Chemical Modification of Chitosan with Bioactive Molecules: A Sustainable Approach for Advanced Film Development
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of CS Modified with Eugenol
2.1.1. Nuclear Magnetic Resonance Spectroscopy
2.1.2. Fourier-Transform Infrared Spectroscopy
2.1.3. Elemental Analysis
2.1.4. Thermal Gravimetric Analysis
2.1.5. Differential Scanning Calorimetry
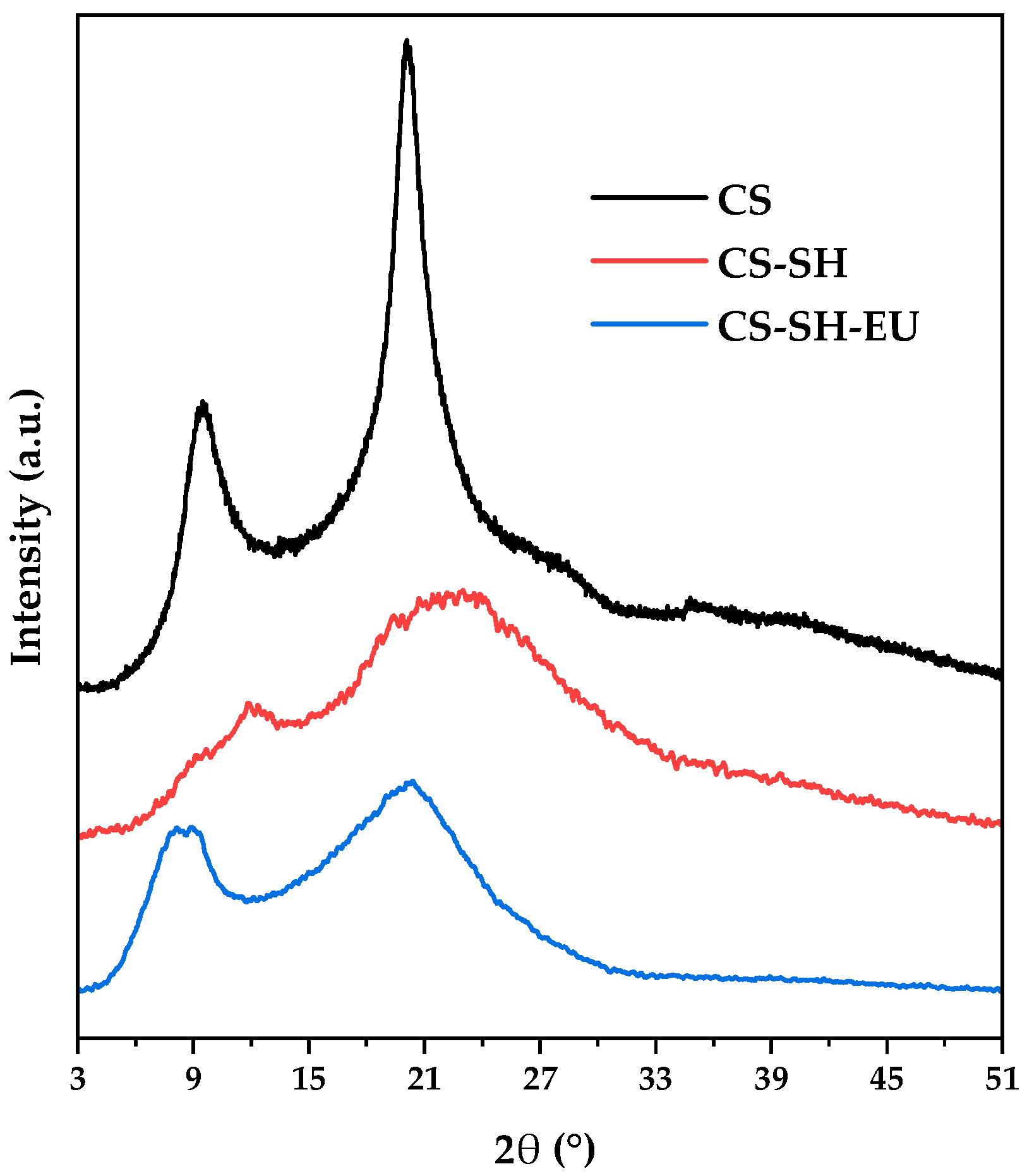
2.1.6. X-Ray Diffraction
2.2. Chitosan Films Containing Functionalized Chitosan Derivatives CS-SH-EU and CS-MTBAQ
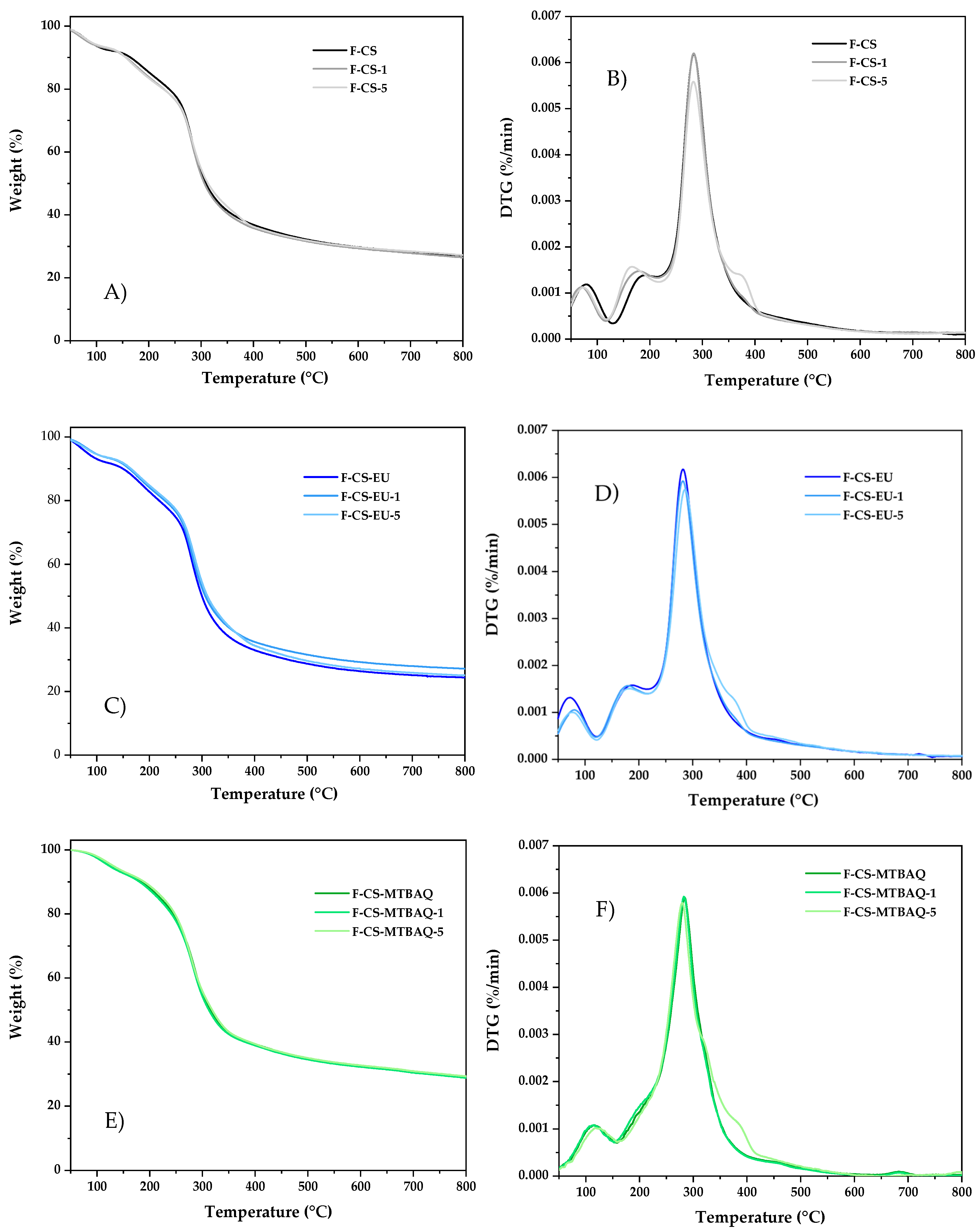
2.2.1. Thermal Gravimetric Analysis of Films
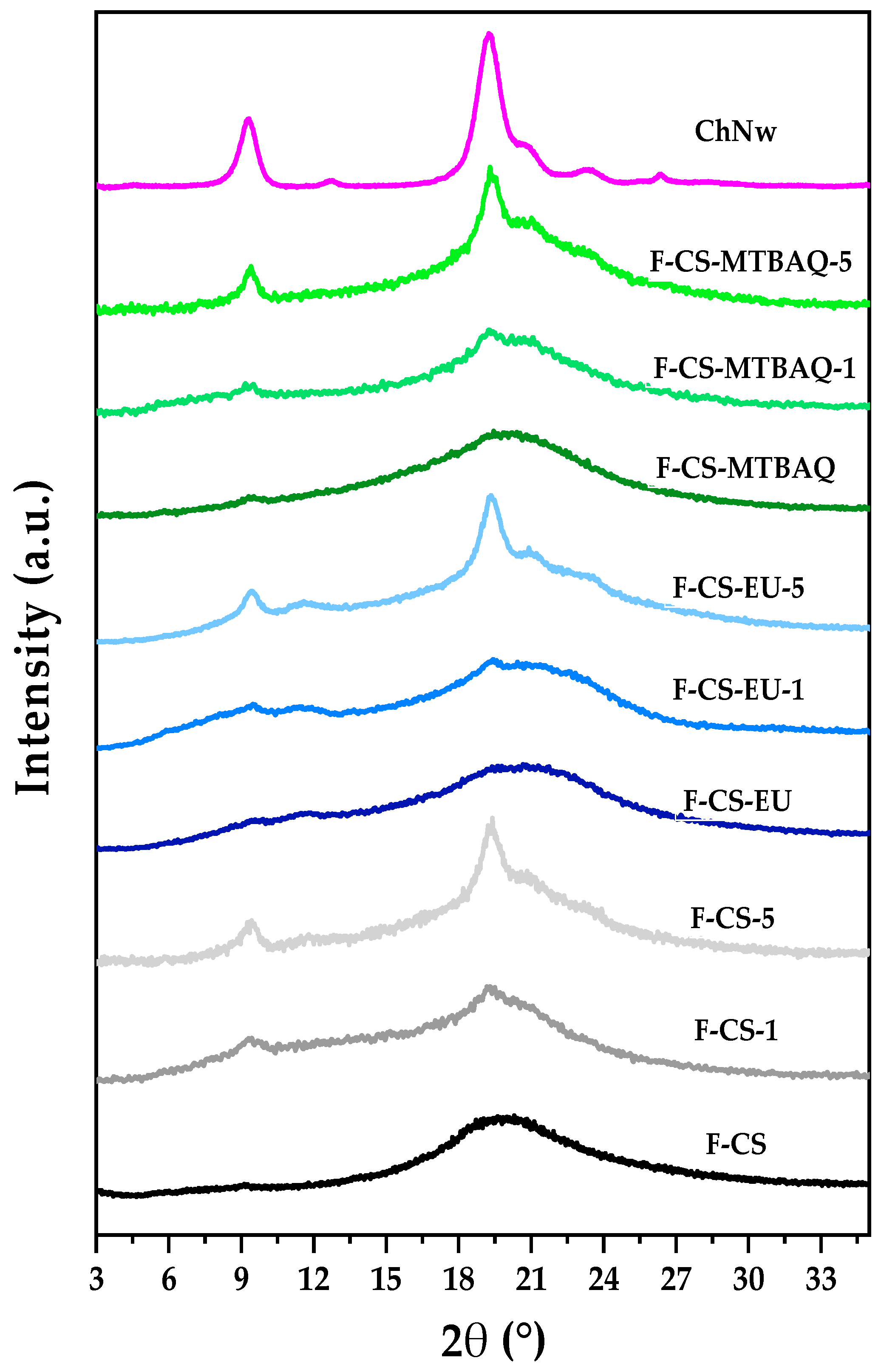
2.2.2. X-Ray Diffractions of the Films
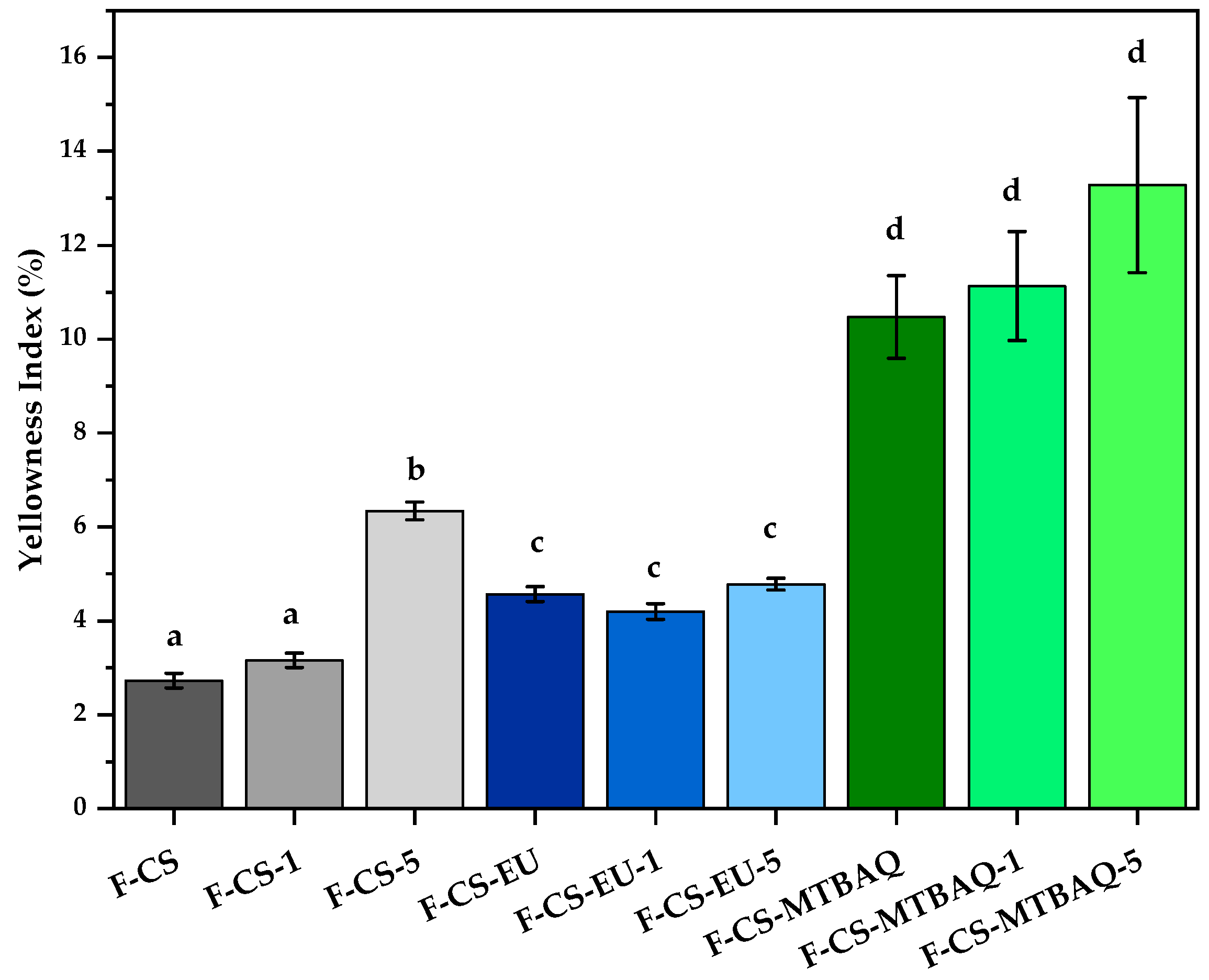
2.2.3. Yellowness Index
2.2.4. Water Vapor Transmission
2.2.5. Mechanical Properties
| Film | E (MPa) | TS (MPa) | ε (%) | WVT 10−3 (g/(d × m) |
|---|---|---|---|---|
| F-CS | 11.2 ± 1.6 a | 28.7 ± 3.5 a | 10.3 ± 3.0 a | 81.4 ± 5.4 a |
| F-CS-1 | 13.9 ± 2.1 a | 24.0 ± 4.9 a | 6.4 ± 1.5 ab | 78.6 ± 5.5 a |
| F-CS-5 | 19.0 ± 2.1 b | 24.3 ± 5.7 a | 8.9 ± 3.8 ab | 70.4 ± 8.7 ab |
| F-CS-MTBAQ | 15.2 ± 3.0 ab | 36.9 ± 5.4 ab | 15.4 ± 1.9 ac | 313.7 ± 32.3 c |
| F-CS-MTBAQ-1 | 17.4 ± 2.6 ab | 38.8 ± 4.6 b | 13.9 ± 3.5 c | 103.1 ± 5.6 d |
| F-CS-MTBAQ-5 | 16.3 ± 1.6 ab | 42.5 ± 4.8 b | 17.3 ± 3.0 c | 105.7 ± 4.3 d |
| F-CS-EU | 15.8 ± 5.0 ab | 30.2 ± 2.7 a | 2.8 ± 1.6 d | 37.6 ± 0.5 e |
| F-CS-EU-1 | 17.6 ± 3.6 ab | 29.7 ± 2.6 a | 3.3 ± 1.5 d | 33.5 ± 1.4 f |
| F-CS-EU-5 | 18.2 ± 1.1 ab | 25.5 ± 4.8 a | 2.1 ± 0.3 d | 32.2 ± 0.7 f |
2.2.6. Antioxidant Properties
2.2.7. Coating Application
3. Materials and Methods
3.1. Materials
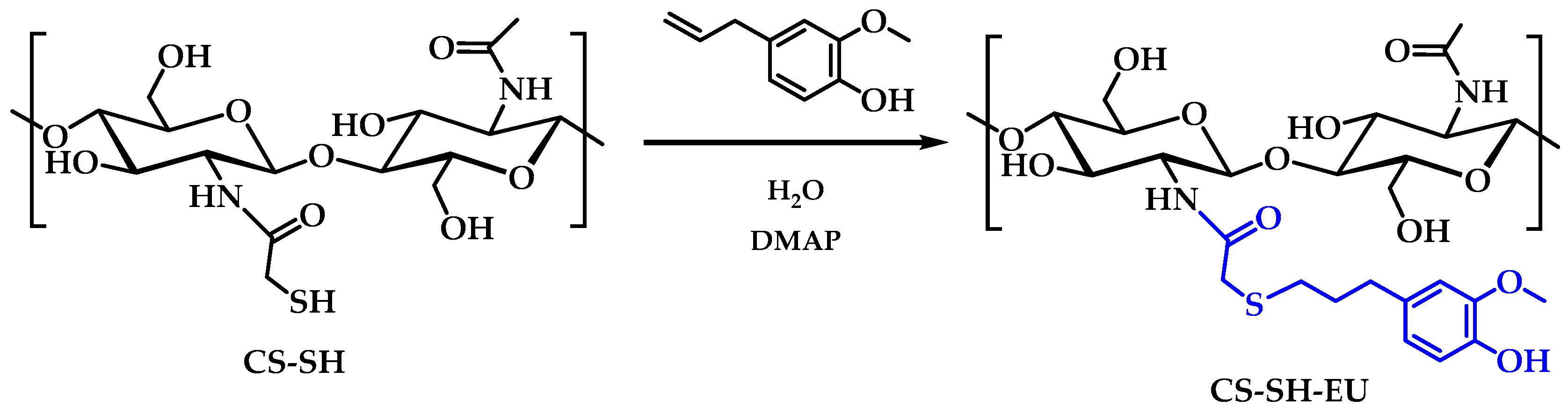
3.2. Synthesis of Chitosan Derivative Modified with Eugenol
3.3. Thiol-Group Determination
3.4. Film Preparation
| Films | CS (%) | CS-EU (%) | CS-MTBAQ (%) | ChNw (%) | Gly (%) |
|---|---|---|---|---|---|
| F-CS | 85 | - | - | 0 | 15 |
| F-CS-1 | 84 | - | - | 1 | 15 |
| F-CS-5 | 80 | - | - | 5 | 15 |
| F-CS-EU | 75 | 10 | - | 0 | 15 |
| F-CS-EU-1 | 74 | 10 | - | 1 | 15 |
| F-CS-EU-5 | 70 | 10 | - | 5 | 15 |
| F-CS-MTBAQ | 75 | - | 10 | 0 | 15 |
| F-CS-MTBAQ-1 | 74 | - | 10 | 1 | 15 |
| F-CS-MTBAQ-5 | 70 | - | 10 | 5 | 15 |
3.5. Characterization
3.5.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.5.2. Fourier-Transform Infrared Spectroscopy
3.5.3. Elemental Analysis
3.5.4. Zeta Potential
3.5.5. Thermal Gravimetric Analysis
3.5.6. Differential Scanning Calorimetry
3.5.7. X-Ray Diffraction
3.5.8. Antioxidant Capacity
3.5.9. Yellowness Index
3.5.10. Water Vapor Transmission
3.5.11. Mechanical Properties
3.6. Coating Application
