Urinary Uremic Toxin Signatures and the Metabolic Index of Gut Dysfunction (MIGD) in Autism Spectrum Disorder: A Stool-Phenotype-Stratified Analysis
Abstract
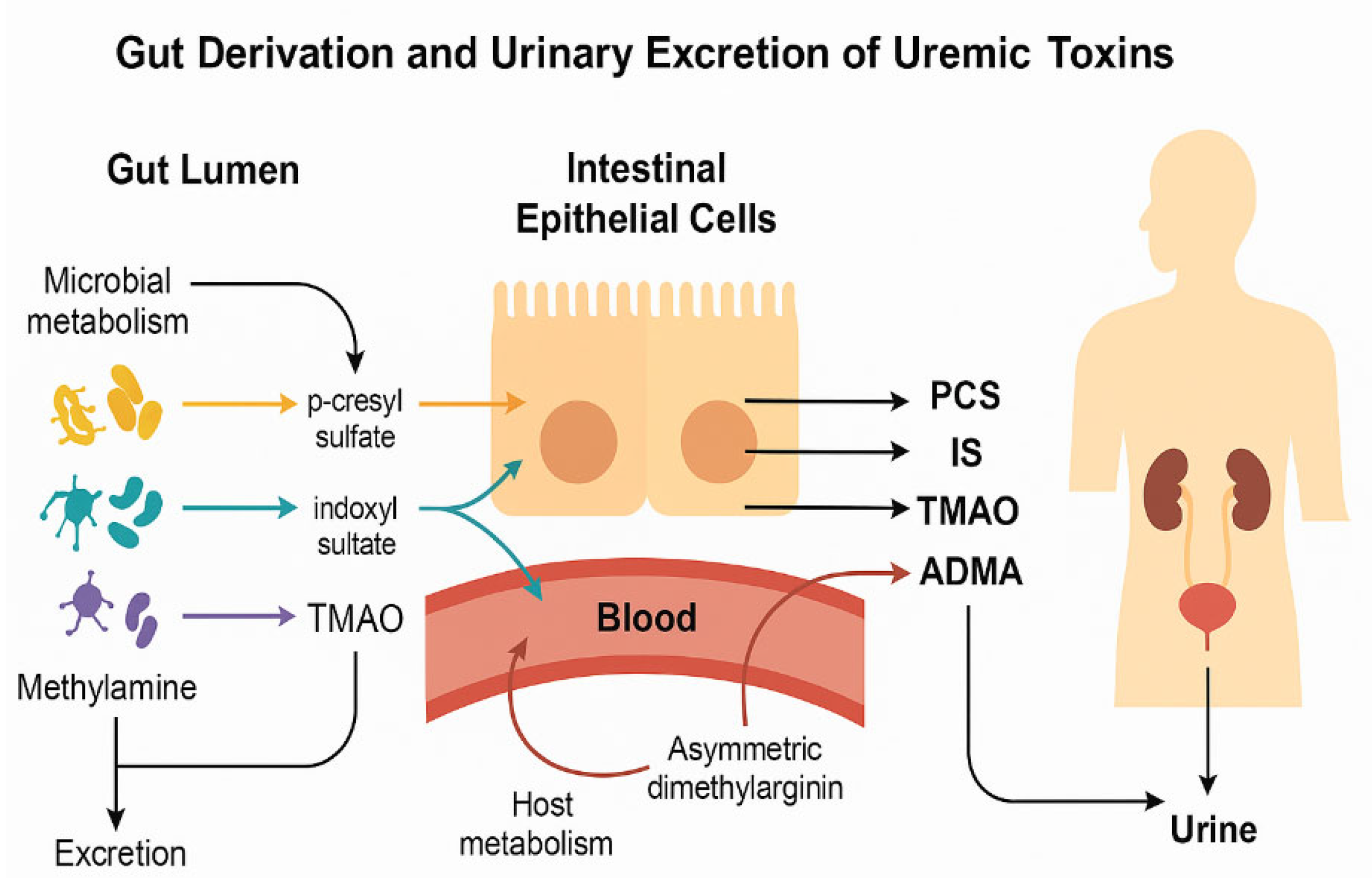
1. Introduction
2. Results
3. Discussion
3.1. Extended Applications of MIGD/OGI
3.2. Strengths
3.3. Limitations
4. Materials and Methods
4.1. Participants
4.2. Stool Assessment
4.3. Urine Collection and Normalization
4.4. Methods
4.4.1. Toxin Quantification
4.4.2. Quantification of TMAO, ADMA, and SDMA
4.4.3. Quantification of PCS and IS
4.4.4. Data Processing and Interpretation
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefter, R.; Ciobica, A.; Timofte, D.; Stanciu, C.; Trifan, A. A Descriptive Review on the Prevalence of Gastrointestinal Disturbances and Their Multiple Associations in Autism Spectrum Disorder. Med. Mex. 2019, 56, 11. [Google Scholar] [CrossRef]
- Warner, B.B. The Contribution of the Gut Microbiome to Neurodevelopment and Neuropsychiatric Disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut Microbiota-Derived Metabolites and Their Importance in Neurological Disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.I.; Evenepoel, P. The Gut-Kidney Axis: Indoxyl Sulfate, p-Cresyl Sulfate and CKD Progression. Nephrol. Dial. Transplant. 2011, 26, 759–761. [Google Scholar] [CrossRef]
- Garrido-Moreno, A.; García-Morales, V.J.; Lockett, N.; King, S. The Missing Link: Creating Value with Social Media Use in Hotels. Int. J. Hosp. Manag. 2018, 75, 94–104. [Google Scholar] [CrossRef]
- Cunha, R.S.D.; Santos, A.F.; Barreto, F.C.; Stinghen, A.E.M. How Do Uremic Toxins Affect the Endothelium? Toxins 2020, 12, 412. [Google Scholar] [CrossRef]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.C.F.M.J.; Noels, H. Impact of Uremic Toxins on Endothelial Dysfunction in Chronic Kidney Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef]
- Osredkar, J.; Baškovič, B.Ž.; Finderle, P.; Bobrowska-Korczak, B.; Gątarek, P.; Rosiak, A.; Giebułtowicz, J.; Vrhovšek, M.J.; Kałużna-Czaplińska, J. Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD. Int. J. Mol. Sci. 2023, 24, 7078. [Google Scholar] [CrossRef]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Velasquez, M.; Ramezani, A.; Manal, A.; Raj, D. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Guerra-Ojeda, S.; Suarez, A.; Valls, A.; Verdú, D.; Pereda, J.; Ortiz-Zapater, E.; Carretero, J.; Mauricio, M.D.; Serna, E. The Role of Aryl Hydrocarbon Receptor in the Endothelium: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 13537. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Yi, J.; Zhao, Y.; Zhang, F.; Shi, X.