An Update on Dynamic Changes in Cytokine Expression and Dysbiosis Due to Radiation Combined Injury
Abstract
1. Introduction
2. Whole-Body Radiation Combined with Physical Trauma
2.1. Radiation Combined with Hemorrhage
2.1.1. Whole-Body Radiation Combined with Hemorrhage
2.1.2. Whole-Body Radiation Combined with Extremity Trauma and Hemorrhage
2.2. Whole-Body Radiation Combined with Skin Wound
2.3. Whole-Body Radiation Combined with Skin Burning
2.4. Whole-Body Radiation Combined with Bacterial Exposure
2.4.1. In Vivo Studies
2.4.2. In Vitro Studies
3. Impacts of Radiation Combined with Skin Wounding on the Microbiome
4. Gut Microbiota Links to Other Organs
5. A Biomolecule Panel for Estimation of Radiation Dose
6. Drugs for Treating Radiation Combined Injury
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFRRI | Armed Forces Radiobiology Research Institute |
| AKT | Serine/threonine-specific protein kinase |
| ARS | Acute radiation syndrome |
| Bcl-2 | B-cell lymphoma-2 |
| C3 | Complement protein 3 |
| CBC | Complete blood count |
| Cdh6 | Cadherin 6 |
| RCI | Radiation combined injury |
| CL | Confidence limits |
| CNS | Central nervous system |
| CRP | C-reactive protein |
| Flt-3 | FMS-like tyrosine kinase 3 |
| FMT | Fecal microbiota transplant |
| G-CSF | Granulocyte colony-stimulating factor |
| H-ARS | Hematopoiesis-acute radiation syndrome |
| Hemo | Hemorrhage |
| HJF | Henry Jackson Foundation |
| IAA | Inter-agency agreement |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IR | Ionizing irradiation |
| Itga7 | Integrin alpha-7 |
| KC | Keratinocyte chemoattractant |
| LC3 | Light chain 3 |
| MAPK | Mitogen-activated protein kinase |
| MCP | Monocyte chemoattractant protein |
| MIG | Monokine induced gamma interferon |
| MIP | Macrophage inflammatory protein |
| mmp | Matrix metallopeptidase |
| Mre11 | Meiotic recombination 11 |
| Myd88 | Myeloid differentiation primary response 88 |
| NO | Nitric oxide |
| NF-1L6 | Nuclear factor interleukin 6 |
| NF-κB p50 | Nuclear factor-keppaB protein 50 kDa |
| NF-κB p65 | Nuclear factor-keppaB protein 65 kDa |
| NIH | National Institute of Health |
| NIAID | National Institute of Allergy and Infectious Diseases |
| p62/SQSM1 | Protein 62 kda/sequestosome-1 |
| PDGF-bb | Platelet-derived growth factor subunit BB |
| RAD | Radiation |
| RBC | Red blood cell |
| RI | Radiation injury |
| RCI | Radiation combined injury |
| Sirt | Sirtuin |
| SUMO1 | Small ubiquitin-related modifier 1 |
| Timp | Metalloproteinase inhibitor |
| TGF | Tumor growth factor |
| TJ | Tight junction |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| USUHS | Uniformed Services University of the Health Sciences |
| WBC | White blood cell |
References
- Kiang, J.G.; Blakely, W.F. Combined Radiation Injury and Its Impacts on Radiation Countermeasures and Biodosimetry. Int. J. Radiat. Biol. 2023, 99, 1055–1065. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Ramakrishnan, N.; Hatchett, R.J. Radiation Combined Injury: Overview of NIAID Research. Health Phys. 2010, 98, 863–867. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Bandremer, A.C.; Hollingsworth, B.A.; Kasim, S.; Laniyonu, A.; Todd, N.F.; Wang, S.-J.; Wertheimer, E.R.; Rios, C.I. Cutaneous Radiation Injuries: Models, Assessment and Treatments. Radiat. Res. 2020, 194, 315–344. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Olabisi, A.O. Radiation: A Poly-Traumatic Hit Leading to Multi-Organ Injury. Cell Biosci. 2019, 9, 25. [Google Scholar] [CrossRef]
- Ledney, G.D.; Exum, E.D.; Sheehy, P.A. Survival Enhanced by Skin-Wound Trauma in Mice Exposed to 60CO Radiation. Experientia 1981, 37, 193–194. [Google Scholar] [CrossRef]
- Ledney, G.D.; Exum, E.D.; Jackson, W.E. Wound-Induced Alterations in Survival of 60CO Irradiated Mice: Importance of Wound Timing. Experientia 1985, 41, 614–616. [Google Scholar] [CrossRef]
- Ledney, G.D.; Elliott, T.B.; Moore, M.M. Modulations of Mortality by Tissue Trauma and Sepsis in Mice after Radiation Injury. In Biological Basis of Radiation Protection Practice; Mossman, K.I., Mills, W.A., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1992; pp. 202–217. [Google Scholar]
