Ventilator-Associated Lung Injury: Pathophysiology, Prevention, and Emerging Therapeutic Strategies
Abstract
1. Introduction
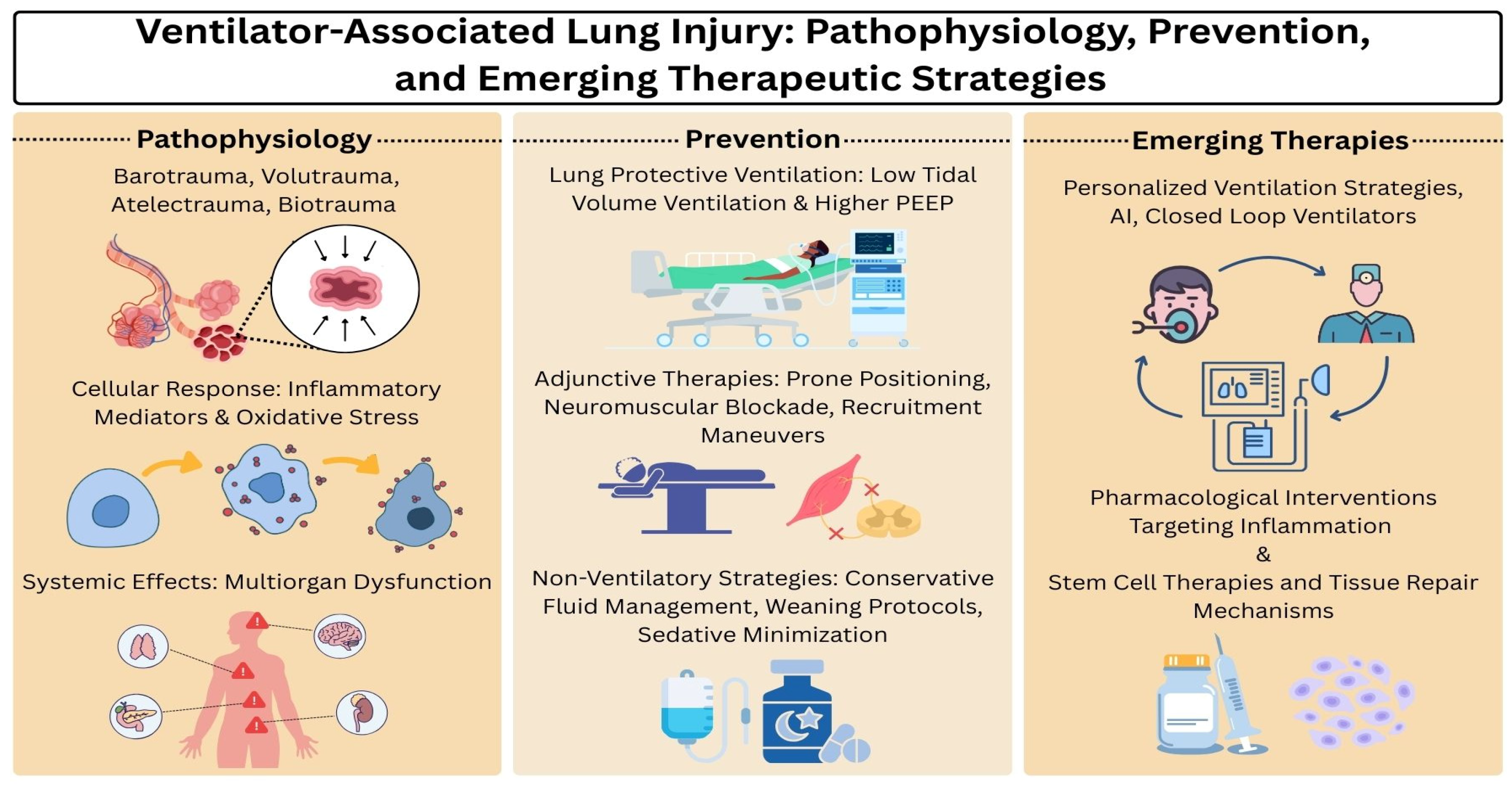
2. Pathophysiology of Ventilator-Associated Lung Injury
2.1. Mechanisms of Injury
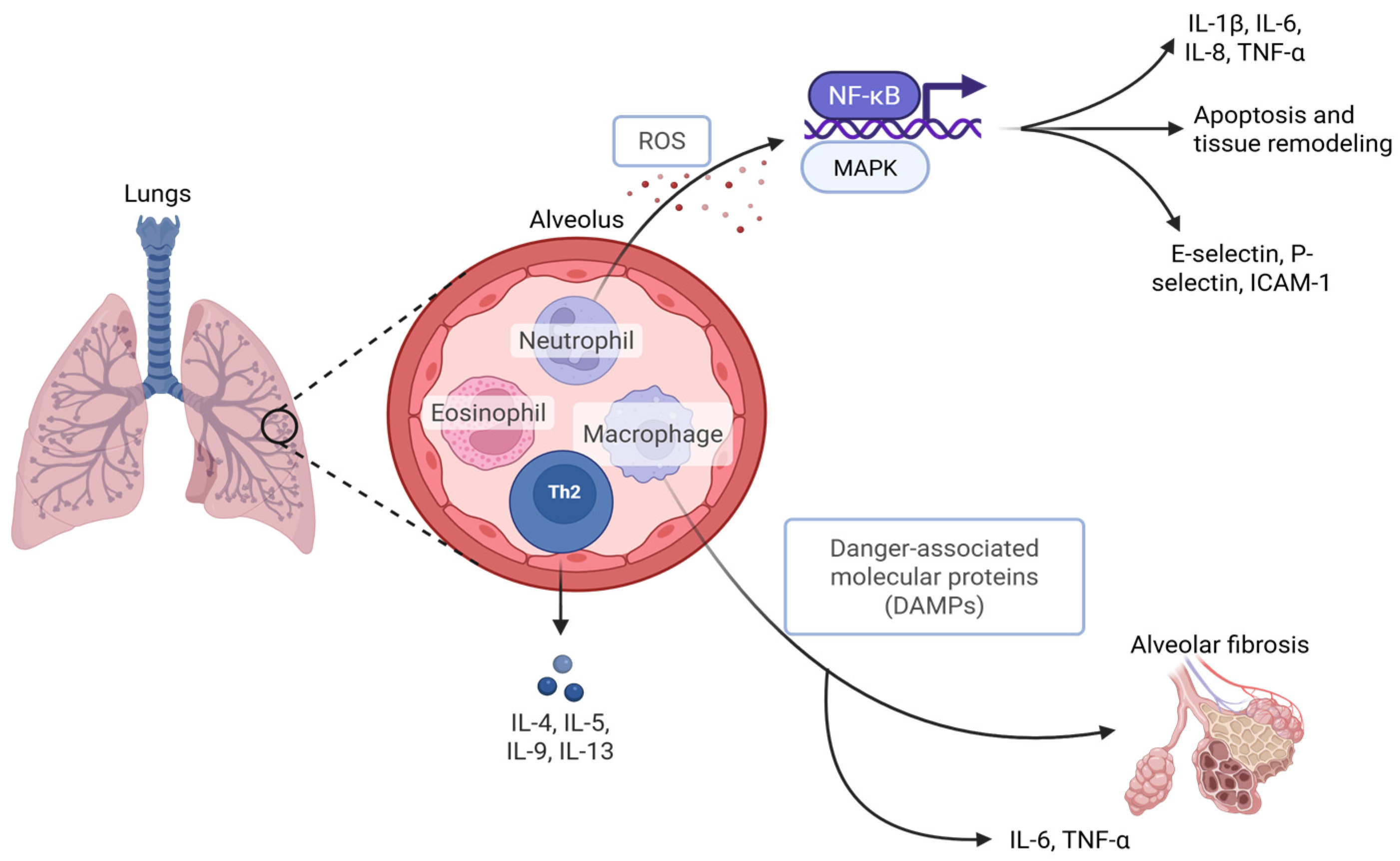
2.2. Cellular and Molecular Responses
2.3. Systemic Effects
3. Risk Factors and Patient Susceptibility
4. Clinical Presentation and Diagnosis
4.1. Clinical Signs and Symptoms
4.2. Radiographic Findings
4.3. Biomarkers
4.4. Differentiating Ventilator-Associated Lung Injury from Disease Progression
5. Prevention and Protective Strategies
5.1. Lung-Protective Ventilation
5.2. Adjunctive Therapies
5.3. Non-Ventilatory Strategies
6. Emerging Therapies and Research
6.1. Personalized Ventilation Strategies
6.2. ArtificiaI Intelligence and Closed-Loop Ventilators
6.3. Pharmacological Interventions Targeting Inflammation
6.4. Stem Cell Therapies and Tissue Repair Mechanisms
7. Controversies and Challenges
7.1. Optimal PEEP Levels and Individualized Settings
7.2. Use of High-Frequency Oscillatory Ventilation
7.3. Debates Around Permissive Hypercapnia
7.4. Ventilation in COVID-19 and Lessons Learned
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| AI | Artificial intelligence |
| COPD | Chronic obstructive pulmonary disease |
| DAMPs | Damage-associated molecular patterns |
| EIT | Electrical impedance tomography |
| ICU | Intensive care unit |
| HFOV | High-frequency oscillatory ventilation |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| MSCs | Mesenchymal stem cells |
| NK-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NMBAs | Neuromuscular blocking agents |
| PEEP | Positive end-expiratory pressure |
| RAGE | Receptor for advanced glycation end products |
| ROS | Reactive oxygen species |
| SP-D | Surfactant protein D |
| TCAV | Time-controlled adaptive ventilation |
| TNF-α | Tumor necrosis factor-alpha |
| TSPO | Translocator protein |
| VALI | Ventilator associated lung injury |
| VE-cadherin | Vascular endothelial cadherin |
| VILI | Ventilator induced lung injury |
| YAP | Yes-associated protein |
References
- Pinhu, L.; Whitehead, T.; Evans, T.; Griffiths, M. Ventilator-associated lung injury. Lancet 2003, 361, 332–340. [Google Scholar] [CrossRef]
- American Thoracic Society. International consensus conferences in intensive care medicine: Ventilator-associated Lung Injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, The European Society of Intensive Care Medicine, and The Societe de Reanimation de Langue Francaise, and was approved by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 1999, 160, 2118–2124. [Google Scholar] [CrossRef]