-T.; Feng, Z.; Miller, H.L. Plasma Trimethylamine N-Oxide, a Gut Microbe–Generated Phosphatidylcholine Metabolite, Is Associated with Autism Spectrum Disorders. NeuroToxicology 2020, 76, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Scalera, F.; Borlak, J.; Beckmann, B.; Martens-Lobenhoffer, J.; Thum, T.; Täger, M.; Bode-Böger, S.M. Endogenous Nitric Oxide Synthesis Inhibitor Asymmetric Dimethyl l-Arginine Accelerates Endothelial Cell Senescence. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1816–1822. [Google Scholar] [CrossRef]
- Masania, J.; Faustmann, G.; Anwar, A.; Hafner-Giessauf, H.; Rajpoot, N.; Grabher, J.; Rajpoot, K.; Tiran, B.; Obermayer-Pietsch, B.; Winklhofer-Roob, B.M.; et al. Urinary Metabolomic Markers of Protein Glycation, Oxidation, and Nitration in Early-Stage Decline in Metabolic, Vascular, and Renal Health. Oxid. Med. Cell. Longev. 2019, 2019, 4851323. [Google Scholar] [CrossRef]
- Persico, A.M.; Napolioni, V. Autism Genetics. Behav. Brain Res. 2013, 251, 95–112. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Sanz-Landaluce, J.; Pérez-Corona, M.T.; Madrid, Y. Fate and Effect of In-House Synthesized Tellurium Based Nanoparticles on Bacterial Biofilm Biomass and Architecture. Challenges for Nanoparticles Characterization in Living Systems. Sci. Total Environ. 2020, 719, 137501. [Google Scholar] [CrossRef]
- Evenepoel, P.; Meijers, B.K.I.; Bammens, B.R.M.; Verbeke, K. Uremic Toxins Originating from Colonic Microbial Metabolism. Kidney Int. 2009, 76, S12–S19. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric Dimethylarginine (ADMA): A Novel Risk Factor for Endothelial Dysfunction: Its Role in Hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- Swann, J.R.; Spitzer, S.O.; Diaz Heijtz, R. Developmental Signatures of Microbiota-Derived Metabolites in the Mouse Brain. Metabolites 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex Interactions Among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Leeming, E.R.; Dimidi, E.; Mazidi, M.; Franks, P.W.; Al Khatib, H.; Valdes, A.M.; Davies, R.; Bakker, E.; Francis, L.; et al. Blue Poo: Impact of Gut Transit Time on the Gut Microbiome Using a Novel Marker. Gut 2021, 70, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool Consistency Is Strongly Associated with Gut Microbiota Richness and Composition, Enterotypes and Bacterial Growth Rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef]
- Vork, L.; Penders, J.; Jalanka, J.; Bojic, S.; Van Kuijk, S.M.J.; Salonen, A.; De Vos, W.M.; Rajilic-Stojanovic, M.; Weerts, Z.Z.R.M.; Masclee, A.A.M.; et al. Does Day-to-Day Variability in Stool Consistency Link to the Fecal Microbiota Composition? Front. Cell. Infect. Microbiol. 2021, 11, 639667. [Google Scholar] [CrossRef]