- Kiang, J.G.; Jiao, W.; Cary, L.H.; Mog, S.R.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. Wound Trauma Increases Radiation-Induced Mortality by Activation of Inos Pathway and Elevation of Cytokine Concentrations and Bacterial Infection. Radiat. Res. 2010, 173, 319–332. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, D.; Jiao, Y.; Ross, J.R.; Corbin, K.; Xiao, Q.; Toncheva, G.; Anderson-Evans, C.; Yoshizumi, T.T.; Chen, B.J.; Chao, N.J. An Ear Punch Model for Studying the Effect of Radiation on Wound Healing. Int. J. Radiat. Biol. 2011, 87, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Ledney, G.D.; Gelston, H.M.; Weinberg, S.R.; Exum, E.D. Survival and Endogenous Spleen Colonies of Irradiated Mice after Skin Wounding and Hydroxyurea Treatment. Experientia 1982, 38, 1228–1230. [Google Scholar] [CrossRef]
- Elliott, T.B.; Bolduc, D.L.; Ledney, G.D.; Kiang, J.G.; Fatanmi, O.O.; Wise, S.Y.; Romaine, P.L.P.; Newman, V.L.; Singh, V.K. Combined Immunomodulator and Antimicrobial Therapy Eliminates Polymicrobial Sepsis and Modulates Cytokine Production in Combined Injured Mice. Int. J. Radiat. Biol. 2015, 91, 690–702. [Google Scholar] [CrossRef]
- Kiang, J.G.; Ledney, G.D. Skin Injuries Reduce Survival and Modulate Corticosterone, C-Reactive Protein, Complement Component 3, Igm, and Prostaglandin E 2 after Whole-Body Reactor-Produced Mixed Field (N + Gamma-Photons) Irradiation. Oxid. Med. Cell. Longev. 2013, 2013, 821541. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-M.; Qu, J.-F.; Cheng, T.-M. Effects of the Nerve Growth Factor on the Survival and Wound Healing in Mice with Combined Radiation and Wound Injury. J. Radiat. Res. 2003, 44, 223–228. [Google Scholar] [CrossRef]
- Alpen, E.L.; Sheline, G.E.M. The Combined Effects of Thermal Burns and Whole Body X Irradiation on Survival Time and Mortality. Ann. Surg. 1954, 140, 113–118. [Google Scholar] [CrossRef]
- Jacob, A.; Shah, K.G.; Wu, R.; Wang, P. Ghrelin as a Novel Therapy for Radiation Combined Injury. Mol. Med. 2010, 16, 137–143. [Google Scholar] [CrossRef]
- Shah, K.G.; Wu, R.; Jacob, A.; Blau, S.A.; Ji, Y.; Dong, W.; Marini, C.P.; Ravikumar, T.S.; Coppa, G.F.; Wang, P. Human Ghrelin Ameliorates Organ Injury and Improves Survival after Radiation Injury Combined with Severe Sepsis. Mol. Med. 2009, 15, 407–414. [Google Scholar] [CrossRef]
- Valeriote, F.A.; Baker, D.G. The Combined Effects of Thermal Trauma and X-Irradiation on Early Mortality. Radiat. Res. 1964, 22, 693–702. [Google Scholar] [CrossRef]
- Dhingra, N.; Gupta, V.; Tyagi, A.; Agrawala, P.K.; Gupta, V. Trichostatin a Ameliorated Combined Radiation and Skin Wound Injury-Induced Mortality and Hematopoietic Suppression in a Rat Model. Int. J. Radiat. Biol. 2025, 101, 952–972. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, D.; Cui, C.; Gao, Y.; Xia, G.; Cui, X. Effects of Radiation on Wound Healing. J. Environ. Pathol. Toxicol. Oncol. 1998, 17, 117–123. [Google Scholar] [PubMed]
- Qu, J.; Cheng, T.; Shi, C.; Lin, Y.; Yan, G.; Ran, X. Reduced Presence of Tissue-Repairing Cells in Wounds Combined with Whole-Body Irradiation Injury Is Associated with Both Suppression of Proliferation and Increased Apoptosis. Med. Sci. Monit. 2003, 9, BR370-7. [Google Scholar]
- Dantzer, D.; Ferguson, P.; Hill, R.P.; Keating, A.; Kandel, R.A.; Wunder, J.S.; O’SUllivan, B.; Sandhu, J.; Waddell, J.; Bell, R.S. Effect of Radiation and Cell Implantation on Wound Healing in a Rat Model. J. Surg. Oncol. 2003, 83, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, M.; Weimer, W.; Wider, S.; Stülten, C.; Bongartz, M.; Budach, W.; Becker, H.-D. Differential Expression of Inflammatory Mediators in Radiation-Impaired Wound Healing. J. Surg. Res. 2002, 107, 93–100. [Google Scholar] [CrossRef]
- Ran, X.; Cheng, T.; Shi, C.; Xu, H.; Qu, J.; Yan, G.; Su, Y.; Wang, W.; Xu, R. The Effects of Total-Body Irradiation on the Survival and Skin Wound Healing of Rats with Combined Radiation-Wound Injury. J. Trauma. 2004, 57, 1087–1093. [Google Scholar] [CrossRef]
- Korlof, B. Infection of Burns. I. A Bacteriological and Clinical Study of 99 Cases. In. Animal Experiments; Burns and Total Body X-Irradiation. Acta Chir. Scand. Suppl. 1956, 209, 1–144. [Google Scholar]
