TFAM Loss Induces Oxidative Stress and Divergent Phenotypes in Glioblastoma Metabolic Subtypes
Abstract
1. Introduction
2. Results
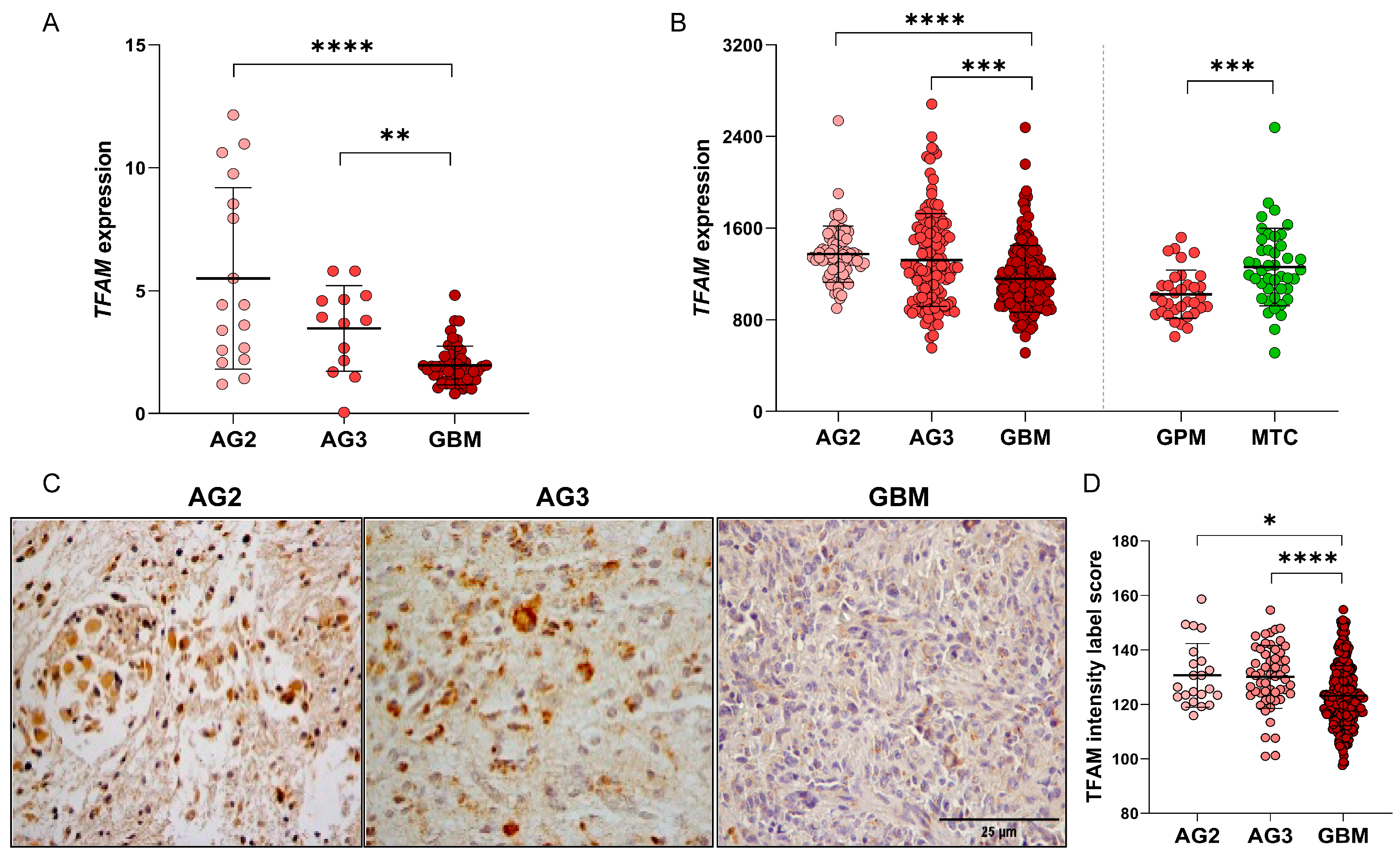
2.1. Subsection TFAM Expression Analysis in Human Astrocytomas Shows Significantly Higher Expression in Lower-Grade Astrocytomas
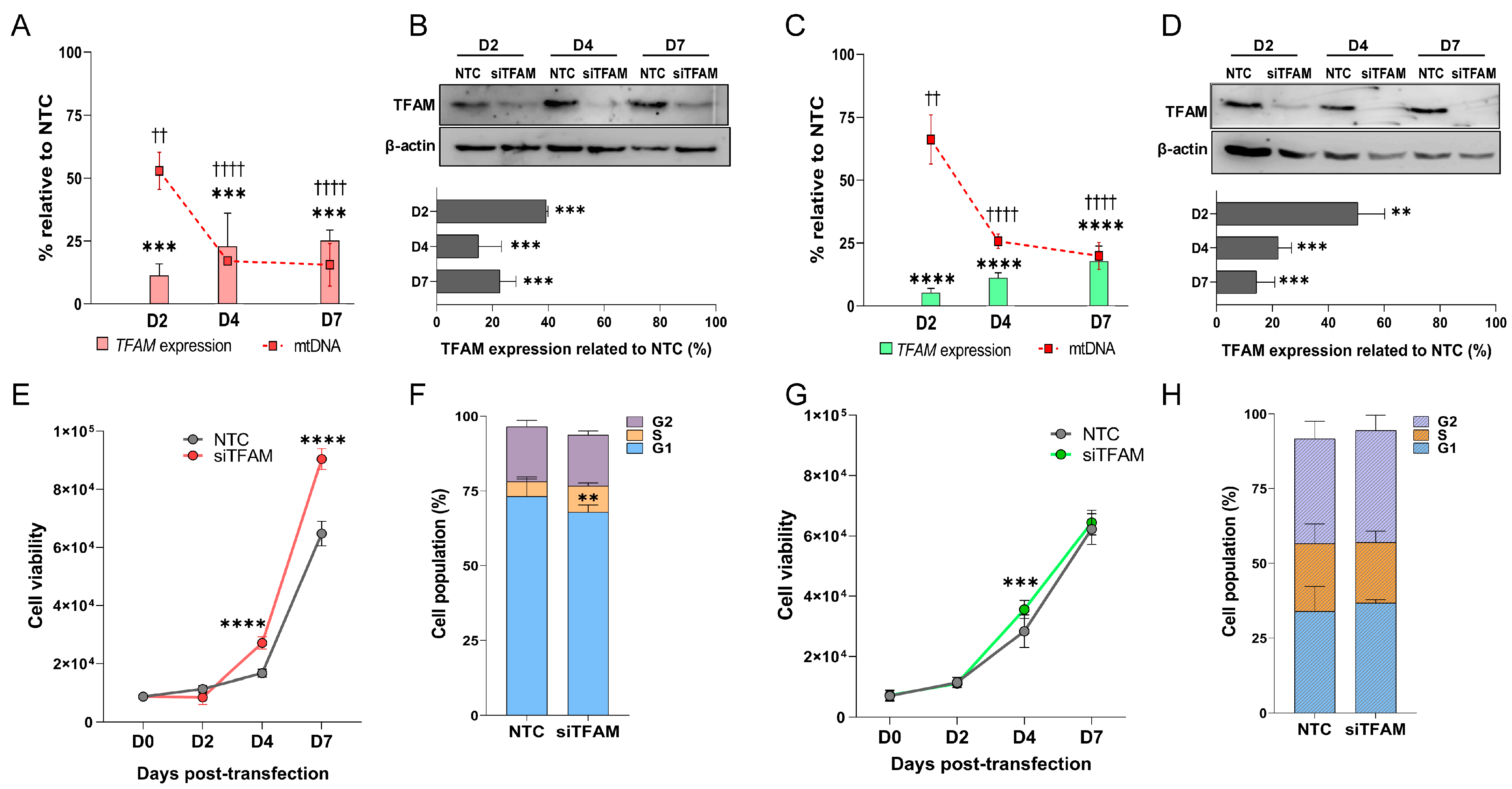
2.2. TFAM Transient Silencing Characterization
2.3. U87MG-siTFAM Cells Exhibit Enhanced Proliferation and Increased S-Phase Entry
2.4. U87MG-siTFAM Cells Exhibit Impaired Motility and Increased Adhesion
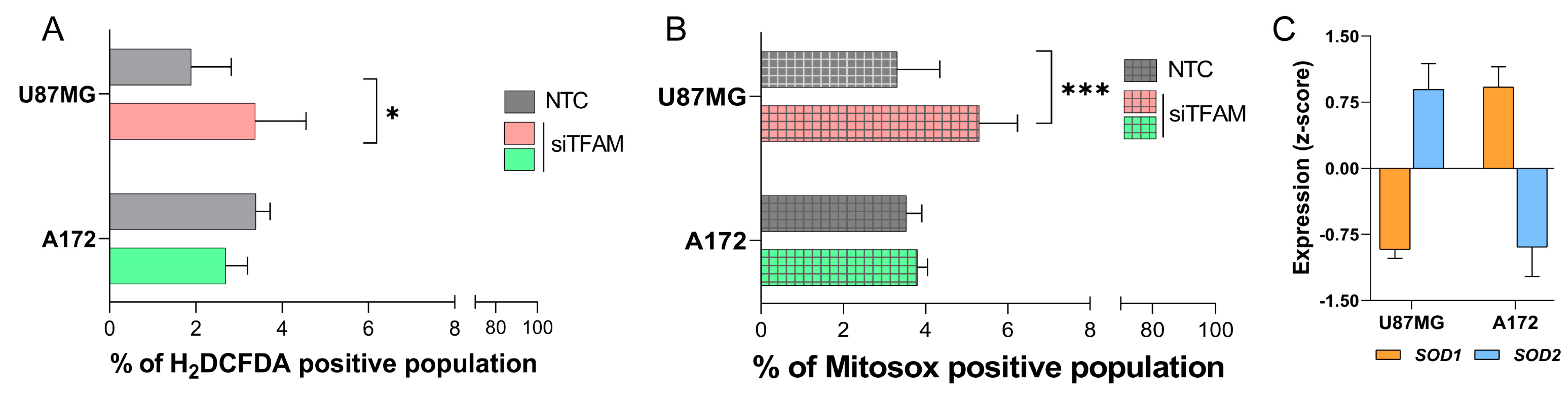
2.5. U87MG-siTFAM and A172-siTFAM Display Differential Responses to Oxidative Stress
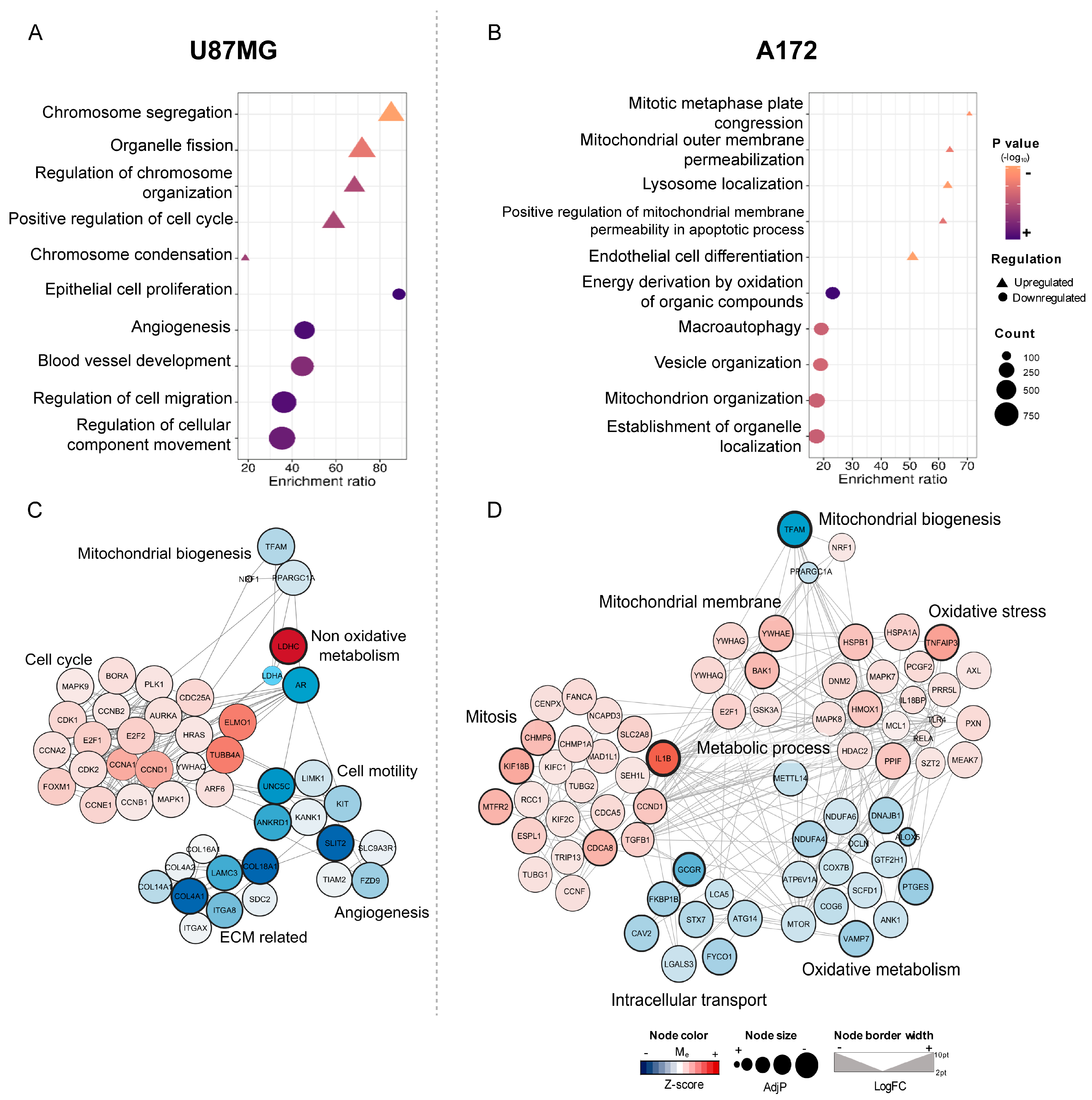
2.6. Transcriptomic Profiles of U87MG and A172-siTFAM Cells
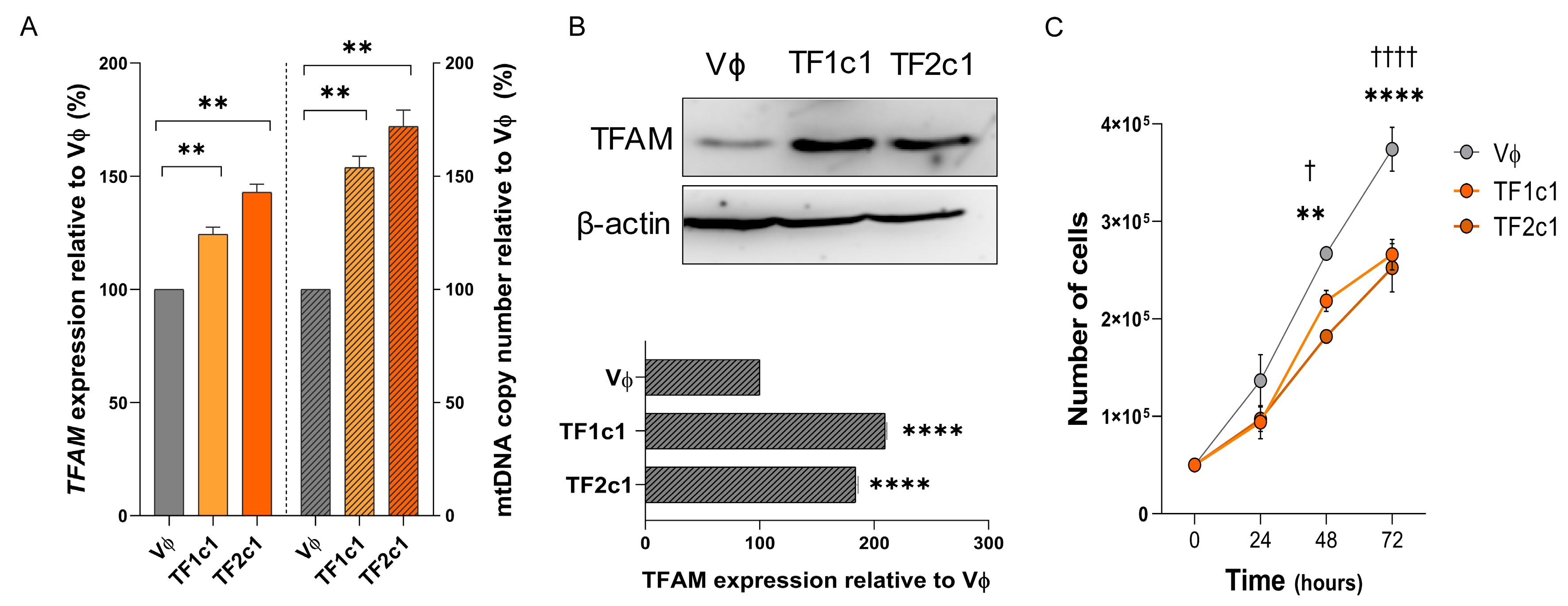
2.7. TFAM Overexpression
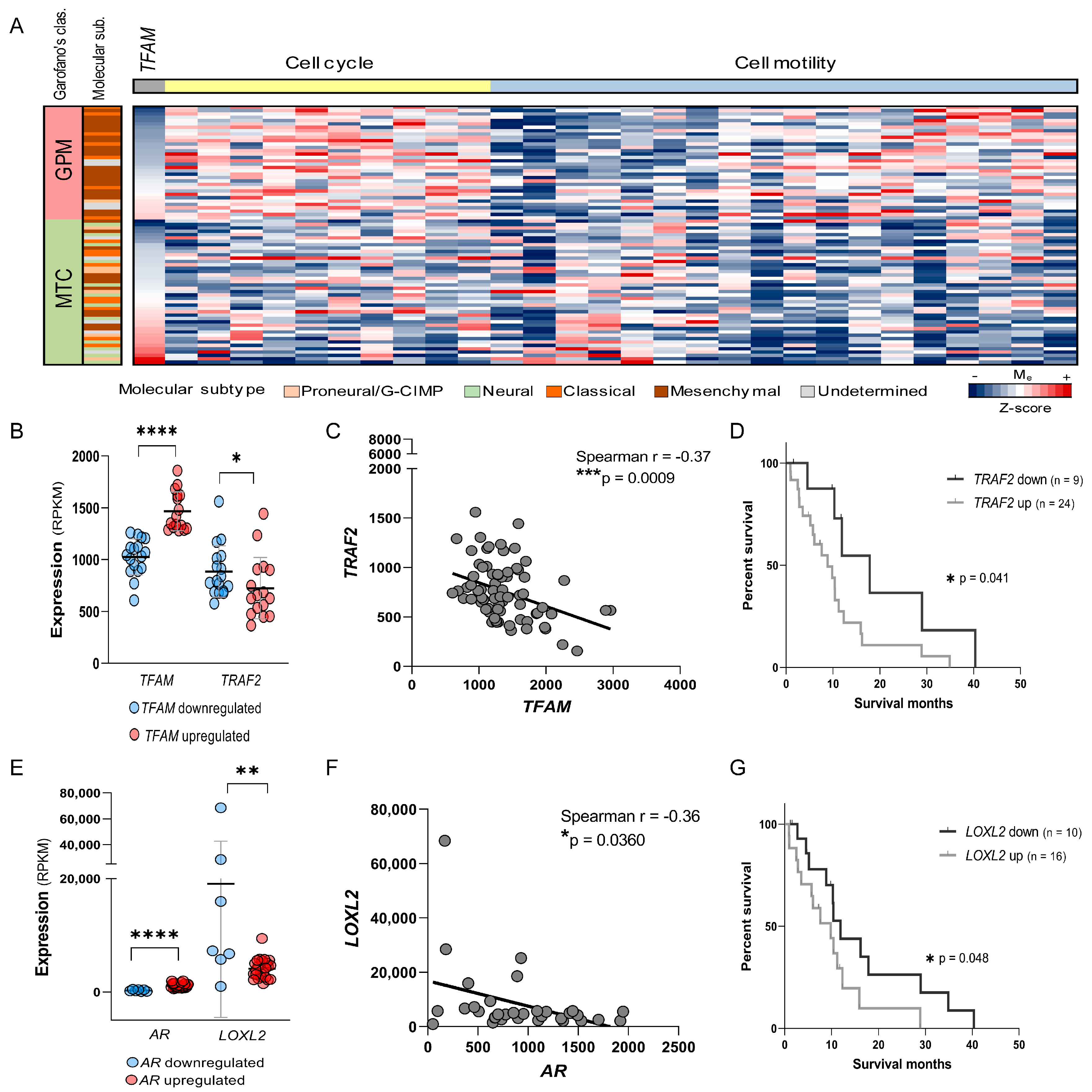
2.8. TFAM Levels Predict Cell Cycle and Motility Pathway Activity and Identify TRAF2 as a Potential Therapeutic Target in GPM-GBM
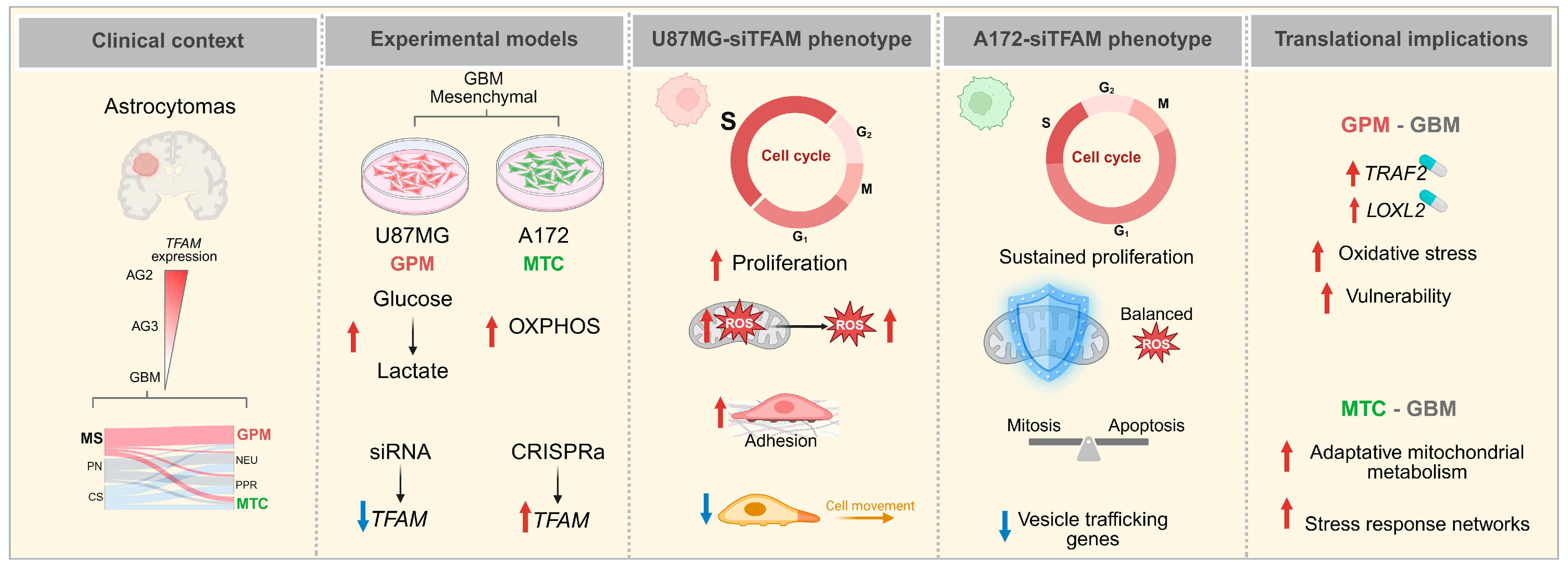
3. Discussion
4. Materials and Methods
4.1. Tumor Sample Collection and Processing, and TCGA Data
4.2. TCGA Gene Expression Analysis
4.3. Cell Culture
4.4. TFAM Silencing by siRNA
4.5. TFAM Activation by CRISPRa
4.6. Nucleic Acid Extraction and cDNA Synthesis
4.7. Quantitative PCR and mtDNA Copy Number
4.8. Western Blot
4.9. Immunohistochemistry
4.10. Cell Viability and Proliferation
4.11. Cell Cycle Analysis
4.12. Cell Motility Analyses
4.13. Cell Adhesion Assay
4.14. General ROS Detection
4.15. Mitochondrial Superoxide Detection
4.16. RNA Sequencing and Bioinformatics
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Peralta, S.; Wang, X.; Moraes, C.T. Mitochondrial transcription: Lessons from mouse models. Biochim. Biophys. Acta 2012, 1819, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.C.; Holt, I.J. Clinical and molecular aspects of diseases of mitochondrial DNA instability. Chang Gung Med. J. 2009, 32, 354–369. [Google Scholar] [PubMed]
- Alam, T.I.; Kanki, T.; Muta, T.; Ukaji, K.; Abe, Y.; Nakayama, H.; Takio, K.; Hamasaki, N.; Kang, D. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003, 31, 1640–1645. [Google Scholar] [CrossRef]
- Bogenhagen, D.F. Mitochondrial DNA nucleoid structure. Biochim. Biophys. Acta 2012, 1819, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Hamasaki, N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: Overview of its multiple roles. Ann. N. Y. Acad. Sci. 2005, 1042, 101–108. [Google Scholar] [CrossRef]
