Bioprotective Strategies to Control Listeria monocytogenes in Food Products and Processing Environments
Abstract
1. Introduction
2. Listeria monocytogenes: Resilience and Pathogenicity Mechanisms
2.1. Generalities on Listeria monocytogenes
| Lineage | Serotypes | Characteristics | Distribution and Origins | References |
|---|---|---|---|---|
| I | 1/2b, 3b, 4b, 4d, 4e and 7 |
| Predominantly isolated from humans and infrequently detected in the environment or food products. | [28] |
| II | 1/2a, 1/2c, 3a and 3c |
| Frequently occurs in processed foods and food processing equipment. | [25,28,29] |
| III | 1/2a, 4a, 4b and 4c |
| Frequently detected in livestock and agricultural environment. | [25] |
| IV | 4a, 4c and 4b |
| Detected in agricultural and livestock environments. | [25,28] |
2.2. Human Listeriosis
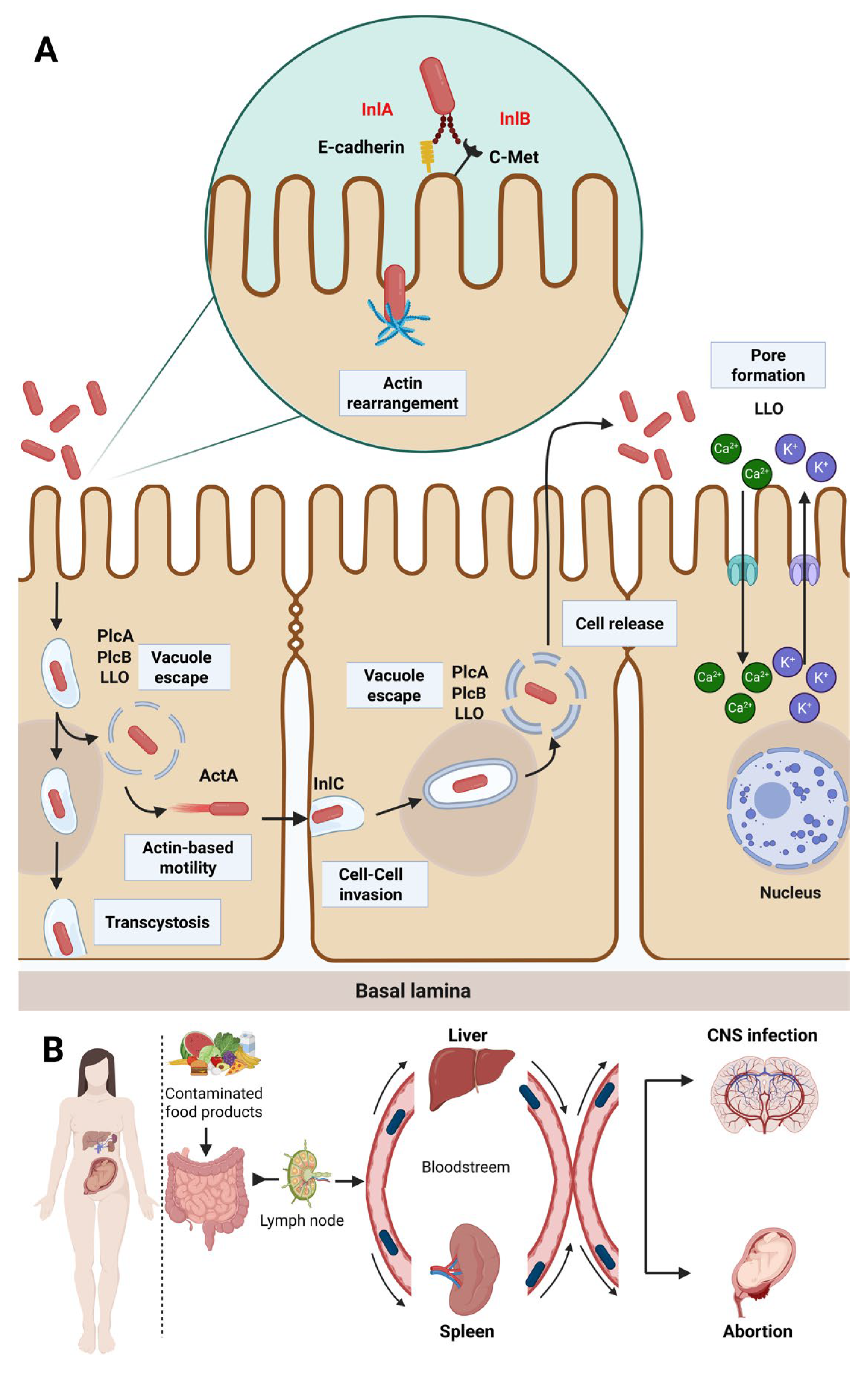
2.3. Virulence Factors and Pathogenicity
2.3.1. Brain
2.3.2. Maternal–Fetal Listeriosis
2.3.3. Liver
2.4. Antibiotic Resistance
2.4.1. Resistance to Quinolones
2.4.2. Resistance to ß-Lactams
2.4.3. Resistance to Tetracyclines
2.4.4. Resistance to Phenicols
2.4.5. Resistance to Macrolides
2.4.6. Resistance to Trimethoprim
2.4.7. Resistance to Aminoglycosides
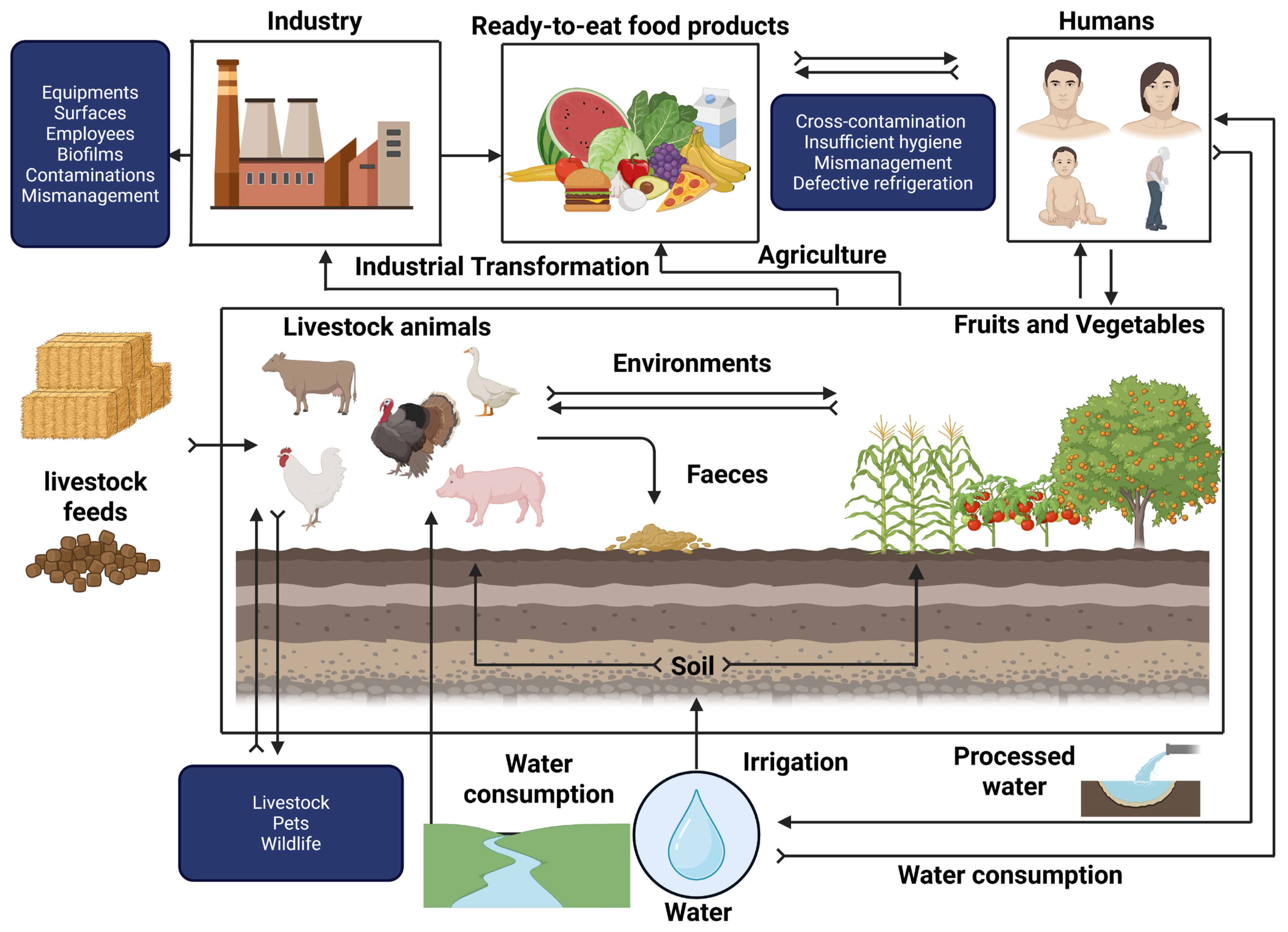
2.5. Survival of Listeria monocytogenes Under Food Processing Conditions
3. Protective Cultures to Control Listeria monocytogenes
3.1. Modes of Action of Protective Cultures
3.2. Uses of Protective Cultures in the Agrifood Industry to Control Listeria
| Protective Culture | Bacteriocins | Food Products | References |
|---|---|---|---|
| Lactococcus lactis DF04Mi | Nisin | Fresh goat’s cheese | [143] |
| Lactococcus lactis LL56 | Nisin | Fresh cheese | [144] |
| Lactococcus lactis DPC4275 | Lactacin 3147 | Cottage cheese | [143] |
| Lactobacillus sakei CTC494 | Sakacin (G/P) | Vacuum-cooked ham | [145] |
| Lactobacillus sakei LAK-23 | Sakacin (non-specified) | Smoked fish | [146] |
| Latilactobacillus curvatus CRL705 | Lactocin 705 and Lactocin AL705 | Cooked vacuum-packed beef meat | [147] |
| Lactobacillus curvatus ACU-1 | Sakacin Q | Cooked meat | [148] |
| Lactobacillus plantarum | Plantaricin A, EF, JK and S | Apples, fresh vegetables, lettuce, cold meats | [149] |
| Lactobacillus pentosus MS031 | Non-specified | Fresh chopped fruits | [150] |
| Lactobacillus casei | Non-specified | Ready-to-eat salads | [151] |
| Pediococcus pentosaceus DT016 | Pediocin (non-specified) | Fresh vegetables | [152] |
| Enterococcus hirae ST57ACC | Non-specified | Skim milk | [153] |
| Leuconostoc mesenteroides | Leucocin C | Apples and lettuce | [154] |
| Carnobacterium maltaromaticum | Carnocin and piscicolin | Fish and smoked salmon | [155] |
| Carnobacterium divergens M35 | Divergicin M35 | Smoked salmon | [156] |
| Paenibacillus polymyxa | Polymyxin-like peptides | Canned vegetables, meat | [157] |
| Streptococcus salivarius K12 | Salivaricin and Subtilin A | Fermented food products | [158] |
| Lacticaseibacillus rhamnosus GG | Rhamnosin-like peptides | Fresh lettuce and ready-to-eat products | [159] |
| Enterococcus durans M3-3 | Duracin-like | Raw milk | [160] |
| Leuconostoc carnosum 4010 | Carnosin | Refrigerated raw meat and meat products | [161] |
| Lactobacillus helveticus CNRZ32 | Helveticin J | Pressed cheese | [162] |
| Weissella hellenica 4M13 | Weissellin A and B | Pickled vegetables | [163] |
4. Antimicrobial Peptides Produced by Bacteria: Promising Biocontrol Agents
4.1. Bacteriocins and Lipopeptides
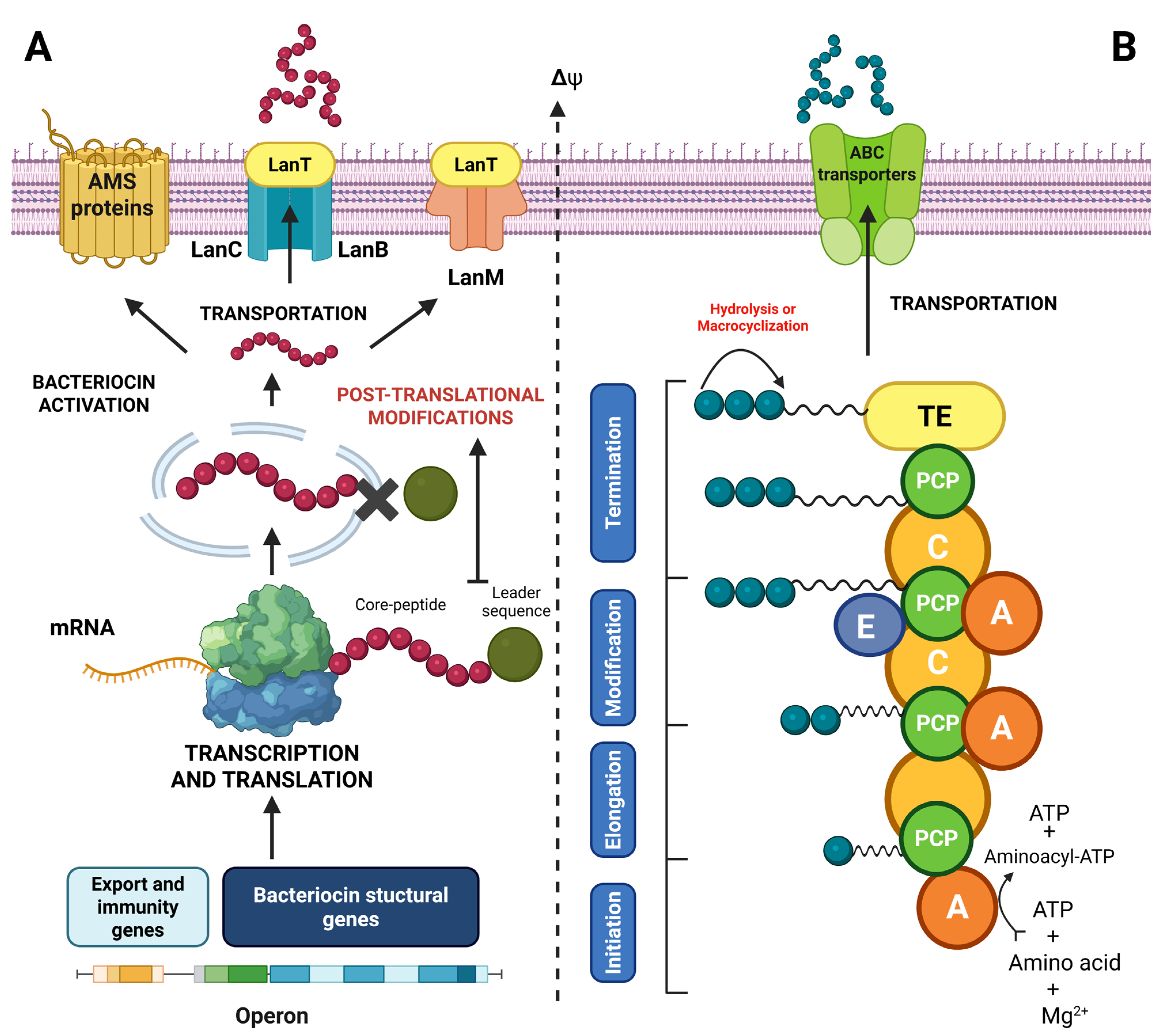
4.2. Classification of Bacteriocins and Lipopeptides
4.2.1. Class I Bacteriocins
4.2.2. Class II Bacteriocins
4.2.3. Class III Bacteriocins
| Class | Subclass | Characteristics | Bacteriocins | References |
|---|---|---|---|---|
| I | Lantibiotics | Post-translational modification | Nisin Z | [194] |
| Labyrinthopeptins | Labyrithopeptin A2 | [195] | ||
| Sactibiotics | Subtilisin A | [196] | ||
| Glycocins | Pallidocin | [197] | ||
| Darobactin | Darobactin A | [198] | ||
| Cayanobactin | Kawaguchipeptin B | [187] | ||
| Circular | Pumilarin | [199] | ||
| Bottromycin | Bottromycin A2 | [200] | ||
| Lasso peptide | Ubonodin | [189] | ||
| II | IIa | Unmodified, low-molecular-weight peptides (<10 KDa) | Pediocin PA-1 | [201] |
| IIb | Lactacin F | [202] | ||
| IIc | Carnocyclin A | [203] | ||
| IId | Enterocin L50A/B | [204] | ||
| III | Bacteriolysins | Thermolabile, unmodified, high-molecular-weight peptides | Helveticin J | [205] |
| Tailocins | Phage tail structure | Monocin J25 | [206] |
4.2.4. Lipopeptides
| Type | Lipopeptide | Structure and Characteristic | Origin | References |
|---|---|---|---|---|
| Cyclic | Surfactin | Cyclic heptapeptide bonded to β-hydroxy-C13-C15 fatty acid through lactone bridge | Bacillus subtilis | [210] |
| Iturin A | β-Amino fatty acid-linked cyclic heptapeptide | Bacillus subtilis | [211] | |
| Daptomycin | Cyclic decapeptide containing branched fatty acids | Streptomyces roseosporus | [212] | |
| Lichenysin | Cyclic, surfactin-like | Bacillus licheniformis | [213] | |
| Gramicidin S | Cyclic decapeptide compound with double symmetrical ring | Bacillus brevis | [214] | |
| Tyrocidin A | Cyclic decapeptide rich in hydrophobic amino acids | Bacillus brevis | [215] | |
| Polymyxin B/E | Cyclic decapeptide containing N-terminal fatty acid, diaminobutyric acid-rich (Dab) | Paenibacillus polymyxa | [216] | |
| Linear | Ramoplanin A | Linear glycosylated lipopeptide | Actinoplanes spp. | [217] |
| Actagardine | Modified linear lipopeptide (lanthionine) | Actinoplanes garbadinensis | [218] | |
| Fusaricidin A | Linear with a lipid tail | Paenibacillus polymyxa | [219] | |
| Taromycin B | Linear lipopeptide | Actinomadura spp. | [220] | |
| Telomycin | Linear peptide, post-translationally modified | Streptomyces spp. | [221] |
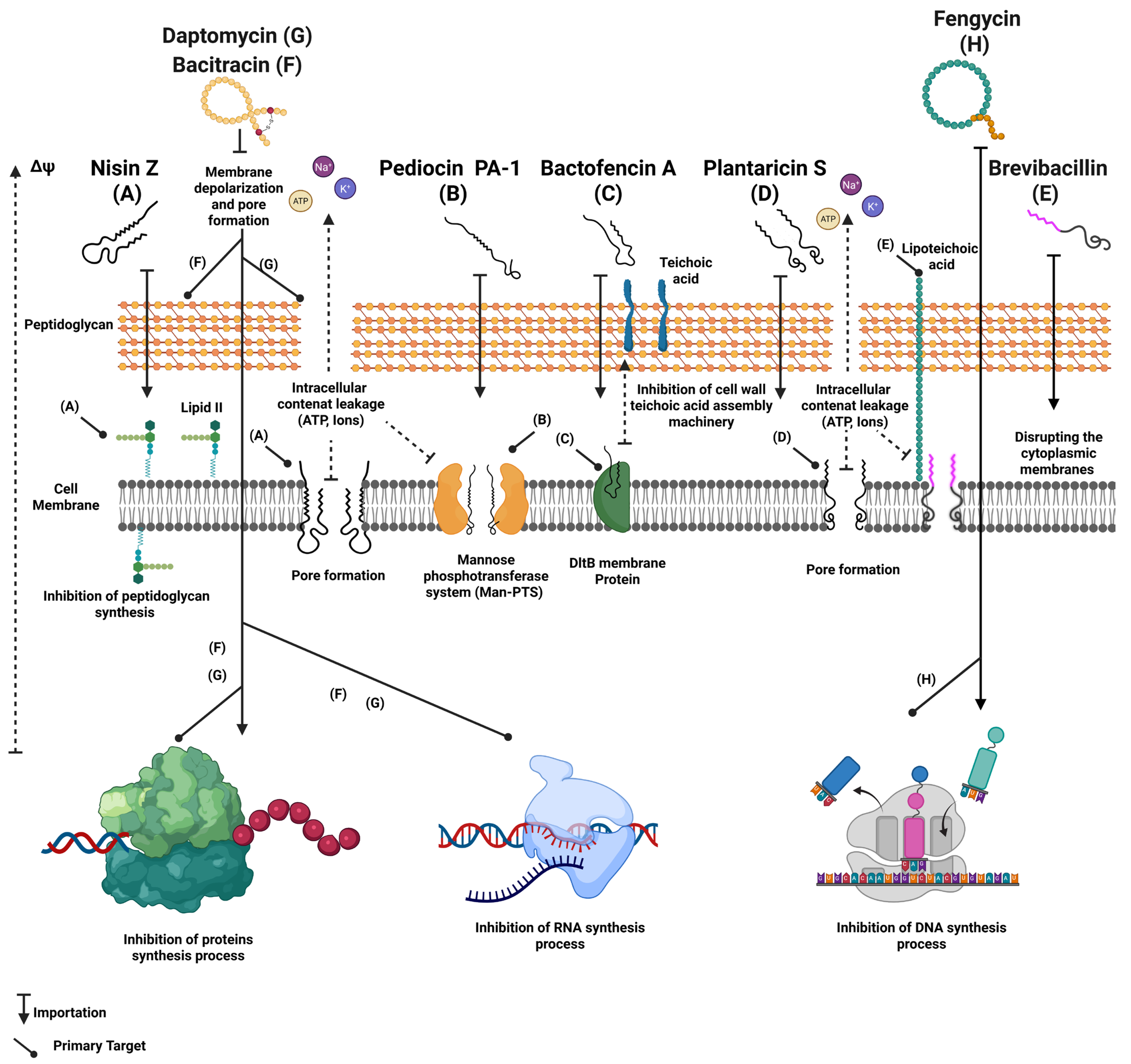
4.3. Mechanisms of Action
4.4. Use of Antimicrobial Peptides for the Biocontrol of Listeria monocytogenes in Foods
| Peptides | Producer Strains | Products | Application | Efficacy | References |
|---|---|---|---|---|---|
| Nisin A | Lactococcus lactis | Nisaplin™ | Ingredients used through direct incorporation, active films or encapsulation in cheeses and UHT milk. | 2–4 log reduction in L. monocytogenes levels in Ricotta, Cottage and Galotiri cheeses. | [124] |
| Nisin Z | Lactococcus lactis | Niseen®-Siveele | Incorporation in Galotyri and Minas fresh cheeses. | Extends products shelf-life up to 21 days under refrigeration conditions. | [139] |
| Micocin | Carnobacterium maltaromaticum | Micocin® | Surface application or coating on soft, raw milk cheeses. Brining or direct application to chopped meat. | Complete reduction in L. monocytogenes under refrigeration for 14 days. L. monocytogenes elimination for 14 days at 4 °C (<102 CFU/g). | [11] |
| Pediocin PA-1 | Pediococcus acidilactici | Alta™ 2341 | Direct incorporation or cultivation in pasteurized milk and soft cheeses. Injections or incorporation into RTE sausages, hams and cooked meats. | L. monocytogenes elimination at 4 °C in 7–10 days. 3 to 5 log reduction in Listeria in ham at 4 °C in 10 days. | [11] |
| Surfactin | Bacillus subtilis | InoviaTech, under development | Food surface cleaning and disinfectant (stainless steel and plastic) | L. monocytogenes biofilm inhibition, 4-log reduction in mature biofilm. | [166] |
| Fengycin | Bacillus subtilis | BioBoom® Clean | Production line and sensitive surfaces decontamination. | Synergistic effect and prolonged anti-Listeria activity with nisin. | [243] |
| Iturine A | Bacillus subtilis | Under investigation | Active packaging formulations and cutting surfaces. | anti-listeria, suitable for encapsulation or coating. | [243] |