- Brooks, J.W.; Evans, E.I.; Ham, W.T., Jr.; Reid, J.D. The Influence of External Body Radiation on Mortality from Thermal Burns. Ann. Surg. 1952, 136, 533–545. [Google Scholar]
- Reid, J.D.; JBrooks, W.; Ham, W.T.; Evans, E.I. The Influence of X-Radiation on Mortality Following Thermal Flash Burns: The Site of Tissue Injury as a Factor Determining the Type of Invading Bacteria. Ann. Surg. 1955, 142, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Garrison, B.R.; Burns, T.M.; Zhai, M.; Dews, I.C.; Ney, P.H.; Cary, L.H.; Fukumoto, R.; Elliott, T.B.; Ledney, G.D. Wound Trauma Alters Ionizing Radiation Dose Assessment. Cell Biosci. 2012, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, X.; Wang, L.; Lin, B.; Zhai, M.; Hull, L.; Zizzo, A.; Cui, W.; Kiang, J.G. Skin Wound Following Irradiation Aggravates Radiation-Induced Brain Injury in a Mouse Model. Int. J. Mol. Sci. 2023, 24, 16. [Google Scholar] [CrossRef]
- Kiang, J.G.; Cannon, G.; Olson, M.G.; Zhai, M.; Woods, A.K.; Xu, F.; Lin, B.; Li, X.; Hull, L.; Jiang, S.; et al. Ciprofloxacin and Pegylated G-CSF Combined Therapy Mitigates Brain Hemorrhage and Mortality Induced by Ionizing Irradiation. Front. Public Health 2023, 11, 1268325. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Woods, A.K.; Cannon, G. Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers. Int. J. Mol. Sci. 2024, 25, 2988. [Google Scholar] [CrossRef]
- Lipiec, S.M.; Torres, L.N.; Ryan, K.L.; Walters, T.J.; Klemcke, H.G.; Xiang, L. A Combat-Relevant Model of Traumatic Hemorrhage in Rats for the Study of Combined Radiation Injury: A Pilot Study. Radiat. Res. 2025, 204, 253–258. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Swift, J.M.; Christensen, C.L.; Gupta, P.; Balakathiresan, N.; Maheshwari, R.K. Hemorrhage Exacerbates Radiation Effects on Survival, Leukocytopenia, Thrombopenia, Erythropenia, Bone Marrow Cell Depletion and Hematopoiesis, and Inflammation-Associated MicroRNAs Expression in Kidney. PLoS ONE 2015, 10, e0139271. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Yacoub, A.; Contessa, J.; Caron, R.; Amorino, G.; Valerie, K.; Hagan, M.P.; Grant, S.; Schmidt-Ullrich, R. Stress and Radiation-Induced Activation of Multiple Intracellular Signaling Pathways. Radiat. Res. 2003, 159, 283–300. [Google Scholar] [CrossRef]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. MAPK Pathways in Radiation Responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef]
- Ledney, G.D.; Exum, E.D.; Stewart, D.A.; Gelston, H.M., Jr.; Weinberg, S.R. Survival and Hematopoietic Recovery in Mice after Wound Trauma and Whole Body -Body Irradiation. Exp. Hematol 1982, 10, 263–278. [Google Scholar]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Umali, M.V.; Ho, C.; Zhai, M.; Lin, B.; Jiang, S. A Novel Therapy, Using Ghrelin with Pegylated G-CSF, Inhibits Brain Hemorrhage from Ionizing Radiation or Combined Radiation Injury. Pharm. Pharmacol. Int. J. 2019, 7, 133–145. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Kiang, J.G. Ghrelin Therapy Decreases Incidents of Intracranial Hemorrhage in Mice after Whole-Body Ionizing Irradiation Combined with Burn Trauma. Int. J. Mol. Sci. 2017, 18, 1693. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced Pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q. Toll-Like Receptor 4: A Promising Therapeutic Target for Pneumonia Caused by Gram-Negative Bacteria. J. Cell. Mol. Med. 2019, 23, 5868–5875. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.K.; Kirti; Kalonia, A.; Shaw, P.; Yashvarddhan, M.H.; Vibhuti, A.; Shukla, S.K. Understanding Innate and Adaptive Responses during Radiation Combined Burn Injuries. Int. Rev. Immunol. 2025, 44, 31–43. [Google Scholar] [CrossRef]
- Kiang, J.G.; Cannon, G.; Zhai, M.; Olson, M.G.; Woods, A.K.; Cleveland, K.S.; Ellery, H.; Xu, F.; Xiao, M. A Combined Therapy of Pegylated G-CSF with Ciprofloxacin Mitigates Damage Induced by Lethal Ionizing Radiation to the Bone Marrow, Spleen, and Ileum by Increasing Akt Activation but Decreasing IL-18, C3, and MiR-34a. Radiat. Res. 2025, 203, 341–356. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Perrucci, F.; Morini, R.; Erreni, M.; Mahoney, M.; Witkowska, A.; Carey, A.; Faggiani, E.; Schuetz, L.T.; Mason, S.; et al. Local Externalization of Phosphatidylserine Mediates Developmental Synaptic Pruning by Microglia. EMBO J. 2020, 39, e105380. [Google Scholar] [CrossRef]