- Mishmar, D.; Levin, R.; Naeem, M.M.; Sondheimer, N. Higher Order Organization of the mtDNA: Beyond Mitochondrial Transcription Factor A. Front. Genet. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Falkenberg, M.; Larsson, N.G.; Gustafsson, C.M. DNA replication and transcription in mammalian mitochondria. Annu. Rev. Biochem. 2007, 76, 679–699. [Google Scholar] [CrossRef]
- Lee, E.J.; Kang, Y.C.; Park, W.H.; Jeong, J.H.; Pak, Y.K. Negative Transcriptional Regulation of Mitochondrial Transcription Factor A (TFAM) by Nuclear TFAM. Biochem. Biophys. Commun. 2014, 450, 166–171. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J. Biol. Chem. 2006, 281, 324–333. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Parker, N.R.; Khong, P.; Parkinson, J.F.; Howell, V.M.; Wheeler, H.R. Molecular heterogeneity in glioblastoma: Potential clinical implications. Front. Oncol. 2015, 5, 55. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Garofano, L.; Migliozzi, S.; Oh, Y.T.; D’Angelo, F.; Najac, R.D.; Ko, A.; Frangaj, B.; Caruso, F.P.; Yu, K.; Yuan, J.; et al. Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat. Cancer 2021, 2, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.E.; Coomber, B.L.; Bridle, B.W. Metabolic reprogramming in the tumour microenvironment: A hallmark shared by cancer cells and T lymphocytes. Immunology 2017, 152, 175–184. [Google Scholar] [CrossRef]
- Correia, R.L.; Oba-Shinjo, S.M.; Uno, M.; Huang, H.; Marie, S.K.N. Mitochondrial DNA Depletion and Its Correlation with TFAM, TFB1M, TFB2M and POLG in Human Diffusely Infiltrating Astrocytomas. Mitochondrion 2011, 11, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Moretti, I.F.; Lerario, A.M.; Sola, P.R.; Macedo-da-Silva, J.; Baptista, M.D.S.; Palmisano, G.; Oba-Shinjo, S.M.; Marie, S.K.N. GBM Cells Exhibit Susceptibility to Metformin Treatment According to TLR4 Pathway Activation and Metabolic and Antioxidant Status. Cancers 2023, 15, 587. [Google Scholar] [CrossRef]
- Katsuki, T.; Nakayama, Y.; Akiyama, M.; Sawatsubashi, Y.; Nagata, J.; Minagawa, N.; Torigoe, T.; Izumi, H.; Kohono, K.; Hirata, K. Prognostic Significance of Mitochondrial Transcription Factor A Expression in Patients with Right-or Left-sided Colorectal Cancer. Anticancer Res. 2018, 38, 569–575. [Google Scholar] [PubMed]
- Gabrielson, M.; Björklund, M.; Carlson, J.; Shoshan, M. Expression of Mitochondrial Regulators PGC1α and TFAM as Putative Markers of Subtype and Chemoresistance in Epithelial Ovarian Carcinoma. PLoS ONE 2014, 9, e107109. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.F.; Siena, A.D.D.; Plaça, J.R.; Brotto, D.B.; Barros, I.I.; Muys, B.R.; Biagi, C.A.O., Jr.; Peronni, K.C.; Sousa, J.F.; Molfetta, G.A.; et al. Mitochondrial Transcription Factor A (TFAM) Shapes Metabolic and Invasion Gene Signatures in Melanoma. Sci. Rep. 2018, 8, 14190. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Nakayama, Y.; Minagawa, N.; Torigoe, T.; Shibao, K.; Yamaguchi, K. Mitochondrial Transcription Factor a Worsens the Clinical Course of Patients with Pancreatic Cancer Through Inhibition of Apoptosis of Cancer Cells. Pancreas 2014, 43, 405–410. [Google Scholar] [CrossRef]