| Lacticin 481 | Lactococcus lactis L3A21M1 | Under investigation | Application of purified lacticin 481 on fresh cheese | 3-log reduction in L. monocytogenes following 3 to 7 days at 4 °C. | [244] |
| Leucocin K7 | Leuconostoc mesenteroides K7 | Under investigation | Incorporated in UHT whole-fat milk | 80 UA/mL of Leucocin K7 combined with 5 mg/mL of glycine effectively inhibited L. monocytogenes growth for 7 days. | [245] |
| Aureocin A70 | Staphylococcus aureus A70 | Under investigation | Incorporated in UHT skim milk | Partially purified aureocin formulation showed 5.5 log inhibition of L. monocytogenes after 7 days at refrigeration conditions. | [124] |
4.5. Synergy and Combination of Different Approaches
| Peptide | Culture and Compounds | Technology and Support | Synergetic Effect | Application | References |
|---|---|---|---|---|---|
| Nisin | Lactococcus lactis | High pressure (400 MPa) | Over 4 log CFU/g L. monocytogenes reduction. | ham | [124] |
| Nisin | Thymol and carvacrol essential oils | Nonencapsulated antimicrobial packaging | Extended L. monocytogenes inhibition effect during storage. | Soft cheeses | [266] |
| Pediocin PA-1 | Pediococcus acidilactici | Modified atmosphere | Increased L. monocytogenes inhibition under modified atmosphere. | Packaged meats | [266] |
| Enterocin A | Enterococcus faecium | Directed fermentation | Selective inhibition of Listeria and preserving technological microflora. | Ripened cheeses | [124] |
| Divergicin V41 | Carnobacterium divergens V41 | Combination of protective culture and AMPs | Specific L. monocytogenes inhibition while maintaining product sensory properties. | Smoked salmon | [267,268] |
| Divergicin M35 | Carnobacterium divergens M35 | Direct application under refrigeration conditions | Reducing L. monocytogenes levels at low temperatures while preserving organoleptic characteristics | Smoked fish | [156] |
| Nisin | Citric acid | AMPs and citric acid combination | Effective antimicrobial effect against L. monocytogenes. | Dairy products | [269] |
| Nisin | Reuterin | Synergistic effect between AMPs and microbial secondary metabolite (aldehyde) | Enhanced antimicrobial effectiveness against foodborne pathogens including L. monocytogenes. | Raw milk | [270] |
| Enterocin 416K1 | Polyethylene terephthalate (PET) films | Active package | Significant decrease in viable L. monocytogenes bacterial cells. | Seasoned cheese | [11] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, V.; Nag, R.; Burgess, C.M.; Gaffney, M.; Celayeta, J.M.F.; Cummins, E. Microbial risks associated with Ready-to-Eat Fresh Produce (RTEFP)—A focus on temperate climatic conditions. Postharvest Biol. Technol. 2024, 213, 112924. [Google Scholar] [CrossRef]
- Benito, S.; López, A.; Lizana, X.; Lope, S.; Carbó, R.; Del Valle, L.J.; Marqués, A.M.; Piqué, N. Presence of Listeria monocytogenes in Prepared Foods: Analysis of Influencing Factors. J. Food Process. Preserv. 2017, 41, e12842. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Sci. Anim. Ind. 2020, 87, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Sobel, J.; Mazaki-Tovi, S.; Kusanovic, J.P.; Vaisbuch, E.; Kim, S.K.; Uldbjerg, N.; Romero, R. Listeriosis in human pregnancy: A systematic review. J. Perinat. Med. 2011, 39, 227–236. [Google Scholar] [CrossRef]
- Doganay, M. Listeriosis: Clinical presentation. FEMS Immunol. Med. Microbiol. 2003, 35, 173–175. [Google Scholar] [CrossRef]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Muncke, J.; Touvier, M.; Trasande, L.; Scheringer, M. Health impacts of exposure to synthetic chemicals in food. Nat. Med. 2025, 31, 1431–1443. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Proestos, C. Food Preservation: Challenges and Efforts for the Future. Foods 2020, 9, 391. [Google Scholar] [CrossRef]
- Castellano, P.; Melian, C.; Burgos, C.; Vignolo, G. Chapter Seven—Bioprotective cultures and bacteriocins as food preservatives. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 106, pp. 275–315. [Google Scholar]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial Activity of Lactic Acid Against Pathogen and Spoilage Microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Carvalho, F.; Sousa, S.; Cabanes, D. How Listeria monocytogenes organizes its surface for virulence. Front. Cell. Infect. Microbiol. 2014, 4, 48. [Google Scholar] [CrossRef]
- Schmid, D.; Allerberger, F.; Huhulescu, S.; Pietzka, A.; Amar, C.; Kleta, S.; Prager, R.; Preußel, K.; Aichinger, E.; Mellmann, A. Whole genome sequencing as a tool to investigate a cluster of seven cases of listeriosis in Austria and Germany, 2011–2013. Clin. Microbiol. Infect. 2014, 20, 431–436. [Google Scholar] [CrossRef]
- Indrawattana, N.; Nibaddhasobon, T.; Sookrung, N.; Chongsa-Nguan, M.; Tungtrongchitr, A.; Makino, S.-I.; Tungyong, W.; Chaicumpa, W. Prevalence of Listeria monocytogenes in raw meats marketed in Bangkok and characterization of the isolates by phenotypic and molecular methods. J. Health Popul. Nutr. 2011, 29, 26–38. [Google Scholar] [CrossRef]
- Holch, A.; Webb, K.; Lukjancenko, O.; Ussery, D.; Rosenthal, B.M.; Gram, L. Genome Sequencing Identifies Two Nearly Unchanged Strains of Persistent Listeria monocytogenes Isolated at Two Different Fish Processing Plants Sampled 6 Years Apart. Appl. Environ. Microbiol. 2013, 79, 2944–2951. [Google Scholar] [CrossRef]
- Liu, D. Identification, subtyping and virulence determination of Listeria monocytogenes, an important foodborne pathogen. J. Med. Microbiol. 2006, 55, 645–659. [Google Scholar] [CrossRef]
- Ducey, T.F.; Page, B.; Usgaard, T.; Borucki, M.K.; Pupedis, K.; Ward, T.J. A single-nucleotide-polymorphism-based multilocus genotyping assay for subtyping lineage I isolates of Listeria monocytogenes. Appl. Environ. Microbiol. 2007, 73, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Barbuddhe, S.; Malik, S.V.S.; Singh, R.K. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.K.; Didelot, X.; Lecuit, M.; Korkeala, H. L monocytogenes MLST Study Group; Achtman, M. The ubiquitous nature of Listeria monocytogenes clones: A large-scale Multilocus Sequence Typing study. Environ. Microbiol. 2014, 16, 405–416. [Google Scholar] [CrossRef]
- Camargo, A.C.; Woodward, J.J.; Nero, L.A. The Continuous Challenge of Characterizing the Foodborne Pathogen Listeria monocytogenes. Foodborne Pathog. Dis. 2016, 13, 405–416. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Moura, A.; Leclercq, A.; Vales, G.; Tessaud-Rita, N.; Bracq-Dieye, H.; Thouvenot, P.; Madec, Y.; Charlier, C.; Lecuit, M. Phenotypic and genotypic antimicrobial resistance of Listeria monocytogenes: An observational study in France. Lancet Reg. Health Eur. 2024, 37, 100800. [Google Scholar] [CrossRef]
- Hilliard, A.; Leong, D.; O’Callaghan, A.; Culligan, E.P.; Morgan, C.A.; DeLappe, N.; Hill, C.; Jordan, K.; Cormican, M.; Gahan, C.G.M. Genomic Characterization of Listeria monocytogenes Isolates Associated with Clinical Listeriosis and the Food Production Environment in Ireland. Genes 2018, 9, 171. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Galvão, J.A.; Oetterer, M. Contamination sources, serogroups, biofilm-forming ability and biocide resistance of Listeria monocytogenes persistent in tilapia-processing facilities. J. Food Sci. Technol. 2017, 54, 3867–3879. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13, Erratum in Food Control 2018, 88, 236. [Google Scholar] [CrossRef]
- Mizuno, S.; Zendejas, I.R.; Reed, A.I.; Kim, R.D.; Howard, R.J.; Hemming, A.W.; Schain, D.C.; Soldevila-Pico, C.; Firpi, R.J.; Fujita, S. Listeria monocytogenes following orthotopic liver transplantation: Central nervous system involvement and review of the literature. World J. Gastroenterol. 2007, 13, 4391–4393. [Google Scholar] [CrossRef]
- Mateus, T.; Silva, J.; Maia, R.L.; Teixeira, P. Listeriosis during Pregnancy: A Public Health Concern. ISRN Obstet. Gynecol. 2013, 2013, 851712. [Google Scholar] [CrossRef] [PubMed]