- Shi, Q.; Chowdhury, S.; Ma, R.; Le, K.X.; Hong, S.; Caldarone, B.J.; Stevens, B.; Lemere, C.A. Complement C3 Deficiency Protects against Neurodegeneration in Aged Plaque-Rich App/Ps1 Mice. Sci. Transl. Med. 2017, 9, eaaf6295. [Google Scholar]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Deng, R.; Tang, J.; Ma, J.-G.; Chen, S.-P.; Xia, L.-P.; Zhou, W.-J.; Li, D.-D.; Feng, G.-K.; Zeng, Y.-X.; Zhu, X.-F. Pkb/Akt Promotes Dsb Repair in Cancer Cells through Upregulating Mre11 Expression Following Ionizing Radiation. Oncogene 2011, 30, 944–955. [Google Scholar]
- Deng, Z.; Huang, H.; Wu, X.; Wu, M.; He, G.; Guo, J. Distinct Expression of Various Angiogenesis Factors in Mice Brain after Whole-Brain Irradiation by X-Ray. Neurochem. Res. 2017, 42, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.E.; Neely, C.J.; Charles, A.G.; Kartchner, L.B.; Brickey, W.J.; Khoury, A.L.; Sempowski, G.D.; Ting, J.P.Y.; Cairns, B.A.; Maile, R. Radiation Combined with Thermal Injury Induces Immature Myeloid Cells. Shock 2012, 38, 532–542. [Google Scholar] [CrossRef]
- Ran, X.Z.; Su, Y.P.; Zong, Z.W.; Guo, C.H.; Zheng, H.E.; Chen, X.H.; Ai, G.P.; Cheng, T.M. Effects of Serum from Rats with Combined Radiation-Burn Injury on the Growth of Hematopoietic Progenitor Cells. J. Trauma. 2007, 62, 193–198. [Google Scholar] [PubMed]
- Budagov, R.S.; Ul’ianova, L.P. Comparative Analysis of Proinflammatory Cytokines in Plasma of Mice Exposed to Radiation or in Combined Radiation Injury. Radiats Biol. Radioecol. 2000, 40, 188–191. [Google Scholar] [PubMed]
- Budagov, R.S.; Ul’ianova, L.P. Cytokine Production by Different Population of Macrophages Following Radiation or Combined Radiation Injury. Radiats Biol. Radioecol. 2000, 40, 684–687. [Google Scholar]
- Budagov, R.S.; Ul’ianova, L.P. Some Consequences of Systemic Inflammatory Response in the Pathogenesis of Aggravation of Outcomes of Combined Radiation and Thermal Injuries. Radiats Biol. Radioecol. 2005, 45, 191–195. [Google Scholar]
- Brook, I.; Elliott, T.B.; Ledney, G.D. Quinolone Therapy of Klebsiella Pneumoniae Sepsis Following Irradiation: Comparison of Pefloxacin, Ciprofloxacin, and Ofloxacin. Radiat. Res. 1990, 122, 215–217. [Google Scholar] [CrossRef]
- Brook, I.; Ledney, G.D. Short and Long Courses of Ofloxacin Therapy of Klebsiella pneumoniae Sepsis Following Irradiation. Radiat. Res. 1992, 130, 61–64. [Google Scholar] [CrossRef]
- Brook, I.; Ledney, G.D. Oral Ofloxacin Therapy of Pseudomonas aeruginosa Sepsis in Mice after Irradiation. Antimicrob. Agents Chemother. 1990, 34, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Elliott, T.B.; Harding, R.A.; Bouhaouala, S.S.; Peacock, S.J.; Ledney, G.D.; Knudson, G.B. Susceptibility of Irradiated Mice to Bacillus anthracis Sterne by the Intratracheal Route of Infection. J. Med. Microbiol. 2001, 50, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Belay, T.; Aviles, H.; Vance, M.; Fountain, K.; Sonnenfeld, G. Effects of the Hindlimb-Unloading Model of Spaceflight Conditions on Resistance of Mice to Infection with Klebsiella pneumoniae. J. Allergy Clin. Immunol. 2002, 110, 262–268. [Google Scholar] [CrossRef]
- Aviles, H.; Belay, T.; Fountain, K.; Vance, M.; Sonnenfeld, G. Increased Susceptibility to Pseudomonas aeruginosa Infection under Hindlimb-Unloading Conditions. J. Appl. Physiol. 2003, 95, 73–80. [Google Scholar] [CrossRef]
- Li, M.; Holmes, V.; Zhou, Y.; Ni, H.; Sanzari, J.K.; Kennedy, A.R.; Weissman, D. Hindlimb Suspension and Spe-Like Radiation Impairs Clearance of Bacterial Infections. PLoS ONE 2014, 9, e85665. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, N.V.; Elliott, T.B.; McDaniel, D.P.; Liao, P.J.; Zhai, M.; Kiang, J.G. Up-Regulation of Autophagy Defense Mechanisms in Mouse Mesenchymal Stromal Cells in Response to Ionizing Irradiation Followed by Bacterial Challenge; InTech: Rijeka, Croatia, 2013; pp. 331–350. [Google Scholar]
- Banerjee, S.; Fu, Q.; Shah, S.K.; Melnyk, S.B.; Sterneck, E.; Hauer-Jensen, M.; Pawar, S.A. C/Ebpdelta Protects from Radiation-Induced Intestinal Injury and Sepsis by Suppression of Inflammatory and Nitrosative Stress. Sci. Rep. 2019, 9, 13953. [Google Scholar] [CrossRef]