- Frye, R.E.; James, S.J. Metabolic Pathology of Autism in Relation to Redox Metabolism. Biomark. Med. 2014, 8, 321–330. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Prince, N.; Bajic, D.; Roussin, L.; Naudon, L.; Rabot, S.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci. 2021, 22, 10052. [Google Scholar] [CrossRef]
- Wang, L.; Conlon, M.A.; Christophersen, C.T.; Sorich, M.J.; Angley, M.T. Gastrointestinal Microbiota and Metabolite Biomarkers in Children with Autism Spectrum Disorders. Biomark. Med. 2014, 8, 331–344. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. P-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Gupta, N.; Buffa, J.A.; Roberts, A.B.; Sangwan, N.; Skye, S.M.; Li, L.; Ho, K.J.; Varga, J.; DiDonato, J.A.; Tang, W.H.W.; et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1239–1255. [Google Scholar] [CrossRef]
- Lano, G.; Burtey, S.; Sallée, M. Indoxyl Sulfate, a Uremic Endotheliotoxin. Toxins 2020, 12, 229. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takayanagi, K.; Kojima, M.; Katome, T.; Taguchi, K.; Kobayashi, T. Direct Impairment of the Endothelial Function by Acute Indoxyl Sulfate through Declined Nitric Oxide and Not Endothelium-Derived Hyperpolarizing Factor or Vasodilator Prostaglandins in the Rat Superior Mesenteric Artery. Biol. Pharm. Bull. 2019, 42, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bi, W.-D.; Shi, Y.-X.; Liang, X.-R.; Wang, H.-Y.; Lai, X.-L.; Bian, X.-L.; Guo, Z.-Y. Derivation and Elimination of Uremic Toxins from Kidney-Gut Axis. Front. Physiol. 2023, 14, 1123182. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Lewis, J.; Cooper, D.; D’Amato, M.; Lim, J.; Gupta, S.; Miranda, A.; Terry, N.; Mehta, D.; Scheimann, A.; et al. Hypomorphic SI Genetic Variants Are Associated with Childhood Chronic Loose Stools. PLoS ONE 2020, 15, e0231891. [Google Scholar] [CrossRef]
- Brydges, C.R.; Bhattacharyya, S.; Dehkordi, S.M.; Milaneschi, Y.; Penninx, B.; Jansen, R.; Kristal, B.S.; Han, X.; Arnold, M.; Kastenmüller, G.; et al. Metabolomic and Inflammatory Signatures of Symptom Dimensions in Major Depression. Brain. Behav. Immun. 2022, 102, 42–52. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 2013; ISBN 978-1-134-74270-7. [Google Scholar]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; De Caro, C.; Comegna, M.; et al. Gut Microbiota Features in Young Children with Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146. [Google Scholar] [CrossRef]