- Poimenidou, S.V.; Dalmasso, M.; Papadimitriou, K.; Fox, E.M.; Skandamis, P.N.; Jordan, K. Virulence Gene Sequencing Highlights Similarities and Differences in Sequences in Listeria monocytogenes Serotype 1/2a and 4b Strains of Clinical and Food Origin From 3 Different Geographic Locations. Front. Microbiol. 2018, 9, 1103. [Google Scholar] [CrossRef]
- Shamloo, E.; Hosseini, H.; Abdi Moghadam, Z.; Halberg Larsen, M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iran. J. Vet. Res. 2019, 20, 241–254. [Google Scholar]
- Bagatella, S.; Tavares-Gomes, L.; Oevermann, A. Listeria monocytogenes at the interface between ruminants and humans: A comparative pathology and pathogenesis review. Vet. Pathol. 2022, 59, 186–210. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Wang, J.; Wu, Q.; Cheng, J.; Zhang, J.; Sun, Q.; Xue, L.; Zeng, H.; Lei, T.; et al. Heterogeneity, Characteristics, and Public Health Implications of Listeria monocytogenes in Ready-to-Eat Foods and Pasteurized Milk in China. Front. Microbiol. 2020, 11, 642. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Domínguez-Bernal, G.; González-Zorn, B.; Kreft, J.; Goebel, W. Pathogenicity islands and virulence evolution in Listeria. Microb. Infect. 2001, 3, 571–584. [Google Scholar] [CrossRef]
- Davis, M.; Ricke, S.; Donaldson, J. Establishment of Listeria monocytogenes in the Gastrointestinal Tract. Microorganisms 2019, 7, 75. [Google Scholar] [CrossRef]
- Smith, J.L.; Liu, Y.; Paoli, G.C. How does Listeria monocytogenes combat acid conditions? Can. J. Microbiol. 2012, 59, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Giaouris, E.; Grigoraki, I.; Skandamis, P.; Nychas, G.-J. Effect of acid tolerance response (ATR) on attachment of Listeria monocytogenes Scott A to stainless steel under extended exposure to acid or/and salt stress and resistance of sessile cells to subsequent strong acid challenge. Int. J. Food Microbiol. 2011, 145, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; Scollard, J.; Meally, A.; Bolton, D.J.; Gahan, C.G.; Cotter, P.D.; Hill, C.; O’Beirne, D. The glutamate decarboxylase acid resistance mechanism affects survival of Listeria monocytogenes LO28 in modified atmosphere-packaged foods. J. Appl. Microbiol. 2007, 103, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, D.; Sousa, S.; Cebriá, A.; Lecuit, M.; García-del Portillo, F.; Cossart, P. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J. 2005, 24, 2827–2838. [Google Scholar] [CrossRef]
- Sleator, R.D.; Wemekamp-Kamphuis, H.H.; Gahan, C.G.M.; Abee, T.; Hill, C. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Mol. Microbiol. 2004, 55, 1183–1195. [Google Scholar] [CrossRef]
- Osek, J.; Wieczorek, K. Listeria monocytogenes-How This Pathogen Uses Its Virulence Mechanisms to Infect the Hosts. Pathogens 2022, 11, 1491. [Google Scholar] [CrossRef]
- Cotter, P.D.; O’Reilly, K.; Hill, C. Role of the glutamate decarboxylase acid resistance system in the survival of Listeria monocytogenes LO28 in low pH foods. J. Food Prot. 2001, 64, 1362–1368. [Google Scholar] [CrossRef]
- Ryan, S.; Begley, M.; Hill, C.; Gahan, C.G. A five-gene stress survival islet (SSI-1) that contributes to the growth of Listeria monocytogenes in suboptimal conditions. J. Appl. Microbiol. 2010, 109, 984–995. [Google Scholar] [CrossRef]
- Cotter, P.D.; Gahan, C.G.; Hill, C. Analysis of the role of the Listeria monocytogenes F0F1-AtPase operon in the acid tolerance response. Int. J. Food Microbiol. 2000, 60, 137–146. [Google Scholar] [CrossRef]
- Begley, M.; Sleator, R.D.; Gahan, C.G.; Hill, C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect. Immun. 2005, 73, 894–904. [Google Scholar] [CrossRef]
- Bourgin, M.; Kriaa, A.; Mkaouar, H.; Mariaule, V.; Jablaoui, A.; Maguin, E.; Rhimi, M. Bile Salt Hydrolases: At the Crossroads of Microbiota and Human Health. Microorganisms 2021, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wemekamp-Kamphuis, H.H.; Wouters, J.A.; de Leeuw, P.P.; Hain, T.; Chakraborty, T.; Abee, T. Identification of sigma factor sigma B-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 2004, 70, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed]
- Drevets, D.A. Dissemination of Listeria monocytogenes by infected phagocytes. Infect. Immun. 1999, 67, 3512–3517. [Google Scholar] [CrossRef]
- Mariscotti, J.F.; Quereda, J.J.; García-del Portillo, F.; Pucciarelli, M.G. The Listeria monocytogenes LPXTG surface protein Lmo1413 is an invasin with capacity to bind mucin. Int. J. Med. Microbiol. 2014, 304, 393–404. [Google Scholar] [CrossRef]
- Dramsi, S.; Cossart, P. Listeriolysin O-Mediated Calcium Influx Potentiates Entry of Listeria monocytogenes into the Human Hep-2 Epithelial Cell Line. Infect. Immun. 2003, 71, 3614–3618. [Google Scholar] [CrossRef]
- Xayarath, B.; Alonzo, F., 3rd; Freitag, N.E. Identification of a peptide-pheromone that enhances Listeria monocytogenes escape from host cell vacuoles. PLoS Pathog. 2015, 11, e1004707. [Google Scholar] [CrossRef]
- Kocks, C.; Gouin, E.; Tabouret, M.; Berche, P.; Ohayon, H.; Cossart, P.L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 1992, 68, 521–531. [Google Scholar] [CrossRef]
- Bonazzi, M.; Lecuit, M.; Cossart, P. Listeria monocytogenes internalin and E-cadherin: From structure to pathogenesis. Cell. Microbiol. 2009, 11, 693–702. [Google Scholar] [CrossRef]
- Witter, A.R.; Okunnu, B.M.; Berg, R.E. The Essential Role of Neutrophils during Infection with the Intracellular Bacterial Pathogen Listeria monocytogenes. J. Immunol. 2016, 197, 1557–1565. [Google Scholar] [CrossRef]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-Del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Paranjape, N. Rhombencephalitis due to Listeria monocytogenes. IDCases 2021, 24, e01081. [Google Scholar] [CrossRef]
- Bartt, R. Listeria and Atypical Presentations of Listeria in the Central Nervous System. Semin. Neurol. 2000, 20, 361–374. [Google Scholar] [CrossRef]
- Disson, O.; Lecuit, M. Targeting of the central nervous system by Listeria monocytogenes. Virulence 2012, 3, 213–221. [Google Scholar] [CrossRef]
- Karlsson, W.K.; Harboe, Z.B.; Roed, C.; Monrad, J.B.; Lindelof, M.; Larsen, V.A.; Kondziella, D. Early trigeminal nerve involvement in Listeria monocytogenes rhombencephalitis: Case series and systematic review. J. Neurol. 2017, 264, 1875–1884. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Wagner, M.; Scortti, M. Why Are Some Listeria monocytogenes Genotypes More Likely To Cause Invasive (Brain, Placental) Infection? mBio 2020, 11, e03126-20. [Google Scholar] [CrossRef]
- McLauchlin, J.; Amar, C.F.L.; Grant, K.A. Neonatal cross-infection due to Listeria monocytogenes. Epidemiol. Infect. 2022, 150, 1–31. [Google Scholar] [CrossRef]
- Faralla, C.; Rizzuto, G.A.; Lowe, D.E.; Kim, B.; Cooke, C.; Shiow, L.R.; Bakardjiev, A.I. InlP, a New Virulence Factor with Strong Placental Tropism. Infect. Immun. 2016, 84, 3584–3596. [Google Scholar] [CrossRef]
- Rogers, H.W.; Unanue, E.R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 1993, 61, 5090–5096. [Google Scholar] [CrossRef]
- Moreno, L.Z.; Paixão, R.; Gobbi, D.D.; Raimundo, D.C.; Ferreira, T.P.; Moreno, A.M.; Hofer, E.; Reis, C.M.; Matté, G.R.; Matté, M.H. Characterization of antibiotic resistance in Listeria spp. isolated from slaughterhouse environments, pork and human infections. J. Infect. Dev. Ctries. 2014, 8, 416–423. [Google Scholar] [CrossRef]