- Han, B.; Izumi, H.; Yasuniwa, Y.; Akiyama, M.; Yamaguchi, T.; Fujimoto, N.; Matsumoto, T.; Wu, B.; Tanimoto, A.; Sasaguri, Y.; et al. Human Mitochondrial Transcription Factor A Functions in Both Nuclei and Mitochondria and Regulates Cancer Cell Growth. Biochem. Biophys. Res. Commun. 2011, 408, 45–51. [Google Scholar] [CrossRef]
- Alán, L.; Špaček, T.; Pajuelo Reguera, D.; Jabůrek, M.; Ježek, P. Mitochondrial Nucleoid Clusters Protect Newly Synthesized mtDNA During Doxorubicin-And Ethidium Bromide-induced Mitochondrial Stress. Toxicol. Appl. Pharmacol. 2016, 302, 31–40. [Google Scholar] [CrossRef]
- Gilkerson, R.W.; Margineantu, D.H.; Capaldi, R.A.; Selker, J.M. Mitochondrial DNA Depletion Causes Morphological Changes in the Mitochondrial Reticulum of Cultured Human Cells. FEBS Lett. 2000, 474, 1–4. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, J. Mitochondrial Nucleoid: Shield and Switch of the Mitochondrial Genome. Oxid. Med. Cell. Longev. 2017, 2017, 8060949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538, Correction in ACS Nano 2021, 15, 20692. [Google Scholar] [CrossRef]
- Zhou, X.; Trinh-Minh, T.; Tran-Manh, C.; Gieβl, A.; Bergmann, C.; Györfi, A.H.; Schett, G.; Distler, J.H.W. Impaired Mitochondrial Transcription Factor A Expression Promotes Mitochondrial Damage to Drive Fibroblast Activation and Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2022, 74, 871–881. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Chen, H.; Yang, X.; Han, J.; Yao, X.; Wei, X.; Si, J.; Yao, H.; Liu, H.; et al. TFAM downregulation promotes autophagy and ESCC survival through mtDNA stress-mediated STING pathway. Oncogene 2022, 41, 3735–3746. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Tu, H.F.; Yang, M.H.; Chen, Y.F.; Lan, X.Y.; Huang, C.L.; Chen, H.M.; Li, W.C. Mitochondrial genome and its regulator TFAM modulates head and neck tumourigenesis through intracellular metabolic reprogramming and activation of oncogenic effectors. Cell Death Dis. 2021, 12, 961. [Google Scholar] [CrossRef]