- Korteniemi, J.; Karlsson, L.; Aatsinki, A. Systematic Review: Autism Spectrum Disorder and the Gut Microbiota. Acta Psychiatr. Scand. 2023, 148, 242–254. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Gómez-Fernández, A.; Chueca, N.; Torre-Aguilar, M.J.D.L.; Gil, Á.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression Is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Salpeter, S.R.; Bode-Boeger, S.M.; Cooke, J.P.; Fliser, D. Symmetric Dimethylarginine (SDMA) as Endogenous Marker of Renal Function—A Meta-Analysis. Nephrol. Dial. Transplant. 2006, 21, 2446–2451. [Google Scholar] [CrossRef]
- Sargent, H.J.; Elliott, J.; Jepson, R.E. The New Age of Renal Biomarkers: Does SDMA Solve All of Our Problems? J. Small Anim. Pract. 2021, 62, 71–81. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- European Medicines Agency Guideline on Quality. Non-Clinical and Clinical Requirements for Investigational Advanced Therapy Medicinal Products in Clinical Trials; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Heinzen, E.; Sinnwell, J.; Atkinson, E.; Gunderson, T.; Dougherty, G. Arsenal: An Arsenal of “R” Functions for Large-Scale Statistical Summaries, 3.6.3, 2016. Available online: https://github.com/mayoverse/arsenal (accessed on 25 October 2025).
| Control (N = 71) | ASD (All) (N = 97) | p Value | 1 (BSC 1,2) (N = 28) | 1 (BSC 6,7) (N = 8) | 1 (BSC 3,4,5 (N = 61) | p Value | p TOT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 0:1 | 2 | 3 | 4 | 0:2 | 0:3 | 0:4 | ||
| ADMA | 0.48 | 0.68 | 0.28 | 0.25 | 0.356 | |||||
| Median (Q1, Q3) | 12.70 (9.73, 18.74) | 14.76 (10.50, 17.03) | 14.79 (10.69, 16.95) | 8.02 (7.57, 15.15) | 15.09 (11.32, 19.54) | |||||
| SDMA | 0.62 | 0.76 | 0.48 | 0.88 | 0.573 | |||||
| Median (Q1, Q3) | 31.32 (23.01, 38.09) | 29.06 (20.10, 39.77) | 29.09 (20.17, 36.91) | 19.73 (14.64, 50.79) | 29.08 (23.16, 44.29) | |||||
| TMAO | 0.51 | 0.11 | 0.29 | 0.76 | 0.115 | |||||
| Median (Q1, Q3) | 3.09 (1.48, 5.19) | 2.64 (1.64, 5.01) | 2.38 (1.69, 3.52) | 2.60 (0.04, 3.81) | 3.19 (1.67, 5.53) | |||||
| IS | 0.25 | 0.43 | 0.91 | 0.26 | 0.869 | |||||
| Median (Q1, Q3) | 63.80 (39.77, 103.18) | 56.77 (28.22, 89.25) | 52.26 (41.88, 79.10) | 89.25 (23.98, 108.77) | 61.52 (27.95, 89.20) | |||||
| PCS | 0.53 | 0.20 | 0.92 | 0.87 | 0.705 | |||||
| Median (Q1, Q3) | 37.74 (20.02, 80.02) | 51.84 (17.07, 86.81) | 46.65 (29.01, 86.73) | 31.85 (9.09, 140.45) | 52.65 (15.80, 84.76) | |||||
| Control (N = 71) | ASD (All) (N = 97) | p Value | 1 (BSC 1,2) (N = 28) | 1 (BSC 6,7) (N = 8) | 1 (BSC 3,4,5) (N = 61) | p Value | p TOT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | ASD | 0:ASD | 1 | 2 | 3 | 0:1 | 0:2 | 0:3 | ||
| MIGD | 0.20 | 0.49 | 0.10 | 0.08 | 0.088 | |||||
| Median (Q1, Q3) | 316.05 (144.24, 475.01) | 342.33 (163.65, 752.00) | 342.33 (172.99, 854.95) | 140.02 (104.96, 271.75) | 374.49 (194.61, 732.45) | |||||
| Group | PCS/TMAO | IS/ADMA | MIGD |
|---|---|---|---|
| Controls (BSC 3–5) | 12.4 | 5.2 | 238.5 |
