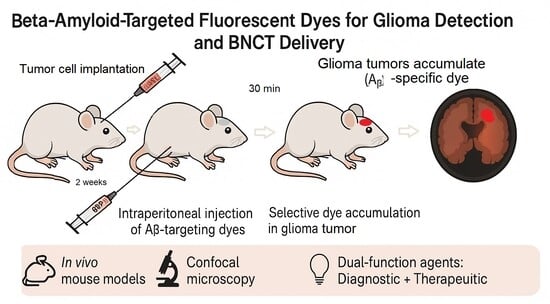
Targeting Gliomas with Beta-Amyloid-Specific Dyes: A Novel Approach for In Vivo Staining and Potential Therapeutic Applications
Abstract
1. Introduction
1.1. Intraoperative Visualization
1.2. Targeted Tumor Destruction via Boron-Neutron Capture Therapy (BNCT)
- High B10 concentration within tumor cells;
- Tumor-to-normal tissue and tumor-to-blood concentration ratios exceeding 3–5;
- Minimal toxicity of boron carriers [19].
2. Results
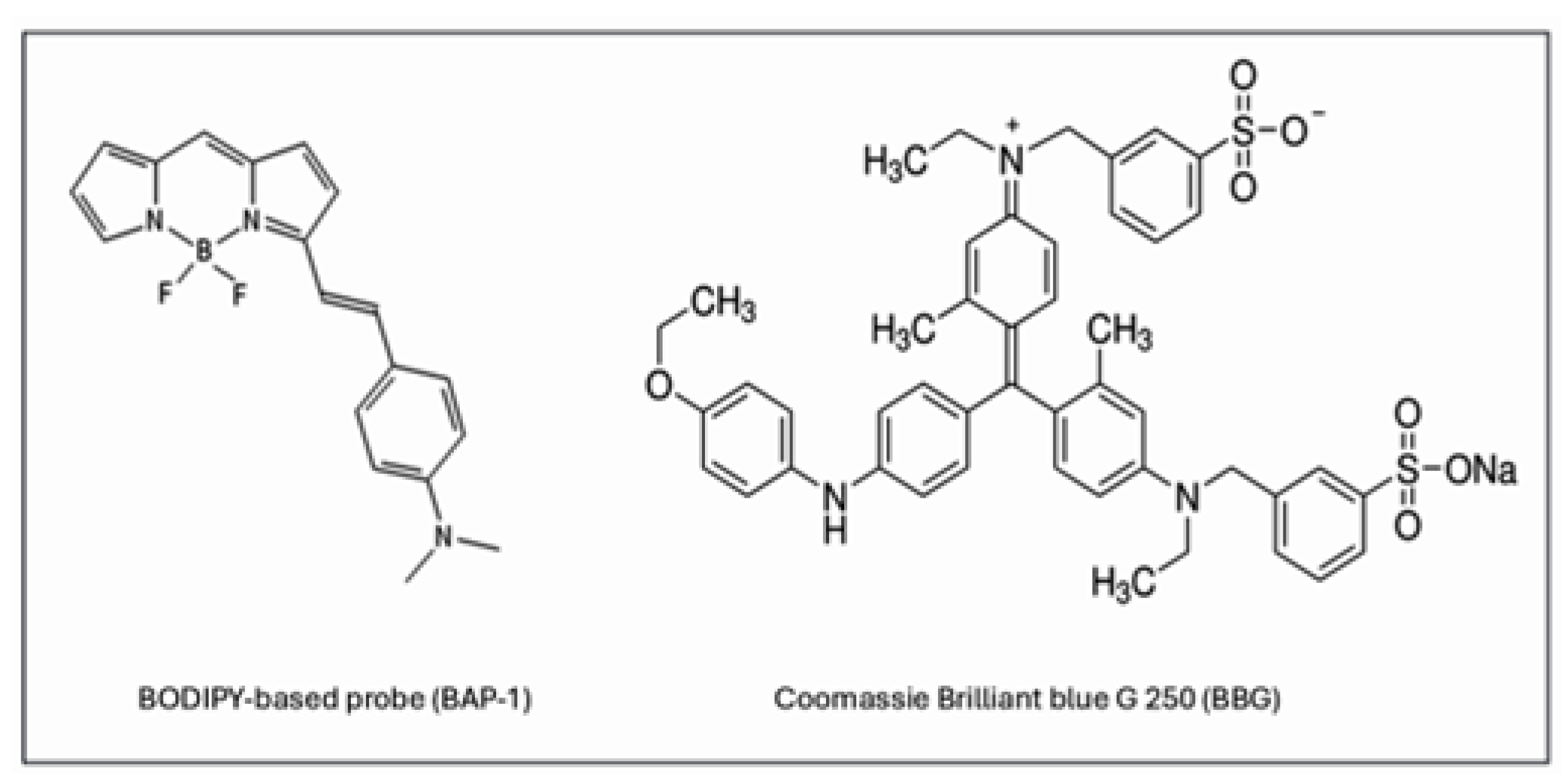