- Lee, W.R.; Na, H.; Lee, S.W.; Lim, W.J.; Kim, N.; Lee, J.E.; Kang, C. Transcriptomic analysis of mitochondrial TFAM depletion changing cell morphology and proliferation. Sci. Rep. 2017, 7, 17841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, J.; Hu, W.; Wang, F.; Zhou, Y.; Xu, W.; Gong, W.; Shao, L. TFAM depletion overcomes hepatocellular carcinoma resistance to doxorubicin and sorafenib through AMPK activation and mitochondrial dysfunction. Gene 2020, 753, 144807. [Google Scholar] [CrossRef]
- Martins, F.; van der Kellen, D.; Gonçalves, L.G.; Serpa, J. Metabolic Profiles Point Out Metabolic Pathways Pivotal in Two Glioblastoma (GBM) Cell Lines, U251 and U-87MG. Biomedicines 2023, 11, 2041. [Google Scholar] [CrossRef]
- Naik, A.; Thomas, R.; Al-Khalifa, A.; Qasem, H.; Decock, J. Immunomodulatory effects of tumor Lactate Dehydrogenase C (LDHC) in breast cancer. Cell Commun. Signal. 2025, 23, 145. [Google Scholar] [CrossRef]
- Naik, A.; Decock, J. Targeting of lactate dehydrogenase C dysregulates the cell cycle and sensitizes breast cancer cells to DNA damage response targeted therapy. Mol. Oncol. 2022, 16, 885–903. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Q.; Xu, X.; Yang, C.; You, J.; Chen, F.; Zeng, Y. Cancer/testis antigen LDHC promotes proliferation and metastasis by activating the PI3K/Akt/GSK-3β-signaling pathway and the in lung adenocarcinoma. Exp. Cell Res. 2021, 398, 112414. [Google Scholar] [CrossRef]
- Hua, Y.; Liang, C.; Zhu, J.; Miao, C.; Yu, Y.; Xu, A.; Zhang, J.; Li, P.; Li, S.; Bao, M.; et al. Expression of lactate dehydrogenase C correlates with poor prognosis in renal cell carcinoma. Tumour Biol. 2017, 39, 1010428317695968. [Google Scholar] [CrossRef]
- Shadbad, M.A.; Baradaran, B. hsa-miR-181a-5p inhibits glioblastoma development via the MAPK pathway: In-silico and in-vitro study. Oncol. Res. 2024, 32, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H.; Benzinger, A. 14-3-3 proteins in cell cycle regulation. Semin. Cancer Biol. 2006, 16, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Sarkar, A.; Mondal, S. Cell cycle protein BORA is associated with colorectal cancer progression by AURORA-PLK1 cascades: A bioinformatics analysis. J. Cell Commun. Signal. 2023, 17, 773–791. [Google Scholar] [CrossRef]
- Baldassarre, G.; Belletti, B. Meet me in the cytoplasm: A role for p27(Kip1) in the control of H-Ras. Small GTPases 2016, 7, 71–75. [Google Scholar] [CrossRef]
- Ershov, P.; Poyarkov, S.; Konstantinova, Y.; Veselovsky, E.; Makarova, A. Transcriptomic Signatures in Colorectal Cancer Progression. Curr. Mol. Med. 2023, 23, 239–249. [Google Scholar] [CrossRef]
- Chiu, J.; Dawes, I.W. Redox control of cell proliferation. Trends Cell Biol. 2012, 22, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kirova, D.G.; Judasova, K.; Vorhauser, J.; Zerjatke, T.; Leung, J.K.; Glauche, I.; Mansfeld, J. ROS-dependent mechanism promotes CDK2 phosphorylation to drive progression through S phase. Dev. Cell. 2022, 57, 1712–1727.e9. [Google Scholar] [CrossRef] [PubMed]