| ASD (BSC 3–5) | 16.5 | 4.1 | 402.4 |
| ASD (BSC 6–7) | 12.3 | 11.1 | 110.8 |
| ASD (BSC 1–2) | 19.6 | 3.5 | 560.0 |
| Control (N = 71) | 1 (All) (N = 97) | 1 (BSC 1,2) (N = 28) | 1 (BSC 6,7) (N = 8) | 1 (BSC 3,4,5) (N = 61) | |
|---|---|---|---|---|---|
| SEX | |||||
| Boys | 37 (52.1%) | 76 (78.4%) | 17 (60.7%) | 5 (62.5%) | 54 (88.5%) |
| Girls | 34 (47.9%) | 21 (21.6%) | 11 (39.3%) | 3 (37.5%) | 7 (11.5%) |
| AGE (years) | |||||
| Mean (SD) | 8.93 (3.82) | 9.44 (3.77) | 9.76 (4.21) | 9.88 (3.15) | 9.24 (3.68) |
| Median (Q1, Q3) | 8.60 (6.20, 11.25) | 8.70 (6.20, 12.40) | 9.05 (6.18, 13.03) | 10.40 (8.05, 12.55) | 8.70 (6.20, 11.30) |
| Min–Max | 2.40–16.70 | 2.50–17.00 | 3.50–17.00 | 4.60–13.20 | 2.50–16.70 |
| MIGD Value Range | Interpretation |
|---|---|
| <50 | Low metabolic disruption or compensatory indole pathway activity |
| 50–150 | Mild to moderate metabolic imbalance |
| 150–300 | Marked microbial–host metabolic disturbance |
| >300 | High dysfunction—skewed fermentation and impaired detoxification |
| >500 | Severe imbalance—indicates high systemic fermentation burden |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osredkar, J.; Fabjan, T.; Kumer, K.; Jekovec-Vrhovšek, M.; Giebułtowicz, J.; Bobrowska-Korczak, B.; Avguštin, G.; Godnov, U. Urinary Uremic Toxin Signatures and the Metabolic Index of Gut Dysfunction (MIGD) in Autism Spectrum Disorder: A Stool-Phenotype-Stratified Analysis. Int. J. Mol. Sci. 2025, 26, 10475. https://doi.org/10.3390/ijms262110475
Osredkar J, Fabjan T, Kumer K, Jekovec-Vrhovšek M, Giebułtowicz J, Bobrowska-Korczak B, Avguštin G, Godnov U. Urinary Uremic Toxin Signatures and the Metabolic Index of Gut Dysfunction (MIGD) in Autism Spectrum Disorder: A Stool-Phenotype-Stratified Analysis. International Journal of Molecular Sciences. 2025; 26(21):10475. https://doi.org/10.3390/ijms262110475
Chicago/Turabian StyleOsredkar, Joško, Teja Fabjan, Kristina Kumer, Maja Jekovec-Vrhovšek, Joanna Giebułtowicz, Barbara Bobrowska-Korczak, Gorazd Avguštin, and Uroš Godnov. 2025. "Urinary Uremic Toxin Signatures and the Metabolic Index of Gut Dysfunction (MIGD) in Autism Spectrum Disorder: A Stool-Phenotype-Stratified Analysis" International Journal of Molecular Sciences 26, no. 21: 10475. https://doi.org/10.3390/ijms262110475
APA StyleOsredkar, J., Fabjan, T., Kumer, K., Jekovec-Vrhovšek, M., Giebułtowicz, J., Bobrowska-Korczak, B., Avguštin, G., & Godnov, U. (2025). Urinary Uremic Toxin Signatures and the Metabolic Index of Gut Dysfunction (MIGD) in Autism Spectrum Disorder: A Stool-Phenotype-Stratified Analysis. International Journal of Molecular Sciences, 26(21), 10475. https://doi.org/10.3390/ijms262110475