- Horseman, T.S.; Frank, A.M.; Cannon, G.; Zhai, M.; Olson, M.G.; Lin, B.; Li, X.; Hull, L.; Xiao, M.; Kiang, J.G.; et al. Effects of Combined Ciprofloxacin and Neulasta Therapy on Intestinal Pathology and Gut Microbiota after High-Dose Irradiation in Mice. Front. Public Health 2024, 12, 1365161. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Cannon, G.; Anderson, M.N.; Ho, C.; Zhai, M.; Cui, W.; Xiao, M. Ghrelin, a Novel Therapy, Corrects Cytokine and NF-κB-Akt-MAPK Network and Mitigates Intestinal Injury Induced by Combined Radiation and Skin-Wound Trauma. Cell Biosci. 2020, 10, 63. [Google Scholar] [CrossRef]
- Mitra, D.; Armijo, G.K.; Ober, E.H.; Baker, S.M.; Turner, H.C.; Broustas, C.G. Miist305 Mitigates Gastrointestinal Acute Radiation Syndrome Injury and Ameliorates Radiation-Induced Gut Microbiome Dysbiosis. Gut Microbes 2025, 17, 2458189. [Google Scholar] [CrossRef]
- Hollingsworth, B.A.; Cassatt, D.R.; DiCarlo, A.L.; Rios, C.I.; Satyamitra, M.M.; Winters, T.A.; Taliaferro, L.P. Acute Radiation Syndrome and the Microbiome: Impact and Review. Front. Pharmacol. 2021, 12, 643283. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180, Erratum in Nature 2011, 474, 666. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut Microbiota and Inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N.; et al. Baseline Human Gut Microbiota Profile in Healthy People and Standard Reporting Template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial Diversity in the Oral Cavity of 10 Healthy Individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef]
- Davis, C.P. Normal Flora. In Medical Microbiology; Baron, S., Ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Charlson, E.S.; Bittinger, K.; Haas, A.R.; Fitzgerald, A.S.; Frank, I.; Yadav, A.; Bushman, F.D.; Collman, R.G. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am. J. Respir. Crit. Care Med. 2011, 184, 957–963. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Huffnagle, G.B. The Role of the Bacterial Microbiome in Lung Disease. Expert. Rev. Respir. Med. 2013, 7, 245–257. [Google Scholar] [CrossRef]
- Liu, N.N.; Ma, Q.; Ge, Y.; Yi, C.X.; Wei, L.Q.; Tan, J.C.; Chu, Q.; Li, J.Q.; Zhang, P.; Wang, H. Microbiome Dysbiosis in Lung Cancer: From Composition to Therapy. npj Precis. Oncol. 2020, 4, 33. [Google Scholar] [CrossRef]
- Frank, D.N.; Feazel, L.M.; Bessesen, M.T.; Price, C.S.; Janoff, E.N.; Pace, N.R. The Human Nasal Microbiota and Staphylococcus aureus Carriage. PLoS ONE 2010, 5, e10598. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; MHerbst-Kralovetz, M. Menopause and the Vaginal Microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Buchta, V. Vaginal Microbiome. Ceska Gynekol. 2018, 83, 371–379. [Google Scholar] [PubMed]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-Intestinal Cross-Talk in Mucosal Inflammatory Disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The Role of the Lung Microbiota and the Gut-Lung Axis in Respiratory Infectious Diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Fagan, A.; Gavis, E.A.; Kassam, Z.; Sikaroodi, M.; Gillevet, P.M. Long-Term Outcomes of Fecal Microbiota Transplantation in Patients with Cirrhosis. Gastroenterology 2019, 156, 1921–1923 e3. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, L.; Yi, M.; Qin, W.; Zhao, W.; Li, F.; Wu, B.; Yuan, X. The Intestinal Microbiota Plays as a Protective Regulator against Radiation Pneumonitis. Radiat. Res. 2020, 194, 52–60. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Jones, C.B.; Davis, C.M.; Sfanos, K.S. The Potential Effects of Radiation on the Gut-Brain Axis. Radiat. Res. 2020, 193, 209–222. [Google Scholar] [CrossRef]
- Jones, C.B.; Peiffer, L.B.; Davis, C.M.; Sfanos, K.S. Examining the Effects of the Exposure on the Gut-Brain Axis. Radiat. Res. 2022, 197, 242–252. [Google Scholar]