- González-Arzola, K.; Díaz-Quintana, A. Mitochondrial Factors in the Cell Nucleus. Int. J. Mol. Sci. 2023, 24, 13656. [Google Scholar] [CrossRef]
- Tao, L.; Ren, X.; Zhai, W.; Chen, Z. Progress and Prospects of Non-Canonical NF-κB Signaling Pathway in the Regulation of Liver Diseases. Molecules 2022, 27, 14275. [Google Scholar] [CrossRef]
- Evans, S.; Tzeng, H.P.; Veis, D.J.; Matkovich, S.; Weinheimer, C.; Kovacs, A.; Barger, P.M.; Mann, D.L. TNF receptor-activated factor 2 mediates cardiac protection through noncanonical NF-κB signaling. JCI Insight 2018, 3, e98278. [Google Scholar] [CrossRef]
- Gentle, I.E.; Wong, W.W.; Evans, J.M.; Bankovacki, A.; Cook, W.D.; Khan, N.R.; Nachbur, U.; Rickard, J.; Anderton, H.; Moulin, M.; et al. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8. J. Biol. Chem. 2011, 286, 13282–13291. [Google Scholar] [CrossRef]
- Etemadi, N.; Chopin, M.; Anderton, H.; Tanzer, M.C.; Rickard, J.A.; Abeysekera, W.; Hall, C.; Spall, S.K.; Wang, B.; Xiong, Y.; et al. TRAF2 regulates TNF and NF-κB signalling to suppress apoptosis and skin inflammation independently of Sphingosine kinase 1. eLife 2015, 4, e10592. [Google Scholar] [CrossRef]
- Borghi, A.; Verstrepen, L.; Beyaert, R. TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death. Biochem. Pharmacol. 2016, 116, 1–10. [Google Scholar] [CrossRef]
- Au, P.Y.; Yeh, W.C. Physiological roles and mechanisms of signaling by TRAF2 and TRAF5. Adv. Exp. Med. Biol. 2007, 597, 32–47. [Google Scholar]
- Shen, R.R.; Zhou, A.Y.; Kim, E.; O’Connell, J.T.; Hagerstrand, D.; Beroukhim, R.; Hahn, W.C. TRAF2 is an NF-κB-activating oncogene in epithelial cancers. Oncogene 2015, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xuan, Y.; Huang, P.; Zhang, C.; Ke, X.; Qu, Y.; Hao, P.; Yan, R. Liquidambaric lactone is a potent inhibitor of TRAF2 for cancer therapy. Pharmacol. Res.-Mod. Chin. Med. 2023, 7, 100265. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Shen, Y.; Zhou, H.; Gao, Y.; Zhu, C.; Qin, X. Unlocking hepatocellular carcinoma aggression: STAMBPL1-mediated TRAF2 deubiquitination activates WNT/PI3K/NF-kb signaling pathway. Biol. Direct. 2024, 19, 18. [Google Scholar] [CrossRef]
- Deen, A.J.; Adinolfi, S.; Härkönen, J.; Patinen, T.; Liu, X.; Laitinen, T.; Takabe, P.; Kainulainen, K.; Pasonen-Seppänen, S.; Gawriyski, L.M.; et al. Oncogenic KEAP1 mutations activate TRAF2-NFκB signaling to prevent apoptosis in lung cancer cells. Redox Biol. 2024, 69, 103031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Zhang, H.H.; Du, X.H.; Gao, J.; Li, C.; Shi, H.R.; Li, S.Z. UCHL3 promotes ovarian cancer progression by stabilizing TRAF2 to activate the NF-κB pathway. Oncogene 2020, 39, 322–333. [Google Scholar] [CrossRef]