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical Characterization of Chitin and Chitosan from Crab Shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent Advances of Chitosan-Based Polymers in Biomedical Applications and Environmental Protection. J. Polym. Res. 2022, 29, 259. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-Solubility of Chitosan and Its Antimicrobial Activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Nagahama, H.; Furuike, T.; Tamura, H. Synthesis of Phosphorylated Chitosan by Novel Method and Its Characterization. Int. J. Biol. Macromol. 2008, 42, 335–339. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, L.; Li, C.; Zhang, D.; Xiao, Y.; Guan, G.; Zhu, W. Modification of Chitosan with Monomethyl Fumaric Acid in an Ionic Liquid Solution. Carbohydr. Polym. 2015, 117, 973–979. [Google Scholar] [CrossRef]
- Piegat, A.; Goszczyńska, A.; Idzik, T.; Niemczyk, A. The Importance of Reaction Conditions on the Chemical Structure of N,O-Acylated Chitosan Derivatives. Molecules 2019, 24, 3047. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.d.R.; Roura, S.I.; Ponce, A. Effectiveness of Chitosan Edible Coatings to Improve Microbiological and Sensory Quality of Fresh Cut Broccoli. LWT-Food Sci. Technol. 2011, 44, 2335–2341. [Google Scholar] [CrossRef]
- Simões, A.D.N.; Tudela, J.A.; Allende, A.; Puschmann, R.; Gil, M.I. Edible Coatings Containing Chitosan and Moderate Modified Atmospheres Maintain Quality and Enhance Phytochemicals of Carrot Sticks. Postharvest Biol. Technol. 2009, 51, 364–370. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for Chitosan Based Antimicrobial Films in Food Applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Yun, S.-M.; Lee, M.-H.; Lee, K.-J.; Ku, H.-O.; Son, S.-W.; Joo, Y.-S. Quantitative Analysis of Eugenol in Clove Extract by a Validated HPLC Method. J. AOAC Int. 2010, 93, 1806–1810. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of Antifungal Activity in Essential Oil of the Syzygium Aromaticum (L.) by Extraction, Purification and Analysis of Its Main Component Eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, Z.; Neoh, K.G.; Kang, E.T. Antioxidant and Antibacterial Activities of Eugenol and Carvacrol-grafted Chitosan Nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An Active Packaging Film Based on Yam Starch with Eugenol and Its Application for Pork Preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jimenez, A.J.; García Martínez, J.C.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Comparative Evaluation of Carvacrol and Eugenol Chitosan Nanoparticles as Eco-Friendly Preservative Agents in Cosmetics. Int. J. Biol. Macromol. 2022, 206, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of Primary Antioxidant Activity to Chitosan for Application as a Multifunctional Food Packaging Material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant Potential of Eugenol and Ginger Essential Oils with Gelatin/Chitosan Films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Y.; Chen, H.; Song, R.; Li, Y. Performance of Eugenol Emulsion/Chitosan Edible Coating and Application in Fresh Meat Preservation. J. Food Process. Preserv. 2022, 46, e16407. [Google Scholar] [CrossRef]