- Gajic, O.; Dara, S.I.; Mendez, J.L.; Adesanya, A.O.; Festic, E.; Caples, S.M.; Rana, R.; St Sauver, J.L.; Lymp, J.F.; Afessa, B.; et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit. Care Med. 2004, 32, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Plotz, F.B.; Slutsky, A.S.; van Vught, A.J.; Heijnen, C.J. Ventilator-induced lung injury and multiple system organ failure: A critical review of facts and hypotheses. Intensive Care Med. 2004, 30, 1865–1872. [Google Scholar] [CrossRef]
- The Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Rocco, P.R.; Dos Santos, C.; Pelosi, P. Pathophysiology of ventilator-associated lung injury. Curr. Opin. Anaesthesiol. 2012, 25, 123–130. [Google Scholar] [CrossRef]
- Kuchnicka, K.; Maciejewski, D. Ventilator-associated lung injury. Anaesthesiol. Intensive Ther. 2013, 45, 164–170. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Gattinoni, L.; Giosa, L.; Bonifazi, M.; Pasticci, I.; Busana, M.; Macri, M.; Romitti, F.; Vassalli, F.; Quintel, M. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev. Respir. Med. 2019, 13, 737–746. [Google Scholar] [CrossRef]
- Blankman, P.; Hasan, D.; Bikker, I.G.; Gommers, D. Lung stress and strain calculations in mechanically ventilated patients in the intensive care unit. Acta Anaesthesiol. Scand. 2016, 60, 69–78. [Google Scholar] [CrossRef]
- Telias, I.; Madorno, M.; Pham, T.; Coudroy, R.; Mellado Artigas, R.; Baedorf-Kassis, E.; Chen, C.W.; Spadaro, S.; Chiumello, D.; Beitler, J.; et al. Physiological Consequences of Breathing Effort According to the Mode of Ventilation During Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2025. [Google Scholar] [CrossRef]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Semin. Respir. Crit. Care Med. 2022, 43, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Carlesso, E.; Brioni, M.; Cressoni, M. Airway driving pressure and lung stress in ARDS patients. Crit. Care 2016, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.M.; Page, D.; Stephens, R.J.; Roberts, B.W.; Drewry, A.M.; Ablordeppey, E.; Mohr, N.M.; Kollef, M.H. Pulmonary Mechanics and Mortality in Mechanically Ventilated Patients Without Acute Respiratory Distress Syndrome: A Cohort Study. Shock 2018, 49, 311–316. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Liu, N.; Tan, X.Y.; Yue, H.; Fang, M.X. The effect of driving pressure-guided ventilation strategy on the patients with mechanical ventilation: A meta-analysis of randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5835–5843. [Google Scholar] [CrossRef]
- Gattinoni, L.; Protti, A.; Caironi, P.; Carlesso, E. Ventilator-induced lung injury: The anatomical and physiological framework. Crit. Care Med. 2010, 38, S539–S548. [Google Scholar] [CrossRef]
- Tsumura, H.; Harris, E.; Brandon, D.; Pan, W.; Vacchiano, C. Review of the Mechanisms of Ventilator Induced Lung Injury and the Principles of Intraoperative Lung Protective Ventilation. AANA J. 2021, 89, 227–233. [Google Scholar]
