watson, K.E.; Horn, S.; Wortham, C.; Herman, I.M.; Demer, L.L. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Investig. 1993, 91, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Biomarkers of vascular calcification in serum. Adv. Clin. Chem. 2020, 98, 91–147. [Google Scholar] [PubMed]
- McCoy, C.M.; Nicholas, D.Q.; Masters, K.S. Sex-related differences in gene expression by porcine aortic valvular interstitial cells. PLoS ONE 2012, 7, e39980. [Google Scholar] [CrossRef] [PubMed]
- Matilla, L.; Jover, E.; Garaikoetxea, M.; Martín-Nuñez, E.; Arrieta, V.; García-Peña, A.; Navarro, A.; Fernández-Celis, A.; Gainza, A.; Álvarez, V.; et al. Sex-Related Signaling of Aldosterone/Mineralocorticoid Receptor Pathway in Calcific Aortic Stenosis. Hypertension 2022, 79, 1724–1737. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Munroe, P.B.; Olgunturk, R.O.; Fryns, J.P.; van Maldergem, L.; Ziereisen, F.; Yuksel, B.; Gardiner, R.M.; Chung, E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef]
- Teebi, A.S.; Lambert, D.M.; Kaye, G.M.; Al-Fifi, S.; Tewfik, T.L.; Azouz, E.M. Keutel syndrome: Further characterization and review. Am. J. Med. Genet. 1998, 78, 182–187. [Google Scholar] [CrossRef]
- Boström, K.; Tsao, D.; Shen, S.; Wang, Y.; Demer, L.L. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J. Biol. Chem. 2001, 276, 14044–14052. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.F.; Knapen, M.H.J.; Kwaijtaal, M.; van Diest, R.; Appels, A.; Reutelingsperger, C.P.; Cleutjens, J.P.; Vermeer, C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1629–1633. [Google Scholar] [CrossRef]
- Zebboudj, A.F.; Imura, M.; Boström, K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J. Biol. Chem. 2002, 277, 4388–4394. [Google Scholar] [CrossRef]
- Fleury, M.-A.; Annabi, M.-S.; Voisine, M.; Hervault, M.; Boilard, A.-J.; Shen, M.; Marette, A.; Côté, N.; Clavel, M.A. Impact of sex and sex hormones on pathophysiology and progression of aortic stenosis in a murine model. Physiol. Rep. 2022, 10, e15433. [Google Scholar] [CrossRef]
- Abdoun, K.; Tastet, L.; Bedard, E.; Arsenault, M.; Pibarot, P.; Clavel, M.-A. Abstract 4138306: Impact of Early Menopause and Hormonal Replacement Therapy on Aortic Stenosis Progressio. Circulation 2024, 150, A4138306. [Google Scholar] [CrossRef]
- Vanberg, P.; Atar, D. Androgenic Anabolic Steroid Abuse and the Cardiovascular System; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 195, pp. 411–457. [Google Scholar]
- Son, B.-K.; Akishita, M.; Iijima, K.; Ogawa, S.; Maemura, K.; Yu, J.; Takeyama, K.; Kato, S.; Eto, M.; Ouchi, Y. Androgen receptor-dependent transactivation of growth arrest-specific gene 6 mediates inhibitory effects of testosterone on vascular calcification. J. Biol. Chem. 2010, 285, 7537–7544. [Google Scholar] [CrossRef]
- Moncla, L.-H.M.; Briend, M.; Bossé, Y.; Mathieu, P. Calcific aortic valve disease: Mechanisms, prevention and treatment. Nat. Rev. Cardiol. 2023, 20, 546–559. [Google Scholar] [CrossRef]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HPRT1 | CTCATGGACTGATTATGGACAGGAC | GCAGGTCAGCAAAGAACTTATAGCC |
| MGP | CCCTATTGAGCTCGTGGACA | ACCTTCATATCCCCTCAGCAGA |
| FN1 | TCGTGCTTTGACCCCTACAC | TTCCCAAGACATGTGCAGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neussl, K.; Werner, S.; Thiele, H.; Schlotter, F.; Borger, M.A.; Büttner, P.; Böttner, J. Hormonal and Sex-Specific Regulation of Key Players in Fibro-Calcific Aortic Valve Disease. Int. J. Mol. Sci. 2025, 26, 10517. https://doi.org/10.3390/ijms262110517
Neussl K, Werner S, Thiele H, Schlotter F, Borger MA, Büttner P, Böttner J. Hormonal and Sex-Specific Regulation of Key Players in Fibro-Calcific Aortic Valve Disease. International Journal of Molecular Sciences. 2025; 26(21):10517. https://doi.org/10.3390/ijms262110517
Chicago/Turabian StyleNeussl, Katherina, Sarah Werner, Holger Thiele, Florian Schlotter, Michael A. Borger, Petra Büttner, and Julia Böttner. 2025. "Hormonal and Sex-Specific Regulation of Key Players in Fibro-Calcific Aortic Valve Disease" International Journal of Molecular Sciences 26, no. 21: 10517. https://doi.org/10.3390/ijms262110517
APA StyleNeussl, K., Werner, S., Thiele, H., Schlotter, F., Borger, M. A., Büttner, P., & Böttner, J. (2025). Hormonal and Sex-Specific Regulation of Key Players in Fibro-Calcific Aortic Valve Disease. International Journal of Molecular Sciences, 26(21), 10517. https://doi.org/10.3390/ijms262110517