- Araki, J.; Yamanaka, Y.; Ohkawa, K. Chitin-Chitosan Nanocomposite Gels: Reinforcement of Chitosan Hydrogels with Rod-like Chitin Nanowhiskers. Polym. J. 2012, 44, 713–717. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Nunes, C.S.; Rubira, A.F.; Muniz, E.C.; Fajardo, A.R. Effect of Chitin Nanowhiskers on Mechanical and Swelling Properties of Gum Arabic Hydrogels Nanocomposites. Carbohydr. Polym. 2021, 266, 118116. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of Chitin Nano-Whiskers on the Antibacterial and Physicochemical Properties of Maize Starch Films. Carbohydr. Polym 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Peng, C.; Chen, G. Preparation and Assessment of Heat-Treated α-Chitin Nanowhiskers Reinforced Poly(Viny Alcohol) Film for Packaging Application. Materials 2018, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, H. Reinforcement of Poly (Vinyl Alcohol) Films with Alpha-Chitin Nanowhiskers. Polímeros 2018, 28, 69–75. [Google Scholar] [CrossRef]
- Yanat, M.; Colijn, I.; Schroën, K. Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films. Polymers 2022, 14, 2965. [Google Scholar] [CrossRef]
- Muñoz-Nuñez, C.; Cuervo-Rodríguez, R.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A. Synthesis and Characterization of Thiazolium Chitosan Derivative with Enhanced Antimicrobial Properties and Its Use as Component of Chitosan Based Films. Carbohydr. Polym. 2023, 302, 120438. [Google Scholar] [CrossRef]
- Danyliuk, I.; Kovalenko, N.; Tolmachova, V.; Kovtun, O.; Saliyeva, L.; Slyvka, N.; Holota, S.; Kutrov, G.; Tsapko, M.; Vovk, M. Synthesis and Antioxidant Activity Evaluation of Some New 4-Thiomethyl Functionalised 1,3-Thiazoles. Curr. Chem. Lett. 2023, 12, 667–676. [Google Scholar] [CrossRef]
- Sauperl, O.; Zemljic, L.F.; Valh, J.V.; Tompa, J. Assessment of Chemically and Enzymatically Modified Chitosan with Eugenol as a Coating for Viscose Functionalization for Potential Medical Use. Text. Res. J. 2021, 91, 2813–2832. [Google Scholar] [CrossRef]
- Muñoz-Núñez, C.; Hevilla, V.; Zágora, J.; Plachá, D.; Muñoz-Bonilla, A.; Fernández-García, M. Functionalization of Chitosan-Chitin Nanowhiskers Films By Impregnation with Essential Oils Via Supercritical CO2. J. Polym. Environ. 2024, 33, 96–111. [Google Scholar] [CrossRef]
- Chen, T.C.; Ho, Y.Y.; Tang, R.C.; Ke, Y.C.; Lin, J.N.; Yang, I.H.; Lin, F.H. Thiolated Chitosan as an Intestinal Absorption Carrier with Hesperidin Encapsulation for Obesity Treatment. Nutrients 2021, 13, 4405. [Google Scholar] [CrossRef]
- Kwon, W.; Jeong, E. Detoxification Properties of Guanidinylated Chitosan Against Chemical Warfare Agents and Its Application to Military Protective Clothing. Polymers 2020, 12, 1461. [Google Scholar] [CrossRef]