- Zheng, M.; Morgan-Lappe, S.E.; Yang, J.; Bockbrader, K.M.; Pamarthy, D.; Thomas, D.; Fesik, S.W.; Sun, Y. Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008, 68, 7570–7578. [Google Scholar] [CrossRef]
- Vredevoogd, D.W.; Kuilman, T.; Ligtenberg, M.A.; Boshuizen, J.; Stecker, K.E.; de Bruijn, B.; Krijgsman, O.; Huang, X.; Kenski, J.C.N.; Lacroix, R.; et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell 2019, 178, 585–599.e15. [Google Scholar] [CrossRef]
- Yan, R.; Zhu, H.; Huang, P.; Yang, M.; Shen, M.; Pan, Y.; Zhang, C.; Zhou, X.; Li, H.; Ke, X.; et al. Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep. 2022, 38, 110319. [Google Scholar] [CrossRef]
- BioInvent International AB. BI-1808 As a Single Agent and with Pembrolizumab (KEYTRUDA®) in Treatment of Advanced Malignancies (Keynote-D20). Available online: https://clinicaltrials.gov/study/NCT04752826#study-overview (accessed on 8 August 2025).
- Rodríguez-Lozano, D.C.; Piña-Medina, A.G.; Hansberg-Pastor, V.; Bello-Alvarez, C.; Camacho-Arroyo, I. Testosterone Promotes Glioblastoma Cell Proliferation, Migration, and Invasion Through Androgen Receptor Activation. Front. Endocrinol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, Y.; Kong, Z.; Zhang, Y.; Fu, F.; Su, X.; Huang, Y.; Wan, X.; Li, Y. Androgen-responsive lncRNA LINC00304 promotes cell cycle and proliferation via regulating CCNA1. Prostate 2019, 79, 994–1006. [Google Scholar] [CrossRef]
- Tortorella, E.; Giantulli, S.; Sciarra, A.; Silvestri, I. AR and PI3K/AKT in Prostate Cancer: A Tale of Two Interconnected Pathways. Int. J. Mol. Sci. 2023, 24, 2046. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Manin, M.; Beaudoin, C.; Leotoing, L.; Communal, Y.; Veyssiere, G.; Morel, L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J. Biol. Chem. 2004, 279, 14579–14586. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Soares, C.; Montersino, A.; Beique, J.C.; Thomas, G.M. Palmitoylation of LIM Kinase-1 ensures spine-specific actin polymerization and morphological plasticity. eLife 2015, 4, e06327. [Google Scholar] [CrossRef]
- Ribba, A.S.; Fraboulet, S.; Sadoul, K.; Lafanechère, L. The Role of LIM Kinases during Development: A Lens to Get a Glimpse of Their Implication in Pathologies. Cells 2022, 11, 403. [Google Scholar] [CrossRef]