- Oeckler, R.A.; Hubmayr, R.D. Ventilator-associated lung injury: A search for better therapeutic targets. Eur. Respir. J. 2007, 30, 1216–1226. [Google Scholar] [CrossRef]
- Carvalho, C.G.; Silveira, R.C.; Procianoy, R.S. Ventilator-induced lung injury in preterm infants. Rev. Bras. Ter. Intensiv. 2013, 25, 319–326. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Lynch, J.P., 3rd; Strieter, R.M. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin. Respir. Crit. Care Med. 2006, 27, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, M.T.; van der Poll, T.; Schultz, M.J.; Wieland, C.W. Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit. Care 2011, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Penesova, A.; Galusova, A.; Vigas, M.; Vlcek, M.; Imrich, R.; Majek, M. The role of endocrine mechanisms in ventilator-associated lung injury in critically ill patients. Endocr. Regul. 2012, 46, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Parodo, J.; Kajikawa, O.; de Perrot, M.; Fischer, S.; Edwards, V.; Cutz, E.; Liu, M.; Keshavjee, S.; Martin, T.R.; et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003, 289, 2104–2112. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.; Alam, A.; Mi, E.; Eguchi, S.; Yao, S.; Ma, D. Postoperative remote lung injury and its impact on surgical outcome. BMC Anesthesiol. 2019, 19, 30. [Google Scholar] [CrossRef]
- Merola, R.; Vargas, M.; Battaglini, D. Ventilator-Induced Lung Injury: The Unseen Challenge in Acute Respiratory Distress Syndrome Management. J. Clin. Med. 2025, 14, 3910. [Google Scholar] [CrossRef]
- von During, S.; Parhar, K.K.S.; Adhikari, N.K.J.; Urner, M.; Kim, S.J.; Munshi, L.; Liu, K.; Fan, E. Understanding ventilator-induced lung injury: The role of mechanical power. J. Crit. Care 2025, 85, 154902. [Google Scholar] [CrossRef]
- Marini, J.J. Ventilator-associated problems related to obstructive lung disease. Respir. Care 2013, 58, 938–949. [Google Scholar] [CrossRef]
- Rouze, A.; Cottereau, A.; Nseir, S. Chronic obstructive pulmonary disease and the risk for ventilator-associated pneumonia. Curr. Opin. Crit. Care 2014, 20, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, W.; Kang, Y.; He, Q.; Zhang, H.; Deng, Y.; Cai, L.; Zhang, R.; Sun, X.; Zong, Z. Clinical outcomes and risk factors for mortality from ventilator-associated events: A registry-based cohort study among 30,830 intensive care unit patients. Infect. Control Hosp. Epidemiol. 2022, 43, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Rocco, P.R.M.; Gattinoni, L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am. J. Respir. Crit. Care Med. 2020, 201, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Chen, R. Neutrophil gelatinase-associated lipocalin as a potential novel biomarker for ventilator-associated lung injury. Mol. Med. Rep. 2017, 15, 3535–3540. [Google Scholar] [CrossRef]
