A New Ursane-Type Pentacyclic Triterpenoid from the Tree Bark of Sandoricum koetjape: Antibacterial, DFT, and Molecular Docking Study
Abstract
1. Introduction
2. Results
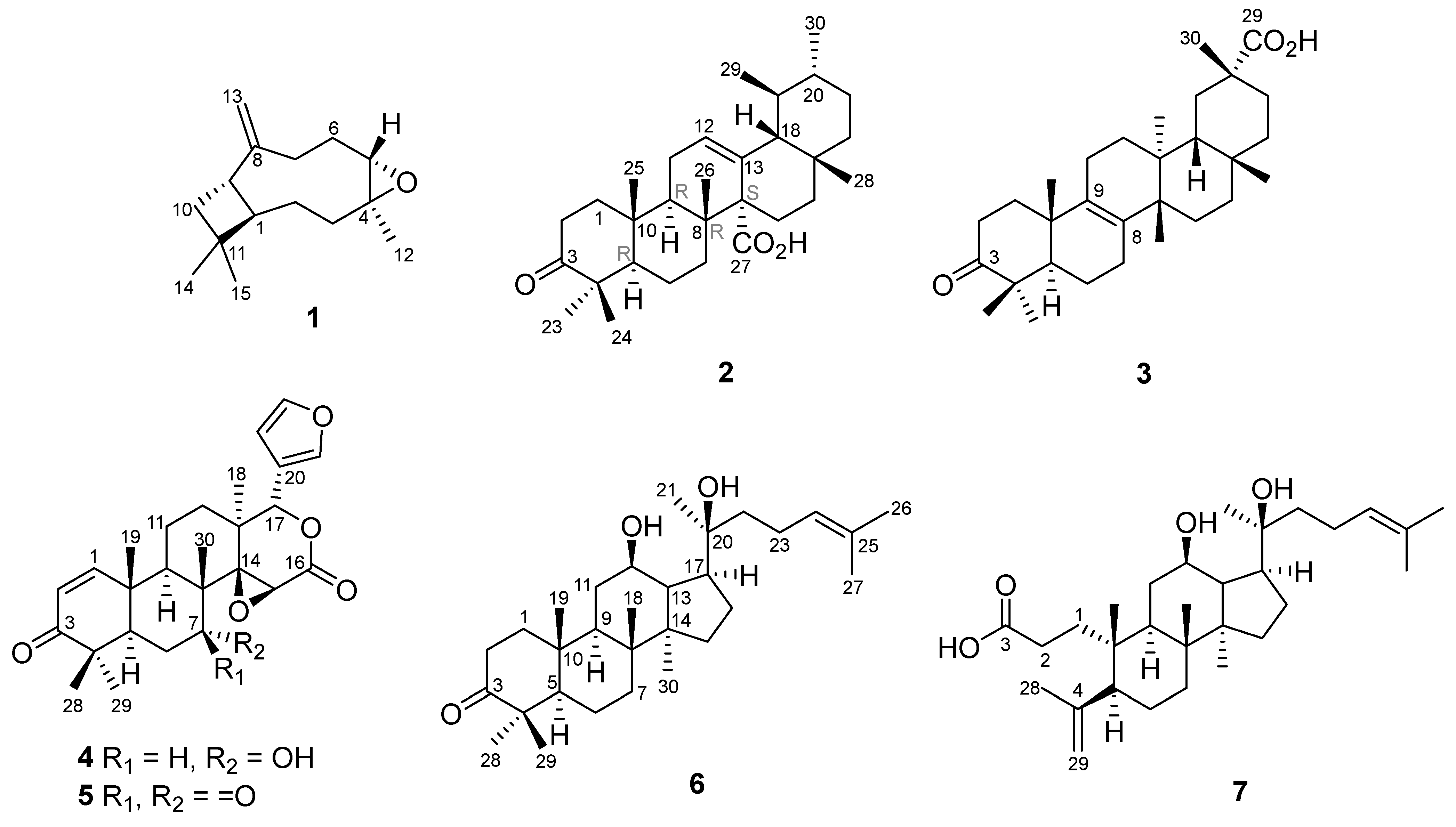
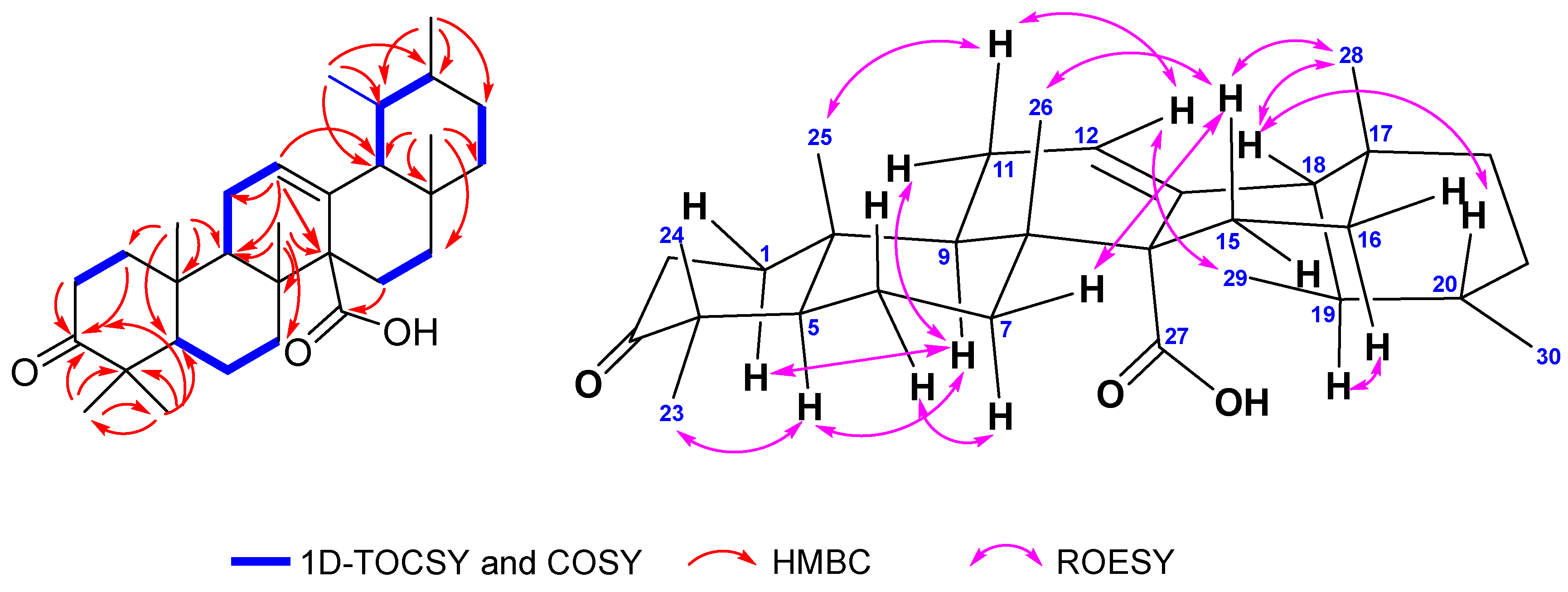
2.1. Identification of Isolated Compounds from S. koetjape
2.2. Antibacterial Evaluation
2.3. Study of Density Functional Theory (DFT)
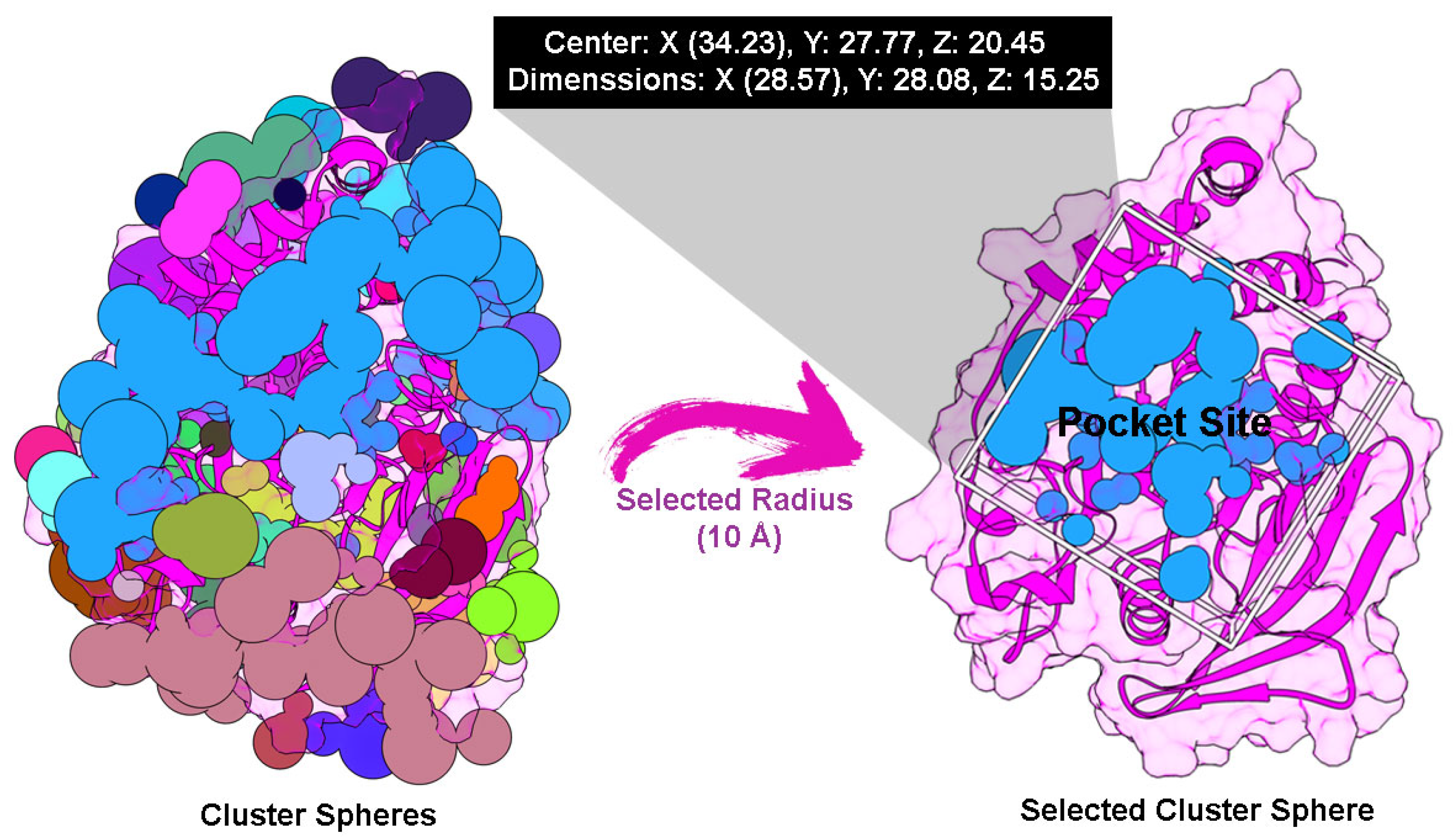
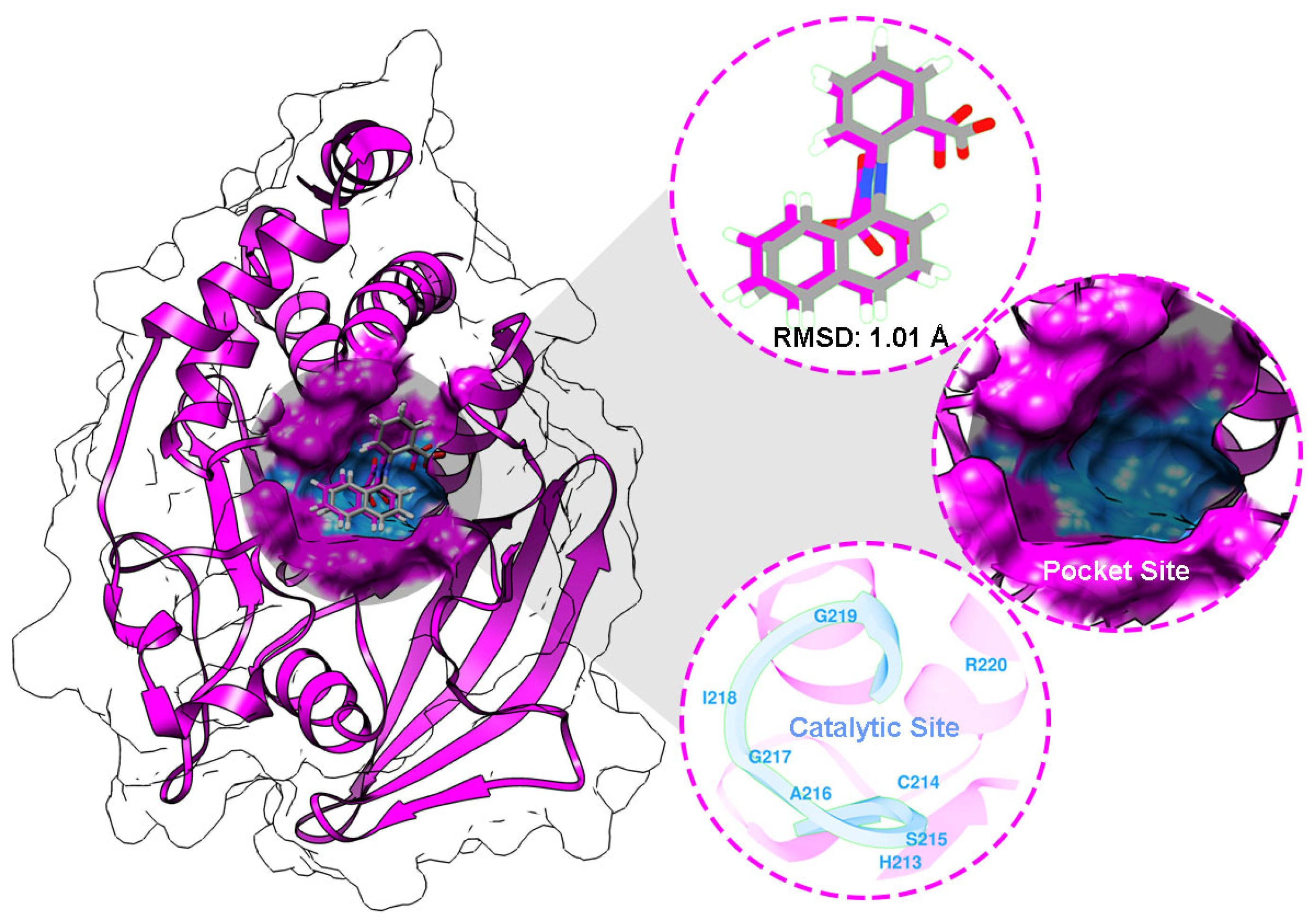
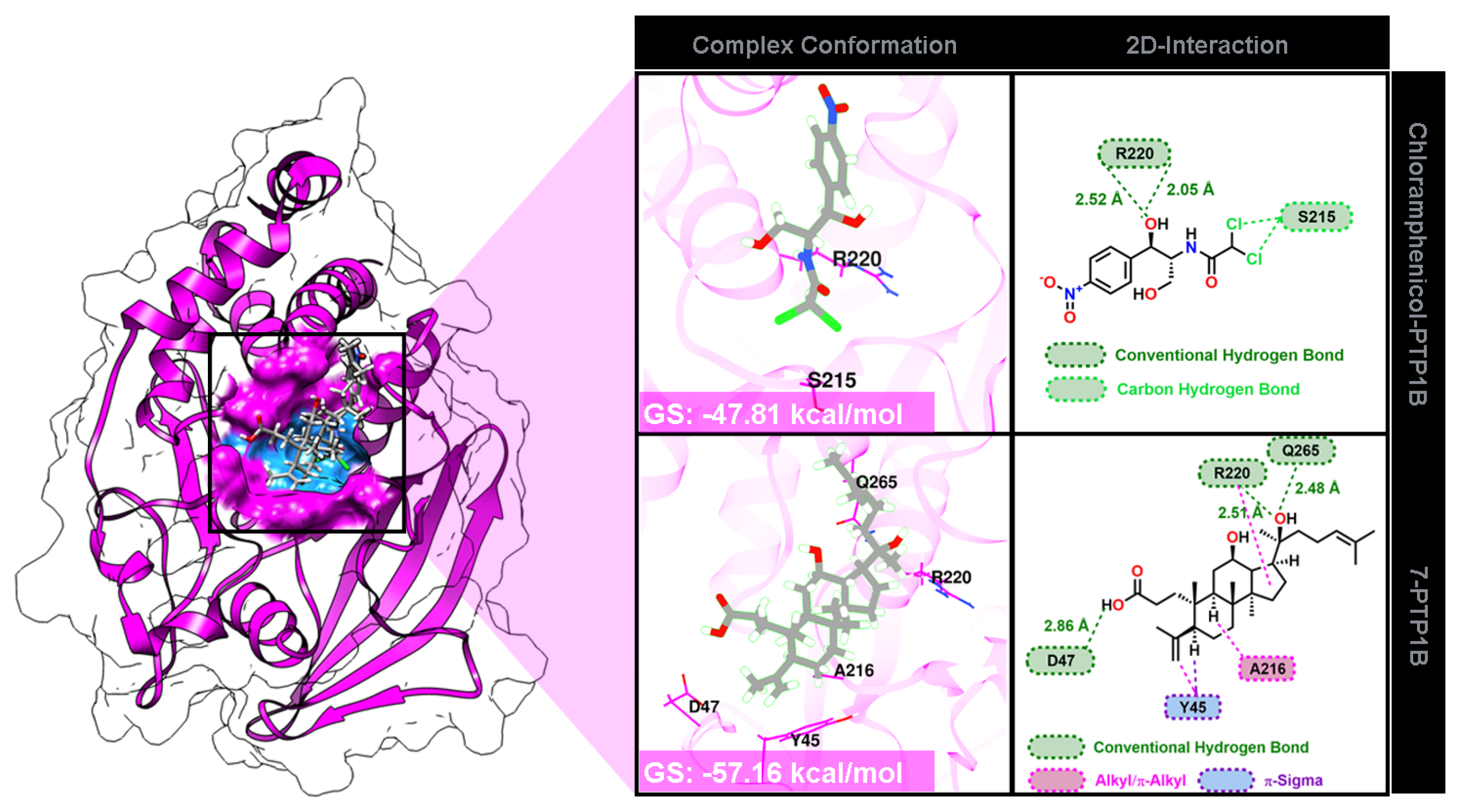
2.4. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Materials
4.3. Extraction and Isolation