- Jensen, A.K.; Björkman, J.; Ethelberg, S.; Kiil, K.; Kemp, M.; Nielsen, E.M. Molecular Typing and Epidemiology of Human Listeriosis Cases, Denmark, 2002–2012. Emerg. Infect. Dis. J. 2016, 22, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Duffy, G.; Sheridan, J.J.; Blair, I.S.; McDowell, D.A. Antibiotic resistance among Listeria, including Listeria monocytogenes, in retail foods. J. Appl. Microbiol. 2001, 90, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Lungu, B.; O’Bryan, C.A.; Muthaiyan, A.; Milillo, S.R.; Johnson, M.G.; Crandall, P.G.; Ricke, S.C. Listeria monocytogenes: Antibiotic Resistance in Food Production. Foodborne Pathog. Dis. 2010, 8, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Conter, M.; Paludi, D.; Zanardi, E.; Ghidini, S.; Vergara, A.; Ianieri, A. Characterization of antimicrobial resistance of foodborne Listeria monocytogenes. Int. J. Food Microbiol. 2009, 128, 497–500. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and Food Processing Environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Jones, F.T.; Ricke, S.C. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult. Sci. 2003, 82, 613–617. [Google Scholar] [CrossRef]
- Kode, D.; Nannapaneni, R.; Bansal, M.; Chang, S.; Cheng, W.H.; Sharma, C.S.; Kiess, A. Low-Level Tolerance to Fluoroquinolone Antibiotic Ciprofloxacin in QAC-Adapted Subpopulations of Listeria monocytogenes. Microorganisms 2021, 9, 1052. [Google Scholar] [CrossRef]
- Lampidis, R.; Kostrewa, D.; Hof, H. Molecular characterization of the genes encoding DNA gyrase and topoisomerase IV of Listeria monocytogenes. J. Antimicrob. Chemother. 2002, 49, 917–924. [Google Scholar] [CrossRef]
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial Resistance in Listeria Species. Microbiol. Spectr. 2018, 6, 1128. [Google Scholar] [CrossRef]
- Zawadzka-Skomiał, J.; Markiewicz, Z.; Nguyen-Disteche, M.; Devreese, B.; Frère, J.-M.; Terrak, M. Characterization of the Bifunctional Glycosyltransferase/Acyltransferase Penicillin-Binding Protein 4 of Listeria monocytogenes. J. Bacteriol. 2006, 188, 1875–1881. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of antibiotic resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef]
- Goh, Y.-X.; Anupoju, S.M.B.; Nguyen, A.; Zhang, H.; Ponder, M.; Krometis, L.-A.; Pruden, A.; Liao, J. Evidence of horizontal gene transfer and environmental selection impacting antibiotic resistance evolution in soil-dwelling Listeria. Nat. Commun. 2024, 15, 10034. [Google Scholar] [CrossRef] [PubMed]
- Troxler, R.; Von Graevenitz, A.; Funke, G.; Wiedemann, B.; Stock, I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin. Microbiol. Infect. 2000, 6, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.F.; Pérez-Dáz, J.C.; Baquero, F.; Angel de Pedro, M.; Berenguer, J. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob. Agents Chemother. 1990, 34, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Goulard, C.; Nahori, M.-A.; Cayet, N.; Decalf, J.; Sachse, M.; Boneca, I.G.; Cossart, P.; Dussurget, O. OatA, a Peptidoglycan O-Acetyltransferase Involved in Listeria monocytogenes Immune Escape, Is Critical for Virulence. J. Infect. Dis. 2011, 204, 731–740. [Google Scholar] [CrossRef]
- Kallipolitis, B.H.; Ingmer, H.; Gahan, C.G.; Hill, C.; Søgaard-Andersen, L. CesRK, a two-component signal transduction system in Listeria monocytogenes, responds to the presence of cell wall-acting antibiotics and affects beta-lactam resistance. Antimicrob. Agents Chemother. 2003, 47, 3421–3429. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Bustamante, F.; Lepuschitz, S.; Pietzka, A.; Contreras-Fernández, A.; Castillo, C.; Ovalle, C.; Alarcón-Lavín, M.P.; Cruz-Córdova, A.; et al. Virulence and Antibiotic Resistance Genes in Listeria monocytogenes Strains Isolated From Ready-to-Eat Foods in Chile. Front. Microbiol. 2022, 12, 3389. [Google Scholar] [CrossRef]
- Lancaster, H.; Roberts, A.P.; Bedi, R.; Wilson, M.; Mullany, P. Characterization of Tn916S, a Tn916-like element containing the tetracycline resistance determinant tet(S). J. Bacteriol. 2004, 186, 4395–4398. [Google Scholar] [CrossRef]
- Novais, C.; Freitas, A.R.; Silveira, E.; Baquero, F.; Peixe, L.; Roberts, A.P.; Coque, T.M. A tet(S/M) hybrid from CTn6000 and CTn916 recombination. Microbiology 2012, 158, 2710–2711. [Google Scholar] [CrossRef]
- van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef]
- Li, L.; Olsen, R.H.; Shi, L.; Ye, L.; He, J.; Meng, H. Characterization of a plasmid carrying cat, ermB and tetS genes in a foodborne Listeria monocytogenes strain and uptake of the plasmid by cariogenic Streptococcus mutans. Int. J. Food Microbiol. 2016, 238, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E.; Gerbaud, G.R.; Courvalin, P. Conjugative Mobilization of the Rolling-Circle Plasmid pIP823 from Listeria monocytogenes BM4293 among Gram-Positive and Gram-Negative Bacteria. J. Bacteriol. 1999, 181, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Nam, H.M.; Nguyen, L.T.; Tamilselvam, B.; Murinda, S.E.; Oliver, S.P. Prevalence of Antimicrobial Resistance Genes in Listeria monocytogenes Isolated from Dairy Farms. Foodborne Pathog. Dis. 2005, 2, 201–211. [Google Scholar] [CrossRef]
- Roberts, M.C. Resistance to tetracycline, macrolide-lincosamide-streptogramin, trimethoprim, and sulfonamide drug classes. Mol. Biotechnol. 2002, 20, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Granier, S.A.; Moubareck, C.; Colaneri, C.; Lemire, A.; Roussel, S.; Dao, T.T.; Courvalin, P.; Brisabois, A. Antimicrobial resistance of Listeria monocytogenes isolates from food and the environment in France over a 10-year period. Appl. Environ. Microbiol. 2011, 77, 2788–2790. [Google Scholar] [CrossRef]
- Maung, A.T.; Mohammadi, T.N.; Nakashima, S.; Liu, P.; Masuda, Y.; Honjoh, K.-i.; Miyamoto, T. Antimicrobial resistance profiles of Listeria monocytogenes isolated from chicken meat in Fukuoka, Japan. Int. J. Food Microbiol. 2019, 304, 49–57. [Google Scholar] [CrossRef]
- Yan, S.; Li, M.; Luque-Sastre, L.; Wang, W.; Hu, Y.; Peng, Z.; Dong, Y.; Gan, X.; Nguyen, S.; Anes, J.; et al. Susceptibility (re)-testing of a large collection of Listeria monocytogenes from foods in China from 2012 to 2015 and WGS characterization of resistant isolates. J. Antimicrob. Chemother. 2019, 74, 1786–1794. [Google Scholar] [CrossRef]
- Morvan, A.; Moubareck, C.; Leclercq, A.; Hervé-Bazin, M.; Bremont, S.; Lecuit, M.; Courvalin, P.; Le Monnier, A. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob. Agents Chemother. 2010, 54, 2728–2731. [Google Scholar] [CrossRef]
- Bertsch, D.; Uruty, A.; Anderegg, J.; Lacroix, C.; Perreten, V.; Meile, L.J. Tn6198, a novel transposon containing the trimethoprim resistance gene dfrG embedded into a Tn916 element in Listeria monocytogenes. J. Antimicrob. Chemother. 2013, 68, 986–991. [Google Scholar] [CrossRef]
- Korsak, D.; Krawczyk-Balska, A. Identification of the Molecular Mechanism of Trimethoprim Resistance in Listeria monocytogenes. Foodborne Pathog. Dis. 2017, 14, 696–700. [Google Scholar] [CrossRef]
- Tsakris, A.; Papa, A.; Douboyas, J.; Antoniadis, A. Neonatal meningitis due to multi-resistant Listeria monocytogenes. J. Antimicrob. Chemother. 1997, 39, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Matereke, L.T.; Okoh, A.I. Listeria monocytogenes Virulence, Antimicrobial Resistance and Environmental Persistence: A Review. Pathogens 2020, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Aslan Kayiran, M.; Karadag, A.S.; Al-Khuzaei, S.; Chen, W.; Parish, L.C. Antibiotic Resistance in Acne: Mechanisms, Complications and Management. Am. J. Clin. Dermatol. 2020, 21, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Ferreira, S.; Domingues, F. The antimicrobial action of resveratrol against Listeria monocytogenes in food-based models and its antibiofilm properties. J. Sci. Food Agric. 2016, 96, 4531–4535. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Lou, Y.; Yousef, A.E. Resistance of Listeria monocytogenes to Heat after Adaptation to Environmental Stresses. J. Food Prot. 1996, 59, 465–471. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Thomas, J.; Govender, N.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; Sekwadi, P.; Ntshoe, G.; et al. Outbreak of Listeriosis in South Africa Associated with Processed Meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef]