- Salman, D.; Finney, S.J.; Griffiths, M.J. Strategies to reduce ventilator-associated lung injury (VALI). Burns 2013, 39, 200–211. [Google Scholar] [CrossRef]
- Matuschak, G.M.; Lechner, A.J. Acute lung injury and the acute respiratory distress syndrome: Pathophysiology and treatment. Mo. Med. 2010, 107, 252–258. [Google Scholar]
- Anjum, A.K.A.F. Ventilator-Induced Lung Injury (VILI); StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Calfee, C.S.; Ware, L.B.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Wickersham, N.; Matthay, M.A.; Network, N.A. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008, 63, 1083–1089. [Google Scholar] [CrossRef]
- Eisner, M.D.; Parsons, P.; Matthay, M.A.; Ware, L.; Greene, K.; The Acute Respiratory Distress Syndrome Network. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003, 58, 983–988. [Google Scholar] [CrossRef]
- de Prost, N.; Ricard, J.D.; Saumon, G.; Dreyfuss, D. Ventilator-induced lung injury: Historical perspectives and clinical implications. Ann. Intensive Care 2011, 1, 28. [Google Scholar] [CrossRef]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Brower, R.G.; Lanken, P.N.; MacIntyre, N.; Matthay, M.A.; Morris, A.; Ancukiewicz, M.; Schoenfeld, D.; Thompson, B.T.; The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004, 351, 327–336. [Google Scholar] [CrossRef]
- Meade, M.O.; Cook, D.J.; Guyatt, G.H.; Slutsky, A.S.; Arabi, Y.M.; Cooper, D.J.; Davies, A.R.; Hand, L.E.; Zhou, Q.; Thabane, L.; et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008, 299, 637–645. [Google Scholar] [CrossRef]
- Mercat, A.; Richard, J.C.; Vielle, B.; Jaber, S.; Osman, D.; Diehl, J.L.; Lefrant, J.Y.; Prat, G.; Richecoeur, J.; Nieszkowska, A.; et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA 2008, 299, 646–655. [Google Scholar] [CrossRef]
- Sud, S.; Sud, M.; Friedrich, J.O.; Adhikari, N.K. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis. CMAJ 2008, 178, 1153–1161. [Google Scholar] [CrossRef]
- Guerin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Rocco, P.R.; Pelosi, P.; de Abreu, M.G. Pros and cons of recruitment maneuvers in acute lung injury and acute respiratory distress syndrome. Expert Rev. Respir. Med. 2010, 4, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Valentine, S.L.; Sapru, A.; Higgerson, R.A.; Spinella, P.C.; Flori, H.R.; Graham, D.A.; Brett, M.; Convery, M.; Christie, L.M.; Karamessinis, L.; et al. Fluid balance in critically ill children with acute lung injury. Crit. Care Med. 2012, 40, 2883–2889. [Google Scholar] [CrossRef]
