Three-Dimensional Culture of Epithelial Cells on Electrospun Nanofibrous Scaffolds
Abstract
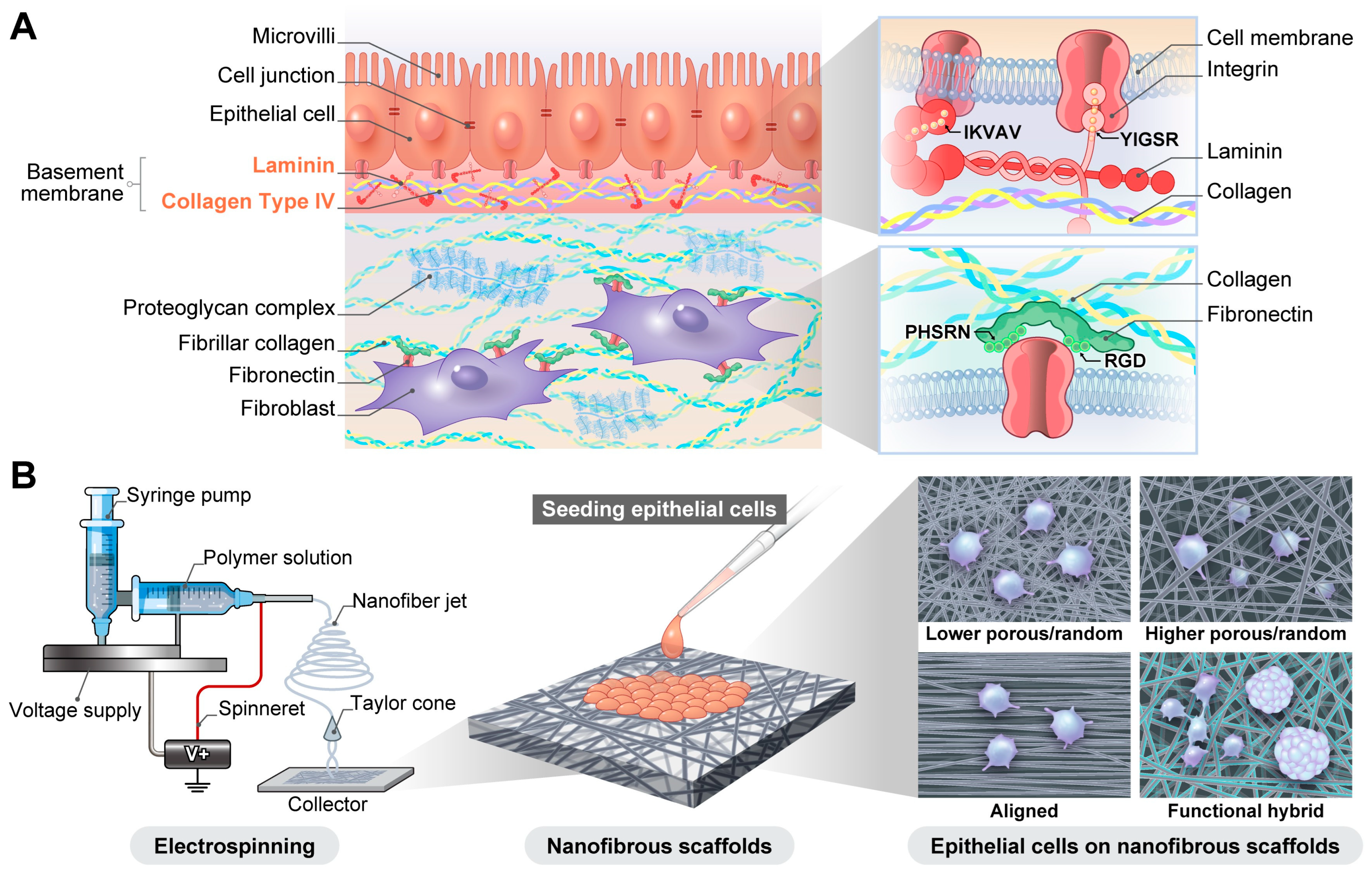
1. Introduction
2. Nanofibrous Scaffold Fabrication by Electrospinning
3. Polymer Scaffolds for Epithelial Cell Adhesion and Growth
3.1. Natural Polymers
3.2. Synthetic Polymers
3.3. Hybrid Polymers
3.4. Laminin and Laminin-Derived Peptide-Blended Nanofibers for Epithelial Cell Culture
4. Tissue-Specific Application of Nanofibrous Scaffolds
4.1. Bronchial and Lung Epithelial Cell Culture on Nanofibrous Scaffolds
4.1.1. Inadequacy of Conventional Models and Emergence of a 3D Culture of Bronchial and Lung Epithelial Cells
4.1.2. Cell Sources: Building Blocks of the Model
4.1.3. Nanofibrous Scaffolds for 3D Cultures of Bronchial and Lung Epithelial Cells
4.1.4. Coculture Systems Formed Using Nanofibrous Scaffolds
4.1.5. Applications of Nanofibrous Scaffolds in ALI Systems
4.2. 3D Culture of Retinal Epithelial Cells on Nanofibrous Scaffolds
4.2.1. Sources of RPE Cells
4.2.2. Nanofibrous Scaffolds for 3D Cultures of RPE Cells
4.2.3. Laminin-Attached Nanofibrous Scaffolds for 3D Cultures of RPE Cells
4.2.4. 3D Cultures of RPE Cells on Nanofibrous Scaffolds for Retinal Transplantation
4.3. 3D Culture of Other Ocular Epithelial Cells on Nanofibrous Scaffolds
4.3.1. 3D Culture of Corneal Epithelial Cells on Nanofibrous Scaffolds
4.3.2. 3D Culture of Conjunctival Epithelial Cells on Nanofibrous Scaffolds
4.3.3. 3D Culture of Limbal Epithelial Cells on Nanofibrous Scaffolds
4.4. 3D Culture of Esophageal, Intestinal, and Colon Epithelial Cells on Nanofibrous Scaffolds
4.4.1. 3D Cultures of Intestinal and Colon Epithelial Cells on Nanofibrous Scaffolds
4.4.2. 3D Culture of Esophageal Epithelial Cells on Nanofibrous Scaffolds
4.5. 3D Culture of Kidney Epithelial Cells on Nanofibrous Scaffolds
4.5.1. Effects of Physical and Chemical Properties of Nanofibrous Scaffolds on 3D Cultures of Kidney Epithelial Cells
4.5.2. 3D Culture of Kidney Epithelial Cells on Functionalized Scaffolds
4.6. 3D Culture of Skin Epithelial Cells on Nanofibrous Scaffolds
4.6.1. 3D Culture of Keratinocytes on Nanofibrous Scaffolds
4.6.2. Nanofibrous Scaffolds in Skin Regeneration
4.7. 3D Culture of Salivary Gland Epithelial Cells on Nanofibrous Scaffolds
Culture of Submandibular Ductal Salivary Gland Cells on Nanofibrous Scaffolds
5. Basic Research and Clinical Applications of Nanofiber Scaffolds
6. Future Outlook
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- LeBleu, V.S.; Macdonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Chen, C.S. Cell biology. Deconstructing dimensionality. Science 2013, 339, 402–404. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef]
- Rosso, F.; Giordano, A.; Barbarisi, M.; Barbarisi, A. From cell-ECM interactions to tissue engineering. J. Cell. Physiol. 2004, 199, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Wise, J.; Lechner, J.F. Normal Human Bronchial Epithelial Cell Culture. In Culture of Epithelial Cells; Wiley: Hoboken, NJ, USA, 2002; pp. 257–276. [Google Scholar]
- Jain, P.; Rauer, S.B.; Möller, M.; Singh, S. Mimicking the Natural Basement Membrane for Advanced Tissue Engineering. Biomacromolecules 2022, 23, 3081–3103. [Google Scholar] [CrossRef]
- Rodriguez-Boulan, E.; Kreitzer, G.; Müsch, A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell. Biol. 2005, 6, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, L.; Vijayavenkataraman, S.; Yuan, Y.; Tan, E.C.K.; Kang, L. Recent applications of three-dimensional bioprinting in drug discovery and development. Adv. Drug Deliv. Rev. 2024, 214, 115456. [Google Scholar] [CrossRef]
- Perry, G.; Xiao, W.; Welsh, G.I.; Perriman, A.W.; Lennon, R. Engineered basement membranes: From in vivo considerations to cell-based assays. Integr. Biol. 2018, 10, 680–695. [Google Scholar] [CrossRef]
- Yang, X.; Shi, J.; Shi, B.; Li, J.; Xue, C.; Ma, J.; Gao, X. Micro- and nano-fibers for organ-on-a-chip: Construction, applications, and prospects. Mater. Today Bio. 2024, 29, 101322. [Google Scholar] [CrossRef]
- Scuto, M.; Lombardo, C.M.G.; Lo Sasso, B.; Di Fatta, E.; Ferri, R.; Trovato Salinaro, A. Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric-Colon-Brain Axis Cancer. Toxics 2025, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, H.; Chen, Y.; Venkateswaran, S.; Hsiao, B.S. Functionalization of cellulose acetate nanofibrous membranes for removal of particulate matters and dyes. Int. J. Biol. Macromol. 2024, 269, 131852. [Google Scholar] [CrossRef]
- Zhu, G.; Li, X.; Li, X.P.; Wang, A.; Li, T.; Zhu, X.; Tang, D.; Zhu, J.; He, X.; Li, H.; et al. Nanopatterned Electroactive Polylactic Acid Nanofibrous MOFilters for Efficient PM(0.3) Filtration and Bacterial Inhibition. ACS Appl. Mater. Interfaces 2023, 15, 47145–47157. [Google Scholar] [CrossRef]
- Anusiya, G.; Rengarajan Jaiganesh, R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Guo, F.; Ren, Z.; Wang, S.; Xie, Y.; Pan, J.; Huang, J.; Zhu, T.; Cheng, S.; Lai, Y. Recent Progress of Electrospun Nanofiber-Based Composite Materials for Monitoring Physical, Physiological, and Body Fluid Signals. Nanomicro. Lett. 2025, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol. 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Simşek, M.; Capkın, M.; Karakeçili, A.; Gümüşderelioğlu, M. Chitosan and polycaprolactone membranes patterned via electrospinning: Effect of underlying chemistry and pattern characteristics on epithelial/fibroblastic cell behavior. J. Biomed. Mater. Res. A 2012, 100, 3332–3343. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef]
- Saberian, E.; Jenča, A.; Petrášová, A.; Zare-Zardini, H.; Ebrahimifar, M. Application of Scaffold-Based Drug Delivery in Oral Cancer Treatment: A Novel Approach. Pharmaceutics 2024, 16, 802. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. Heliyon 2023, 9, e16228. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Abadi, B.; Goshtasbi, N.; Bolourian, S.; Tahsili, J.; Adeli-Sardou, M.; Forootanfar, H. Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 986975. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Youssef, A.M.; El-Khonezy, M.I.; Kotob, S.E.; Kishta, M.S. Anti-Inflammatory/Antioxidant Features of Adipose Tissue Mesenchymal Stem Cells Conditioned Media for Doped TiO2 Nanoparticles in Induced Inflammation. ChemistryOpen 2025, 14, e202500261. [Google Scholar] [CrossRef]