- Hu, L.; Chen, H.; Zhang, X.; Feng, Z.; Zhang, H.; Meng, Q. Rosiglitazone Ameliorates Radiation-Induced Intestinal Inflammation in Rats by Inhibiting Nlrp3 Inflammasome and TNF-alpha Production. J. Radiat. Res. 2020, 61, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Vera, G.; Nurgali, K.; Abalo, R. Chemotherapy-Induced Neuropathy Affecting the Gastrointestinal Tract. Neurogastroenterol. Motil. 2025, 37, e14976. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Zhu, W.; Li, X.; Fu, M.; Peng, X.; Yang, F.; Zhang, Y.; Yin, H.; Yang, C.; Zhao, J.; et al. Impact of Gut Microbiota on Radiation-Associated Cognitive Dysfunction and Neuroinflammation in Mice. Radiat. Res. 2022, 197, 350–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Song, X.; Dai, J.; Tang, Z.; Huang, G.; Jiao, W.; Wu, Y.; Wang, C.; Du, L.; et al. The Effects of Lactobacillus reuteri Microcapsules on Radiation-Induced Brain Injury by Regulating the Gut Microenvironment. Food Funct. 2023, 14, 10041–10051. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xiao, H.; Li, Y.; Dong, J.; Luo, D.; Li, H.; Feng, G.; Wang, H.; Fan, S. Total Abdominal Irradiation Exposure Impairs Cognitive Function Involving Mir-34a-5p/Bdnf Axis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2333–2341. [Google Scholar]
- Luo, X.X.; Yang, C.; Zhan, G.F.; Li, S.; Hua, D.Y.; Luo, A.L.; Yuan, X.L. Whole Brain Radiotherapy Induces Cognitive Dysfunction in Mice: Key Role of Gut Microbiota. Psychopharmacology 2020, 237, 2089–2101. [Google Scholar] [CrossRef]
- Song, C.; Yin, Y.; Qin, Y.; Li, T.; Zeng, D.; Ju, T.; Duan, F.; Zhang, Y.; Lu, W. Acanthopanax senticosus Extract Alleviates Radiation-Induced Learning and Memory Impairment Based on Neurotransmitter-Gut Microbiota Communication. CNS Neurosci. Ther. 2023, 29 (Suppl. S1), 129–145. [Google Scholar] [CrossRef]
- Franchi, L.; Kamada, N.; Nakamura, Y.; Burberry, A.; Kuffa, P.; Suzuki, S.; Shaw, M.H.; Kim, Y.G.; Nunez, G. Nlrc4-Driven Production of IL-1beta Discriminates between Pathogenic and Commensal Bacteria and Promotes Host Intestinal Defense. Nat. Immunol. 2012, 13, 449–456. [Google Scholar] [CrossRef]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human Epidermal Langerhans Cells Maintain Immune Homeostasis in Skin by Activating Skin Resident Regulatory T Cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-Omics Analyses of Radiation Survivors Identify Radioprotective Microbes and Metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Zhao, T.S.; Xie, L.W.; Cai, S.; Xu, J.Y.; Zhou, H.; Tang, L.F.; Yang, C.; Fang, S.; Li, M.; Tian, Y. Dysbiosis of Gut Microbiota Is Associated with the Progression of Radiation-Induced Intestinal Injury and Is Alleviated by Oral Compound Probiotics in Mouse Model. Front. Cell Infect. Microbiol. 2021, 11, 717636. [Google Scholar] [CrossRef]
- Pinkerton, J.W.; Kim, R.Y.; Robertson, A.A.B.; Hirota, J.A.; Wood, L.G.; Knight, D.A.; Cooper, M.A.; O’Neill, L.A.J.; Horvat, J.C.; Hansbro, P.M. Inflammasomes in the Lung. Mol. Immunol. 2017, 86, 44–55. [Google Scholar] [CrossRef]
- Hu, J.; Jiao, W.; Tang, Z.; Wang, C.; Li, Q.; Wei, M.; Song, S.; Du, L.; Jin, Y. Quercetin Inclusion Complex Gels Ameliorate Radiation-Induced Brain Injury by Regulating Gut Microbiota. Biomed. Pharmacother. 2023, 158, 114142. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yoshida, N.; Emoto, T.; Saito, Y.; Hirata, K.I. Two Gut Microbiota-Derived Toxins Are Closely Associated with Cardiovascular Diseases: A Review. Toxins 2021, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Tan, H. Gut Microbiota and Heart, Vascular Injury. Adv. Exp. Med. Biol. 2020, 1238, 107–141. [Google Scholar] [PubMed]
- Liu, G.; Li, J.; Li, Y.; Hu, Y.; Franke, A.A.; Liang, L.; Hu, F.B.; Chan, A.T.; Mukamal, K.J.; Rimm, E.B.; et al. Gut Microbiota-Derived Metabolites and Risk of Coronary Artery Disease: A Prospective Study among Us Men and Women. Am. J. Clin. Nutr. 2021, 114, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Renesteen, E.; Boyajian, J.L.; Islam, P.; Kassab, A.; Abosalha, A.; Makhlouf, S.; Santos, M.; Chen, H.; Shum-Tim, C.; Prakash, S. Microbiome Engineering for Biotherapeutic in Alzheimer’s Disease through the Gut-Brain Axis: Potentials and Limitations. Int. J. Mol. Sci. 2025, 26, 5351. [Google Scholar] [CrossRef]
- Yin, L.; Li, X.; Ghosh, S.; Xie, C.; Chen, J.; Huang, H. Role of Gut Microbiota-Derived Metabolites on Vascular Calcification in Ckd. J. Cell Mol. Med. 2021, 25, 1332–1341. [Google Scholar] [CrossRef]