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; deBoisblanc, B.; Connors, A.F., Jr.; Hite, R.D.; Winston-Salem, N.C.; et al. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Herbert, J.A.; Valentine, M.S.; Saravanan, N.; Schneck, M.B.; Pidaparti, R.; Fowler, A.A., 3rd; Reynolds, A.M.; Heise, R.L. Conservative fluid management prevents age-associated ventilator induced mortality. Exp. Gerontol. 2016, 81, 101–109. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Constantin, J.M.; Jabaudon, M.; Lefrant, J.Y.; Jaber, S.; Quenot, J.P.; Langeron, O.; Ferrandiere, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Sinnige, J.S.; Smit, M.R.; Ghose, A.; de Grooth, H.J.; Itenov, T.S.; Ischaki, E.; Laffey, J.; Paulus, F.; Povoa, P.; Pierrakos, C.; et al. Personalized mechanical ventilation guided by ultrasound in patients with acute respiratory distress syndrome (PEGASUS): Study protocol for an international randomized clinical trial. Trials 2024, 25, 308. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Ball, L.; Barbas, C.S.V.; Bellomo, R.; Burns, K.E.A.; Einav, S.; Gattinoni, L.; Laffey, J.G.; Marini, J.J.; Myatra, S.N.; et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit. Care 2021, 25, 250. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Deliberato, R.O.; Johnson, A.E.W.; Bos, L.D.; Amorim, P.; Pereira, S.M.; Cazati, D.C.; Cordioli, R.L.; Correa, T.D.; Pollard, T.J.; et al. Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts. Intensive Care Med. 2018, 44, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Santer, P.; Wachtendorf, L.J.; Suleiman, A.; Houle, T.T.; Fassbender, P.; Costa, E.L.; Talmor, D.; Eikermann, M.; Baedorf-Kassis, E.; Schaefer, M.S. Mechanical Power during General Anesthesia and Postoperative Respiratory Failure: A Multicenter Retrospective Cohort Study. Anesthesiology 2022, 137, 41–54. [Google Scholar] [CrossRef]
- El-Khatib, M.; Zeeni, C.; Shebbo, F.M.; Karam, C.; Safi, B.; Toukhtarian, A.; Nafeh, N.A.; Mkhayel, S.; Shadid, C.A.; Chalhoub, S.; et al. Intraoperative mechanical power and postoperative pulmonary complications in low-risk surgical patients: A prospective observational cohort study. BMC Anesthesiol. 2024, 24, 82. [Google Scholar] [CrossRef]
- Azizi, B.A.; Munoz-Acuna, R.; Suleiman, A.; Ahrens, E.; Redaelli, S.; Tartler, T.M.; Chen, G.; Jung, B.; Talmor, D.; Baedorf-Kassis, E.N.; et al. Mechanical power and 30-day mortality in mechanically ventilated, critically ill patients with and without Coronavirus Disease-2019: A hospital registry study. J. Intensive Care 2023, 11, 14. [Google Scholar] [CrossRef]
- Silva, P.L.; Scharffenberg, M.; Rocco, P.R.M. Understanding the mechanisms of ventilator-induced lung injury using animal models. Intensive Care Med. Exp. 2023, 11, 82. [Google Scholar] [CrossRef]
- Munoz, J.; Cedeno, J.A.; Castaneda, G.F.; Visedo, L.C. Personalized ventilation adjustment in ARDS: A systematic review and meta-analysis of image, driving pressure, transpulmonary pressure, and mechanical power. Heart Lung 2024, 68, 305–315. [Google Scholar] [CrossRef]