- Doshi, B.; Repo, E.; Heiskanen, J.P.; Sirviö, J.A.; Sillanpää, M. Effectiveness of N,O-Carboxymethyl Chitosan on Destabilization of Marine Diesel, Diesel and Marine-2T Oil for Oil Spill Treatment. Carbohydr. Polym. 2017, 167, 326–336. [Google Scholar] [CrossRef]
- Khadim, H.; Zeeshan, R.; Riaz, S.; Tabassum, S.; Ansari, A.A.; Zulfiqar, S.; Yar, M.; Asif, A. Development of Eugenol Loaded Cellulose-Chitosan Based Hydrogels; In-Vitro and In-Vivo Evaluation for Wound Healing. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 693, 134033. [Google Scholar] [CrossRef]
- Kazemi, M.S.; Mohammadi, Z.; Amini, M.; Yousefi, M.; Tarighi, P.; Eftekhari, S.; Rafiee Tehrani, M. Thiolated Chitosan-Lauric Acid as a New Chitosan Derivative: Synthesis, Characterization and Cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, T.; Ovchinnikov, O.; Grevtseva, I.; Smirnov, M.; Erina, O.; Khokhlov, V.; Darinsky, B.; Tatianina, E. Thioglycolic Acid FTIR Spectra on Ag2S Quantum Dots Interfaces. Materials 2020, 13, 909. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2004; ISBN 978-0824754808. [Google Scholar]
- Cerrada, M.L.; Sánchez-Chaves, M.; Ruiz, C.; Fernández-García, M. Glycopolymers Resultant from Ethylene-Vinyl Alcohol Copolymers: Degradation and Rheological Behavior in Bulk. Eur. Polym. J. 2008, 44, 2194–2201. [Google Scholar] [CrossRef]
- Hakimi, S.; Mortazavian, E.; Mohammadi, Z.; Samadi, F.Y.; Samadikhah, H.; Taheritarigh, S.; Tehrani, N.R.; Rafiee-Tehrani, M. Thiolated Methylated Dimethylaminobenzyl Chitosan: A Novel Chitosan Derivative as a Potential Delivery Vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-Loaded Chitosan Nanoparticles: I. Thermal Stability Improvement of Eugenol through Encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Peralta, Y.; López-Cervantes, J.; Madera Santana, T.J.; Sánchez-Duarte, R.G.; Sánchez-Machado, D.I.; Martínez-Macías, M.d.R.; Correa-Murrieta, M.A. Impact of the Molecular Weight on the Size of Chitosan Nanoparticles: Characterization and Its Solid-State Application. Polym. Bull. 2021, 78, 813–832. [Google Scholar] [CrossRef]
- Mukherjee, D.; Srinivasan, B.; Anbu, J.; Azamthulla, M.; Banala, V.T.; Ramachandra, S.G. Improvement of Bone Microarchitecture in Methylprednisolone Induced Rat Model of Osteoporosis by Using Thiolated Chitosan-Based Risedronate Mucoadhesive Film. Drug Dev. Ind. Pharm. 2018, 44, 1845–1856. [Google Scholar] [CrossRef]
- Zulcaif; Zafar, N.; Mahmood, A.; Sarfraz, R.M.; Elaissari, A. Simvastatin Loaded Dissolvable Microneedle Patches with Improved Pharmacokinetic Performance. Micromachines 2022, 13, 1304. [Google Scholar] [CrossRef]
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Thatte, M.; Daly, W.H. Synthesis and Characterization of N-Aryl Chitosan Derivatives. Int. J. Biol. Macromol. 2008, 43, 79–87. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-Acorn Starch-Eugenol Edible Film: Physico-Chemical, Barrier, Antimicrobial, Antioxidant and Structural Properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Kennedy, J.F.; Nie, J. Synthesize and Characterization of Organic-Soluble Acylated Chitosan. Carbohydr. Polym. 2009, 75, 390–394. [Google Scholar] [CrossRef]