- Aumailley, M.; Smyth, N. The role of laminins in basement membrane function. J. Anat. 1998, 193, 1–21. [Google Scholar] [CrossRef]
- Matlin, K.S.; Myllymäki, S.M.; Manninen, A. Laminins in Epithelial Cell Polarization: Old Questions in Search of New Answers. Cold Spring Harb. Perspect. Biol. 2017, 9, a027920. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.A.; McClugage, S.G.; Link, M.C.; Sefcik, L.S.; Ogle, R.C.; Botchwey, E.A. Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng. Part C Methods 2009, 15, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Baskapan, B.; Callanan, A. Electrospinning Fabrication Methods to Incorporate Laminin in Polycaprolactone for Kidney Tissue Engineering. Tissue Eng. Regen. Med. 2022, 19, 73–82. [Google Scholar] [CrossRef]
- Tran, T.X.T.; Sun, G.M.; Tran, H.V.A.; Jeong, Y.H.; Slama, P.; Chang, Y.C.; Lee, I.J.; Kwak, J.Y. Synthetic Extracellular Matrix of Polyvinyl Alcohol Nanofibers for Three-Dimensional Cell Culture. J. Funct. Biomater. 2024, 15, 262. [Google Scholar] [CrossRef]
- Cantara, S.I.; Soscia, D.A.; Sequeira, S.J.; Jean-Gilles, R.P.; Castracane, J.; Larsen, M. Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity. Biomaterials 2012, 33, 8372–8382. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef]
- Tran, B.M.; Grimley, S.L.; McAuley, J.L.; Hachani, A.; Earnest, L.; Wong, S.L.; Caly, L.; Druce, J.; Purcell, D.F.J.; Jackson, D.C.; et al. Air-Liquid-Interface Differentiated Human Nose Epithelium: A Robust Primary Tissue Culture Model of SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 835. [Google Scholar] [CrossRef]
- Tuncer, S.; Subasi Can, S.; Akel Bilgic, H.; Kilic, B.; Gunal, G.; Aydin, H.M.; Karaaslan, C. Biodegradable Nanofiber Membranes for Air–Liquid Interface Culture: Advancing Airway In Vitro Models. ACS Omega 2025, 10, 39693–39705. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef]
- Cao, X.; Coyle, J.P.; Xiong, R.; Wang, Y.; Heflich, R.H.; Ren, B.; Gwinn, W.M.; Hayden, P.; Rojanasakul, L. Invited review: Human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. Vitr. Cell. Dev. Biol.-Anim. 2021, 57, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kim, H.J.; Hwang, S.; Park, J.; Park, J.; Lee, J.W.; Son, K.H. The Modulation of Respiratory Epithelial Cell Differentiation by the Thickness of an Electrospun Poly-ε-Carprolactone Mesh Mimicking the Basement Membrane. Int. J. Mol. Sci. 2024, 25, 6650. [Google Scholar] [CrossRef]
- Yu, Y.S.; Park, S.H.; Choi, S.Y.; Lee, H.J.; Son, K.H.; Lee, J.W.; Kim, S.W. Cell-Free Biomimetic Tracheal Graft via Hybrid 3D Printing for Enhanced Tracheal Reconstruction. Adv. Healthc. Mater. 2025, 14, e2404648. [Google Scholar] [CrossRef]
- Radiom, M.; He, Y.; Peng-Wang, J.; Baeza-Squiban, A.; Berret, J.F.; Chen, Y. Alveolar mimics with periodic strain and its effect on the cell layer formation. Biotechnol. Bioeng. 2020, 117, 2827–2841. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Sousa de Almeida, M.; Milinkovic, D.; Septiadi, D.; Taladriz-Blanco, P.; Loussert, C.; Balog, S.; Bazzoni, A.; Rothen-Rutishauser, B.; Fink, A.S. Substrate stiffness reduces particle uptake by epithelial cells and macrophages in a size-dependent manner through mechanoregulation. Nanoscale 2022, 14, 15141–15155. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Lee, A.; Itel, F.; Maniura-Weber, K.; Petri-Fink, A.; Rothen-Rutishauser, B. The Effect of Substrate Properties on Cellular Behavior and Nanoparticle Uptake in Human Fibroblasts and Epithelial Cells. Nanomaterials 2024, 14, 342. [Google Scholar] [CrossRef]
- Tran, T.X.T.; Tran, H.V.A.; Lee, I.J.; Kwak, J.Y. Establishing a Three-Dimensional Coculture Module of Epithelial Cells Using Nanofibrous Membranes. J. Vis. Exp. 2024, 214, e67780. [Google Scholar] [CrossRef]
- Yan, S.; Li, X.; Dai, J.; Wang, Y.; Wang, B.; Lu, Y.; Shi, J.; Huang, P.; Gong, J.; Yao, Y. Electrospinning of PVA/sericin nanofiber and the effect on epithelial-mesenchymal transition of A549 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 436–444. [Google Scholar] [CrossRef]
- Li, X.; Yan, S.; Dai, J.; Lu, Y.; Wang, Y.; Sun, M.; Gong, J.; Yao, Y. Human lung epithelial cells A549 epithelial-mesenchymal transition induced by PVA/Collagen nanofiber. Colloids Surf. B Biointerfaces 2018, 162, 390–397. [Google Scholar] [CrossRef]
- Thuringer, M.; Zent, R.; Lennon, R.; Plosa, E.J. Basement membranes in lung development, disease, and repair. Matrix Biol. 2025, 140, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Choi, M.H.; Shin, J.I.; Maza, P.; Kwak, J.Y. Co-Culturing of Endothelial and Cancer Cells in a Nanofibrous Scaffold-Based Two-Layer System. Int. J. Mol. Sci. 2020, 21, 4128. [Google Scholar] [CrossRef] [PubMed]
- Malakpour-Permlid, A.; Buzzi, I.; Hegardt, C.; Johansson, F.; Oredsson, S. Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Sci. Rep. 2021, 11, 6655. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber Membrane supported lung-on-a-chip Microdevice for Anti-cancer Drug Testing. Lab Chip 2018, 18, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Singh, S.; Nishigushi, A.; Fischer, T.; Wessling, M.; Möller, M.; Sader, R.; Kasper, J.; Ghanaati, S.; Kirkpatrick, C.J. Human Co- and Triple-Culture Model of the Alveolar-Capillary Barrier on a Basement Membrane Mimic. Tissue Eng. Part C Methods 2018, 24, 495–503. [Google Scholar] [CrossRef]
- Capandova, M.; Sedlakova, V.; Vorac, Z.; Kotasova, H.; Dumkova, J.; Moran, L.; Jaros, J.; Antol, M.; Bohaciakova, D.; Hampl, A. Using Polycaprolactone Nanofibers for the Proof-of-Concept Construction of the Alveolar-Capillary Interface. J. Biomed. Mater. Res. A 2025, 113, e37824. [Google Scholar] [CrossRef] [PubMed]