- Samayawardhena, L.A.; Kapur, R.; Craig, A.W.B. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood 2007, 109, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Aureille, J.; Prabhu, S.S.; Barnett, S.F.; Farrugia, A.J.; Arnal, I.; Lafanechère, L.; Low, B.C.; Kanchanawong, P.; Mogilner, A.; Bershadsky, A.D. Focal adhesions are controlled by microtubules through local contractility regulation. EMBO J. 2024, 43, 2715–2732. [Google Scholar] [CrossRef]
- Weng, Z.; Shang, Y.; Yao, D.; Zhu, J.; Zhang, R. Structural analyses of key features in the KANK1·KIF21A complex yield mechanistic insights into the cross-talk between microtubules and the cell cortex. J. Biol. Chem. 2018, 293, 215–225. [Google Scholar] [CrossRef]
- Takai, Y.; Naito, S.; Ito, H.; Horie, S.; Ushijima, M.; Narisawa, T.; Yagi, M.; Ichiyanagi, O.; Tsuchiya, N. Ankrd1 Promotes Lamellipodia Formation and Cell Motility via Interaction with Talin-1 in Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 4232. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.; Wang, Q.; Zhou, F.; Liu, Y.; Zhang, Y.; Ding, H.; Yuan, M.; Li, F.; Chen, Y. Involvement of c-Fos in cell proliferation, migration, and invasion in osteosarcoma cells accompanied by altered expression of Wnt2 and Fzd9. PLoS ONE 2017, 12, e0180558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Niu, G.; Wu, C.; Tu, X.; Xiao, J.; Li, X.; Chen, J.G.; Cao, H. Cell-autonomous action of Slit2 in radial migration of cortical projection neurons. Front. Mol. Neurosci. 2024, 17, 1505434. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, J.; Bodmer, R. Slit and Robo control cardiac cell polarity and morphogenesis. Curr. Biol. 2005, 15, 2271–2278. [Google Scholar] [CrossRef]
- Arakawa, H. Netrin-1 and its receptors in tumorigenesis. Nat. Rev. Cancer 2004, 4, 978–987. [Google Scholar] [CrossRef]
- Akkermans, O.; Delloye-Bourgeois, C.; Peregrina, C.; Carrasquero-Ordaz, M.; Kokolaki, M.; Berbeira-Santana, M.; Chavent, M.; Reynaud, F.; Raj, R.; Agirre, J.; et al. GPC3-Unc5 receptor complex structure and role in cell migration. Cell 2022, 185, 3931–3949.e26. [Google Scholar] [CrossRef]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31, Erratum in Curr. Opin. Cell Biol. 2015, 37, 119. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Pan, S.; Chu, M.; Wang, Z.W.; Zhu, X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 2020, 215, 107633. [Google Scholar] [CrossRef]
- Gu, J.; Sun, H.; Shao, J.; Zhang, H.; Zhu, Z.; Ma, D.; Duan, Y. Lysyl oxidase-like 2 promotes the survival, migration, and ferroptosis of endometrial cancer cells by activating the phosphoinositide 3-kinase/protein kinase B pathway. Iran. J. Basic Med. Sci. 2025, 28, 72–79. [Google Scholar] [PubMed]