- β-caryophyllene oxide (1)—yellowish oil, [α]25D −40.0 (c 0.1; CHCl3) 1H-NMR (CDCl3, 500 MHz), δH (ppm) 1.76 (1H, t; 9.92 Hz, H-1); 1.43 (1H, m, H-2); 1.65 (1H, m, H-2); 0.97 (1H, m, H-3); 2.09 (1H, dt; 13.15 Hz; 4.19 Hz, H-3); 2.88 (1H, dd; 10.6 Hz; 4.3 Hz, H-5); 2.34 (1H, ddd, 12.70 Hz; 8.07 Hz; 4.46 Hz, H-6); 1.25 (1H, d; 3.58 Hz, H-6); 1.33 (2H, m, H-7); 2.61 (1H, q; 9.5 Hz, H-9); 1.65 (2H, m, H-10); 1.20 (3H, s, H-12); 4.86 (1H, s, H-13); 4.97 (1H, s, H-13); 0.98 (3H, s, H-14); 1.01 (3H, s, H-15). 13C-NMR (CDCl3, 125 MHz), δC (ppm) 50.8 (C-1); 27.2 (C-2); 39.2 (C-3); 59.8 (C-4); 63.8 (C-5); 29.8 (C-6); 30.2 (C-7); 151.8 (C-8); 