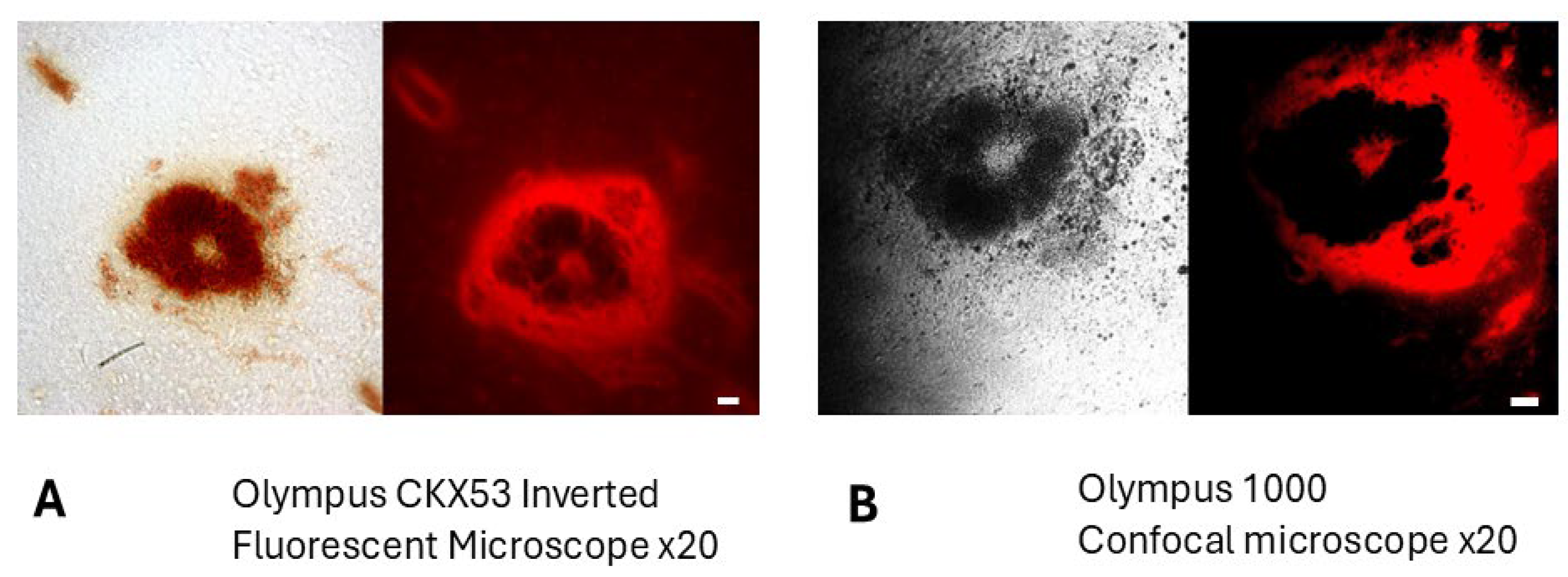
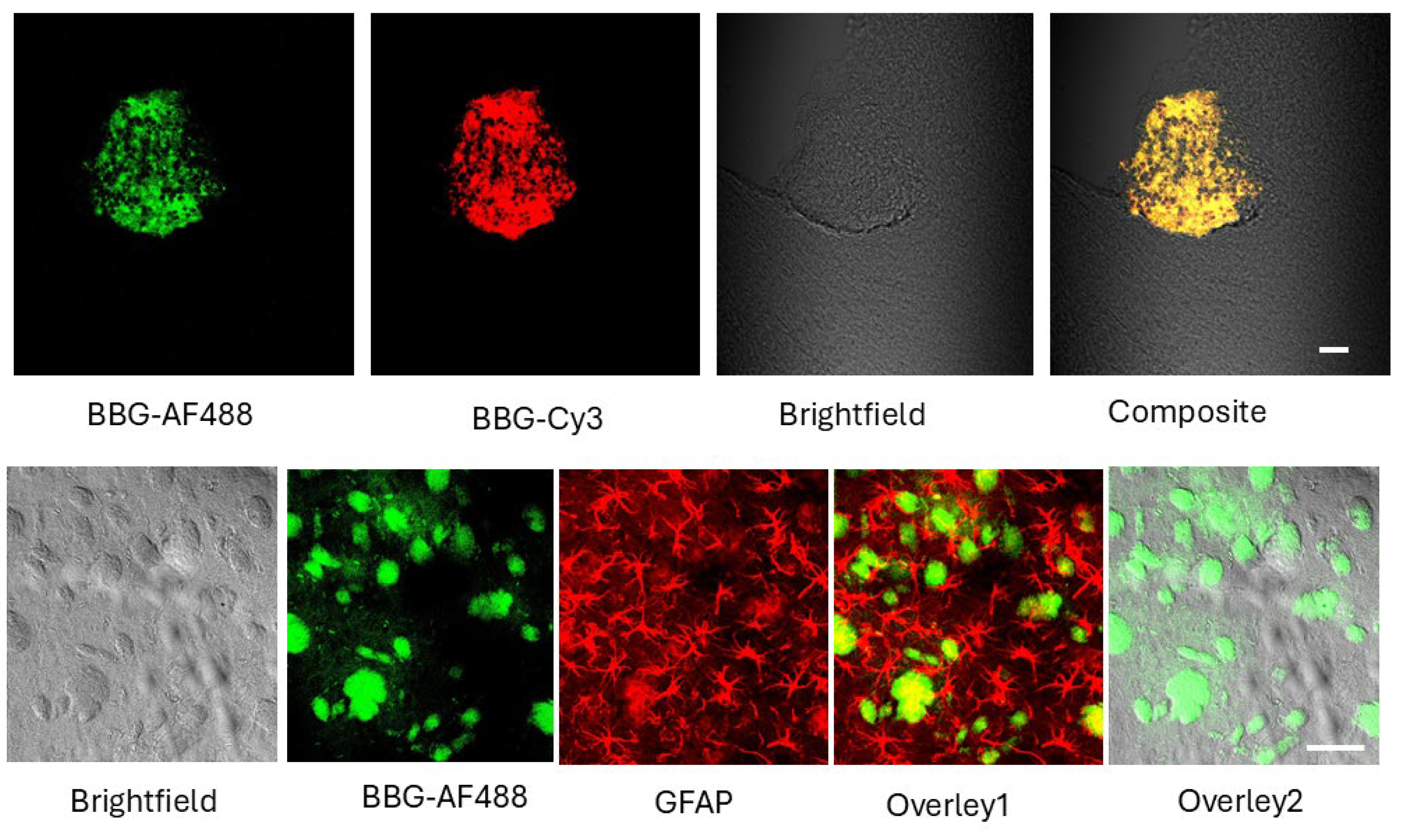
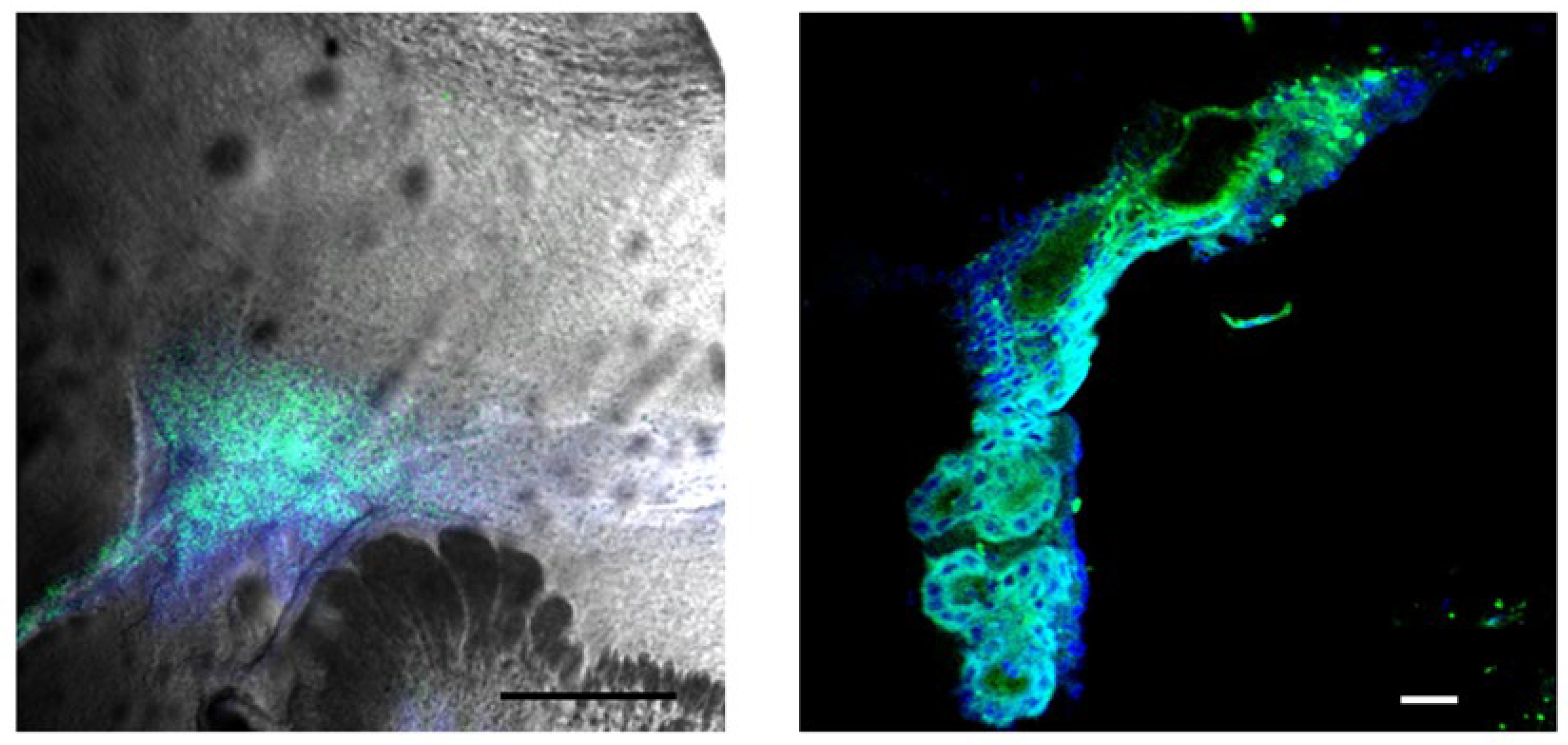
2.1. BBG Selectively Accumulates in Glioma Tissue
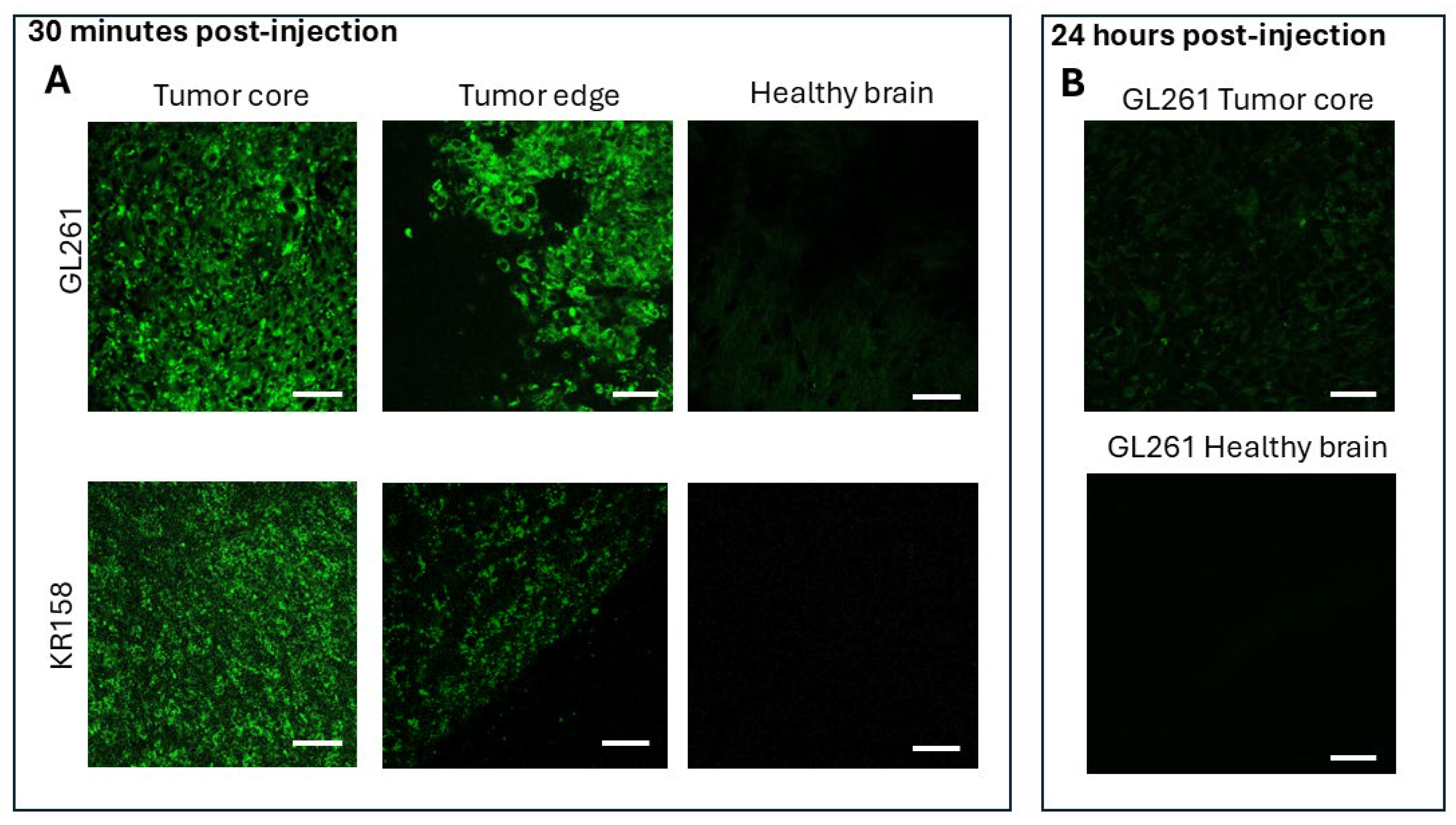
2.2. BAP-1 Preferentially Accumulates in Glioma Cells with High Contrast
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Animals and Study Design
- BBG-treated group: 5 animals received intraperitoneal (IP) BBG injection. A total of 2 animals were used to prepare brain slices containing tumors for post-staining.
