Enhancement of Photosynthetic Efficiency and Antioxidant Response in Wheat Under Drought Stress by Quercetin–Copper Complex
Abstract
1. Introduction
2. Results
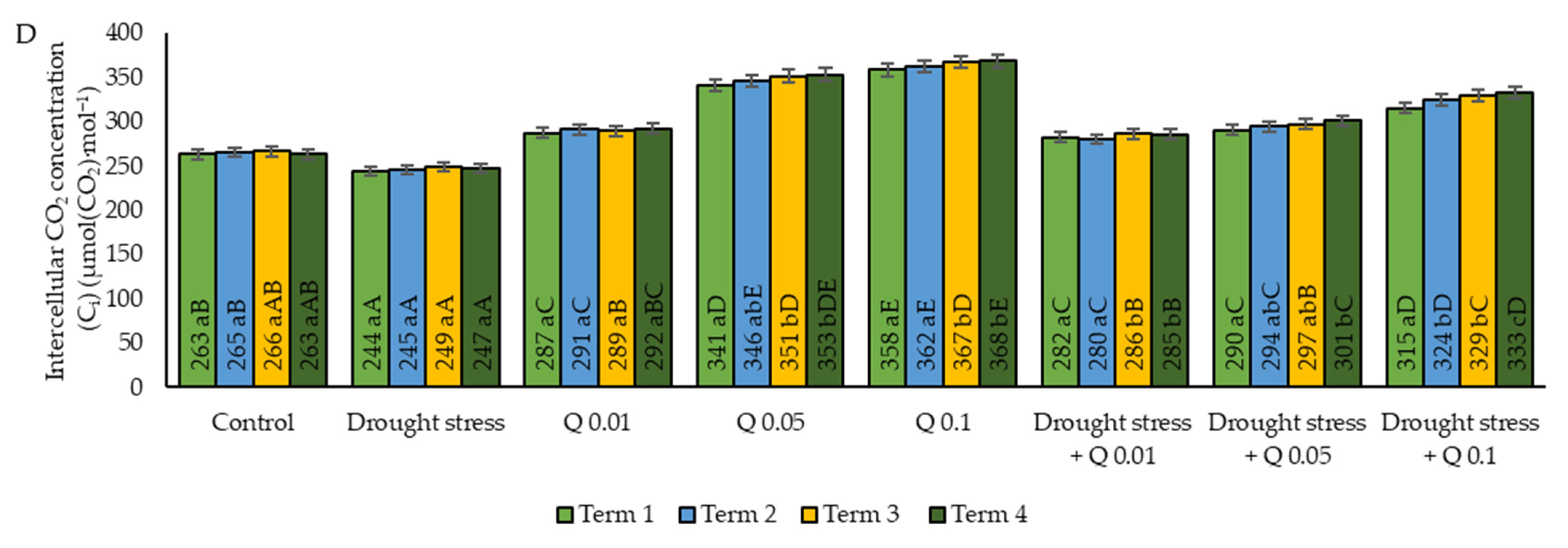