48.7 (C-9); 39.8 (C-10); 34.0 (C-11); 17.0 (C-12); 112.8 (C-13); 29.9 (C-14); 21.6 (C-15). HRESITOF-MS: [M+H]+ ion m/z 221.1908 (calcd. [M+H]+ ion for C15H24O: m/z 221.1905; Δ 1.4 ppm). The NMR and mass spectra of compound 1 are available in the supplementary file (Figures S1–S5).
- (18β,19αH)-3-oxo-urs-12-en-27α-oic acid (2)—white powders, [α]23D +142.0 (c 0.1; CHCl3). 13C-NMR (in CDCl3) see Table 2; 1H-NMR see Table 1. HRESITOF-MS: [M-H]− ion m/z 453.3367 (calcd. [M-H]− ion for C30H46O3: m/z 453.3369; Δ 0.4 ppm). The NMR and mass spectra of compound 2 are available in the supplementary file (Figures S6–S12).
- Bryononic acid (3)—white powders, [α]23D +36.0 (c 0.1; CHCl3). 13C-NMR (in CDCl3) see Table 2; 1H-NMR see Table 1. HRESITOF-MS: [M-H]− ion m/z 453.3356 (calcd. [M-H]− ion for C30H46O3: m/z 453.3369; Δ 2.9 ppm). The NMR and mass spectra of compound 3 are available in the supplementary file (Figures S13–S18).
- 7-deacetyl gedunin (4)—white powders, [α]23D +46.0 (c 0.1; CHCl3). 13C-NMR (in CDCl3) see Table 2; 1H-NMR see Table 1. HRESITOF-MS: [M+H]+ ion m/z 441.2285 (calcd. [M+H]+ ion for C26H32O6: m/z 441.2277; Δ 1.8 ppm). The NMR and mass spectra of compound 4 are available in the supplementary file (Figures S19–S23).