- Chan, Y.C.; Raengpradub, S.; Boor, K.J.; Wiedmann, M. Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 2007, 73, 6484–6498. [Google Scholar] [CrossRef]
- Flegler, A.; Iswara, J.; Mänz, A.T.; Schocke, F.S.; Faßbender, W.A.; Hölzl, G.; Lipski, A. Exogenous fatty acids affect membrane properties and cold adaptation of Listeria monocytogenes. Sci. Rep. 2022, 12, 1499. [Google Scholar] [CrossRef] [PubMed]
- Chastanet, A.; Derre, I.; Nair, S.; Msadek, T. clpB, a novel member of the Listeria monocytogenes CtsR regulon, is involved in virulence but not in general stress tolerance. J. Bacteriol. 2004, 186, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.; Abee, T. Importance of SigB for Listeria monocytogenes static and continuous-flow biofilm formation and disinfectant resistance. Appl. Environ. Microbiol. 2010, 76, 7854–7860. [Google Scholar] [CrossRef]
- Müller, A.; Rychli, K.; Muhterem-Uyar, M.; Zaiser, A.; Stessl, B.; Guinane, C.M.; Cotter, P.D.; Wagner, M.; Schmitz-Esser, S. Tn6188—A novel transposon in Listeria monocytogenes responsible for tolerance to benzalkonium chloride. PLoS ONE 2013, 8, e76835. [Google Scholar] [CrossRef]
- Ratani, S.S.; Siletzky, R.M.; Dutta, V.; Yildirim, S.; Osborne, J.A.; Lin, W.; Hitchins, A.D.; Ward, T.J.; Kathariou, S. Heavy metal and disinfectant resistance of Listeria monocytogenes from foods and food processing plants. Appl. Environ. Microbiol. 2012, 78, 6938–6945. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Ivy, R.A.; Ho, A.J.; Fortes, E.D.; Njaa, B.L.; Peters, R.M.; Wiedmann, M. inlA premature stop codons are common among Listeria monocytogenes isolates from foods and yield virulence-attenuated strains that confer protection against fully virulent strains. Appl. Environ. Microbiol. 2008, 74, 6570–6583. [Google Scholar] [CrossRef]
- Bland, R.; Brown, S.R.B.; Waite-Cusic, J.; Kovacevic, J. Probing antimicrobial resistance and sanitizer tolerance themes and their implications for the food industry through the Listeria monocytogenes lens. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1777–1802. [Google Scholar] [CrossRef]
- Conficoni, D.; Losasso, C.; Cortini, E.; Di Cesare, A.; Cibin, V.; Giaccone, V.; Corno, G.; Ricci, A. Resistance to Biocides in Listeria monocytogenes Collected in Meat-Processing Environments. Front. Microbiol. 2016, 7, 1627. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes Biofilms in the Wonderland of Food Industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef]
- Lee, B.-H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm Formation of Listeria monocytogenes Strains Under Food Processing Environments and Pan-Genome-Wide Association Study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- García-Díez, J.; Saraiva, C. Use of Starter Cultures in Foods from Animal Origin to Improve Their Safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.B. Commercial bacterial starter cultures for fermented foods of the future. Int. J. Food Microbiol. 2002, 78, 119–131. [Google Scholar] [CrossRef]
- Castellano, P.; Pérez Ibarreche, M.; Blanco Massani, M.; Fontana, C.; Vignolo, G.M. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef]
- Scatassa, M.L.; Gaglio, R.; Cardamone, C.; Macaluso, G.; Arcuri, L.; Todaro, M.; Mancuso, I. Anti-Listeria Activity of Lactic Acid Bacteria in Two Traditional Sicilian Cheeses. Ital. J. Food. Saf. 2017, 6, 6191. [Google Scholar] [CrossRef][Green Version]
- Souza, L.V.; Martins, E.; Moreira, I.; de Carvalho, A.F. Strategies for the Development of Bioprotective Cultures in Food Preservation. Int. J. Microbiol. 2022, 2022, 6264170. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Jay, J.M. Antimicrobial properties of diacetyl. Appl. Environ. Microbiol. 1982, 44, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Schwab, C.; Zhang, J.; Stevens, M.J.A.; Bieri, C.; Ebert, M.-O.; McNeill, K.; Sturla, S.J.; Lacroix, C. Acrolein contributes strongly to antimicrobial and heterocyclic amine transformation activities of reuterin. Sci. Rep. 2016, 6, 36246. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, K.; Kriksic, V.; Kovacevic, I.; Kovacevic, D. Influence of Oral Probiotic Streptococcus salivarius K12 on Ear and Oral Cavity Health in Humans: Systematic Review. Probiotics Antimicrob. Proteins 2017, 9, 102–110. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Todorov, S.D. Bacteriocinogenic LAB Strains for Fermented Meat Preservation: Perspectives, Challenges, and Limitations. Probiotics Antimicrob. Proteins 2017, 9, 444–458. [Google Scholar] [CrossRef]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in vivo Models. Front. Microbiol. 2021, 12, 695. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Aljasir, S.F.; D’Amico, D.J. Effect of pre-exposure to protective bacterial cultures in food on Listeria monocytogenes virulence. LWT 2021, 152, 112373. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ramos, I.M.; Rodríguez-Pérez, M.; Poveda, J.M.; Seseña, S.; Palop, M.L. Lactic acid bacteria as biocontrol agents to reduce Staphylococcus aureus growth, enterotoxin production and virulence gene expression. LWT 2022, 170, 114025. [Google Scholar] [CrossRef]
- Meireles, D.; Pombinho, R.; Cabanes, D. Signals behind Listeria monocytogenes virulence mechanisms. Gut Microbes 2024, 16, 2369564. [Google Scholar] [CrossRef]
- Ross, R.P.; Galvin, M.; McAuliffe, O.; Morgan, S.M.; Ryan, M.P.; Twomey, D.P.; Meaney, W.J.; Hill, C. Developing applications for lactococcal bacteriocins. Antonie Van Leeuwenhoek 1999, 76, 337–346. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Chapter 5—“Green Preservatives”: Combating Fungi in the Food and Feed Industry by Applying Antifungal Lactic Acid Bacteria. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 66, pp. 217–238. [Google Scholar]
- Katla, T.; Møretrø, T.; Aasen, I.M.; Holck, A.; Axelsson, L.; Naterstad, K. Inhibition of Listeria monocytogenes in cold smoked salmon by addition of sakacin P and/or liveLactobacillus sakei cultures. Food Microbiol. 2001, 18, 431–439. [Google Scholar] [CrossRef]
- Iacumin, L.; Cappellari, G.; Pellegrini, M.; Basso, M.; Comi, G. Analysis of the Bioprotective Potential of Different Lactic Acid Bacteria Against Listeria monocytogenes in Cold-Smoked Sea Bass, a New Product Packaged Under Vacuum and Stored at 6 ± 2 °C. Front. Microbiol. 2021, 12, 796655. [Google Scholar] [CrossRef]
- Vignolo, G.; Fadda, S.; de Kairuz, M.N.; de Ruiz Holgado, A.A.P.; Oliver, G. Control of Listeria monocytogenes in ground beef by ‘Lactocin 705’, a bacteriocin produced by Lactobacillus casei CRL 705. Int. J. Food Microbiol. 1996, 29, 397–402. [Google Scholar] [CrossRef]
- Rivas, F.P.; Castro, M.P.; Vallejo, M.; Marguet, E.; Campos, C.A. Sakacin Q produced by Lactobacillus curvatus ACU-1: Functionality characterization and antilisterial activity on cooked meat surface. Meat Sci. 2014, 97, 475–479. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Montesinos, E.; Badosa, E. Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int. J. Microbiol. 2009, 11, 231–236. [Google Scholar] [CrossRef]
- Yi, L.; Qi, T.; Ma, J.; Zeng, K. Genome and metabolites analysis reveal insights into control of foodborne pathogens in fresh-cut fruits by Lactobacillus pentosus MS031 isolated from Chinese Sichuan Paocai. Postharvest Biol. Technol. 2020, 164, 111150. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei Group: History and Health Related Applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; Camargo, A.C.; Todorov, S.D.; Nero, L.A. Potential Control of Listeria monocytogenes by Bacteriocinogenic Enterococcus hirae ST57ACC and Pediococcus pentosaceus ST65ACC Strains Isolated from Artisanal Cheese. Probiotics Antimicrob. Proteins 2019, 11, 696–704. [Google Scholar] [CrossRef]