- Gabela-Zuniga, B.; Shukla, V.C.; Bobba, C.; Higuita-Castro, N.; Powell, H.M.; Englert, J.A.; Ghadiali, S.N. A micro-scale humanized ventilator-on-a-chip to examine the injurious effects of mechanical ventilation. Lab Chip 2024, 24, 4390–4402. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.; Soriano, L.; Fagan-Murphy, A.; Ivankovic, I.; Cavanagh, B.; O’Brien, F.J.; Cryan, S.A. The Fabrication and in vitro Evaluation of Retinoic Acid-Loaded Electrospun Composite Biomaterials for Tracheal Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Maza, P.; Sun, G.M.; Slama, P.; Lee, I.J.; Kwak, J.Y. Bacterial Infection-Mimicking Three-Dimensional Phagocytosis and Chemotaxis in Electrospun Poly(ε-caprolactone) Nanofibrous Membrane. Membranes 2021, 11, 569. [Google Scholar]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15, 384–389. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.; Bergen, A.A. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Murali, A.; Krishnakumar, S.; Subramanian, A.; Parameswaran, S. Bruch’s membrane pathology: A mechanistic perspective. Eur. J. Ophthalmol. 2020, 30, 1195–1206. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Khristov, V.; Wan, Q.; Sharma, R.; Jha, B.S.; Lotfi, M.; Maminishkis, A.; Simon, C.G., Jr.; Bharti, K. Nanofiber Scaffold-Based Tissue-Engineered Retinal Pigment Epithelium to Treat Degenerative Eye Diseases. J. Ocul. Pharmacol. Ther. 2016, 32, 272–285. [Google Scholar] [CrossRef]
- White, C.E.; Olabisi, R.M. Scaffolds for retinal pigment epithelial cell transplantation in age-related macular degeneration. J. Tissue. Eng. 2017, 8, 2041731417720841. [Google Scholar]
- Xiang, P.; Wu, K.C.; Zhu, Y.; Xiang, L.; Li, C.; Chen, D.L.; Chen, F.; Xu, G.; Wang, A.; Li, M.; et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials 2014, 35, 9777–9788. [Google Scholar] [CrossRef]
- Wang, S.; Lin, S.; Xue, B.; Wang, C.; Yan, N.; Guan, Y.; Hu, Y.; Wen, X. Bruch’s-Mimetic Nanofibrous Membranes Functionalized with the Integrin-Binding Peptides as a Promising Approach for Human Retinal Pigment Epithelium Cell Transplantation. Molecules 2022, 27, 1429. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Alamein, M.; Skabo, S.; Stephens, S.; Bourke, R.; Heiner, P.; Liu, Q. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013, 9, 9414–9422. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, N.; Holz, F.G.; Yang, F.; Stanzel, B.V. Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography. Biomaterials 2014, 35, 2837–2850. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; Irlenbusch, L.; Hansen, U.; Himmler, M.; Zeng, C.; Eter, N.; Fuchsluger, T.; Heiduschka, P. Long-term cultivation of retinal pigment epithelium cells on nanofiber scaffolds. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 1327–1336. [Google Scholar] [CrossRef]
- Abbasi, N.; O’Neill, H. Cytocompatibility of electrospun poly-L-lactic acid membranes for Bruch’s membrane regeneration using human embryonic stem cell-derived retinal pigment epithelial cells. J. Biomed. Mater. Res. A 2024, 112, 1902–1920. [Google Scholar] [CrossRef]
- Thieltges, F.; Stanzel, B.V.; Liu, Z.; Holz, F.G. A nanofibrillar surface promotes superior growth characteristics in cultured human retinal pigment epithelium. Ophthalmic. Res. 2011, 46, 133–140. [Google Scholar] [CrossRef]
- Tichotová, L.; Studenovska, H.; Petrovski, G.; Popelka, Š.; Nemesh, Y.; Sedláčková, M.; Drutovič, S.; Rohiwal, S.; Jendelová, P.; Erceg, S.; et al. Advantages of nanofibrous membranes for culturing of primary RPE cells compared to commercial scaffolds. Acta Ophthalmol. 2022, 100, e1172–e1185. [Google Scholar] [CrossRef] [PubMed]
- Egbowon, B.F.; Fornari, E.; Pally, J.M.; Hargreaves, A.J.; Stevens, B.; McGinnity, T.M.; Pierscionek, B.K. Retinal pigment epithelial cells can be cultured on fluocinolone acetonide treated nanofibrous scaffold. Mater. Des. 2023, 232, 112152. [Google Scholar] [CrossRef]
- Surrao, D.C.; Greferath, U.; Chau, Y.Q.; Skabo, S.J.; Huynh, M.; Shelat, K.J.; Limnios, I.J.; Fletcher, E.L.; Liu, Q. Design, development and characterization of synthetic Bruch’s membranes. Acta Biomater. 2017, 64, 357–376. [Google Scholar] [CrossRef]
- Faura, G.; Studenovska, H.; Sekac, D.; Ellederova, Z.; Petrovski, G.; Eide, L. The Effects of the Coating and Aging of Biodegradable Polylactic Acid Membranes on In Vitro Primary Human Retinal Pigment Epithelium Cells. Biomedicines 2024, 12, 966. [Google Scholar] [CrossRef] [PubMed]
- Treharne, A.J.; Thomson, H.A.; Grossel, M.C.; Lotery, A.J. Developing methacrylate-based copolymers as an artificial Bruch’s membrane substitute. J. Biomed. Mater. Res. A 2012, 100, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, V.K.; Sugino, I.K.; Zarbin, M.A. Culture-induced increase in alpha integrin subunit expression in retinal pigment epithelium is important for improved resurfacing of aged human Bruch’s membrane. Exp. Eye. Res. 2008, 86, 189–200. [Google Scholar] [PubMed]
- Trousil, J.; Cabral, J.V.; Voukali, E.; Nováčková, J.; Pop-Georgievski, O.; Vacík, T.; Studený, P.; Studenovska, H.; Jirsova, K. Electrospun poly(l-lactide-co-dl-lactide) nanofibrous scaffold as substrate for ex vivo limbal epithelial cell cultivation. Heliyon 2024, 10, e30970. [Google Scholar] [CrossRef]
- Seiler, M.J.; Aramant, R.B. Cell replacement and visual restoration by retinal sheet transplants. Prog. Retin. Eye Res. 2012, 31, 661–687. [Google Scholar] [CrossRef] [PubMed]
- Bagewadi, S.; Parameswaran, S.; Subramanian, K.; Sethuraman, S.; Subramanian, A. Tissue Engineering approaches towards the regeneration of Biomimetic Scaffolds for Age-Related Macular Degeneration. J. Mater. Chem. B 2021, 9, 5935–5953. [Google Scholar] [CrossRef]
- Popelka, Š.; Studenovská, H.; Abelová, L.; Ardan, T.; Studený, P.; Straňák, Z.; Klíma, J.; Dvořánková, B.; Kotek, J.; Hodan, J.; et al. A frame-supported ultrathin electrospun polymer membrane for transplantation of retinal pigment epithelial cells. Biomed. Mater. 2015, 10, 045022. [Google Scholar] [CrossRef]
- Lytvynchuk, L.; Ebbert, A.; Studenovska, H.; Nagymihály, R.; Josifovska, N.; Rais, D.; Popelka, Š.; Tichotová, L.; Nemesh, Y.; Čížková, J.; et al. Subretinal Implantation of Human Primary RPE Cells Cultured on Nanofibrous Membranes in Minipigs. Biomedicines 2022, 10, 669. [Google Scholar] [CrossRef]
- Sorkio, A.; Porter, P.J.; Juuti-Uusitalo, K.; Meenan, B.J.; Skottman, H.; Burke, G.A. Surface Modified Biodegradable Electrospun Membranes as a Carrier for Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Tissue Eng. Part A 2015, 21, 2301–2314. [Google Scholar] [CrossRef]