- BAP-1-treated groups:
- 5 animals received a 0.2 mg dose of BAP-1 via IP injection, analyzed 30 min post-injection (GL-261).
- 5 animals received a 0.04 mg dose of BAP-1 via IP injection, analyzed 30 min post-injection (GL-261).
- 5 animals received a 0.02 mg dose of BAP-1 via IP injection, analyzed 30 min post-injection (GL-261).
- 3 animals received a 0.2 mg dose of BAP-1 via IP injection, analyzed 24 h post-injection (GL-261).
- 3 animals received a 0.2 mg dose of BAP-1 via tail vein injection, analyzed 30 min post-injection (GL-261).
- 3 animals received a 0.02 mg dose of BAP-1 via IP injection, analyzed 30 min post-injection (KR-158).
4.3. Intracranial Implantation of Glioma Cells
4.4. Tumor Staining After IPO Injections
4.5. Glioma Cell Culture
4.6. Hematoxylin and Eosin Staining
4.7. Tissue Preparation and Immunofluorescence Staining
4.8. Microscopy
4.9. Reagents
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulenic acid |
| APP | amyloid precursor protein |
| Aβ | amyloid beta |
| BAP-1 | BODIPY-based Amyloid Probe-1 |
| BBG | Brilliant Blue G |
| BBB | Blood-brain barrier |
| BNCT | boron-neutron capture therapy |
| GBM | glioblastoma |
| GTR | Gross total resection |
| H&E | hematoxylin and eosin |
| HGG | high-grade gliomas |
| ICG | indocyanine green |
| ICP-MS | Inductively coupled plasma Mass-Spec |
| IP | intraperitoneal |
| PpIX | protoporphyrin IX |
| SF | fluorescein sodium |
References
- Bruno, F.; Pellerino, A.; Pronello, E.; Palmiero, R.; Bertero, L.; Mantovani, C.; Bianconi, A.; Melcarne, A.; Garbossa, D.; Rudà, R. Elderly Gliobastoma Patients: The Impact of Surgery and Adjuvant Treatments on Survival. A Single Institution Experience. Brain Sci. 2022, 12, 632. [Google Scholar] [CrossRef]
- Kucheryavykh, L.Y.; Ortiz-Rivera, J.; Kucheryavykh, Y.V.; Zayas-Santiago, A.; Diaz-Garcia, A.; Inyushin, M.Y. Accumulation of Innate Amyloid Beta Peptide in Glioblastoma Tumors. Int. J. Mol. Sci. 2019, 20, 2482. [Google Scholar] [CrossRef]
- Zayas-Santiago, A.; Díaz-García, A.; Nuñez-Rodríguez, R.; Inyushin, M. Accumulation of amyloid beta in human glioblastomas. Clin. Exp. Immunol. 2020, 202, 325–334. [Google Scholar] [CrossRef]
- Morato, E.; Mayor, F. Production of the Alzheimer’s beta-amyloid peptide by C6 glioma cells. FEBS Lett. 1993, 336, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, R.; Kim, Y.; Miller, C.; Pacheco-Quinto, J.; Eckman, E.A.; Levy, E. Extracellular vesicles: Where the amyloid precursor protein carboxyl-terminal fragments accumulate and amyloid-β oligomerizes. FASEB J. 2020, 34, 12922–12931. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.R.; Lind, M.; Wagen, A.Z.; Rembach, A.; Frugier, T.; Li, Q.X.; Ryan, T.M.; McLean, C.A.; Doecke, J.D.; Rowe, C.C.; et al. Biochemically-defined pools of amyloid-β in sporadic Alzheimer’s disease: Correlation with amyloid PET. Brain 2017, 140, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Belousov, A.; Titov, S.; Shved, N.; Garbuz, M.; Malykin, G.; Gulaia, V.; Kagansky, A.; Kumeiko, V. The Extracellular Matrix and Biocompatible Materials in Glioblastoma Treatment. Front. Bioeng. Biotechnol. 2019, 7, 341. [Google Scholar] [CrossRef]
- Bianconi, A.; Aruta, G.; Rizzo, F.; Salvati, L.F.; Zeppa, P.; Garbossa, D.; Cofano, F. Systematic Review on Tumor Microenvironment in Glial Neoplasm: From Understanding Pathogenesis to Future Therapeutic Perspectives. Int. J. Mol. Sci. 2022, 23, 4166. [Google Scholar] [CrossRef]
- Marino, S.; Menna, G.; Di Bonaventura, R.; Lisi, L.; Mattogno, P.; Figà, F.; Bilgin, L.; D’Alessandris, Q.G.; Olivi, A.; Della Pepa, G.M. The Extracellular Matrix in Glioblastomas: A Glance at Its Structural Modifications in Shaping the Tumoral Microenvironment—A Systematic Review. Cancers 2023, 15, 1879. [Google Scholar] [CrossRef]