- Trias, R.; Badosa, E.; Montesinos, E.; Bañeras, L. Bioprotective Leuconostoc strains against Listeria monocytogenes in fresh fruits and vegetables. Int. J. Food Microbiol. 2008, 127, 91–98. [Google Scholar] [CrossRef]
- Dalgaard, P.; Vigel Jørgensen, L. Predicted and observed growth of Listeria monocytogenes in seafood challenge tests and in naturally contaminated cold-smoked salmon. Int. J. Food Microbiol. 1998, 40, 105–115. [Google Scholar] [CrossRef]
- Tahiri, I.; Desbiens, M.; Kheadr, E.; Lacroix, C.; Fliss, I. Comparison of different application strategies of divergicin M35 for inactivation of Listeria monocytogenes in cold-smoked wild salmon. Food Microbiol. 2009, 26, 783–793. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Naziębło, A. Paenibacillus as a Biocontrol Agent for Fungal Phytopathogens: Is P. polymyxa the Only One Worth Attention? Microb. Ecol. 2024, 87, 134. [Google Scholar] [CrossRef]
- Burton, J.P.; Wescombe, P.A.; Moore, C.J.; Chilcott, C.N.; Tagg, J.R. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl. Environ. Microbiol. 2006, 72, 3050–3053. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Scharlack, N.K.; Pena, F.d.L.; Antunes, A.E.C. Effects of Lacticaseibacillus rhamnosus GG supplementation, via food and non-food matrices, on children’s health promotion: A scoping review. Food Res. Int. 2022, 158, 111518. [Google Scholar] [CrossRef]
- Acar, B.C.; Yuksekdag, Z.; Yilmaz, E.S. Probiotic potential of three Enterococcus spp. isolated from raw milk: An in vitro assessment. Int. Dairy J. 2025, 164, 106196. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Chapter 3—Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 53–107. [Google Scholar]
- El-Sayed, H.S.; Salama, H.H.; Edris, A.E. Survival of Lactobacillus helveticus CNRZ32 in spray dried functional yogurt powder during processing and storage. J. Saudi Soc. Agric. Sci. 2020, 19, 461–467. [Google Scholar] [CrossRef]
- Teixeira, C.G.; da Silva, R.R.; Fusieger, A.; Martins, E.; de Freitas, R.; de Carvalho, A.F. The Weissella genus in the food industry: A review. Res. Soc. Dev. 2021, 10, e8310514557. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Bucur, F.I.; Mihalache, O.A.; Nicolau, A.I. Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products. Foods 2024, 13, 734. [Google Scholar] [CrossRef]
- Heir, E.; Liland, K.H.; Carlehög, M.; Holck, A.L. Reduction and inhibition of Listeria monocytogenes in cold-smoked salmon by Verdad N6, a buffered vinegar fermentate, and UV-C treatments. Int. J. Food Microbiol. 2019, 291, 48–58. [Google Scholar] [CrossRef]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Probiotics and its functionally valuable products—A review. Crit. Rev. Food. Sci. Nutr. 2013, 53, 641–658. [Google Scholar] [CrossRef] [PubMed]
- de Freire Bastos, M.d.C.; Coelho, M.L.V.; Da Silva Santos, O.C. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 2015, 161, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Bountra, K.; Hagelueken, G.; Choudhury, H.G.; Corradi, V.; El Omari, K.; Wagner, A.; Mathavan, I.; Zirah, S.; Yuan Wahlgren, W.; Tieleman, D.P.; et al. Structural basis for antibacterial peptide self-immunity by the bacterial ABC transporter McjD. EMBO J. 2017, 36, 3062–3079. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Strieker, M.; Tanović, A.; Marahiel, M.A. Nonribosomal peptide synthetases: Structures and dynamics. Curr. Opin. Struct. Biol. 2010, 20, 234–240. [Google Scholar] [CrossRef]
- Süssmuth, R.D.; Mainz, A. Nonribosomal Peptide Synthesis—Principles and Prospects. Angew. Chem. Int. Ed. 2017, 56, 3770–3821. [Google Scholar] [CrossRef]
- Dini, S.; Oz, F.; Bekhit, A.E.-D.A.; Carne, A.; Agyei, D. Production, characterization, and potential applications of lipopeptides in food systems: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13394. [Google Scholar] [CrossRef]
- Walsh, C.T. Polyketide and Nonribosomal Peptide Antibiotics: Modularity and Versatility. Science 2004, 303, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhang, W.; Zhang, B.; Navaz, S.; Wang, F.; Liao, Y. Biosynthesis and modification strategies of novel cyclic lipopeptide secreted by Bacillus spp.: Research progress. Process Biochem. 2025, 151, 27–42. [Google Scholar] [CrossRef]
- Schneider, T.; Müller, A.; Miess, H.; Gross, H. Cyclic lipopeptides as antibacterial agents—Potent antibiotic activity mediated by intriguing mode of actions. Int. J. Med. Microbiol. 2014, 304, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 2017, 8, 3389. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Alvarez Sieiro, P.; Montalban-Lopez, M.; Mu, D.; Kuipers, O. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Bierbaum, G.; Sahl, H.G. Lantibiotics: Mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18. [Google Scholar] [CrossRef]
- Meindl, K.; Schmiederer, T.; Schneider, K.; Reicke, A.; Butz, D.; Keller, S.; Gühring, H.; Vértesy, L.; Wink, J.; Hoffmann, H.; et al. Labyrinthopeptins: A New Class of Carbacyclic Lantibiotics. Angew. Chem. Int. Ed. 2010, 49, 1151–1154. [Google Scholar] [CrossRef]
- Lohans, C.T.; Vederas, J.C. Structural characterization of thioether-bridged bacteriocins. J. Antibiot. 2014, 67, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.E.; Patchett, M.L. The glycocins: In a class of their own. Curr. Opin. Struct. Biol. 2016, 40, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Sharma, P.; Dass, D.; Yarlagadda, V. Exploring the Darobactin Class of Antibiotics: A Comprehensive Review from Discovery to Recent Advancements. ACS Infect. Dis. 2024, 10, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—Ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Cheung-Lee, W.L.; Parry, M.E.; Zong, C.; Cartagena, A.J.; Darst, S.A.; Connell, N.D.; Russo, R.; Link, A.J. Discovery of Ubonodin, an Antimicrobial Lasso Peptide Active against Members of the Burkholderia cepacia Complex. ChemBioChem 2020, 21, 1335–1340. [Google Scholar] [CrossRef]
- Nissen-Meyer, J.; Oppegård, C.; Rogne, P.; Haugen, H.S.; Kristiansen, P.E. The Two-Peptide (Class-IIb) Bacteriocins: Genetics, Biosynthesis, Structure, and Mode of Action. In Prokaryotic Antimicrobial Peptides: From Genes to Applications; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011; pp. 197–212. [Google Scholar]
- Nissen-Meyer, J.; Rogne, P.; Oppegård, C.; Haugen, H.S.; Kristiansen, P.E. Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by gram-positive bacteria. Curr. Pharm. Biotechnol. 2009, 10, 19–37. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, X.; Wu, H.; Zou, Y.; Li, P.; Sun, C.; Xu, W.; Liu, F.; Wang, D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J. Ind. Microbiol. Biotechnol. 2018, 45, 213–227. [Google Scholar] [CrossRef]
- Príncipe, A.; Fernandez, M.; Torasso, M.; Godino, A.; Fischer, S. Effectiveness of tailocins produced by Pseudomonas fluorescens SF4c in controlling the bacterial-spot disease in tomatoes caused by Xanthomonas vesicatoria. Microbiol. Res. 2018, 212–213, 94–102. [Google Scholar] [CrossRef]
- Saraiva, M.A.F.; Birri, D.J.; Brede, D.A.; Baracat-Pereira, M.C.; de Queiroz, M.V.; Nes, I.F.; de Moraes, C.A. Nisin Z Production by Wild Strains of Lactococcus lactis Isolated from Brazilian (Italian Type) Fermented Sausage. Int. J. Microbiol. 2020, 2020, 9309628. [Google Scholar] [CrossRef]
- Müller, W.M.; Schmiederer, T.; Ensle, P.; Süssmuth, R.D. In vitro biosynthesis of the prepeptide of type-III lantibiotic labyrinthopeptin A2 including formation of a C-C bond as a post-translational modification. Angew. Chem. Int. Ed. 2010, 49, 2436–2440. [Google Scholar] [CrossRef]