- Phelan, M.A.; Kruczek, K.; Wilson, J.H.; Brooks, M.J.; Drinnan, C.T.; Regent, F.; Gerstenhaber, J.A.; Swaroop, A.; Lelkes, P.I.; Li, T. Soy Protein Nanofiber Scaffolds for Uniform Maturation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Tissue Eng. Part C Methods 2020, 26, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Yao, Q.; Yu, F.; Chen, L.; Zhang, S.; Sun, H.; Lin, J.; Fu, Y. Surface modified electrospun poly(lactic acid) fibrous scaffold with cellulose nanofibrils and Ag nanoparticles for ocular cell proliferation and antimicrobial application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110767. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, D.; Mohanty, S.; Jassal, M.; Agrawal, A.K.; Tandon, R. Surface-modified electrospun poly(epsilon-caprolactone) scaffold with improved optical transparency and bioactivity for damaged ocular surface reconstruction. Invest. Ophthalmol. Vis. Sci. 2014, 55, 899–907. [Google Scholar] [CrossRef]
- Stafiej, P.; Küng, F.; Thieme, D.; Czugala, M.; Kruse, F.E.; Schubert, D.W.; Fuchsluger, T.A. Adhesion and metabolic activity of human corneal cells on PCL based nanofiber matrices. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 764–770. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef]
- Aslan, B.; Guler, S.; Tevlek, A.; Aydin, H.M. Evaluation of collagen foam, poly(l-lactic acid) nanofiber mesh, and decellularized matrices for corneal regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qiang, L.; Gao, Y.; Cui, X.; Zhou, H.; Zhong, S.; Wang, Q.; Wang, H. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J. Biomed. Mater. Res. A 2012, 100, 527–535. [Google Scholar] [CrossRef]
- He, M.; Storr-Paulsen, T.; Wang, A.L.; Ghezzi, C.E.; Wang, S.; Fullana, M.; Karamichos, D.; Utheim, T.P.; Islam, R.; Griffith, M.; et al. Artificial Polymeric Scaffolds as Extracellular Matrix Substitutes for Autologous Conjunctival Goblet Cell Expansion. Invest. Ophthalmol. Vis. Sci. 2016, 57, 6134–6146. [Google Scholar] [CrossRef]
- Salehi, S.; Czugala, M.; Stafiej, P.; Fathi, M.; Bahners, T.; Gutmann, J.S.; Singer, B.B.; Fuchsluger, T.A. Poly (glycerol sebacate)-poly (ε-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair. Acta Biomater. 2017, 50, 370–380. [Google Scholar] [CrossRef]
- Yao, Q.; Hu, Y.; Yu, F.; Zhang, W.; Fu, Y. A novel application of electrospun silk fibroin/poly(l-lactic acid-co-ε-caprolactone) scaffolds for conjunctiva reconstruction. RSC Adv. 2018, 8, 18372–18380. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, S.; Yu, F.; Gong, D.; Lin, J.; Yao, Q.; Fu, Y. Insight into levofloxacin loaded biocompatible electrospun scaffolds for their potential as conjunctival substitutes. Carbohydr. Polym. 2021, 269, 118341. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Patient, J.D.; Hajiali, H.; Harris, K.; Abrahamsson, B.; Tannergren, C.; White, L.J.; Ghaemmaghami, A.M.; Williams, P.M.; Roberts, C.J.; Rose, F. Nanofibrous Scaffolds Support a 3D in vitro Permeability Model of the Human Intestinal Epithelium. Front. Pharmacol. 2019, 10, 456. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.; Shan, H.; Jiao, Y.; Zhang, Q.; Li, X.; Yu, J.; Ding, B. Deoxycholic acid-modified microporous SiO(2)nanofibers mimicking colorectal microenvironment to optimize radiotherapy-chemotherapy combined therapy. Biomed. Mater. 2021, 16, 065020. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.; Tarnawski, A.S.; Tran, T. Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol. Biol. Cell 2004, 15, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhang, M.; Wei, W.; Wang, H.; Zhang, W.; Li, Z.; Tan, M.; Chen, Z. Microfluidics-assisted electrospinning of aligned nanofibers for modeling intestine barriers. PeerJ 2022, 10, e13513. [Google Scholar] [CrossRef]
- Poling, H.M.; Singh, A.; Krutko, M.; Reza, A.A.; Srivastava, K.; Wells, J.M.; Helmrath, M.A.; Esfandiari, L. Promoting Human Intestinal Organoid Formation and Stimulation Using Piezoelectric Nanofiber Matrices. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, H.M.; Guo, Y.X.; Ma, X.K.; Hu, M.X.; Han, J.Z.; Qin, Y.M. Crypt-like patterned electrospun nanofibrous membrane and probiotics promote intestinal epithelium models close to tissues. Appl. Microbiol. Biotechnol. 2023, 107, 4395–4408. [Google Scholar] [CrossRef]
- Fardous, R.S.; Alshmmari, S.; Tawfik, E.; Khadra, I.; Ramadan, Q.; Zourob, M. An Integrated and Modular Compartmentalized Microfluidic System with Tunable Electrospun Porous Membranes for Epithelialized Organs-on-a-Chip. ACS Applied. Mater. Interfaces 2024, 16, 40767–40786. [Google Scholar] [CrossRef]
- Hu, M.; Li, Y.; Huang, J.; Wang, X.; Han, J. Electrospun Scaffold for Biomimic Culture of Caco-2 Cell Monolayer as an In Vitro Intestinal Model. ACS Appl. Bio. Mater. 2021, 4, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Elia, E.; Brownell, D.; Chabaud, S.; Bolduc, S. Tissue Engineering for Gastrointestinal and Genitourinary Tracts. Int. J. Mol. Sci. 2022, 24, 9. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. PCL and PCL-gelatin nanofibers as esophageal tissue scaffolds: Optimization, characterization and cell-matrix interactions. J. Biomed. Nanotechnol. 2013, 9, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-based nanofibrous scaffolds to support functional esophageal epithelial cells towards engineering the esophagus. J. Biomater. Sci. Polym. Ed. 2014, 25, 574–593. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. In vitro co-culture of epithelial cells and smooth muscle cells on aligned nanofibrous scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Blanchette, A.; Vadasz, S.; Dave, A.; Canfarotta, M.; Sayej, W.N.; Finck, C. Biomimetic and synthetic esophageal tissue engineering. Biomaterials 2015, 57, 133–141. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, J.; Wu, Y.; Li, L.; Li, D.; Deng, H.; Liu, J.; Zhang, L. Chitosan/collagen layer-by-layer deposition for improving the esophageal regeneration ability of nanofibrous mats. Carbohydr. Polym. 2022, 286, 119269. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Aida, T.; Taguchi, Y. Advances in tissue engineering technology for kidney regeneration and construction. J. Artif. Organs 2022, 25, 191–194. [Google Scholar] [CrossRef]