- 7-deacetyl-7-oxogedunin (5)—white powders, [α]23D −62.0 (c 0.1; CHCl3). 13C-NMR (in CDCl3) see Table 2; 1H-NMR see Table 1. HRESITOF-MS: [M+H]+ ion m/z 439.2123 (calcd. [M+H]+ ion for C26H30O6: m/z 439.2121; Δ 0.5 ppm). The NMR and mass spectra of compound 5 are available in the supplementary file (Figures S24–S28).
- 12,20-dihydroxydammar-24-en-3-one (6)—white powders, [α]23D +24.0 (c 0.1; CHCl3). 13C-NMR (in CDCl3) see Table 2; 1H-NMR see Table 1. HRESITOF-MS: [M+H]+ ion m/z 459.3835 (calcd. [M+H]+ ion for C30H50O3: m/z 459.3838; Δ 0.7 ppm). The NMR and mass spectra of compound 6 are available in the supplementary file (Figures S29–S33).
4.4. In Vitro Antibacterial Activity
4.5. DFT Calculation
4.6. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, G.; Sajid, A.; Kalia, V.C. Drug Resistance in Bacteria, Fungi, Malaria, and Cancer; Springer: Cham, Switzerland, 2017; pp. 1–629. [Google Scholar] [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List, 2024; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem. Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef]
- Moloney, M.G. Natural Products as a Source for Novel Antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Erin Lim, S.H.; Lai, K.S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Chen, H.T.; Wu, Z.Y.; Jhan, Y.L.; Shyu, C.L.; Chou, C.H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia Scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Tret’yakova, E.; Baev, D. Evaluation of A-Azepano-Triterpenoids and Related Derivatives as Antimicrobial and Antiviral Agents. J. Antibiot. 2021, 74, 559–573. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Brunel, J.M.; Khusnutdinova, E.F.; Negrel, S.; Giniyatullina, G.V.; Lopatina, T.V.; Petrova, A.V. A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity. Molbank 2019, 2019, M1078. [Google Scholar] [CrossRef]
- Bailly, C. The Health Benefits of Santol Fruits and Bioactive Products Isolated from Sandoricum Koetjape Merr.: A Scoping Review. J. Food Biochem. 2022, 46, e14152. [Google Scholar] [CrossRef]
- Bumi, M.B.; Heliawaty, L.; Hermawati, E.; Syah, Y.M. Four Limonoids from the Seeds Extract of Sandoricum Koetjape. J. Nat. Med. 2019, 73, 641–647. [Google Scholar] [CrossRef]
- Efdi, M.; Ninomiya, M.; Suryani, E.; Tanaka, K.; Ibrahim, S.; Watanabe, K.; Koketsu, M. Sentulic Acid: A Cytotoxic Ring A-Seco Triterpenoid from Sandoricum Koetjape Merr. Bioorganic Med. Chem. Lett. 2012, 22, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Rachmadhaningtiyas, D.A.; Heliawati, L.; Hermawati, E.; Syah, Y.M. A Cipadesin Limonoid and a Tirucallane Triterpene from the Fruit of Sandoricum Koetjape and Their Inhibitory Properties against Receptor Tyrosine Kinases. Nat. Prod. Sci. 2021, 27, 134–139. [Google Scholar] [CrossRef]
- Kaneda, N.; Pezzuto, J.M.; Kinghorn, D.; Farnsworth, N.R.; Santisuk, T.; Tuchinda, P.; Udchachon, J.; Reutrakul, V. Plant Anticancer Agents, l. Cytotoxic Triterpenes from Sandoricum Koetjape Stems. J. Nat. Prod. 1992, 55, 654–659. [Google Scholar] [CrossRef]
- Ismail, I.S.; Ito, H.; Hatano, T.; Taniguchi, S.; Yoshida, T. Modified Limonoids from the Leaves of Sandoricum Koetjape. Phytochemistry 2003, 64, 1345–1349. [Google Scholar] [CrossRef]
- Ismail, I.S.; Ito, H.; Hatano, T.; Taniguchi, S.; Yoshida, T. Two New Analogues of Trijugin-Type Limonoids from the Leaves of Sandoricum Koetjape. Chem. Pharm. Bull. 2004, 52, 1145–1147. [Google Scholar] [CrossRef]
- Kosela, S.; Yulizar, Y.; Chairul, C.; Tori, M.; Asakawa, Y. Secomultiflorane-Type Triterpenoid Acids from Stem Bark of Sandoricum Koetjape. Phytochemistry 1995, 38, 691–694. [Google Scholar] [CrossRef]
- Nagakura, Y.; Nugroho, A.E.; Hirasawa, Y.; Hosoya, T.; Rahman, A.; Kusumawati, I.; Zaini, N.C.; Morita, H. Sanjecumins A and B: New Limonoids from Sandoricum Koetjape. J. Nat. Med. 2013, 67, 381–385. [Google Scholar] [CrossRef]