- Babasaki, K.; Takao, T.; Shimonishi, Y.; Kurahashi, K. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: Isolation, structural analysis, and biogenesis. J. Biochem. 1985, 98, 585–603. [Google Scholar] [CrossRef]
- Kaunietis, A.; Buivydas, A.; Čitavičius, D.J.; Kuipers, O.P. Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nat. Commun. 2019, 10, 1115. [Google Scholar] [CrossRef]
- Wuisan, Z.G.; Kresna, I.D.M.; Böhringer, N.; Lewis, K.; Schäberle, T.F. Optimization of heterologous Darobactin A expression and identification of the minimal biosynthetic gene cluster. Metab. Eng. 2021, 66, 123–136. [Google Scholar] [CrossRef]
- van Heel, A.J.; Montalban-Lopez, M.; Oliveau, Q.; Kuipers, O.P. Genome-guided identification of novel head-to-tail cyclized antimicrobial peptides, exemplified by the discovery of pumilarin. Microb. Genom. 2017, 3, e000134. [Google Scholar] [CrossRef]
- Shimamura, H.; Gouda, H.; Nagai, K.; Hirose, T.; Ichioka, M.; Furuya, Y.; Kobayashi, Y.; Hirono, S.; Sunazuka, T.; Ōmura, S. Structure Determination and Total Synthesis of Bottromycin A2: A Potent Antibiotic against MRSA and VRE. Angew. Chem. Int. Ed. 2009, 48, 914–917. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Fliss, I.; Drider, D.; Lacroix, C. Pediocin PA-1 production during repeated-cycle batch culture of immobilized Pediococcus acidilactici UL5 cells. J. Biosci. Bioeng. 2008, 105, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Muriana, P.M.; Klaenhammer, T.R. Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl. Environ. Microbiol. 1991, 57, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Martin-Visscher, L.A.; Gong, X.; Duszyk, M.; Vederas, J.C. The three-dimensional structure of carnocyclin A reveals that many circular bacteriocins share a common structural motif. J. Biol. Chem. 2009, 284, 28674–28681. [Google Scholar] [CrossRef] [PubMed]
- García-Vela, S.; Guay, L.D.; Rahman, M.R.T.; Biron, E.; Torres, C.; Fliss, I. Antimicrobial Activity of Synthetic Enterocins A, B, P, SEK4, and L50, Alone and in Combinations, against Clostridium perfringens. Int. J. Mol. Sci. 2024, 25, 1597. [Google Scholar] [CrossRef]
- Joerger, M.C.; Klaenhammer, T.R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J. Bacteriol. 1986, 167, 439–446. [Google Scholar] [CrossRef]
- Curtis, G.D.W.; Mitchell, R.G. Bacteriocin (monocin) interactions among Listeria monocytogenes strains. Int. J. Food Microbiol. 1992, 16, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Götze, S.; Stallforth, P. Structure, properties, and biological functions of nonribosomal lipopeptides from pseudomonads. Nat. Prod. Rep. 2020, 37, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Biopolymers 2015, 104, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Qin, W.-Q.; Li, J.-Y.; Liu, Y.-F.; Zhou, L.; Yang, S.-Z.; Mu, B.-Z. Structural diversity and applications of lipopeptide biosurfactants as biocontrol agents against phytopathogens: A review. Microbiol. Res. 2024, 278, 127518. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Shan, M.; Zhao, H.; Lu, Z.; Lu, Y. A mini-review: Mechanism of antimicrobial action and application of surfactin. World J. Microbiol. Biotechnol. 2022, 38, 143. [Google Scholar] [CrossRef]
- Yaraguppi, D.A.; Bagewadi, Z.K.; Patil, N.R.; Mantri, N. Iturin: A Promising Cyclic Lipopeptide with Diverse Applications. Biomolecules 2023, 13, 1515. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef]
- Konz, D.; Doekel, S.; Marahiel, M.A. Molecular and biochemical characterization of the protein template controlling biosynthesis of the lipopeptide lichenysin. J. Bacteriol. 1999, 181, 133–140. [Google Scholar] [CrossRef]
- Swierstra, J.; Kapoerchan, V.; Knijnenburg, A.; van Belkum, A.; Overhand, M. Structure, toxicity and antibiotic activity of gramicidin S and derivatives. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 763–769. [Google Scholar] [CrossRef]
- Mootz, H.D.; Marahiel, M.A. The tyrocidine biosynthesis operon of Bacillus brevis: Complete nucleotide sequence and biochemical characterization of functional internal adenylation domains. J. Bacteriol. 1997, 179, 6843–6850. [Google Scholar] [CrossRef] [PubMed]
- Kwa, A.; Kasiakou, S.K.; Tam, V.H.; Falagas, M.E. Polymyxin B: Similarities to and differences from colistin (polymyxin E). Expert Rev. Anti Infect. Ther. 2007, 5, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Farver, D.K.; Hedge, D.D.; Lee, S.C. Ramoplanin: A Lipoglycodepsipeptide Antibiotic. Ann. Pharmacother. 2005, 39, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; Metzger, J.W.; Jung, G. The Tetracyclic Lantibiotic Actagardine 1H-NMR and 13C-NMR Assignments and Revised Primary Structure. Eur. J. Biochem. 1995, 228, 786–797. [Google Scholar] [CrossRef]
- Kajimura, Y.; Kaneda, M. Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Luhavaya, H.; Li, J.; Dahesh, S.; Nizet, V.; Yamanaka, K.; Moore, B.S. Isolation and structure elucidation of lipopeptide antibiotic taromycin B from the activated taromycin biosynthetic gene cluster. J. Antibiot. 2018, 71, 333–338. [Google Scholar] [CrossRef]
- Resa, S.; González, M.; Reyes, F.; Pérez-Victoria, I. Revision of the full stereochemistry of telomycin. Org. Chem. Front. 2024, 11, 306–314. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D. Bacteriocins from lactic acid bacteria as an alternative to antibiotics. Postep. Hig. Med. Dosw. 2017, 71, 328–338. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; de Melo, M.R.; da Silva, C.M.R.; Jain, S.; Dolabella, S.S. Nisin resistance in Gram-positive bacteria and approaches to circumvent resistance for successful therapeutic use. Crit. Rev. Microbiol. 2021, 47, 376–385. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Martínez, M.I.; Kok, J. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2002, 42, 91–121. [Google Scholar] [CrossRef] [PubMed]
- Diep, D.B.; Skaugen, M.; Salehian, Z.; Holo, H.; Nes, I.F. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. USA 2007, 104, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.; Wang, L.; Bhattacharjee, S.; Kaur, K. Structure−Activity Relationships of an Antimicrobial Peptide Plantaricin S from Two-Peptide Class IIb Bacteriocins. J. Med. Chem. 2011, 54, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, R.; Rios-Sánchez, R.M.; Desmazeaud, M.; Ruiz-Barba, J.L.; Piard, J.C. Plantaricins S and T, Two New Bacteriocins Produced by Lactobacillus plantarum LPCO10 Isolated from a Green Olive Fermentation. Appl. Environ. Microbiol. 1993, 59, 1416–1424. [Google Scholar] [CrossRef]
- Bédard, F.; Fliss, I.; Biron, E. Structure-Activity Relationships of the Bacteriocin Bactofencin A and Its Interaction with the Bacterial Membrane. ACS Infect. Dis. 2019, 5, 199–207. [Google Scholar] [CrossRef]
- Héchard, Y.; Sahl, H.G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 2002, 84, 545–557. [Google Scholar] [CrossRef]
- Janek, T.; Rodrigues, L.R.; Gudiña, E.J.; Czyżnikowska, Ż. Structure and mode of action of cyclic lipopeptide pseudofactin II with divalent metal ions. Colloids Surf. B Biointerfaces 2016, 146, 498–506. [Google Scholar] [CrossRef]