- Misseri, G.; Piattoli, M.; Cuttone, G.; Gregoretti, C.; Bignami, E.G. Artificial Intelligence for Mechanical Ventilation: A Transformative Shift in Critical Care. Ther. Adv. Pulm. Crit. Care Med. 2024, 19, 29768675241298918. [Google Scholar] [CrossRef]
- Arnal, J.M.; Wysocki, M.; Novotni, D.; Demory, D.; Lopez, R.; Donati, S.; Granier, I.; Corno, G.; Durand-Gasselin, J. Safety and efficacy of a fully closed-loop control ventilation (IntelliVent-ASV®) in sedated ICU patients with acute respiratory failure: A prospective randomized crossover study. Intensive Care Med. 2012, 38, 781–787. [Google Scholar] [CrossRef]
- Bialais, E.; Wittebole, X.; Vignaux, L.; Roeseler, J.; Wysocki, M.; Meyer, J.; Reychler, G.; Novotni, D.; Sottiaux, T.; Laterre, P.F.; et al. Closed-loop ventilation mode (IntelliVent®-ASV) in intensive care unit: A randomized trial. Minerva Anestesiol. 2016, 82, 657–668. [Google Scholar]
- Al-Khalisy, H.; Nieman, G.F.; Kollisch-Singule, M.; Andrews, P.; Camporota, L.; Shiber, J.; Manougian, T.; Satalin, J.; Blair, S.; Ghosh, A.; et al. Time-Controlled Adaptive Ventilation (TCAV): A personalized strategy for lung protection. Respir. Res. 2024, 25, 37. [Google Scholar] [CrossRef]
- Su, K.; Wang, J.; Lv, Y.; Tian, M.; Zhao, Y.Y.; Minshall, R.D.; Hu, G. YAP expression in endothelial cells prevents ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L568–L582. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, D.; Lv, H.; Jiang, X. Remimazolam Alleviates Ventilator-Induced Lung Injury by Activating TSPO to Inhibit Macrophage Pyroptosis. Discov. Med. 2024, 36, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.X.; Zhang, J.H.; Zhao, B.C.; Liu, K.X.; Bai, Y.W. Dexmedetomidine attenuates one-lung ventilation associated lung injury by suppressing inflammatory responses: A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2012, 67, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef]
- Zheng, G.; Huang, L.; Tong, H.; Shu, Q.; Hu, Y.; Ge, M.; Deng, K.; Zhang, L.; Zou, B.; Cheng, B.; et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study. Respir. Res. 2014, 15, 39. [Google Scholar] [CrossRef]
- Wang, W.; Lei, W.; Jiang, L.; Gao, S.; Hu, S.; Zhao, Z.G.; Niu, C.Y.; Zhao, Z.A. Therapeutic mechanisms of mesenchymal stem cells in acute respiratory distress syndrome reveal potentials for Covid-19 treatment. J. Transl. Med. 2021, 19, 198. [Google Scholar] [CrossRef]
- Liou, J.; Doherty, D.; Gillin, T.; Emberger, J.; Yi, Y.; Cardenas, L.; Benninghoff, M.; Vest, M.; Deitchman, A. Retrospective Review of Transpulmonary Pressure Guided Positive End-Expiratory Pressure Titration for Mechanical Ventilation in Class II and III Obesity. Crit. Care Explor. 2022, 4, e0690. [Google Scholar] [CrossRef] [PubMed]