- Habib, R.; Azad, A.K.; Akhlaq, M.; Al-Joufi, F.A.; Shahnaz, G.; Mohamed, H.R.H.; Naeem, M.; Almalki, A.S.A.; Asghar, J.; Jalil, A.; et al. Thiolated Chitosan Microneedle Patch of Levosulpiride from Fabrication, Characterization to Bioavailability Enhancement Approach. Polymers 2022, 14, 415. [Google Scholar] [CrossRef]
- Luo, Q.; Han, Q.; Wang, Y.; Zhang, H.; Fei, Z.; Wang, Y. The Thiolated Chitosan: Synthesis, Gelling and Antibacterial Capability. Int. J. Biol. Macromol. 2019, 139, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Anbukkarasi, M.; Thomas, P.A.; Geraldine, P. A Comparison between Plain Eugenol and Eugenol-Loaded Chitosan Nanoparticles for Prevention of in Vitro Selenite-Induced Cataractogenesis. J. Drug Deliv. Sci. Technol. 2021, 65, 102696. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Castro, J.I.; Valencia Llano, C.H.; Navia Porras, D.P.; Delgado Ospina, J.; Valencia Zapata, M.E.; Herminsul Mina Hernandez, J.; Chaur, M.N. Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films. Molecules 2020, 25, 1688. [Google Scholar] [CrossRef]
- Debandi, M.V.; Bernal, C.; Francois, N. Development of Biodegradable Films Based on Chitosan/Glycerol Blends Suitable for Biomedical Applications. J. Tissue Sci. Eng. 2016, 7, 1–9. [Google Scholar]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; De La Caba, K. Characterization and Antimicrobial Analysis of Chitosan-Based Films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and Characterization of Antioxidant and PH-Sensitive Films Based on Chitosan and Black Soybean Seed Coat Extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Muñoz-Nuñez, C.; Quiroz-Pereira, Y.; Muñoz-Bonilla, A.; Fernández-García, M. Enhancing Antimicrobial and Antioxidant Properties of Chitosan-Based Films with 1-Methylimidazolium-Chitosan. Polymers 2025, 17, 2608. [Google Scholar] [CrossRef]
- Etxabide, A.; Kilmartin, P.A.; Maté, J.I.; Gómez-Estaca, J. Characterization of Glucose-Crosslinked Gelatin Films Reinforced with Chitin Nanowhiskers for Active Packaging Development. LWT 2022, 154, 112833. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Eze, R.C.; Ovatlarnporn, C. Construction of Carboxymethyl Chitosan/PVA/Chitin Nanowhiskers Multicomponent Film Activated with Cotylelobium Lanceolatum Phenolics and in Situ SeNP for Enhanced Packaging Application. Int. J. Biol. Macromol. 2024, 255, 128073. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Ben Messaoud, G.; Sánchez-González, L.; Tehrany, E.A.; Vargas, M.; Atarés, L.; Chiralt, A. Chitosan Films Containing Encapsulated Eugenol in Alginate Microspheres. Food Hydrocoll. 2024, 151, 109791. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.-J.; Tu, Q.; Jian, Y.-Y.; Xie, D.-M.; Bai, T.; Li, S.-S.; Liu, Y.-T.; Li, C.; Wang, C.-X.; et al. Preparation and Characterization of Carboxymethyl Chitosan/Pullulan Composite Film Incorporated with Eugenol and Its Application in the Preservation of Chilled Meat. Meat Sci. 2023, 198, 109085. [Google Scholar] [CrossRef]