- Omardien, S.; Brul, S.; Zaat, S.A. Antimicrobial Activity of Cationic Antimicrobial Peptides against Gram-Positives: Current Progress Made in Understanding the Mode of Action and the Response of Bacteria. Front. Cell Dev. Biol. 2016, 4, 111. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, M.; Chen, M.; Luo, X.; Xing, J.; Zhang, X.; Li, C.; Liu, Y. FengycinA-M3 Inhibits Listeria monocytogenes by Binding to Penicillin-Binding Protein 2B Targets to Disrupt Cell Structure. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Fliss, O.; Guay, L.-D.; Fliss, I.; Biron, É. Synthesis and structure–activity study of the antimicrobial lipopeptide brevibacillin. RSC Med. Chem. 2024, 15, 4168–4179. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Spanjaard, L.; Vandenbroucke-Grauls, C.M. Activity of daptomycin against Listeria monocytogenes isolates from cerebrospinal fluid. Antimicrob. Agents Chemother. 2008, 52, 1850–1851. [Google Scholar] [CrossRef]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.-G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Abee, T.; Krockel, L.; Hill, C. Bacteriocins: Modes of action and potentials in food preservation and control of food poisoning. Int. J. Food Microbiol. 1995, 28, 169–185. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Acharya, A.; Hegde, V.; Prakash, B. Rational design of hyperstable antibacterial peptides for food preservation. npj Sci. Food 2021, 5, 26. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Li, P.; Qu, Y. Pentocin 31-1, a novel meat-borne bacteriocin and its application as biopreservative in chill-stored tray-packaged pork meat. Food Control 2010, 21, 198–202. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; O’Connor, P.M.; Ross, R.P.; Stanton, C.; Silva, C.C.G. An anti-listerial Lactococcus lactis strain isolated from Azorean Pico cheese produces lacticin 481. Int. Dent. J. 2016, 63, 18–28. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.; Li, Y.; Wang, X. Mode of action of leucocin K7 produced by Leuconostoc mesenteroides K7 against Listeria monocytogenes and its potential in milk preservation. Biotechnol. Lett. 2016, 38, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Guitián, M.V.; Ibarguren, C.; Soria, M.C.; Hovanyecz, P.; Banchio, C.; Audisio, M.C. Anti-Listeria monocytogenes effect of bacteriocin-incorporated agar edible coatings applied on cheese. Int. Dent. J. 2019, 97, 92–98. [Google Scholar] [CrossRef]
- Yildirim, Z.; Oncul, N.; Yıldırım, M.; Karabiyikli, S. Application of lactococcin BZ and enterocin KP against Listeria monocytogenes in milk as biopreservation agents. Acta Aliment. 2016, 45, 486–492. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef]
- Yang, X.; Yousef, A.E. Antimicrobial peptides produced by Brevibacillus spp.: Structure, classification and bioactivity: A mini review. World J. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, L.; Ding, J.; Wang, M.; Ge, R.; Zhao, H.; Zhang, B.; Fan, J. Natural antimicrobial lipopeptides secreted by Bacillus spp. and their application in food preservation, a critical review. Trends Food Sci. Technol. 2022, 127, 26–37. [Google Scholar] [CrossRef]
- Abhyankar, I.; Hirlekar, S.; Prabhune, A.; Nisal, A. Bridging the gap: An investigation of biosurfactants-polymer systems. Curr. Opin. Colloid Interface Sci. 2024, 72, 101806. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-based functional films for packaging applications: A review. Front. Nutri. 2022, 9, 1000116. [Google Scholar] [CrossRef]
- Adhikari, M.K.; Yadav, C.K.; Chaudhary, S.; Nath, D.; Bhattarai, A. An overview of novel biosurfactants and their potential for industrial applications from a biosurfactant perspective. Next Res. 2025, 2, 100305. [Google Scholar] [CrossRef]
- Krebs, B.; Höding, B.; Kübart, S.; Workie, M.; Junge, H.; Schmiedeknecht, G.; Grosch, R.; Bochow, H.; Hevesi, M. Use of Bacillus subtilis as Biocontrol Agent. I. Activities and Characterization of Bacillus subtilis Strains. J. Plant Dis. Prot. 1998, 105, 181–197. [Google Scholar]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Aljasir, S.F.; Gensler, C.; Sun, L.; D’Amico, D.J. The efficacy of individual and combined commercial protective cultures against Listeria monocytogenes, Salmonella, O157 and non-O157 shiga toxin-producing Escherichia coli in growth medium and raw milk. Food Control 2020, 109, 106924. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Afzia, N.; Ghosh, T.; Zhang, W. Recent progress in the antimicrobial and antioxidant peptide activated film/coatings for food packaging applications: A Review. Food Biosci. 2024, 62, 105288. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Fu, L.; Li, S.; Chen, Z.; Ouyang, J.; Shang, X.; Liu, Y.; Gao, L.; Wang, Y. Synergistic collaboration between AMPs and non-direct antimicrobial cationic peptides. Nat. Commun. 2024, 15, 7319. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Z.; Liang, Z.; Fu, X.; Li, D.; Zhu, C.; Kong, Q.; Mou, H. Current challenges and development strategies of bacteriocins produced by lactic acid bacteria applied in the food industry. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70038. [Google Scholar] [CrossRef]
- Fischer, S.W.; Titgemeyer, F. Protective Cultures in Food Products: From Science to Market. Foods 2023, 12, 1541. [Google Scholar] [CrossRef]
- Rossi, F.; Lathrop, A. Effects of Lactobacillus plantarum, Pediococcus acidilactici, and Pediococcus pentosaceus on the Growth of Listeria monocytogenes and Salmonella on Alfalfa Sprouts. J. Food Prot. 2019, 82, 522–527. [Google Scholar] [CrossRef]
- Castellano, P.; Aristoy, M.C.; Sentandreu, M.A.; Vignolo, G.; Toldrá, F. Lactobacillus sakei CRL1862 improves safety and protein hydrolysis in meat systems. J. Appl. Microbiol. 2012, 113, 1407–1416. [Google Scholar] [CrossRef]
- Pujato, S.A.; Mercanti, D.J.; Briggiler Marcó, M.; Capra, M.L.; Quiberoni, A.; Guglielmotti, D.M. Bacteriocins from lactic acid bacteria: Strategies for the bioprotection of dairy foods. Front. Food Sci. Technol. 2024, 4, 1439891. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-Strain Probiotics: Synergy among Isolates Enhances Biological Activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef]
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M.; Barba, F.J.; Sant’Ana, A.S. Combining reformulation, active packaging and non-thermal post-packaging decontamination technologies to increase the microbiological quality and safety of cooked ready-to-eat meat products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Connil, N.; Plissoneau, L.; Onno, B.; Pilet, M.-F.; Prévost, H.; Dousset, X. Growth of Carnobacterium divergens V41 and Production of Biogenic Amines and Divercin V41 in Sterile Cold-Smoked Salmon Extract at Varying Temperatures, NaCl Levels, and Glucose Concentrations. J. Food Prot. 2002, 65, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Connil, N.; Prévost, H.; Dousset, X. Production of biogenic amines and divercin V41 in cold smoked salmon inoculated with Carnobacterium divergens V41, and specific detection of this strain by multiplex-PCR. J. Appl. Microbiol. 2002, 92, 611–617. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Zhen, Z.; Wang, X.; Guo, N. Synergy of a combination of nisin and citric acid against Staphylococcus aureus and Listeria monocytogenes. Food Addit. Contam. 2017, 34, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, V.; Waheed, S.M.; Pradhan, D. Efficacy of Reuterin and Bacteriocins Nisin and Pediocin in the Preservation of Raw Milk from Dairy Farms. Food Technol. Biotechnol. 2020, 58, 359–369. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fliss, O.; Fliss, I.; Biron, E. Bioprotective Strategies to Control Listeria monocytogenes in Food Products and Processing Environments. Int. J. Mol. Sci. 2025, 26, 10481. https://doi.org/10.3390/ijms262110481
Fliss O, Fliss I, Biron E. Bioprotective Strategies to Control Listeria monocytogenes in Food Products and Processing Environments. International Journal of Molecular Sciences. 2025; 26(21):10481. https://doi.org/10.3390/ijms262110481
Chicago/Turabian StyleFliss, Omar, Ismail Fliss, and Eric Biron. 2025. "Bioprotective Strategies to Control Listeria monocytogenes in Food Products and Processing Environments" International Journal of Molecular Sciences 26, no. 21: 10481. https://doi.org/10.3390/ijms262110481
APA StyleFliss, O., Fliss, I., & Biron, E. (2025). Bioprotective Strategies to Control Listeria monocytogenes in Food Products and Processing Environments. International Journal of Molecular Sciences, 26(21), 10481. https://doi.org/10.3390/ijms262110481