- Singh, S.; Joshi, V.; Upadhyay, A. Amyloids and brain cancer: Molecular linkages and crossovers. Biosci. Rep. 2023, 43, BSR20230489. [Google Scholar] [CrossRef]
- Mazurek, M.; Kulesza, B.; Stoma, F.; Osuchowski, J.; Mańdziuk, S.; Rola, R. Characteristics of Fluorescent Intraoperative Dyes Helpful in Gross Total Resection of High-Grade Gliomas-A Systematic Review. Diagnostics 2020, 10, 1100. [Google Scholar] [CrossRef]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Crasto, S.G.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci. 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Jinli, S.; Jianyao, M.; Yan, C.; Huijuan, L.; Zhongjie, S.; Zhangyu, L.; Liwei, Z.; Yukui, L.; Sifang, C.; et al. Fluorescein-guided surgery for high-grade glioma resection: A five-year-long retrospective study at our institute. Front. Oncol. 2023, 13, 1191470. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.C.; Krabbenhoft, M.G.; Rasmus, W.H.; Mikic, N.; Pedersen, C.B.; Poulsen, F.R.; Korshoej, A.R. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers 2022, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, A.; La Cava, P.; Bonada, M.; Zeppa, P.; Melcarne, A.; Cofano, F.; Fiaschi, P.; Garbossa, D.; Bianconi, A. Combined Fluorescence-Guided Surgery with 5-Aminolevulinic Acid and Fluorescein in Glioblastoma: Technical Description and Report of 100 Cases. Cancers 2024, 16, 2771. [Google Scholar] [CrossRef]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let me see: Correlation between 5-ala fluorescence and molecular pathways in glioblastoma: A single center experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Kodjikian, L.; Richter, T.; Halberstadt, M.; Beby, F.; Flueckiger, F.; Boehnke, M.; Garweg, J.G. Toxic effects of indocyanine green, infracyanine green, and trypan blue on the human retinal pigmented epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 917–925. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, E.B.; Meyer, C.H.; Mennel, S.; Farah, M.E. Mechanisms of intravitreal toxicity of indocyanine green dye: Implications for chromovitrectomy. Retina 2007, 27, 958–970. [Google Scholar] [CrossRef]
- Miyatake, S.I.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K. Boron Neutron Capture Therapy for Malignant Brain Tumors. Neurol. Med. Chir. 2016, 56, 361–371. [Google Scholar] [CrossRef]
- Farr, L.E.; Sweet, W.H.; Robertson, J.S.; Foster, C.G.; Locksley, H.B.; Sutherland, D.L.; Mendelsohn, M.L.; Stickley, E.E. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1954, 71, 279–293. [Google Scholar]
- Hughes, A.M.; Hu, N. Optimizing Boron Neutron Capture Therapy (BNCT) to Treat Cancer: An Updated Review on the Latest Developments on Boron Compounds and Strategies. Cancers 2023, 15, 4091. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Li, R.; Lin, J.; Gui, L.; Wang, Y.; Jin, Z.; Xia, W.; Liu, Y.; Cheng, S.; et al. Novel promising boron agents for boron neutron capture therapy: Current status and outlook on the future. Coord. Chem. Rev. 2024, 511, 215795. [Google Scholar] [CrossRef]
- Hatanaka, H.; Nakagawa, Y. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Congdon, R.W.; Muth, G.W.; Splittgerber, A.G. The binding interaction of Coomassie blue with proteins. Anal. Biochem. 1993, 213, 407–413. [Google Scholar] [CrossRef]
- Iwamaru, Y.; Takenouchi, T.; Murayama, Y.; Okada, H.; Imamura, M.; Shimizu, Y.; Hashimoto, M.; Mohri, S.; Yokoyama, T.; Kitani, H. Anti-Prion Activity of Brilliant Blue G. PLoS ONE 2012, 7, e37896. [Google Scholar] [CrossRef]