- Miranda, C.C.; Gomes, M.R.; Moço, M.; Cabral, J.M.S.; Ferreira, F.C.; Sanjuan-Alberte, P. A Concise Review on Electrospun Scaffolds for Kidney Tissue Engineering. Bioengineering 2022, 9, 554. [Google Scholar] [CrossRef]
- Vermue, I.M.; Begum, R.; Castilho, M.; Rookmaaker, M.B.; Masereeuw, R.; Bouten, C.V.C.; Verhaar, M.C.; Cheng, C. Renal Biology Driven Macro- and Microscale Design Strategies for Creating an Artificial Proximal Tubule Using Fiber-Based Technologies. ACS Biomater. Sci. Eng. 2021, 7, 4679–4693. [Google Scholar] [CrossRef]
- Dukes, J.D.; Whitley, P.; Chalmers, A.D. The MDCK variety pack: Choosing the right strain. BMC Cell Biol. 2011, 12, 43. [Google Scholar] [CrossRef]
- Burton, T.P.; Corcoran, A.; Callanan, A. The effect of electrospun polycaprolactone scaffold morphology on human kidney epithelial cells. Biomed. Mater. 2017, 13, 015006. [Google Scholar] [CrossRef]
- Sobreiro-Almeida, R.; Fonseca, D.R.; Neves, N.M. Extracellular matrix electrospun membranes for mimicking natural renal filtration barriers. Mater. Sci. Eng. C Mater. Biol Appl. 2019, 103, 109866. [Google Scholar] [CrossRef]
- Kapadia, R.; Yurchenco, P.D.; Amsler, K. Binding of the renal epithelial cell line LLC-PK1 to laminin is regulated by protein kinase C. J. Am. Soc. Nephrol. 1999, 10, 1214–1223. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Rasekhian, P.; Karbasi, S.; Karbalaie, K.; Karamali, F.; Abedi, D.; Razavi, S.; Jafarian-Dehkordi, A.; Nasr-Esfahani, M.H.; et al. Does the tissue engineering architecture of poly(3-hydroxybutyrate) scaffold affects cell-material interactions? J. Biomed. Mater. Res. A 2012, 100, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Morshed, M.; Karbalaie, K.; Fesharaki, M.A.; Nematallahi, M.; Nasr-Esfahani, M.H.; Baharvand, H. The thickness of electrospun poly (epsilon-caprolactone) nanofibrous scaffolds influences cell proliferation. Int. J. Artif. Organs 2009, 32, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dankers, P.Y.; Boomker, J.M.; Huizinga-van der Vlag, A.; Smedts, F.M.; Harmsen, M.C.; van Luyn, M.J. The use of fibrous, supramolecular membranes and human tubular cells for renal epithelial tissue engineering: Towards a suitable membrane for a bioartificial kidney. Macromol. Biosci. 2010, 10, 1345–1354. [Google Scholar] [CrossRef]
- Dankers, P.Y.; Boomker, J.M.; Huizinga-van der Vlag, A.; Wisse, E.; Appel, W.P.; Smedts, F.M.; Harmsen, M.C.; Bosman, A.W.; Meijer, W.; van Luyn, M.J. Bioengineering of living renal membranes consisting of hierarchical, bioactive supramolecular meshes and human tubular cells. Biomaterials 2011, 32, 723–733. [Google Scholar] [CrossRef]
- Jansen, K.; Castilho, M.; Aarts, S.; Kaminski, M.M.; Lienkamp, S.S.; Pichler, R.; Malda, J.; Vermonden, T.; Jansen, J.; Masereeuw, R. Fabrication of Kidney Proximal Tubule Grafts Using Biofunctionalized Electrospun Polymer Scaffolds. Macromol. Biosci. 2019, 19, e1800412. [Google Scholar] [CrossRef] [PubMed]
- Mollet, B.B.; Bogaerts, I.L.J.; van Almen, G.C.; Dankers, P.Y.W. A bioartificial environment for kidney epithelial cells based on a supramolecular polymer basement membrane mimic and an organotypical culture system. J. Tissue Eng. Regen. Med. 2017, 11, 1820–1834. [Google Scholar] [CrossRef]
- van Gaal, R.C.; Fedecostante, M.; Fransen, P.K.H.; Masereeuw, R.; Dankers, P.Y.W. Renal Epithelial Monolayer Formation on Monomeric and Polymeric Catechol Functionalized Supramolecular Biomaterials. Macromol. Biosci. 2019, 19, e1800300. [Google Scholar] [CrossRef]
- van Gaal, R.C.; Vrehen, A.F.; van Sprang, J.F.; Fransen, P.K.H.; van Turnhout, M.C.; Dankers, P.Y.W. Biomaterial screening of protein coatings and peptide additives: Towards a simple synthetic mimic of a complex natural coating for a bio-artificial kidney. Biomater. Sci. 2021, 9, 2209–2220. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Yin, H.; Hu, M.; Li, D. Regulation of epidermal stratification and development by basal keratinocytes. J. Cell. Physiol. 2023, 238, 742–748. [Google Scholar] [CrossRef]
- Schoop, V.M.; Mirancea, N.; Fusenig, N.E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Nanofiber diameter as a critical parameter affecting skin cell response. Eur. J. Pharm. Sci. 2015, 66, 29–35. [Google Scholar] [CrossRef]
- Yildiz, G.; Arslan, Y.; Derkus, B.; Sezgin, B.; Menceloglu, Y.; Bayar, G. Development and characterization of skin substitutes from electrospun polycaprolactone/silk fibroin. J. Bioactiv. Compat. Polym. 2024, 39, 46–62. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Luo, L.; Qi, F.; Jiang, Y.; Atta, O.M.; Kishta, M.S.; Maboruk, M.; Wu, M.; Ge, W.; Shi, Z.; et al. Tree thorns-inspired hyaluronic acid-based dissolving microneedles loaded with salvianolic acid B for effective hypertrophic scar treatment with enhanced long-term stability. Carbohydr. Polym. 2025, 364, 123773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf. B Biointerfaces 2016, 143, 415–422. [Google Scholar] [CrossRef]
- Zhou, T.; Sui, B.; Mo, X.; Sun, J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: Fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3495–3507. [Google Scholar] [CrossRef] [PubMed]
- Baran, E.T.; Tahmasebifar, A.; Yilmaz, B. Co-axial electrospinning of PLLA shell, collagen core nanofibers for skin tissue engineering. J. Biomater. Appl. 2023, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, T.; Xin, Y.; Zhang, Z.; Ren, Z.; Lei, J.; Chu, B.; Wang, Y.; Tang, S. Nanofibrous asymmetric membranes self-organized from chemically heterogeneous electrospun mats for skin tissue engineering. Biomed. Mater. 2016, 11, 035019. [Google Scholar] [CrossRef]
- Rimann, M.; Jüngel, A.; Mousavi, S.; Moeschlin, N.; Calcagni, M.; Wuertz-Kozak, K.; Brunner, F.; Dudli, S.; Distler, O.; Adlhart, C. Acrylonitrile and Pullulan Based Nanofiber Mats as Easily Accessible Scaffolds for 3D Skin Cell Models Containing Primary Cells. Cells 2022, 11, 445. [Google Scholar] [CrossRef]