- Vojvodic, A.; Peric-Hajzler, Z.; Matovic, D.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Sijan, G.; Dimitrijevic, S.; Stepic, N.; Wollina, U.; Badr, B.A.E.; et al. Gut Microbiota and the Alteration of Immune Balance in Skin Diseases: From Nutraceuticals to Fecal Transplantation. Open Access Maced. J. Med. Sci. 2019, 7, 3034–3038. [Google Scholar] [CrossRef]
- Millman, J.F.; Kondrashina, A.; Walsh, C.; Busca, K.; Karawugodage, A.; Park, J.; Sirisena, S.; Martin, F.P.; Felice, V.D.; Lane, J.A. Biotics as Novel Therapeutics in Targeting Signs of Skin Ageing Via the Gut-Skin Axis. Ageing Res. Rev. 2024, 102, 102518. [Google Scholar] [CrossRef]
- Li, Y.; Dong, J.; Xiao, H.; Zhang, S.; Wang, B.; Cui, M.; Fan, S. Gut Commensal Derived-Valeric Acid Protects against Radiation Injuries. Gut Microbes 2020, 11, 789–806. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Dong, J.; Li, Y.; Zhang, S.; Zeng, X.; Xiao, H.; Fan, S.; Cui, M. Gut Microbiota-Derived L-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury. Int. J. Mol. Sci. 2021, 22, 11436. [Google Scholar] [CrossRef]
- Cui, W.; Hull, L.; Zizzo, A.; Wang, L.; Lin, B.; Zhai, M.; Xiao, M. The Gut Microbiome Changes in Wild Type and IL-18 Knockout Mice after 9.0 Gy Total Body Irradiation. Anim. Microbiome 2023, 5, 42. [Google Scholar] [CrossRef]
- Ossetrova, N.I.; Sandgren, D.J.; Blakely, W.F. Protein Biomarkers for Enhancement of Radiation Dose and Injury Assessment in Nonhuman Primate Total-Body Irradiation Model. Radiat. Prot. Dosimetry 2014, 159, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, Z.; Chen, Y.; Cheng, Z.; Shi, C. Characterization of Two Stable Biodosimeters for Absorbed Ionizing Radiation Dose Estimation in Multiple Combined Injury Models. Radiat. Res. 2025, 204, 27–45. [Google Scholar] [CrossRef]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Ho, C.; Gorbunov, N.V.; Elliott, T.B. Thrombopoietin Receptor Agonist Mitigates Hematopoietic Radiation Syndrome and Improves Survival after Whole-Body Ionizing Irradiation Followed by Wound Trauma. Mediators Inflamm. 2017, 2017, 7582079. [Google Scholar] [CrossRef] [PubMed]
- Ledney, G.D.; Elliott, T.B. Combined Injury: Factors with Potential to Impact Radiation Dose Assessments. Health Phys. 2010, 98, 145–152. [Google Scholar] [CrossRef]
- Kiang, J.G.; Gorbunov, N.V. Bone Marrow Mesenchymal Stem Cells Increase Survival after Ionizing Irradiation Combined with Wound Trauma: Characterization and Therapy. J. Cell Sci. Ther. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Fukumoto, R. Ciprofloxacin Increases Survival after Ionizing Irradiation Combined Injury: Gamma-H2AX Formation, Cytokine/Chemokine, and Red Blood Cells. Health Phys. 2014, 106, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Bolduc, D.L.; Smith, J.T.; Anderson, M.N.; Ho, C.; Lin, B.; Jiang, S. Combined Therapy of Pegylated G-CSF and Alxn4100TPO Improves Survival and Mitigates Acute Radiation Syndrome after Whole-Body Ionizing Irradiation alone and Followed by Wound Trauma. Radiat. Res. 2017, 188, 476–490. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, M.; Lin, B.; Cui, W.; Hull, L.; Li, X.; Anderson, M.N.; Smith, J.T.; Umali, M.V.; Jiang, S.; et al. Peg-G-CSF and L-Citrulline Combination Therapy for Mitigating Skin Wound Combined Radiation Injury in a Mouse Model. Radiat. Res. 2021, 196, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Volovat, C.C.; Cosovanu, M.A.; Ostafe, M.-R.; Augustin, I.G.; Volovat, C.; Georgescu, B.; Volovat, S.R. Bacteriophages, Antibiotics and Probiotics: Exploring the Microbial Battlefield of Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 7837. [Google Scholar] [CrossRef]
- Weerakoon, S.; Avula, S.; Mandefro, B.T.; Sundara, S.V.; Lu, X.; Busmail, H.; Malasevskaia, I.A. Microbiota-Based Therapies for Recurrent Clostridium Difficile Infection: A Systematic Review of Their Efficacy and Safety. Cureus 2025, 17, e90737. [Google Scholar] [CrossRef]
- Bradshaw, P.C.; Seeds, W.A.; Miller, A.C.; Mahajan, V.R.; Curtis, W.M. COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm. Oxid. Med. Cell Longev. 2020, 2020, 6401341. [Google Scholar] [CrossRef]