- Muders, T.; Luepschen, H.; Putensen, C. Impedance tomography as a new monitoring technique. Curr. Opin. Crit. Care 2010, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Piedra, C.; Rodriguez-Ortiz-de-Salazar, B.; Roca, O.; Prado-Galbarro, F.J.; Perestelo-Perez, L.; Sanchez-Gomez, L.M. Electrical impedance tomography for PEEP titration in ARDS patients: A systematic review and meta-analysis. J. Clin. Monit. Comput. 2025, 39, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.I.; Jagelman, T.A.; Webster, N.R. Emerging modes of ventilation in the intensive care unit. Br. J. Anaesth. 2011, 107, 74–82. [Google Scholar] [CrossRef]
- Dani, C.; Bertini, G.; Pezzati, M.; Filippi, L.; Pratesi, S.; Caviglioli, C.; Rubaltelli, F.F. Effects of pressure support ventilation plus volume guarantee vs. high-frequency oscillatory ventilation on lung inflammation in preterm infants. Pediatr. Pulmonol. 2006, 41, 242–249. [Google Scholar] [CrossRef]
- Young, D.; Lamb, S.E.; Shah, S.; MacKenzie, I.; Tunnicliffe, W.; Lall, R.; Rowan, K.; Cuthbertson, B.H.; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 806–813. [Google Scholar] [CrossRef]
- Ismaiel, N.; Whynot, S.; Geldenhuys, L.; Xu, Z.; Slutsky, A.S.; Chappe, V.; Henzler, D. Lung-Protective Ventilation Attenuates Mechanical Injury While Hypercapnia Attenuates Biological Injury in a Rat Model of Ventilator-Associated Lung Injury. Front. Physiol. 2022, 13, 814968. [Google Scholar] [CrossRef]
- Fuchs, H.; Rossmann, N.; Schmid, M.B.; Hoenig, M.; Thome, U.; Mayer, B.; Klotz, D.; Hummler, H.D. Permissive hypercapnia for severe acute respiratory distress syndrome in immunocompromised children: A single center experience. PLoS ONE 2017, 12, e0179974. [Google Scholar] [CrossRef]
- Nin, N.; Muriel, A.; Penuelas, O.; Brochard, L.; Lorente, J.A.; Ferguson, N.D.; Raymondos, K.; Rios, F.; Violi, D.A.; Thille, A.W.; et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 200–208. [Google Scholar] [CrossRef]
- Yamamoto, R.; Kaito, D.; Homma, K.; Endo, A.; Tagami, T.; Suzuki, M.; Umetani, N.; Yagi, M.; Nashiki, E.; Suhara, T.; et al. Early intubation and decreased in-hospital mortality in patients with coronavirus disease 2019. Crit. Care 2022, 26, 124. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, C.; Bouadma, L.; de Montmollin, E.; Goldgran-Toledano, D.; Schwebel, C.; Reignier, J.; Neuville, M.; Ursino, M.; Siami, S.; Ruckly, S.; et al. Association Between Early Invasive Mechanical Ventilation and Day-60 Mortality in Acute Hypoxemic Respiratory Failure Related to Coronavirus Disease-2019 Pneumonia. Crit. Care Explor. 2021, 3, e0329. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Sedhai, Y.R.; Budhathoki, P.; Adhikari, A.; Pokharel, N.; Dhakal, R.; Kafle, S.; Yadullahi Mir, W.A.; Acharya, R.; Kashiouris, M.G.; et al. Pulmonary barotrauma in COVID-19: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 73, 103221. [Google Scholar] [CrossRef]