- Fu, X.; Xu, M.; Liu, J.; Qi, Y.; Li, S.; Wang, H. Regulation of migratory activity of human keratinocytes by topography of multiscale collagen-containing nanofibrous matrices. Biomaterials 2014, 35, 1496–1506. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.M.; Park, Y.J.; Hong, S.D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Dutta, D.; Markhoff, J.; Suter, N.; Rezwan, K.; Brüggemann, D. Effect of Collagen Nanofibers and Silanization on the Interaction of HaCaT Keratinocytes and 3T3 Fibroblasts with Alumina Nanopores. ACS Appl. Bio. Mater. 2021, 4, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Noh, H.K.; Lee, S.W.; Kim, J.M.; Oh, J.E.; Kim, K.H.; Chung, C.P.; Choi, S.C.; Park, W.H.; Min, B.M. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 2006, 27, 3934–3944. [Google Scholar] [CrossRef]
- Yoo, C.R.; Yeo, I.S.; Park, K.E.; Park, J.H.; Lee, S.J.; Park, W.H.; Min, B.M. Effect of chitin/silk fibroin nanofibrous bicomponent structures on interaction with human epidermal keratinocytes. Int. J. Biol. Macromol. 2008, 42, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, H.; Sun, W.; Zhang, Y.; Cai, W.; Lu, C.; Luo, K.; Liu, Y.; Wang, Y. Low-Temperature Electrospinning-Fabricated Three-Dimensional Nanofiber Scaffolds for Skin Substitutes. Micromachines 2025, 16, 552. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Unalan, I.; Fernández, K.; Boccaccini, A.R. Fabrication of Biocompatible Electrospun Poly(ε-caprolactone)/Gelatin Nanofibers Loaded with Pinus radiata Bark Extracts for Wound Healing Applications. Polymers 2022, 14, 2331. [Google Scholar] [CrossRef]
- Bacakova, M.; Lopot, F.; Hadraba, D.; Varga, M.; Zaloudkova, M.; Stranska, D.; Suchy, T.; Bacakova, L. Effects of fiber density and plasma modification of nanofibrous membranes on the adhesion and growth of HaCaT keratinocytes. J. Biomater. Appl. 2015, 29, 837–853. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1350–1364. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J. Therapeutic applications of electrospun nanofibers impregnated with various biological macromolecules for effective wound healing strategy—A review. Biomed. Pharmacother. 2023, 157, 113996. [Google Scholar] [CrossRef]
- Hajiabbas, M.; D’Agostino, C.; Simińska-Stanny, J.; Tran, S.D.; Shavandi, A.; Delporte, C. Bioengineering in salivary gland regeneration. J. Biomed. Sci. 2022, 29, 35. [Google Scholar] [CrossRef]
- Sequeira, S.J.; Soscia, D.A.; Oztan, B.; Mosier, A.P.; Jean-Gilles, R.; Gadre, A.; Cady, N.C.; Yener, B.; Castracane, J.; Larsen, M. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials 2012, 33, 3175–3186. [Google Scholar] [CrossRef]
- Soscia, D.A.; Sequeira, S.J.; Schramm, R.A.; Jayarathanam, K.; Cantara, S.I.; Larsen, M.; Castracane, J. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials 2013, 34, 6773–6784. [Google Scholar] [CrossRef] [PubMed]
- Foraida, Z.I.; Kamaldinov, T.; Nelson, D.A.; Larsen, M.; Castracane, J. Elastin-PLGA hybrid electrospun nanofiber scaffolds for salivary epithelial cell self-organization and polarization. Acta Biomater. 2017, 62, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Sfakis, L.; Kamaldinov, T.; Khmaladze, A.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M.; Castracane, J. Mesenchymal Cells Affect Salivary Epithelial Cell Morphology on PGS/PLGA Core/Shell Nanofibers. Int. J. Mol. Sci. 2018, 19, 1031. [Google Scholar] [CrossRef] [PubMed]
- Schramm, R.A.; Koslow, M.H.; Nelson, D.A.; Larsen, M.; Castracane, J. A Novel Impedance Biosensor for Measurement of Trans-Epithelial Resistance in Cells Cultured on Nanofiber Scaffolds. Biosensors 2017, 7, 35. [Google Scholar] [CrossRef]
- Aminu, N.; Ilyasu, S.; Hassan, M.A.; Kurfi, F.S.; Jatau, A.I.; Chan, S.-Y.; Alfred-Ugbenbo, D. Applications of nanofibers drug delivery system in cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 90, 105128. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Omidian, H.; Gill, E.J. Nanofibrous Scaffolds in Biomedicine. J. Compos. Sci. 2024, 8, 269. [Google Scholar] [CrossRef]
- Aglan, H.A.; Abd-Rabou, A.A.; Ahmed, H.H.; Elsayed, G.H.; Kishta, M.S.; Elhinnawi, M.A.; Mahmoud, N.S. Role of CD133 antibody-conjugated nanocarrier in enhancing the targetability of hepatocellular carcinoma stem cells. Sci. Rep. 2025, 15, 30441. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, C.; Conklin, D.; Waterman, J.; Sankar, J.; Bhattarai, N. Electrospun nanofibers of poly(ε-caprolactone)/depolymerized chitosan for respiratory tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 611–625. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Leal, F.; Moreira, A.; Schellhorn, T.; Blahnová, V.H.; Zeiringer, S.; Vocetková, K.; Tetyczka, C.; Simaite, A.; Buzgo, M.; et al. Potential of Electrospun Fibrous Scaffolds for Intestinal, Skin, and Lung Epithelial Tissue Modeling. Advan. NanoBiomed. Res. 2023, 3, 2200104. [Google Scholar]
- Kanabekova, P.; Dauletkanov, B.; Bekezhankyzy, Z.; Toktarkan, S.; Martin, A.; Pham, T.T.; Kostas, K.; Kulsharova, G. A hybrid fluorescent nanofiber membrane integrated with microfluidic chips towards lung-on-a-chip applications. Lab Chip 2024, 24, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Higuita-Castro, N.; Nelson, M.T.; Shukla, V.; Agudelo-Garcia, P.A.; Zhang, W.; Duarte-Sanmiguel, S.M.; Englert, J.A.; Lannutti, J.J.; Hansford, D.J.; Ghadiali, S.N. Using a Novel Microfabricated Model of the Alveolar-Capillary Barrier to Investigate the Effect of Matrix Structure on Atelectrauma. Sci. Rep. 2017, 7, 11623. [Google Scholar] [CrossRef]
- Moghadas, H.; Saidi, M.S.; Kashaninejad, N.; Nguyen, N.T. A high-performance polydimethylsiloxane electrospun membrane for cell culture in lab-on-a-chip. Biomicrofluidics 2018, 12, 024117. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Yazdian, F.; Tabandeh, F.; Soheili, Z.S.; Hatamian Zarami, A.S.; Navaei-Nigjeh, M. Controlled surface morphology and hydrophilicity of polycaprolactone toward human retinal pigment epithelium cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 300–309. [Google Scholar] [CrossRef]
- Noorani, B.; Tabandeh, F.; Yazdian, F.; Soheili, Z.-S.; Shakibaie, M.; Rahmani, S. Thin natural gelatin/chitosan nanofibrous scaffolds for retinal pigment epithelium cells. Int. J. Polymer. Mater. Polymer. Biomater. 2018, 67, 754–763. [Google Scholar] [CrossRef]