- Rios, C.I.; Cassatt, D.R.; Hollingsworth, B.A.; Satyamitra, M.M.; Tadesse, Y.S.; Taliaferro, L.P.; Winters, T.A.; DiCarlo, A.L. Commonalities between COVID-19 and Radiation Injury. Radiat. Res. 2021, 195, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and Serum Amyloid a Synergy Mediates Angiotensin II-Induced Muscle Wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef]
- Ma, L.J.; Corsa, B.A.; Zhou, J.; Yang, H.; Li, H.; Tang, Y.W.; Babaev, V.R.; Major, A.S.; Linton, M.F.; Fazio, S.; et al. Angiotensin Type 1 Receptor Modulates Macrophage Polarization and Renal Injury in Obesity. Am. J. Physiol. Renal Physiol. 2011, 300, F1203–F1213. [Google Scholar] [CrossRef]
- Huss, D.J.; Winger, R.C.; Cox, G.M.; Guerau-de-Arellano, M.; Yang, Y.; Racke, M.K.; Lovett-Racke, A.E. TGF-beta Signaling Via Smad4 Drives IL-10 Production in Effector Th1 Cells and Reduces T-Cell Trafficking in Eae. Eur. J. Immunol. 2011, 41, 2987–2996. [Google Scholar] [CrossRef]
- Lesgards, J.-F.; Cerdan, D.; Perronne, C. Do Long Covid and Covid Vaccine Side Effects Share Pathophysiological Picture and Biochemical Pathways? Int. J. Mol. Sci. 2025, 26, 7837, Erratum in Int. J. Mol. Sci. 2025, 26, 9513. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 Vaccine Development: Milestones, Lessons and Prospects. Signal Transduct. Target. Ther. 2022, 7, 146. [Google Scholar] [CrossRef] [PubMed]
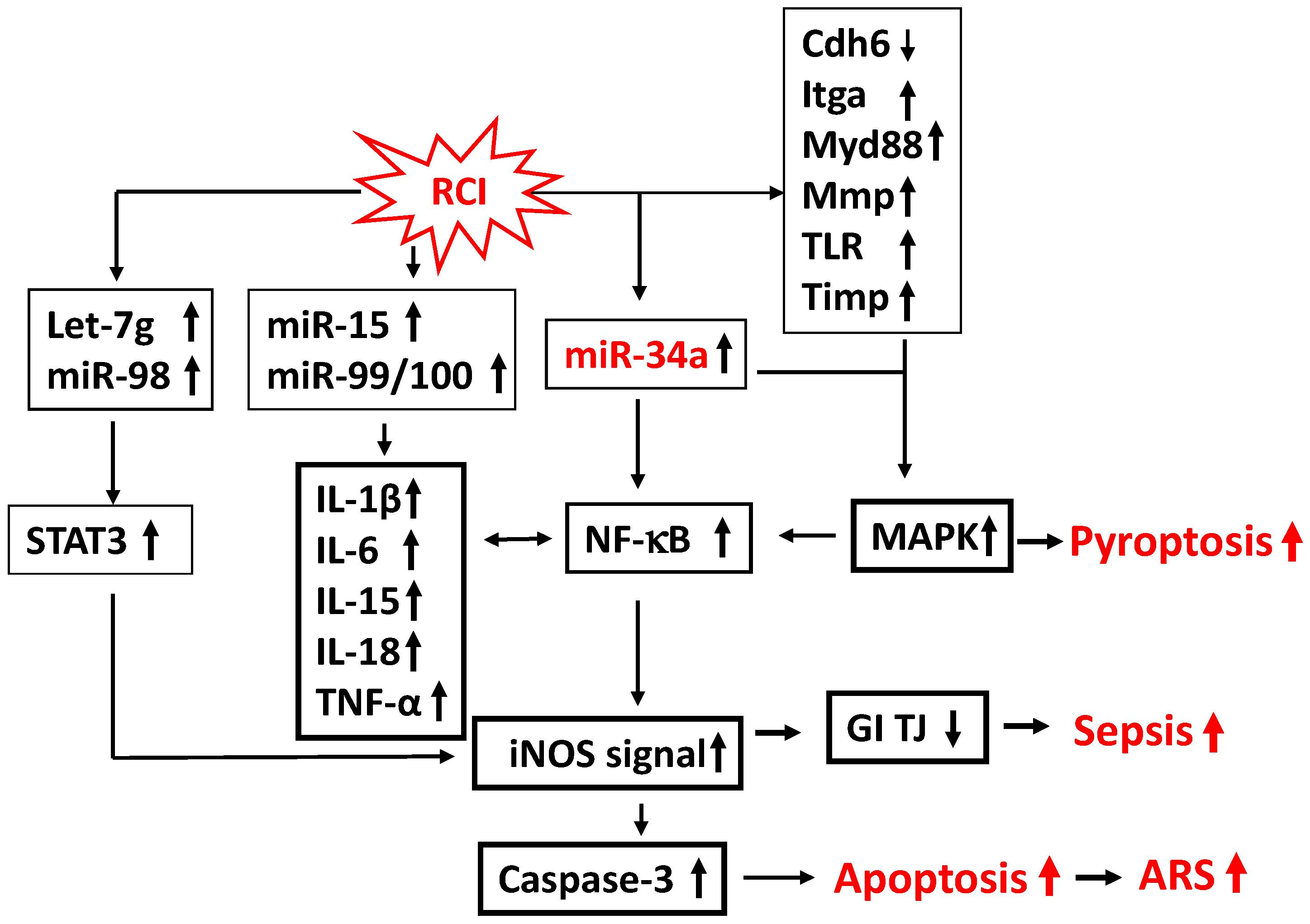
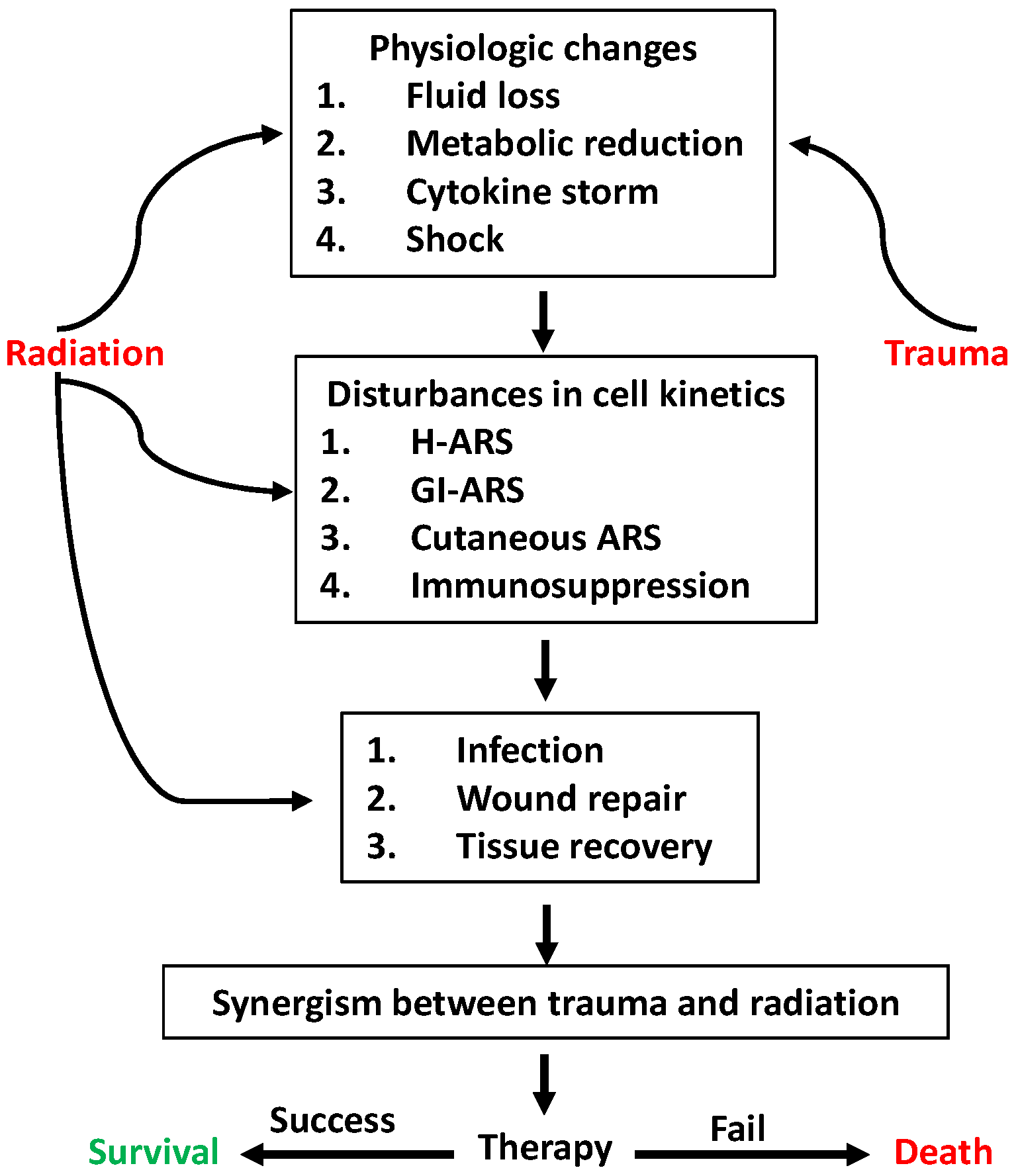
| Type | Species | References |
|---|---|---|
| Rad + 20% Hemo | Mouse | [30] |
| Rad + 37% Hemo + Penetrating soft tissue injury | Rat | [32] |
| Rad + Skin wound | Mouse | [8] |
| Rad + Ear punch wound | Mouse | [9] |
| Rad + Skin burn | Mouse | [12,48] |
| Rat | [14,17,19,20,21,22,23] | |
| Guinea pig | [24] | |
| Dog | [25,26] | |
| Swine | [24] | |
| Rad + 15% total body surface area burn | Mouse | [12] |
| Rad + 20% total body surface area burn | Mouse | [48] |
| Rad + Infection | Mouse | [53,54,55,56] |
| Rad + CLP | Rat | [16] |
| Rad+ hindlimb suspension + bacteria | Mouse | [59] |
| Type | Cytokine/Chemokine | Species | References |
|---|---|---|---|
| Rad + Wound | IL-1β, IL-6, IL-9, IL-10, IL-13, | Mouse | [8,41] |
| IL-18, KC, G-CSF, eotaxin, | |||
| MCP-1, MIP-1α, MIP-1β CRP, C3 | |||
| Rad + Skin burn | IL-1β, IL-6, IL-10, IL-12, TNF-α | Rat | [49,50,51] |
| Rad + CLP | IL-6, TNF-α | Rat | [16] |
| Rad + Infection | KC, TNF-α | Mouse | [64] |
| Drug | Actions | Species | References |
|---|---|---|---|
| Alxn4100TPO | TPO receptor agonist | Mouse | [111] |
| Increased platelets | |||
| BM transplant | Increased bone marrow cells | Mouse | [112] |
| Bone marrow MSCs | Increased bone marrow cells | Mouse | [113] |
| Ghrelin | Decreased IL-1β, IL-6, IL-17A | Mouse | [63] |
| IL-18 levels | |||
| Increased G-CSF, KC, MIP-1α levels | |||
| Vagotomy | Rat | [16] | |
| Ciprofloxacin | Increased IL-3 levels and RBCs | Mouse | [114] |
| WR-151327 | Not identified | Mouse | [112] |
| Silvadene | Not identified | Mouse | [112] |
| Trichostatin | Increased IL-1β, IL-6, CRP | Rat | [18] |
| TNF-α levels | |||
| S-TDCM + Gentamicin | Not identified | Mouse | [112] |
| Neulasta + Alexn4100TPO | Improved H-ARS and GI-ARS | Mouse | [115] |
| Neulasta + Citrulline | Improved endothelium | Mouse | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiang, J.G.; Cannon, G. An Update on Dynamic Changes in Cytokine Expression and Dysbiosis Due to Radiation Combined Injury. Int. J. Mol. Sci. 2025, 26, 10456. https://doi.org/10.3390/ijms262110456
Kiang JG, Cannon G. An Update on Dynamic Changes in Cytokine Expression and Dysbiosis Due to Radiation Combined Injury. International Journal of Molecular Sciences. 2025; 26(21):10456. https://doi.org/10.3390/ijms262110456
Chicago/Turabian StyleKiang, Juliann G., and Georgetta Cannon. 2025. "An Update on Dynamic Changes in Cytokine Expression and Dysbiosis Due to Radiation Combined Injury" International Journal of Molecular Sciences 26, no. 21: 10456. https://doi.org/10.3390/ijms262110456
APA StyleKiang, J. G., & Cannon, G. (2025). An Update on Dynamic Changes in Cytokine Expression and Dysbiosis Due to Radiation Combined Injury. International Journal of Molecular Sciences, 26(21), 10456. https://doi.org/10.3390/ijms262110456