- Pancharoen, O.; Pipatanapatikarn, A.; Charles Taylor, W.; Bansiddhi, J. Two New Limonoids from the Leaves of Sandoricum Koetjape. Nat. Prod. Res. 2009, 23, 10–16. [Google Scholar] [CrossRef]
- Powell, R.G.; Mikolajczak, K.L.; Zilkowski, B.W. Limonoid Antifeedants from Seed of Sandoricum Koetjape. J. Nat. Prod. 1991, 54, 241–246. [Google Scholar] [CrossRef]
- Tanaka, T.; Koyano, T.; Kowithayakorn, T.; Fujimoto, H.; Okuyama, E.; Hayashi, M.; Komiyama, K.; Ishibashi, M. New Multiflorane-Type Triterpenoid Acids from Sandoricum Indicum. J. Nat. Prod. 2001, 64, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Saptanti, K.; Heliawati, L.; Hermawati, E.; Syah, Y.M. Pentacyclic Triterpenes from the Leaves Extract of Sandoricum Koetjape. J. Nat. Med. 2022, 76, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.-X.-H.; Jarupinthusophon, S.; Hairani, R.; Nguyen, V.-K.; Chavasiri, W. Cycloartane-Type Triterpenoids from the Leaves of Sandoricum Koetjape and Their Efficacy on α-Glucosidase Inhibition Activity. J. Nat. Med. 2024, 78, 655–663. [Google Scholar] [CrossRef]
- Heliawati, L.; Khatimah, H.; Hermawati, E.; Syah, Y.M. Four Dammarane Triterpenes and Their Inhibitory Properties against Eight Receptor Tyrosine Kinases. Nat. Prod. Sci. 2020, 26, 345–350. [Google Scholar] [CrossRef]
- Nsiama, T.K.; Okamura, H.; Hamada, T.; Morimoto, Y.; Doe, M.; Iwagawa, T.; Nakatani, M. Rings D-Seco and B,D-Seco Tetranortriterpenoids from Root Bark of Entandrophragma Angolense. Phytochemistry 2011, 72, 1854–1858. [Google Scholar] [CrossRef]
- Nagatomo, A.; Ninomiya, K.; Marumoto, S.; Sakai, C.; Watanabe, S.; Ishikawa, W.; Manse, Y.; Kikuchi, T.; Yamada, T.; Tanaka, R.; et al. A Gedunin-Type Limonoid, 7-Deacetoxy-7-Oxogedunin, from Andiroba (Carapa Guianensis Aublet) Reduced Intracellular Triglyceride Content and Enhanced Autophagy in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 13141. [Google Scholar] [CrossRef]
- Chen, J.J.; Fei, D.Q.; Chen, S.G.; Gao, K. Antimicrobial Triterpenoids from Vladimiria Muliensis. J. Nat. Prod. 2008, 71, 547–550. [Google Scholar] [CrossRef]
- Cai, X.F.; Park, B.Y.; Ahn, K.S.; Kwon, O.K.; Lee, H.K.; Oh, S.R. Cytotoxic Triterpenoids from the Rhizomes of Astilbe Chinensis. J. Nat. Prod. 2009, 72, 1241–1244. [Google Scholar] [CrossRef]
- Razdan, T.K.; Kachroo, V.; Harkar, S.; Koul, G.L. Plectranthoic Acid A & B, Two New Triterpenoids from Plectranthus Rugosus. Tetrahedron 1982, 38, 991–992. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Chen, K.; Zhang, L.; Li, Y. Triterpenoids from the Roots of Sanguisorba Officinalis and Their Nrf2 Stimulation Activity. Phytochemistry 2023, 214, 113803. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.J.; ter Steege, E.; den Hertog, J. Recent Advances in Understanding the Role of Protein-Tyrosine Phosphatases in Development and Disease. Dev. Biol. 2017, 428, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Alfarisi, S.; Santoso, M.; Kristanti, A.N.; Siswanto, I.; Puspaningsih, N.N.T. Synthesis, Antimicrobial Study, and Molecular Docking Simulation of 3,4-Dimethoxy-β-Nitrostyrene Derivatives as Candidate Ptp1b Inhibitor. Sci. Pharm. 2020, 88, 37. [Google Scholar] [CrossRef]