- Jo, S.; Bean, B.P. Inhibition of neuronal voltage-gated sodium channels by brilliant blue G. Mol. Pharmacol. 2011, 80, 247–257. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, H.; Kimura, H.; Saji, H. BODIPY-based molecular probe for imaging of cerebral β-amyloid plaques. ACS Chem. Neurosci. 2012, 3, 319–324. [Google Scholar] [CrossRef]
- Chial, H.J.; Thompson, H.B.; Splittgerber, A.G. A spectral study of the charge forms of Coomassie blue G. Anal. Biochem. 1993, 209, 258–266. [Google Scholar] [CrossRef]
- Shakya, A.K.; Al-Najjar, B.O.; Deb, P.K.; Naik, R.R.; Tekade, R.K. First-Pass Metabolism Considerations in Pharmaceutical Product Development. In Dosage Form Design Considerations; Tekade, R.K., Ed.; Academic Press is an imprint of Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 259–286. ISBN 978-0-12-814423-7. [Google Scholar]
- Jankowsky, J.L.; Younkin, L.H.; Gonzales, V.; Fadale, D.J.; Slunt, H.H.; Lester, H.A.; Younkin, S.G.; Borchelt, D.R. Rodent A beta modulates the solubility and distribution of amyloid deposits in transgenic mice. J. Biol. Chem. 2007, 282, 22707–22720. [Google Scholar] [CrossRef]
- Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Rolón-Reyes, K.; Skatchkov, S.N.; Eaton, M.J.; Cubano, L.A.; Inyushin, M. Visualization of implanted GL261 glioma cells in living mouse brain slices using fluorescent 4-(4-(dimethylamino)-styryl)-N-methylpyridinium iodide (ASP+). Biotechniques 2012, 53, 305. [Google Scholar] [CrossRef]
- Inyushin, M.; Zayas-Santiago, A.; Rojas, L.; Kucheryavykh, L. On the Role of Platelet-Generated Amyloid Beta Peptides in Certain Amyloidosis Health Complications. Front. Immunol. 2020, 11, 571083. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Salveson, P.J.; Haerianardakani, S.; Thuy-Boun, A.; Yoo, S.; Kreutzer, A.G.; Demeler, B.; Nowick, J.S. Repurposing Triphenylmethane Dyes to Bind to Trimers Derived from Aβ. J. Am. Chem. Soc. 2018, 140, 11745–11754, Correction in J. Am. Chem. Soc. 2018, 140, 15546. [Google Scholar] [CrossRef] [PubMed]
- Butt, R.H.; Coorssen, J.R. Coomassie blue as a near-infrared fluorescent stain: A systematic comparison with Sypro Ruby for in-gel protein detection. Mol. Cell. Proteomics 2013, 12, 3834–3850. [Google Scholar] [CrossRef]
- Taylor, S.H.; Thorp, J.M. Properties and Biological Behaviour of Coomassie Blue. Heart 1959, 21, 492–496. [Google Scholar] [CrossRef]
- Luo, S.; Wehr, N.B.; Levine, R.L. Quantitation of protein on gels and blots by infrared fluorescence of Coomassie blue and Fast Green. Anal. Biochem. 2006, 350, 233–238. [Google Scholar] [CrossRef]
- Kiersztyn, B.; Siuda, W.; Chróst, R. Coomassie Blue G250 for Visualization of Active Bacteria from Lake Environment and Culture. Polish J. Microbiol. 2017, 66, 365–373. [Google Scholar] [CrossRef]
- Boyd, W.M.; Chambers, W.A.; Stein, P.P. TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025%: Clinical and Regulatory Review; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019. [Google Scholar]
- Irvine, G.B.; El-Agnaf, O.M.; Shankar, G.M.; Walsh, D.M. Protein Aggregation in the Brain: The Molecular Basis for Alzheimer’s and Parkinson’s Diseases. Mol. Med. 2008, 14, 451–464. [Google Scholar] [CrossRef]
- Jiang, L.H.; Mackenzie, A.B.; North, R.A.; Surprenant, A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol. Pharmacol. 2000, 58, 82–88. [Google Scholar] [CrossRef]
- Kang, S.S.; Keasey, M.P.; Hagg, T. P2X7 receptor inhibition increases CNTF in the subventricular zone, but not neurogenesis or neuroprotection after stroke in adult mice. Transl. Stroke Res. 2013, 4, 533–545. [Google Scholar] [CrossRef]