- Bajto, P.; Saric, I.; Bugarin, J.D.; Delic, N.; Dosenovic, S.; Ilic, D.; Stipic, S.S.; Duplancic, B.; Saric, L. Barotrauma in patients with severe coronavirus disease 2019—Retrospective observational study. J. Thorac. Dis. 2023, 15, 5297–5306. [Google Scholar] [CrossRef] [PubMed]
- Mega, C.; Cavalli, I.; Ranieri, V.M.; Tonetti, T. Protective ventilation in patients with acute respiratory distress syndrome related to COVID-19: Always, sometimes or never? Curr. Opin. Crit. Care 2022, 28, 51–56. [Google Scholar] [CrossRef] [PubMed]
| Intervention | Effect | Evidence/Source |
|---|---|---|
| Low tidal volume ventilation (6 mL/kg PBW, plateau ≤ 30 cm H2O) | Reduced mortality by ~9%, shorter duration of mechanical ventilation, lower inflammatory cytokine levels | ARDSNet (2000) [7] |
| Individualized PEEP titration | Improved oxygenation and reduced ventilator dependency; no consistent mortality benefit | Meade et al. [43], Mercat et al. [44] |
| Prone positioning (early, prolonged sessions in severe ARDS) | Improved oxygenation and significantly reduced 28- and 90-day mortality without major increase in complications | PROSEVA trial [46] |
| Neuromuscular blocking agents (48-h cisatracurium infusion) | Reduced 28- and 90-day mortality, fewer days on ventilation, lower incidence of barotrauma and pneumothorax | ACURASYS trial [47] |
| Conservative fluid management | Reduced pulmonary edema, increased ventilator-free days, improved outcomes | FACTT trial [50] |
| Early mobilization and sedation minimization | Improved functional recovery, increased ventilator-free days, enhanced physical and neurocognitive outcomes | Schweickert et al. [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Sakho, B.; Gomez, S.; Khanyan, B.; Leybengrub, P.; Bergese, S. Ventilator-Associated Lung Injury: Pathophysiology, Prevention, and Emerging Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 10448. https://doi.org/10.3390/ijms262110448
Costa A, Sakho B, Gomez S, Khanyan B, Leybengrub P, Bergese S. Ventilator-Associated Lung Injury: Pathophysiology, Prevention, and Emerging Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(21):10448. https://doi.org/10.3390/ijms262110448
Chicago/Turabian StyleCosta, Ana, Bintia Sakho, Sangel Gomez, Brandon Khanyan, Pamella Leybengrub, and Sergio Bergese. 2025. "Ventilator-Associated Lung Injury: Pathophysiology, Prevention, and Emerging Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 21: 10448. https://doi.org/10.3390/ijms262110448
APA StyleCosta, A., Sakho, B., Gomez, S., Khanyan, B., Leybengrub, P., & Bergese, S. (2025). Ventilator-Associated Lung Injury: Pathophysiology, Prevention, and Emerging Therapeutic Strategies. International Journal of Molecular Sciences, 26(21), 10448. https://doi.org/10.3390/ijms262110448