- Muhammad, I.; El Sayed, K.A.; Mossa, J.S.; Al-Said, M.S.; El-Feraly, F.S.; Clark, A.M.; Hufford, C.D.; Oh, S.; Mayer, A.M.S. Bioactive 12-Oleanene Triterpene and Secotriterpene Acids from Maytenus Undata. J. Nat. Prod. 2000, 63, 605–610. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Wang, X.N.; Yin, S.; Dong, L.; Yue, J.M. Ring A-Seco Triterpenoids with Antibacterial Activity from Dysoxylum Hainanense. Bioorganic Med. Chem. Lett. 2011, 21, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Balius, T.E.; Tan, Y.S.; Chakrabarti, M. DOCK 6: Incorporating Hierarchical Traversal through Precomputed Ligand Conformations to Enable Large-Scale Docking. J. Comput. Chem. 2024, 45, 47–63. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of Diffusion and Dilution Methods to Determine the Antibacterial Activity of Plant Extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.D.; Ene, F.; Duong, T.H.; Mulya, F.; Chavasiri, W. Insights into the Inhibitory Activity and Mechanism of Natural Compounds from Rhinacanthus Nasutus on α-Glucosidase through Kinetic, Molecular Docking, and Molecular Dynamics Studies. J. Mol. Struct. 2025, 1322, 140527. [Google Scholar] [CrossRef]
- Kaennakam, S.; Sukandar, E.R.; Phasuthan, P.; Yahuafai, J.; Onsrisawat, P.; Mulya, F.; Parasuk, V.; Phuwapraisirisan, P.; Tip-pyang, S. Garcowacinols A-J, Cytotoxic Polyprenylated Benzoylphloroglucinol Derivatives from the Twigs of Garcinia Cowa. Phytochemistry 2023, 209, 113622. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Tsuneda, T.; Song, J.W.; Suzuki, S.; Hirao, K. On Koopmans’ Theorem in Density Functional Theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef]
- Szczepankiewicz, B.G.; Liu, G.; Hajduk, P.J.; Abad-Zapatero, C.; Pei, Z.; Xin, Z.; Lubben, T.H.; Trevillyan, J.M.; Stashko, M.A.; Ballaron, S.J.; et al. Discovery of a Potent, Selective Protein Tyrosine Phosphatase 1B Inhibitor Using a Linked-Fragment Strategy. J. Am. Chem. Soc. 2003, 125, 4087–4096. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 56531, 1157–1174. [Google Scholar] [CrossRef]
- Brozell, S.R.; Mukherjee, S.; Balius, T.E.; Roe, D.R.; Case, D.A.; Rizzo, R.C. Evaluation of DOCK 6 as a Pose Generation and Database Enrichment Tool. J. Comput. Aided Mol. Des. 2012, 26, 749–773. [Google Scholar] [CrossRef]
- Allen, W.J.; Balius, T.E.; Mukherjee, S.; Brozell, S.R.; Moustakas, D.T.; Lang, P.T.; Case, D.A.; Kuntz, I.D.; Rizzo, R.C. DOCK 6: Impact of New Features and Current Docking Performance. J. Comput. Chem. 2015, 36, 1132–1156. [Google Scholar] [CrossRef] [PubMed]
| Position | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| 1 | 39.3 | 35.4 | 157.8 | 155.9 | 39.8 |
| 2 | 34.0 | 34.4 | 125.8 | 126.4 | 34.1 |
| 3 | 217.9 | 218.2 | 205.6 | 203.2 | 217.8 |
| 4 | 47.1 | 47.1 | 44.2 | 45.2 | 47.4 |
| 5 | 54.6 | 51.2 | 44.6 | 54.6 | 55.3 |
| 6 | 19.7 | 20.6 | 27.3 | 36.7 | 19.7 |
| 7 | 36.0 | 34.2 | 69.7 | 208.2 | 34.1 |
| 8 | 39.8 | 134.9 | 43.7 | 53.4 | 51.6 |
| 9 | 45.9 | 132.7 | 38.0 | 47.6 | 49.4 |
| 10 | 36.6 | 30.9 | 40.2 | 39.6 | 36.8 |
| 11 | 22.9 | 20.5 | 15.1 | 17.2 | 31.5 |
| 12 | 128.4 | 30.0 | 26.4 | 32.2 | 70.7 |
| 13 | 133.2 | 37.1 | 38.4 | 37.7 | 48.0 |
| 14 | 55.8 | 42.2 | 70.1 | 65.6 | 39.7 |
| 15 | 22.4 | 25.2 | 57.8 | 53.6 | 31.0 |
| 16 | 28.9 | 27.7 | 168.3 | 166.9 | 26.5 |
| 17 | 33.7 | 37.5 | 78.5 | 78.0 | 53.4 |
| 18 | 60.3 | 44.5 | 17.8 | 20.9 | 15.4 |
| 19 | 37.7 | 29.5 | 19.9 | 19.8 | 15.9 |
| 20 | 39.6 | 40.4 | 120.6 | 120.2 | 74.7 |
| 21 | 30.4 | 30.5 | 141.2 | 141.0 | 27.1 |
| 22 | 40.8 | 36.8 | 110.0 | 109.8 | 34.3 |
| 23 | 27.0 | 21.1 | 143.0 | 143.1 | 22.4 |
| 24 | 21.3 | 26.8 | - | - | 124.8 |
| 25 | 16.4 | 19.5 | - | - | 132.0 |
| 26 | 18.1 | 21.7 | - | - | 25.8 |
| 27 | 180.9 | 18.1 | - | - | 17.8 |
| 28 | 29.0 | 31.1 | 27.3 | 27.0 | 21.0 |
| 29 | 17.8 | 185.1 | 21.5 | 20.6 | 26.7 |
| 30 | 21.4 | 32.7 | 18.7 | 17.4 | 16.8 |
| Position | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| 1 | 1.57 (m); 1.93 (m) | 2.02 (m); 1.53 (m) | 7.10 (d, 10.2) | 7.11 (d, 10.2) | 1.47 (m) |
| 2 | 2.46 (m) | 2.45 (m); 2.53(m) | 5.84 (d, 10.2) | 5.92 (d, 10.2) | 2.44 (m); 2.51 (m) |
| 5 | 1.35 (m) | 1.61 (m) | 2.48 (m) | 2.18 (m) | 1.36 (m) |
| 6 | 1.43 (m) | 1.95 (m) | 1.66 (m); 1.90 (m) | 2.41 (dd, 2.9; 13.9); 2.93 (t, 14.2) | 1.52 (m) |