- Fan, Z.; Liu, Y.; Liu, X.; Nian, W.; Huang, X.; Yang, Q.; Hou, S.; Chen, F. Phosphorylation of AKT by lysyl oxidase-like 2 activates the PI3K/AKT signaling pathway to promote proliferation, invasion and metastasis in esophageal squamous carcinoma. Clin. Transl. Oncol. 2023, 25, 2487–2498. [Google Scholar] [CrossRef]
- Li, C.; Tang, Y.; Zhang, R.; Shi, L.; Chen, J.; Zhang, P.; Zhang, N.; Li, W. Inhibiting glycolysis facilitated checkpoint blockade therapy for triple-negative breast cancer. Discov. Oncol. 2025, 16, 550. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, Y.S.; Dong, S.M.; Kim, H.J.; Lee, D.E.; Kang, H.W.; Kim, M.J.; Park, J.S. Neural Wiskott-Aldrich syndrome protein (N-WASP) promotes distant metastasis in pancreatic ductal adenocarcinoma via activation of LOXL2. Oncol. Res. 2024, 32, 615–624. [Google Scholar] [CrossRef]
- Laurentino, T.S.; Soares, R.d.S.; Marie, S.K.N.; Oba-Shinjo, S.M. Correlation of Matrisome-Associatted Gene Expressions with LOX Family Members in Astrocytomas Stratified by IDH Mutation Status. Int. J. Mol. Sci. 2022, 23, 9507. [Google Scholar] [CrossRef]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S. Vitamin D Regulation of a SOD1-to-SOD2 Antioxidative Switch to Prevent Bone Cancer. Appl. Sci. 2020, 10, 2554. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcante, S.G.; Soares, R.d.S.; Uno, M.; Alves, M.J.F.; Cintra, R.C.; Sola, P.R.; Ozaki, C.Y.; Lerário, A.M.; Oba-Shinjo, S.M.; Marie, S.K.N. TFAM Loss Induces Oxidative Stress and Divergent Phenotypes in Glioblastoma Metabolic Subtypes. Int. J. Mol. Sci. 2025, 26, 10446. https://doi.org/10.3390/ijms262110446
Cavalcante SG, Soares RdS, Uno M, Alves MJF, Cintra RC, Sola PR, Ozaki CY, Lerário AM, Oba-Shinjo SM, Marie SKN. TFAM Loss Induces Oxidative Stress and Divergent Phenotypes in Glioblastoma Metabolic Subtypes. International Journal of Molecular Sciences. 2025; 26(21):10446. https://doi.org/10.3390/ijms262110446
Chicago/Turabian StyleCavalcante, Stella G., Roseli da S. Soares, Miyuki Uno, Maria J. F. Alves, Ricardo C. Cintra, Paula R. Sola, Christiane Y. Ozaki, Antonio M. Lerário, Sueli M. Oba-Shinjo, and Suely K. N. Marie. 2025. "TFAM Loss Induces Oxidative Stress and Divergent Phenotypes in Glioblastoma Metabolic Subtypes" International Journal of Molecular Sciences 26, no. 21: 10446. https://doi.org/10.3390/ijms262110446
APA StyleCavalcante, S. G., Soares, R. d. S., Uno, M., Alves, M. J. F., Cintra, R. C., Sola, P. R., Ozaki, C. Y., Lerário, A. M., Oba-Shinjo, S. M., & Marie, S. K. N. (2025). TFAM Loss Induces Oxidative Stress and Divergent Phenotypes in Glioblastoma Metabolic Subtypes. International Journal of Molecular Sciences, 26(21), 10446. https://doi.org/10.3390/ijms262110446