- Ortega, I.; Ryan, A.J.; Deshpande, P.; MacNeil, S.; Claeyssens, F. Combined microfabrication and electrospinning to produce 3-D architectures for corneal repair. Acta Biomater. 2013, 9, 5511–5520. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohanty, S.; Gupta, D.; Jassal, M.; Agrawal, A.K.; Tandon, R. Cellular response of limbal epithelial cells on electrospun poly-ε-caprolactone nanofibrous scaffolds for ocular surface bioengineering: A preliminary in vitro study. Mol. Vis. 2011, 17, 2898–2910. [Google Scholar] [PubMed]
- Leong, M.F.; Chian, K.S.; Mhaisalkar, P.S.; Ong, W.F.; Ratner, B.D. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J. Biomed. Mater. Res. A 2009, 89, 1040–1048. [Google Scholar] [CrossRef]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Jean-Gilles, R.; Soscia, D.; Sequeira, S.; Melfi, M.; Gadre, A.; Castracane, J.; Larsen, M. Novel Modeling Approach to Generate a Polymeric Nanofiber Scaffold for Salivary Gland Cells. J. Nanotechnol. Eng. Med. 2010, 1, 31008. [Google Scholar] [CrossRef]
| Polymer Type | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Natural | Collagen, Gelatin, Chitosan, Silk Fibroin | - Inherently bioactive (e.g., containing RGD cell-binding sequences) - Excellent biocompatibility - Biodegradable | - Poor mechanical strength - Potential for immunogenic response - Significant batch-to-batch variability. |
| Synthetic | PLLA, PCL, PLGA | - Highly tunable mechanical properties - Predictable and controllable degradation rates - High purity - Consistency | - Inherently hydrophobic, which can hinder initial cell attachment - Lack of native bioactive sites, often requiring surface functionalization - Acidic degradation byproducts can induce local inflammation. |
| Hybrid | Blends (e.g., PCL-Gelatin) | - A combination of the mechanical strength and stability of synthetic polymers with the inherent bioactivity of natural polymers creates a superior composite material. | - Fabrication can be complex. - Potential for delamination between material phases - Achieving a homogenous blend can be challenging. |
| Cell Source | Description and Examples | Advantages | Disadvantages |
|---|---|---|---|
| Primary cells | - Harvested directly from donor tissue and used with minimal passaging. - NHBE cells - HSAE cells | - High physiological relevance: capable of forming a fully differentiated and pseudostratified mucociliary epithelium. - Gold standard: considered the most accurate representation of in vivo tissue. - Patient-specific models: enable personalized medicine approaches using cells from patients with specific diseases (e.g., cystic fibrosis). | - Limited lifespan: Finite proliferative capacity restricts the scale and duration of experiments. - Donor-to-donor variability: Significant biological differences between donors can affect reproducibility. - High cost and limited availability: more expensive and difficult to procure than cell lines. |
| Immortalized cell lines | - Cells that have been genetically modified to proliferate indefinitely in culture. - BEAS-2B: SV40-transformed bronchial epithelial cells. - Calu-3: adenocarcinoma-derived, forms tight junctions and secretes mucus. - A549: alveolar adenocarcinoma-derived. | - High reproducibility: genetically homogenous, providing consistent results across experiments. - Ease of culture and scalability: robust and easy to expand to large numbers for high-throughput screening. - Cost-effective: readily available and inexpensive to maintain. | - Altered phenotype: Genetic modifications can lead to non-physiological behavior. - Incomplete differentiation: often fails to differentiate into a complex and pseudostratified epithelium fully. - Tumorigenic origin: Many lines are derived from cancers, which may not reflect normal cell biology. |
Tissue Type | Cell Type | Nanofiber Composites | Specific Purposes | Ref. |
|---|---|---|---|---|
| Bronchial and Lung epithelium | NHBE cells | PCL | Formation of differentiated pseudostratified Epithelium on multilayer scaffolds | [50] |
| PCL | Tracheal frame using 3D printing | [51] | ||
| Primary human alveolar epithelial cell (pneumocyte) | Polyurethane | Coculture of endothelial cells Lung-on-a-chip model | [64] | |
| Porcine tracheobronchial epithelial (PTBE) cell | PCL/Chitosan | Air-liquid interface culture | [166] | |
| Bronchial epithelial cells (16HBE) | PCL, PCL/CA, PCL/CAP, PCL/EC | Culture of various types of epithelial cells | [167] | |
| Bronchial epithelial cell (MLE-12) | PVA | Laminin-coated and peptide-blended scaffold | [43] | |
| PVA | Coculture of fibroblasts | [55] | ||
| PCL | S. aureus infection model | [66] | ||
| Bronchial epithelial cell (Calu-3) | PCL/Chitosan | All-trans Retinoic Acid-loaded | [65] | |
| Lung epithelial cell (A549) | Gelatin | Air–liquid interface, microfluidic | [52] | |
| Polyurethane | Aligned and non-aligned nanofibers | [54] | ||
| PVA/Silk sericin | Epithelial-mesenchymal transition induced on scaffolds | [56] | ||
| PVA/Collagen | Epithelial-mesenchymal transition induced on scaffolds | [57] | ||
| PGLA | Coculture of human fetal lung fibroblasts | [61] | ||
| PCL/Collagen | Nanofiber on a microfluidic chip | [168] | ||
| PCL/Gelatin | Coculture of endothelial cells | [169] | ||
| PDMS/PMMA | Combined with microfluidics | [170] | ||
| Lung epithelial cell (NCI H441) | PCL | Coculture of endothelial and immune cells | [62] | |
| Retinal pigment epithelium | Primary RPE cell | SF/PCL/Gelatin | Similar thickness to native Bruch’s membranes | [73] |
| PLGA/Collagen | Formation of sheet-like microvilli | [75] | ||
| PET/PLCL | Fiber diameter-dependent adhesion | [76] | ||
| PCL/Collagen | Stable long-term culture on scaffolds | [77] | ||
| Polyamide | A colony-like distribution of polygonal cells | [79] | ||
| PDLLA | Compared to the polyester membrane | [80] | ||
| PLLA | Functional RPE monolayer on laminin-coated scaffolds | [82] | ||
| PLA | Matrigel-coated scaffolds | [83] | ||
| PDLLA | Ultrathin scaffold with frame | [89,90] | ||
| PCL | Surface modification by plasma surface treatment | [171] | ||
| Gelatin/Chitosan | Appropriate adhesion of cells on the substrate | [172] | ||