- Cottet, C.; Salvay, A.G.; Peltzer, M.A.; Fernández-García, M. Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging. Polymers 2021, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Patricia Miranda, S.; Garnica, O.; Lara-Sagahon, V.; Cárdenas, G. Water Vapor Permeability and Mechanical Properties of Chitosan Composite Films. J. Chil. Chem. Soc. 2004, 49, 173–178. [Google Scholar] [CrossRef]
- Sridhar, B.; Shivakumar, S.; Wahid, A. Preparation and Evaluation of Transdermal Drug Delivery System of Etoricoxib Using Modified Chitosan. Indian J. Pharm. Sci. 2008, 70, 455. [Google Scholar] [CrossRef]
- Rani, R.; Saini, R.; Patel, M.K.; Gumber, S.; Mazumder, K.; Deep, A.; Nayak, M.K. Fabrication of Antimicrobial Films Based on Cross-Linked Chitosan Nanocomposite in Food Packaging Applications. ACS Food Sci. Technol. 2025, 5, 336–349. [Google Scholar] [CrossRef]
- Chi-Yan Li, S.; Sun, Y.-C.; Guan, Q.; Naguib, H. Effects of Chitin Nanowhiskers on the Thermal, Barrier, Mechanical, and Rheological Properties of Polypropylene Nanocomposites. RSC Adv. 2016, 6, 72086–72095. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, J.; Jiao, Z.; Zhang, Q.; Lv, Z.; Yang, W.; Chen, D.; Liu, H. Polysaccharide-Based Packaging Coatings and Films with Phenolic Compounds in Preservation of Fruits and Vegetables—A Review. Foods 2024, 13, 3896. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.Y.; Mubarak, N.M.; Tanjung, F.A. Structure-Property Relationship of Cellulose Nanowhiskers Reinforced Chitosan Biocomposite Films. J. Environ. Chem. Eng. 2017, 5, 6132–6136. [Google Scholar] [CrossRef]
- Kim, S.J.; Hong, B.M.; Park, W.H. The Effects of Chitin/Chitosan Nanowhiskers on the Thermal, Mechanical and Dye Adsorption Properties of Electrospun PVA Nanofibrous Membranes. Cellulose 2020, 27, 5771–5783. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant Films Based on Cross-Linked Methyl Cellulose and Native Chilean Berry for Food Packaging Applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.J.; Garg, V.K.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Gupta, J.K. Thiazoles: Having Diverse Biological Activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Xing, R.; Yu, H.; Liu, S.; Zhang, W.; Zhang, Q.; Li, Z.; Li, P. Antioxidant Activity of Differently Regioselective Chitosan Sulfates in Vitro. Bioorganic Med. Chem. 2005, 13, 1387–1392. [Google Scholar] [CrossRef]