| 7 | 1.16 (m); 1.75 (m) | 2.03 (m); 0.89 (m) | 3.57 (s) | - | 1.34 (m); 1.55 (m) |
| 9 | 2.33 (dd, 5.45; 11.0) | - | 2.52 (m) | 2.21 (m) | 1.51 (m) |
| 11 | 2.01 (m) | 1.62 (m); 1.44 (m) | 1.80 (m); 1.97 (m) | 1.80 (m); 2.00 (m) | 1.31 (m); 1.85 (m) |
| 12 | 5.58 (qui, 2.4) | 1.67 (m); 1.28 (m) | 1.54 (m); 1.71 (m) | 1.48 (dt, 12.43; 8.84); 1.86 (m) | 3.60 (td, 10.41; 5.08) |
| 13 | - | - | - | - | 1.76 (t, 10.65) |
| 15 | 1.77 (m); 1.98 (m) | 1.46 (m); 1.31 (m) | 3.91 (s) | 3.87 (s) | 1.05 (m); 1.51 (m) |
| 16 | 0.91 (m); 2.11 (td, 13.62; 3.85) | 1.82 (m); 2.13 (m) | - | - | 1.27 (m); 1.87 (m) |
| 17 | - | - | 5.60 (s) | 5.47 (s) | 2.05 (td, 10.53; 7.16) |
| 18 | 1.37 (m) | 1.51 (m) | 1.24 (s) | 1.13 (s) | 1.03 (s) |
| 19 | 0.89 (m) | 2.18 (m); 1.38 (m) | 1.20 (s) | 1.36 (s) | 0.98 (s) |
| 20 | 0.86 (m) | - | - | - | - |
| 21 | 1.24 (m); 1.34 (m) | 2.43 (m); 1.64 (m) | 7.41 (s) | 7.41 (s) | 1.21 (s) |
| 22 | 1.24 (m); 1.41 (m) | 1.31 (m); 1.70 (m) | 6.35 (s) | 6.36 (s) | 1.41 (m); 1.68 (m) |
| 23 | 1.06 (s) | 1.06 (s) | 7.40 (d, 1.70) | 7.39 (d, 0.95) | 2.05 (m); 2.17 (m) |
| 24 | 1.03 (s) | 1.09 (s) | - | - | 5.17 (t, 7.04) |
| 25 | 1.05 (s) | 1.03 (s) | - | - | - |
| 26 | 1.07 (s) | 0.96 (s) | - | - | 1.70 (s) |
| 27 | - | 0.85 (s) | - | - | 1.64 (s) |
| 28 | 0.84 (s) | 1.03 (s) | 1.14 (s) | 1.16 (s) | 1.05 (s) |
| 29 | 0.79 (d, 5.5) | - | 1.10 (s) | 1.14 (s) | 1.08 (s) |
| 30 | 0.86 (brs) | 1.22 (s) | 1.09 (s) | 1.22 (s) | 0.89 (s) |
| Compounds | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | |||
| E. faecalis | S. saprophyticus | S. enterica | C. freundii | |
| Amoxicillin (1) | 28 | 14 | 15 | 30 |
| Chloramphenicol (1) | 8 | 25 | 26 | 25 |
| 1 | - | - | - | - |
| 2 | - | - | - | - |
| 3 | - | - | - | - |
| 4 | - | - | - | - |
| 5 | - | - | - | - |
| 6 | - | - | - | - |
| 7 | 9 | 7.5 | 8 | 9 |
| 5% DMSO-MHB (2) | - | - | - | - |
| Compound | EHOMO | ELUMO | IP | EA | χ | µ | η | ω | Eg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −6.487 | −0.142 | 6.49 | 0.14 | 3.31 | −3.31 | 3.17 | 1.73 | 6.34 |
| 2 | −6.565 | −0.736 | 6.56 | 0.74 | 3.65 | −3.65 | 2.91 | 2.29 | 5.83 |
| 3 | −6.174 | −0.587 | 6.17 | 0.59 | 3.38 | −3.38 | 2.79 | 2.05 | 5.59 |
| 4 | −6.667 | −1.967 | 6.67 | 1.97 | 4.32 | −4.32 | 2.35 | 3.96 | 4.70 |
| 5 | −6.740 | −2.195 | 6.74 | 2.20 | 4.47 | −4.47 | 2.27 | 4.39 | 4.54 |
| 6 | −6.351 | −0.756 | 6.35 | 0.76 | 3.55 | −3.55 | 2.80 | 2.26 | 5.59 |
| 7 | −6.331 | −0.458 | 6.33 | 0.46 | 3.39 | −3.39 | 2.94 | 1.96 | 5.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatimah, H.; Hermawati, E.; Mulya, F.; Abdjan, M.I.; Kuamit, T.; Danova, A. A New Ursane-Type Pentacyclic Triterpenoid from the Tree Bark of Sandoricum koetjape: Antibacterial, DFT, and Molecular Docking Study. Int. J. Mol. Sci. 2025, 26, 10389. https://doi.org/10.3390/ijms262110389
Khatimah H, Hermawati E, Mulya F, Abdjan MI, Kuamit T, Danova A. A New Ursane-Type Pentacyclic Triterpenoid from the Tree Bark of Sandoricum koetjape: Antibacterial, DFT, and Molecular Docking Study. International Journal of Molecular Sciences. 2025; 26(21):10389. https://doi.org/10.3390/ijms262110389
Chicago/Turabian StyleKhatimah, Husnul, Elvira Hermawati, Fadjar Mulya, Muhammad Ikhlas Abdjan, Thanawit Kuamit, and Ade Danova. 2025. "A New Ursane-Type Pentacyclic Triterpenoid from the Tree Bark of Sandoricum koetjape: Antibacterial, DFT, and Molecular Docking Study" International Journal of Molecular Sciences 26, no. 21: 10389. https://doi.org/10.3390/ijms262110389
APA StyleKhatimah, H., Hermawati, E., Mulya, F., Abdjan, M. I., Kuamit, T., & Danova, A. (2025). A New Ursane-Type Pentacyclic Triterpenoid from the Tree Bark of Sandoricum koetjape: Antibacterial, DFT, and Molecular Docking Study. International Journal of Molecular Sciences, 26(21), 10389. https://doi.org/10.3390/ijms262110389