| human RPE cell (ARPE-19) | PCL | Integrin-binding peptides-coated | [74] | |
| PAN | FA-treated nanofiber | [81] | ||
| PEG/methacrylate | Peptide and laminin-attached | [84] | ||
| Stem cell-derived RPE cells | PLLA | Laminin-coated scaffolds | [78] | |
| PLCL | Plasma processing, Collagen IV coating | [91] | ||
| Soy protein/PCL | Blow electrospun soy scaffolds | [92] | ||
| Other ocular epithelium | Human corneal epithelial cells | PCL | Modified by helium-oxygen (He/O2) plasma discharge | [94] |
| PCL/PGS, PCL/chitosan | Random and aligned scaffolds | [95] | ||
| Polyvinyl acetate/ collagen | Random and aligned scaffolds | [96] | ||
| Gelatin/PLLA | Random and aligned scaffolds | [98] | ||
| Rabbit corneal epithelial cells | PLA | Coated by cellulose fibril and Ag nanoparticle | [93] | |
| Primary limbal epithelial cells | PDLLA | Induction of mesenchymal phenotype in fibronectin-coated scaffolds | [86] | |
| PCL | Modified by helium-oxygen (He/O2) plasma discharge | [94] | ||
| PLGA | Combined pattern of nanofiber on microfabrication | [173] | ||
| Rabbit conjunctival epithelial cells (HCjEC) | PLA | Coated by cellulose fibril and Ag nanoparticle | [93] | |
| Conjunctival goblet cells | Collagen/PAA/PCL, PVA | Growth of goblet cells in PAA scaffolds | [99] | |
| human conjunctival epithelial cells (HCjEC) | PGS/PCL | Aligned scaffold | [100] | |
| Rabbit conjunctival epithelial cells | SF/PLCL | Implantation of cell-seeded scaffold | [101] | |
| PLA | Coated by cellulose nanofibrils and/or silk peptide, transplanted in vivo | [102] | ||
| Human corneal epithelial cells (HCE-T), Human limbal epithelial cells | PCL | Limbal epithelial cell expansion | [174] | |
| Esophageal, intestinal and colon epithelium | Esophageal epithelial cells | PHBV/PCL | Gelatin-blended aligned scaffolds | [114,116] |
| PHBV | Gelatin-blended | [115] | ||
| PCL/PGLA | In bioreactor | [117] | ||
| Porcine esophageal epithelial cells | PLA | Nanoporous fiber scaffold | [175] | |
| PLLC | Fibronectin immobilization on the scaffolds | [176] | ||
| Human intestinal epithelial cells | PVA/SiO2 | Modified with deoxycholic acid | [106] | |
| Nylon 6/silk fibroin | Chitosan and collagen-coated | [118] | ||
| Intestinal organoid epithelial cells | PVDF-TrFE | Intestinal organoid on a nanofiber | [109] | |
| Colon epithelial cells (Caco-2) | PET | Collagen-coated scaffolds | [105] | |
| PVP | Aligned nanofiber on a microfluidic | [108] | ||
| PLA | Modified with Matrigel, Crypt-like pattern | [110] | ||
| PMMA-PVP | Scaffold in a microfluidic system | [111] | ||
| PLA | Monolayer | [112] | ||
| PCL/Cellulose | Other epithelial cell culture | [167] | ||
| Kidney epithelium | human primary tubular epithelial cells (PTEC) | PCL | UPy-Urea-modified | [128,129] |
| Conditionally immortalized proximal tubule epithelial cells (ciPTEC) | PCL | Coated with l-DOPA and collagen | [130] | |
| PCL | Incorporation of UPy-DOPA in PCL-diUPy | [132] | ||
| Human kidney-2 (HK-2) cells | PCL | Decellularized kidney ECM-blended | [124] | |
| PCL | UPy-modified, peptide-blended | [131] | ||
| Bis-urea/PCL | Peptide additive | [133] | ||
| human kidney epithelial cells (RC-124) | PCL | Laminin-blended | [42] | |
| Cryogenic electrospun random and aligned scaffolds | [123] | |||
| Madine Darby Bovine Kidney epithelial cells (MDBK) | Chitosan/PCL | Collagen-coated, random, and aligned | [26] | |
| Monkey epithelial kidney cells (Vero) | PHB | Electrospinning and salt-leaching procedures | [126] | |
| PCL | Increased cell proliferation in thick scaffolds | [127] | ||
| Chitosan/PCL | Hyaluronic acid scaffold layered | [177] | ||
| Skin epithelium | Primary human keratinocytes | PVA | Nanofiber diameter-dependent growth | [137] |
| SF/PCL | Increased tensile strength and hydrophilicity | [138] | ||
| P(AN-MA), Pullulan/PVA/PAA | Air-liquid interface | [144] | ||
| Collagen | Collagen, laminin-coated | [146] | ||
| Chitin | Collagen-coated | [149] | ||
| Chitin/SF | Blend and hybrid scaffold | [150] | ||
| Human keratinocytes immortalized | PCL/Collagen | Collagen-coated | [145] | |
| Keratinocytes (HaCat) | Tilapia collagen | Wound healing | [140] | |
| Collagen/bioactive glass | Wound healing | [141] | ||
| PLLA/Collagen | Coaxial, EGF-encapsulated collagen fiber | [142] | ||
| β-glucan ester | Bilayer culture | [143] | ||
| Collagen | Anodic aluminum oxide-modified | [147] | ||
| PLGA/PCL/MAP | Enhanced adhesive properties and biocompatibility | [151] | ||
| PCL/gelatin | Pinus radiata bark extracts (PEs)-incorporated | [152] | ||
| PLA | Plasma-treated scaffolds | [153] | ||
| Gland ductal epithelium | Salivary gland epithelial cells, SIMS and SMGC10 cell line | PLGA | Chitosan-attached Laminin-111-attached | [44] |
| Ductal submandibular epithelial cell | PLGA | Elastin-attached scaffolds by blending and covalent surface conjugation | [159] | |
| Salivary gland ductal epithelial cells (SIMS) | PLGA | Decreased levels of the focal adhesion proteins in scaffold culture | [157] | |
| PLGA | Micropatterned scaffold crater | [158] | ||
| PGS/PLGA | Coculture with fibroblasts | [160] | ||
| PLGA | Nanofiber scaffold integrated into an ECIS-TEER Trans-well system. | [161] | ||
| PGLA | Different solvents for the fabrication | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-J.; Kwak, J.-Y. Three-Dimensional Culture of Epithelial Cells on Electrospun Nanofibrous Scaffolds. Int. J. Mol. Sci. 2025, 26, 10500. https://doi.org/10.3390/ijms262110500
Lee I-J, Kwak J-Y. Three-Dimensional Culture of Epithelial Cells on Electrospun Nanofibrous Scaffolds. International Journal of Molecular Sciences. 2025; 26(21):10500. https://doi.org/10.3390/ijms262110500
Chicago/Turabian StyleLee, In-Jeong, and Jong-Young Kwak. 2025. "Three-Dimensional Culture of Epithelial Cells on Electrospun Nanofibrous Scaffolds" International Journal of Molecular Sciences 26, no. 21: 10500. https://doi.org/10.3390/ijms262110500
APA StyleLee, I.-J., & Kwak, J.-Y. (2025). Three-Dimensional Culture of Epithelial Cells on Electrospun Nanofibrous Scaffolds. International Journal of Molecular Sciences, 26(21), 10500. https://doi.org/10.3390/ijms262110500