- Dong, W.; Su, J.; Chen, Y.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Characterization and Antioxidant Properties of Chitosan Film Incorporated with Modified Silica Nanoparticles as an Active Food Packaging. Food Chem. 2022, 373, 131414. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 73, ISBN 9780128002681. [Google Scholar]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. Biores. Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Luo, Y. In Vitro Antioxidant-Activity Evaluation of Gallic-Acid-Grafted Chitosan Conjugate Synthesized by Free-Radical-Induced Grafting Method. J. Agric. Food Chem. 2016, 64, 5893–5900. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of Gallic Acid onto Chitosan Enhances Antioxidant Activities and Alters Rheological Properties of the Copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Li, Q.; Mi, Y.; Tan, W.; Guo, Z. Highly Efficient Free Radical-Scavenging Property of Phenolic-Functionalized Chitosan Derivatives: Chemical Modification and Activity Assessment. Int. J. Biol. Macromol. 2020, 164, 4279–4288. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H.; Su, W.W. Modification of Different Molecular Weights of Chitosan by P-Coumaric Acid: Preparation, Characterization and Effect of Molecular Weight on Its Water Solubility and Antioxidant Property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Soni, B.; Mahmoud, B.; Chang, S.; El-Giar, E.M.; Hassan, E.B. Physicochemical, Antimicrobial and Antioxidant Properties of Chitosan/TEMPO Biocomposite Packaging Films. Food Packag. Shelf Life 2018, 17, 73–79. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of Calcium Dips and Chitosan Coatings on Postharvest Life of Strawberries (Fragaria x Ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Yang, C.; Lu, J.-H.; Xu, M.-T.; Shi, X.-C.; Song, Z.-W.; Chen, T.-M.; Herrera-Balandrano, D.D.; Zhang, Y.-J.; Laborda, P.; Shahriar, M.; et al. Evaluation of Chitosan Coatings Enriched with Turmeric and Green Tea Extracts on Postharvest Preservation of Strawberries. LWT 2022, 163, 113551. [Google Scholar] [CrossRef]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Käch, A.; Maake, C.; Patzke, G.R. Chitosan-Thioglycolic Acid as a Versatile Antimicrobial Agent. Biomacromolecules 2013, 14, 1010–1017. [Google Scholar] [CrossRef]
- Guaresti, O.; Basasoro, S.; González, K.; Eceiza, A.; Gabilondo, N. In Situ Cross–Linked Chitosan Hydrogels via Michael Addition Reaction Based on Water–Soluble Thiol–Maleimide Precursors. Eur. Polym. J. 2019, 119, 376–384. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Danin-Poleg, Y.; Kashi, Y.; Bianco-Peled, H. Modified Chitosan: A Step toward Improving the Properties of Antibacterial Food Packages. Food Packag. Shelf Life 2014, 1, 160–169. [Google Scholar] [CrossRef]