- Arbeloa, J.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol. Dis. 2012, 45, 954–961. [Google Scholar] [CrossRef]
- Marcillo, A.; Frydel, B.; Bramlett, H.M.; Dietrich, W.D. A reassessment of P2X7 receptor inhibition as a neuroprotective strategy in rat models of contusion injury. Exp. Neurol. 2012, 233, 687–692. [Google Scholar] [CrossRef][Green Version]
- Peng, W.; Cotrina, M.L.; Han, X.; Yu, H.; Bekar, L.; Blum, L.; Takano, T.; Tian, G.F.; Goldman, S.A.; Nedergaard, M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 106, 12489–12493. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hu, C.G.; Zhu, Z.M.; Luo, H.L. Effect of P2X7 receptor on tumorigenesis and its pharmacological properties. Biomed. Pharmacother. 2020, 125, 109844. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Nema, P.; Soni, S.; Yadav, V.; Jain, S.; Soni, V.; Kashaw, S.K. Preparation and in vitro evaluation of BBG-250 loaded liposomal formulation for anticancer potential. Futur. J. Pharm. Sci. 2024, 10, 1–16. [Google Scholar] [CrossRef]
- Kan, L.K.; Seneviratne, S.; Drummond, K.J.; Williams, D.A.; O’Brien, T.J.; Monif, M. P2X7 receptor antagonism inhibits tumour growth in human high-grade gliomas. Purinergic Signal. 2020, 16, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Teng, B.; Xu, Z.; Wan, Y.; Jin, G. A porous form Coomassie brilliant blue G250-isorhamnetin fluorescent composite coated with acrylic resin for tumor cell imaging. Front. Chem. 2023, 11, 1260533. [Google Scholar] [CrossRef]
- Carlsson, N.; Kitts, C.C.; Kerman, B. Spectroscopic characterization of Coomassie blue and its binding to amyloid fibrils. Anal. Biochem. 2012, 420, 33–40. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Wang, L.; Xu, Y.; Zhang, S.; Lv, J.; Ran, C.; Li, Y. Targeting β-amyloid plaques and oligomers: Development of near-IR fluorescence imaging probes. Future Med. Chem. 2017, 9, 179. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucheryavykh, L.; Ortiz Rivera, J.; Ermolinsky, B.; Tsytsarev, V.; Cary, L.; Alves, J.; Reyes, A.; de la Cruz-Rivera, N.; Rosa Gonzalez, K.; Narvaez Irizarry, F.; et al. Targeting Gliomas with Beta-Amyloid-Specific Dyes: A Novel Approach for In Vivo Staining and Potential Therapeutic Applications. Int. J. Mol. Sci. 2025, 26, 10450. https://doi.org/10.3390/ijms262110450
Kucheryavykh L, Ortiz Rivera J, Ermolinsky B, Tsytsarev V, Cary L, Alves J, Reyes A, de la Cruz-Rivera N, Rosa Gonzalez K, Narvaez Irizarry F, et al. Targeting Gliomas with Beta-Amyloid-Specific Dyes: A Novel Approach for In Vivo Staining and Potential Therapeutic Applications. International Journal of Molecular Sciences. 2025; 26(21):10450. https://doi.org/10.3390/ijms262110450
Chicago/Turabian StyleKucheryavykh, Lilia, Jescelica Ortiz Rivera, Boris Ermolinsky, Vassiliy Tsytsarev, Lynnette Cary, Janaina Alves, Adriana Reyes, Noelis de la Cruz-Rivera, Kevin Rosa Gonzalez, Felix Narvaez Irizarry, and et al. 2025. "Targeting Gliomas with Beta-Amyloid-Specific Dyes: A Novel Approach for In Vivo Staining and Potential Therapeutic Applications" International Journal of Molecular Sciences 26, no. 21: 10450. https://doi.org/10.3390/ijms262110450
APA StyleKucheryavykh, L., Ortiz Rivera, J., Ermolinsky, B., Tsytsarev, V., Cary, L., Alves, J., Reyes, A., de la Cruz-Rivera, N., Rosa Gonzalez, K., Narvaez Irizarry, F., Porter, T. R., & Inyushin, M. (2025). Targeting Gliomas with Beta-Amyloid-Specific Dyes: A Novel Approach for In Vivo Staining and Potential Therapeutic Applications. International Journal of Molecular Sciences, 26(21), 10450. https://doi.org/10.3390/ijms262110450