- Bravoosuna, I.; Teutonico, D.; Arpicco, S.; Vauthier, C.; Ponchel, G. Characterization of Chitosan Thiolation and Application to Thiol Quantification onto Nanoparticle Surface. Int. J. Pharm. 2007, 340, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wei, Y.; Jia, X.; Xu, J.; Li, G. Correlation of the Structure, Properties, and Antimicrobial Activity of a Soluble Thiolated Chitosan Derivative. J. Appl. Polym. Sci. 2012, 125, E143–E148. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Luan, F.; Yin, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities. Polymers 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- ASTM-D1925-70; Standard Test Method for Yellowness Indez of Plastics. American Society for Testing and Materials. ASTM International: Philadelphia, PA, USA, 1988.
- ASTM E96-00e1; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2000.
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of Chitosan-based Apple Peel Polyphenols Edible Coating on the Preservation of Strawberry (Fragaria Ananassa Cv Hongyan) Fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
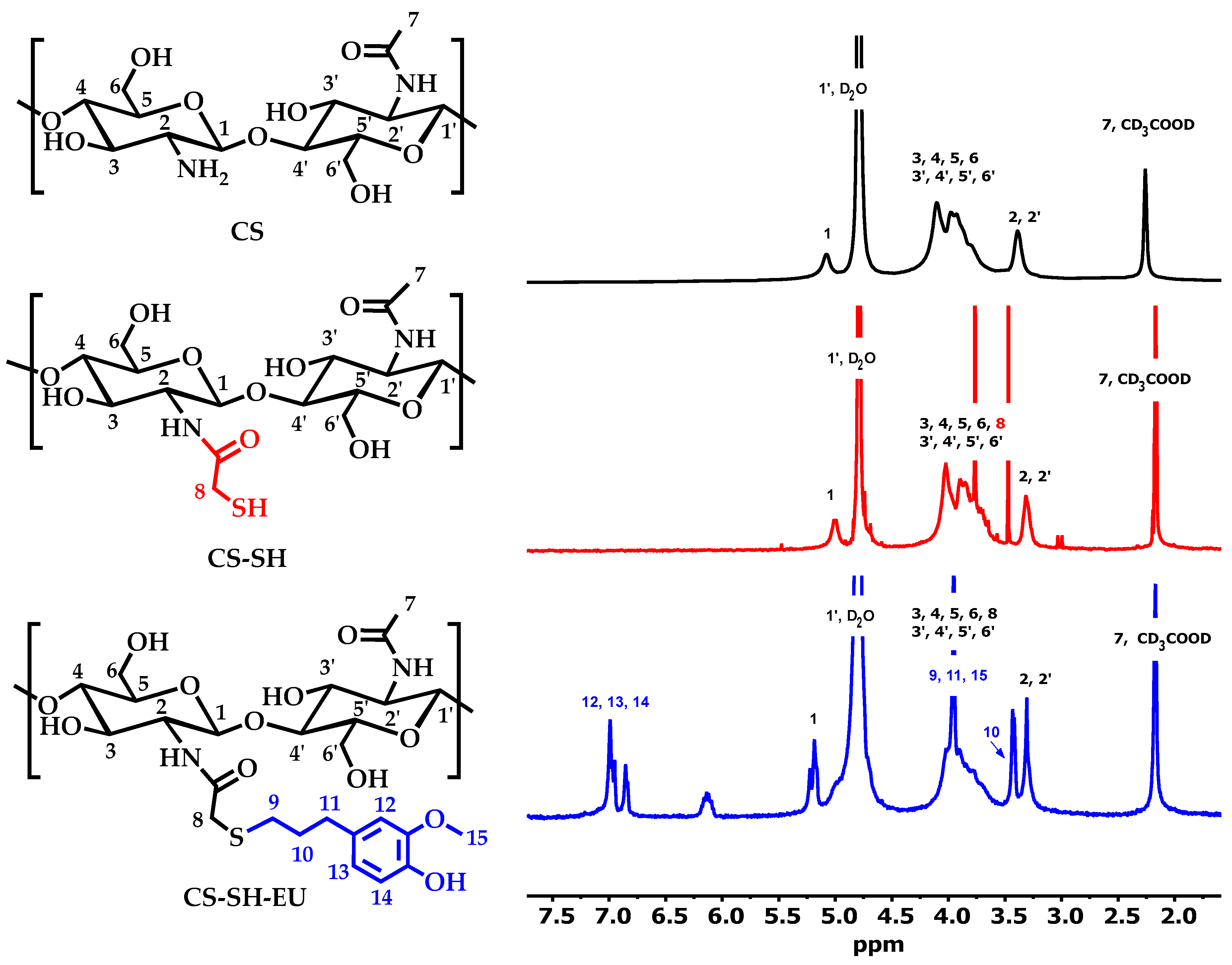
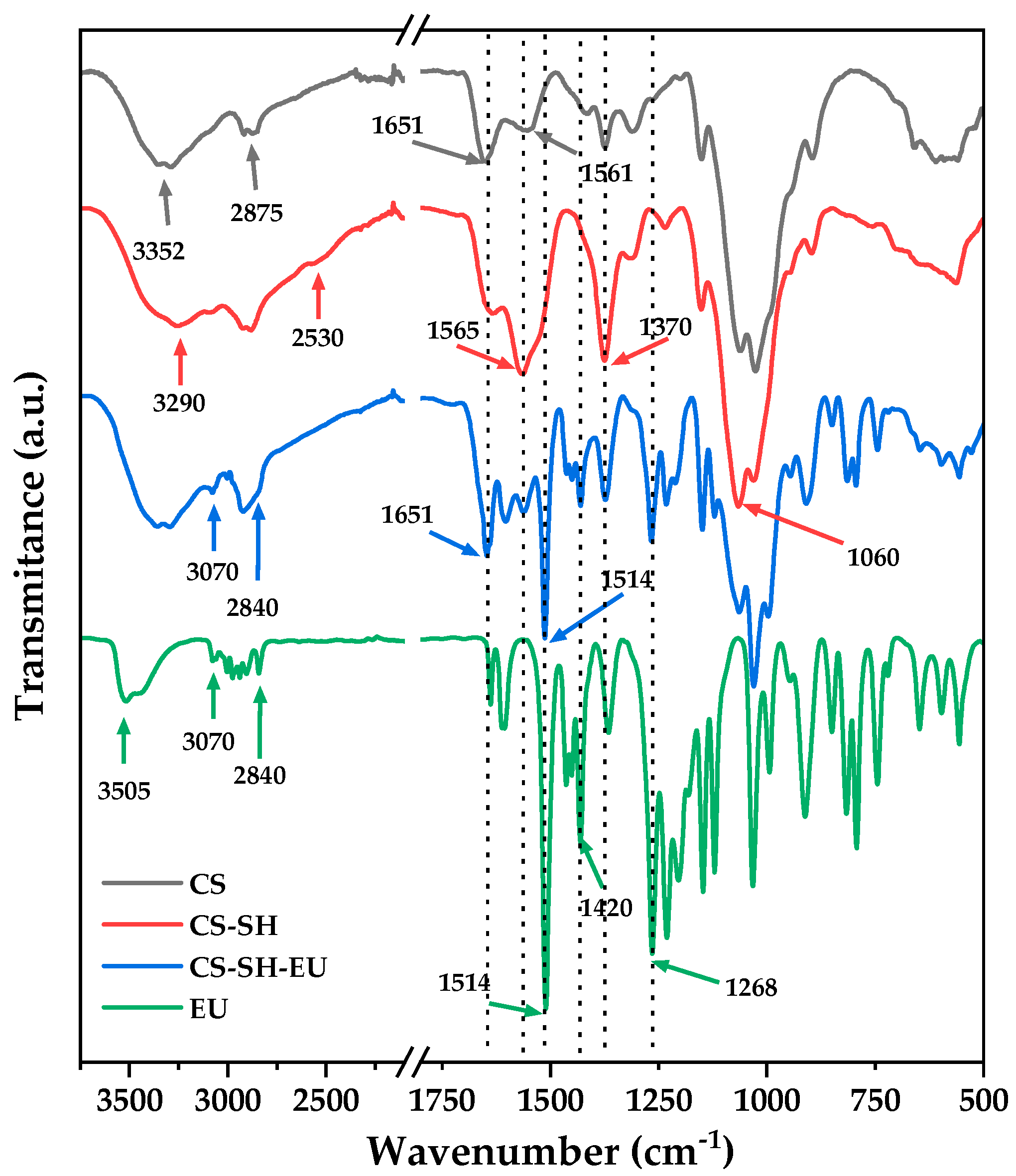
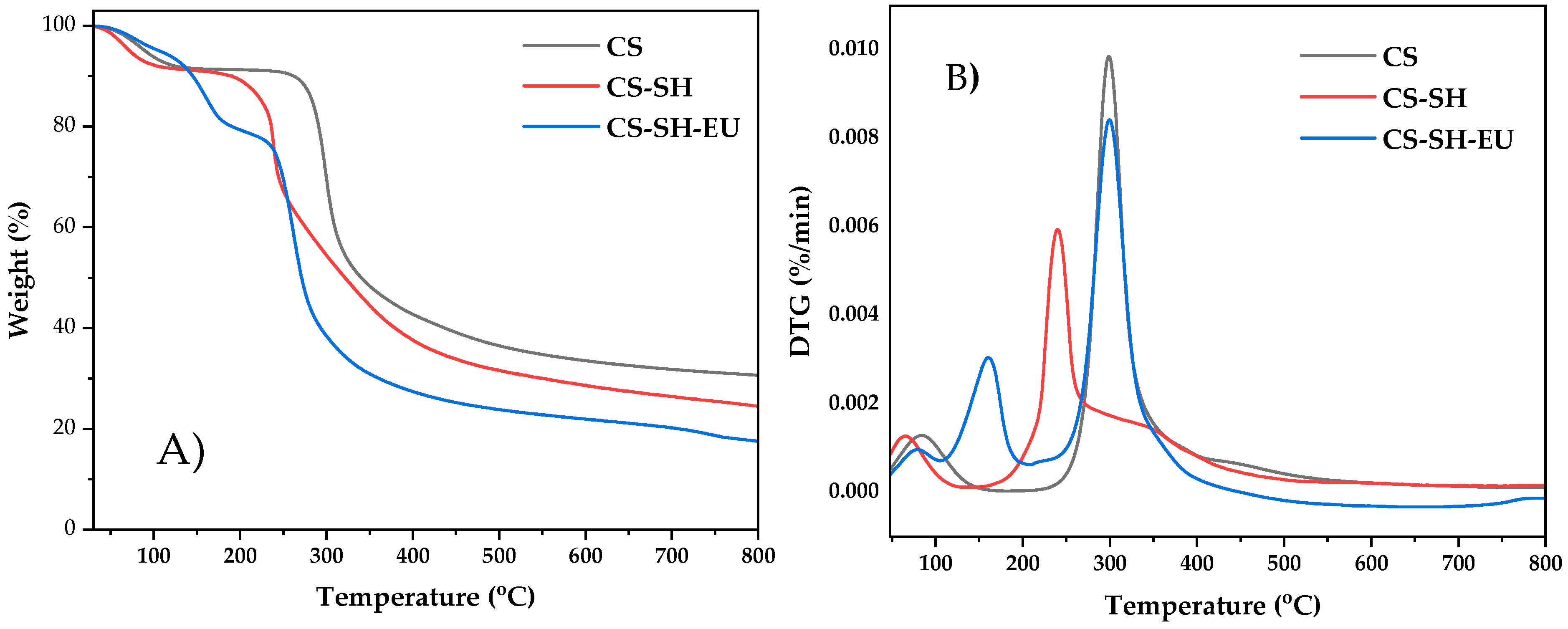
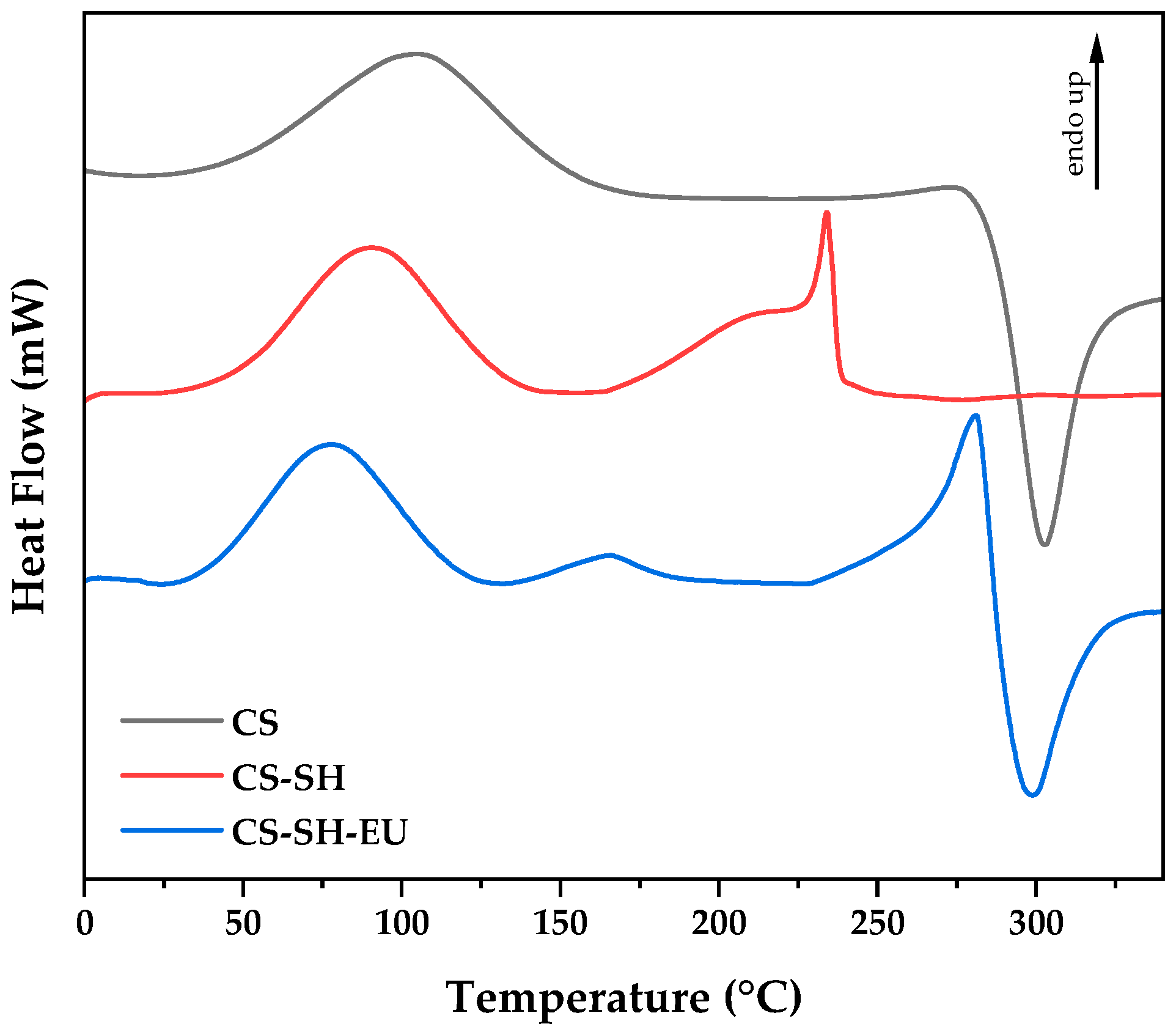
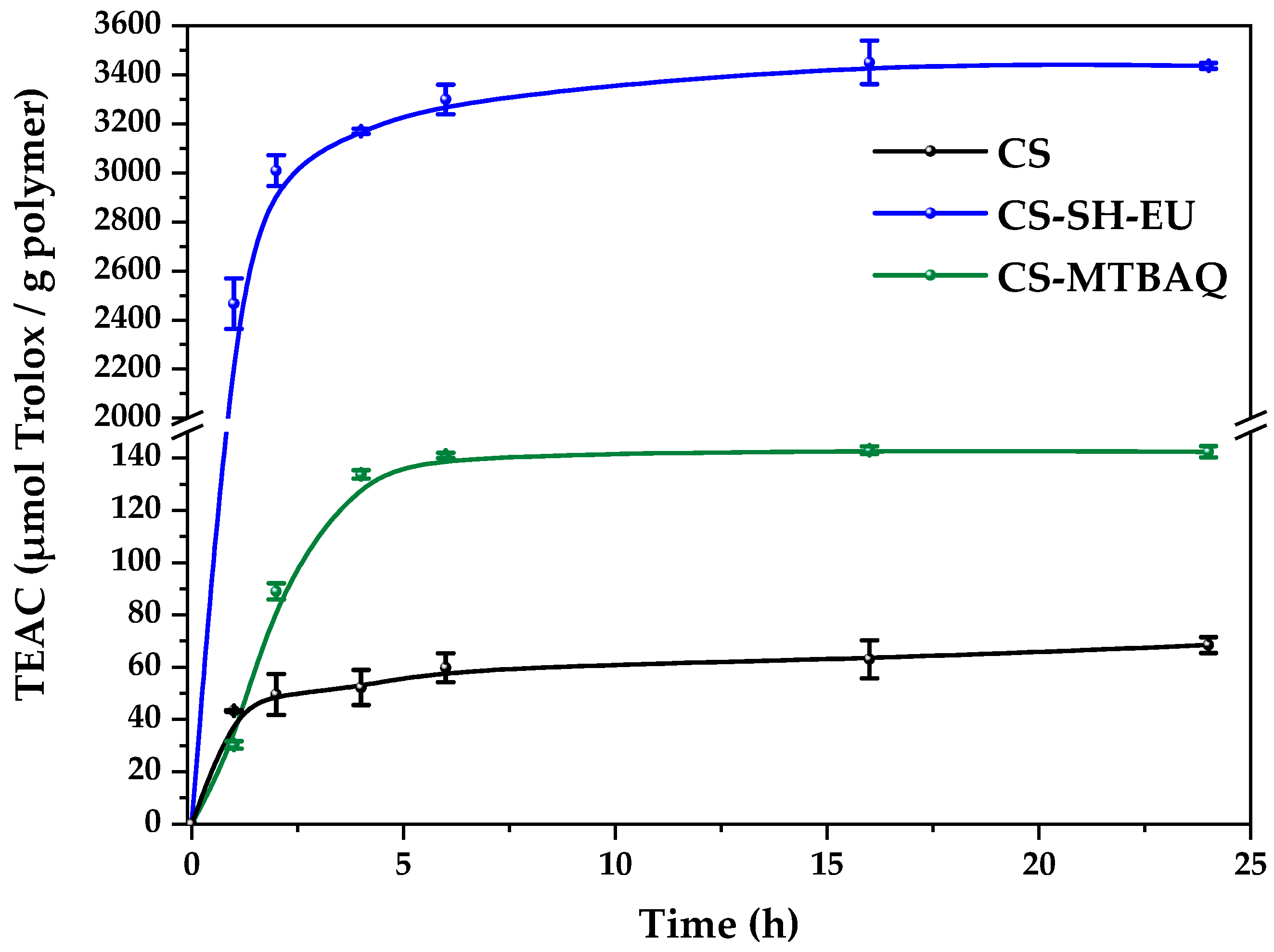
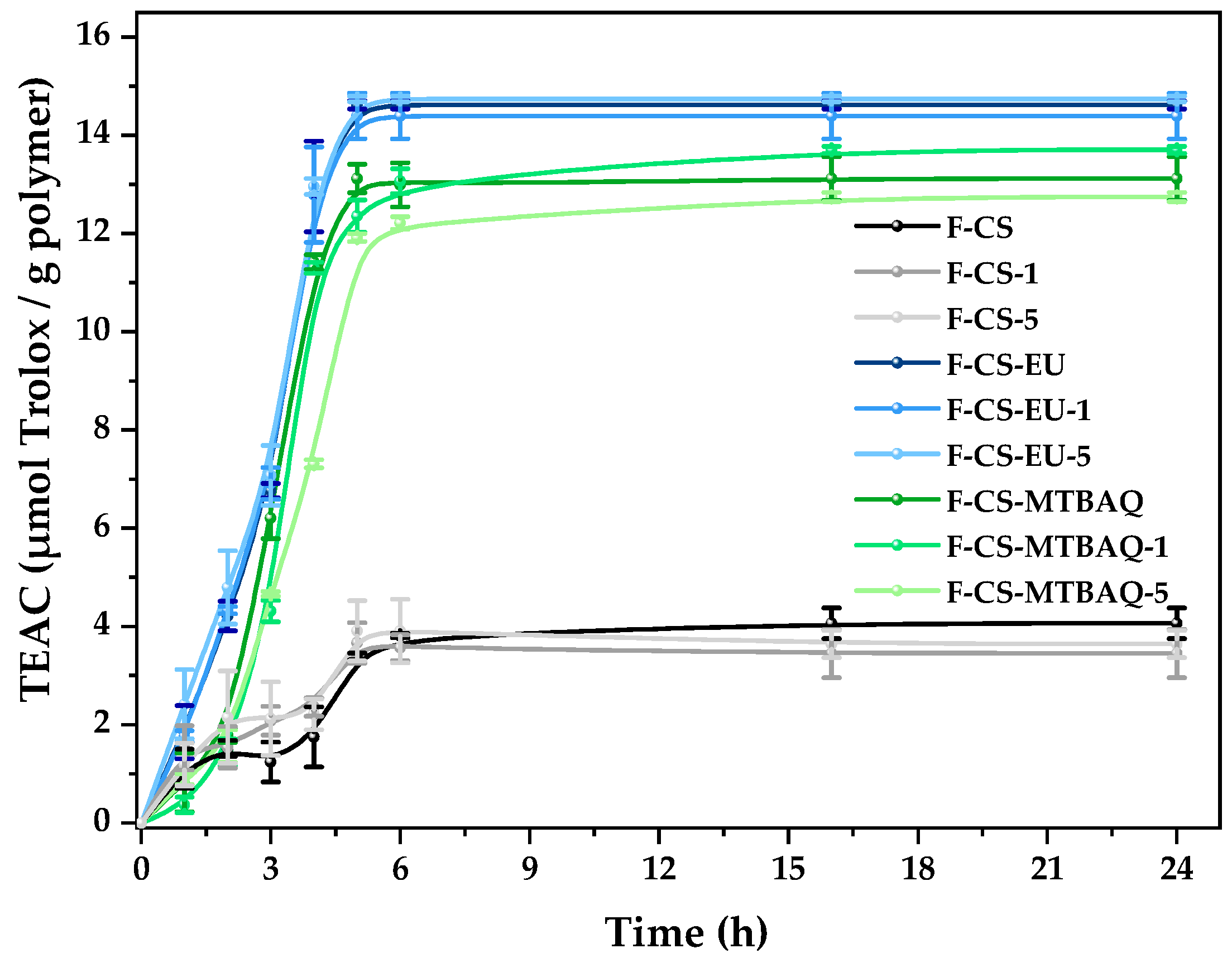
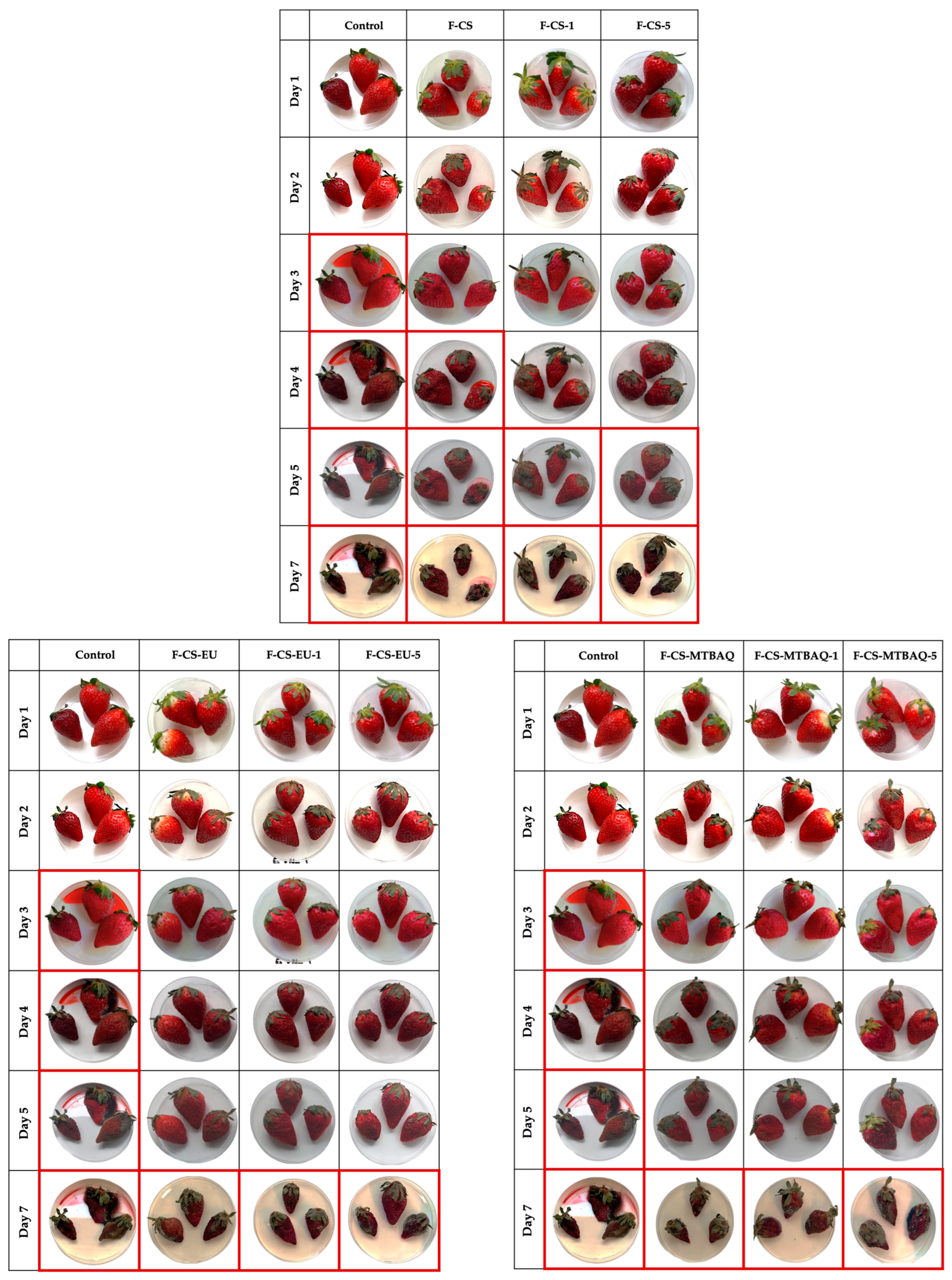
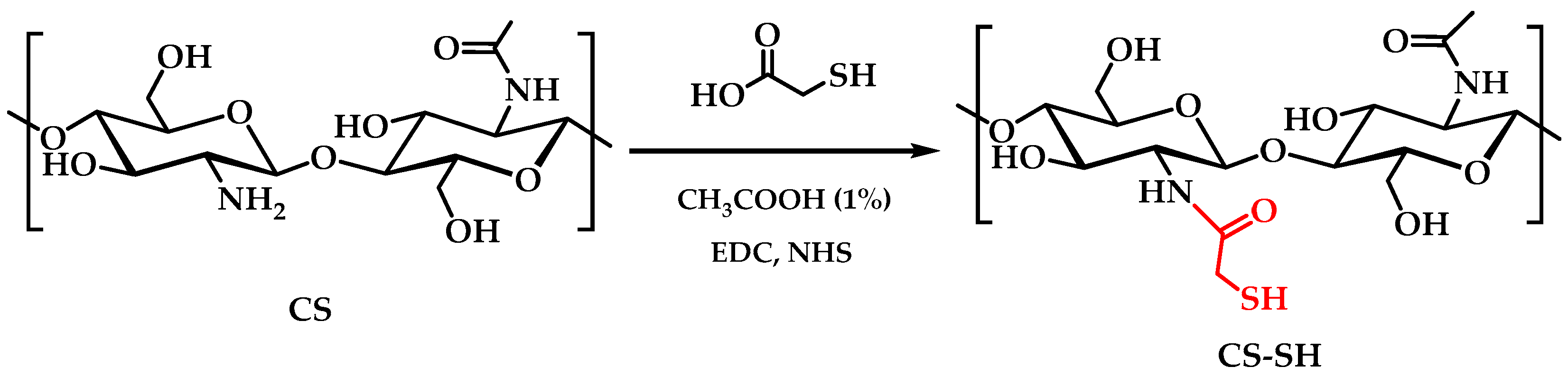
| Sample | C (%) | H (%) | N (%) | S (%) | C/N | DA (%) | DS (%) | DM (%) |
|---|---|---|---|---|---|---|---|---|
| CS | 40.6 ± 0.3 | 6.7 ± 0.3 | 7.2 ± 0.3 | - | 5.6 | 28 | - | - |
| CS-SH | 36.2 ± 0.1 | 6.4 ± 0.1 | 5.8 ± 0.1 | 6.6 ± 0.2 | 6.2 | 28 | 63 | - |
| CS-SH-EU | 53.6 ± 0.2 | 7.1 ± 0.1 | 5.1 ± 0.1 | 5.6 ± 0.2 | 10.5 | 28 | - | 62 |
| CS-MTBAQ [34] | 33.1 ± 0.3 | 5.9 ± 0.3 | 4.8 ± 0.3 | 5.2 ± 0.3 | 6.9 | 28 | - | 68 |
| ChNw | 43.5 ± 0.6 | 6.4 ± 0.6 | 6.4 ± 0.6 | 6.8 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Núñez, C.; Gómez-Fernández, N.; Muñoz-Bonilla, A.; Fernández-García, M. Chemical Modification of Chitosan with Bioactive Molecules: A Sustainable Approach for Advanced Film Development. Int. J. Mol. Sci. 2025, 26, 10403. https://doi.org/10.3390/ijms262110403
Muñoz-Núñez C, Gómez-Fernández N, Muñoz-Bonilla A, Fernández-García M. Chemical Modification of Chitosan with Bioactive Molecules: A Sustainable Approach for Advanced Film Development. International Journal of Molecular Sciences. 2025; 26(21):10403. https://doi.org/10.3390/ijms262110403
Chicago/Turabian StyleMuñoz-Núñez, Carolina, Nuria Gómez-Fernández, Alexandra Muñoz-Bonilla, and Marta Fernández-García. 2025. "Chemical Modification of Chitosan with Bioactive Molecules: A Sustainable Approach for Advanced Film Development" International Journal of Molecular Sciences 26, no. 21: 10403. https://doi.org/10.3390/ijms262110403
APA StyleMuñoz-Núñez, C., Gómez-Fernández, N., Muñoz-Bonilla, A., & Fernández-García, M. (2025). Chemical Modification of Chitosan with Bioactive Molecules: A Sustainable Approach for Advanced Film Development. International Journal of Molecular Sciences, 26(21), 10403. https://doi.org/10.3390/ijms262110403