2.1. Gas Exchange
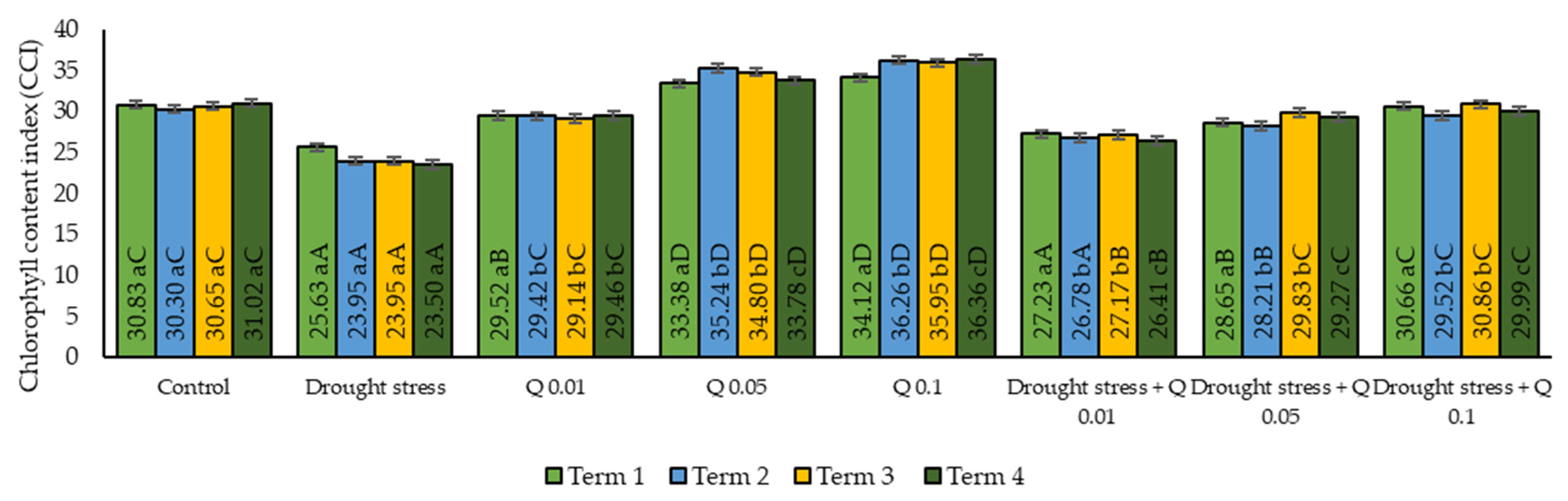
2.2. Chlorophyll Content Index (CCI)
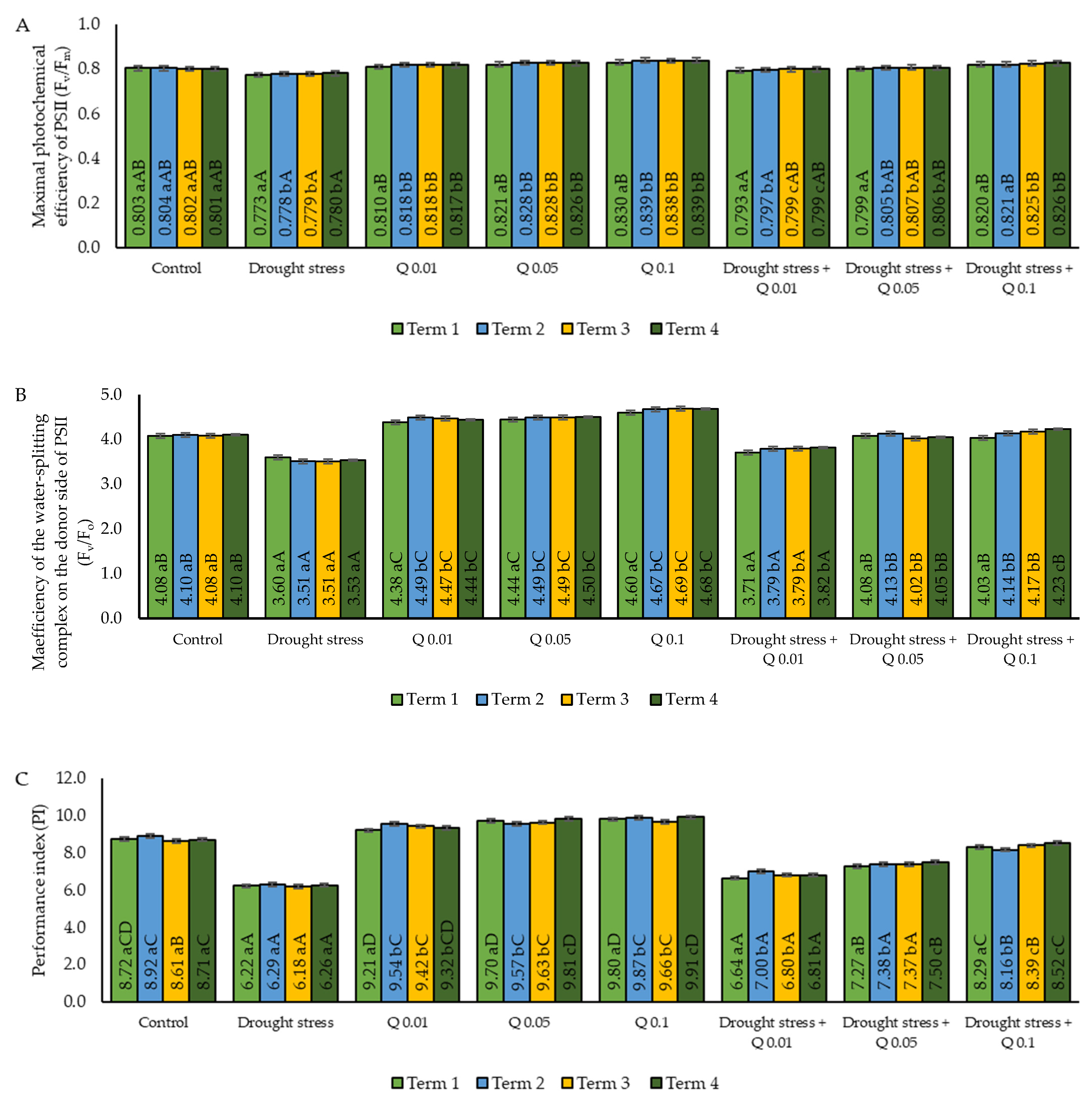
2.3. Chlorophyll Fluorescence
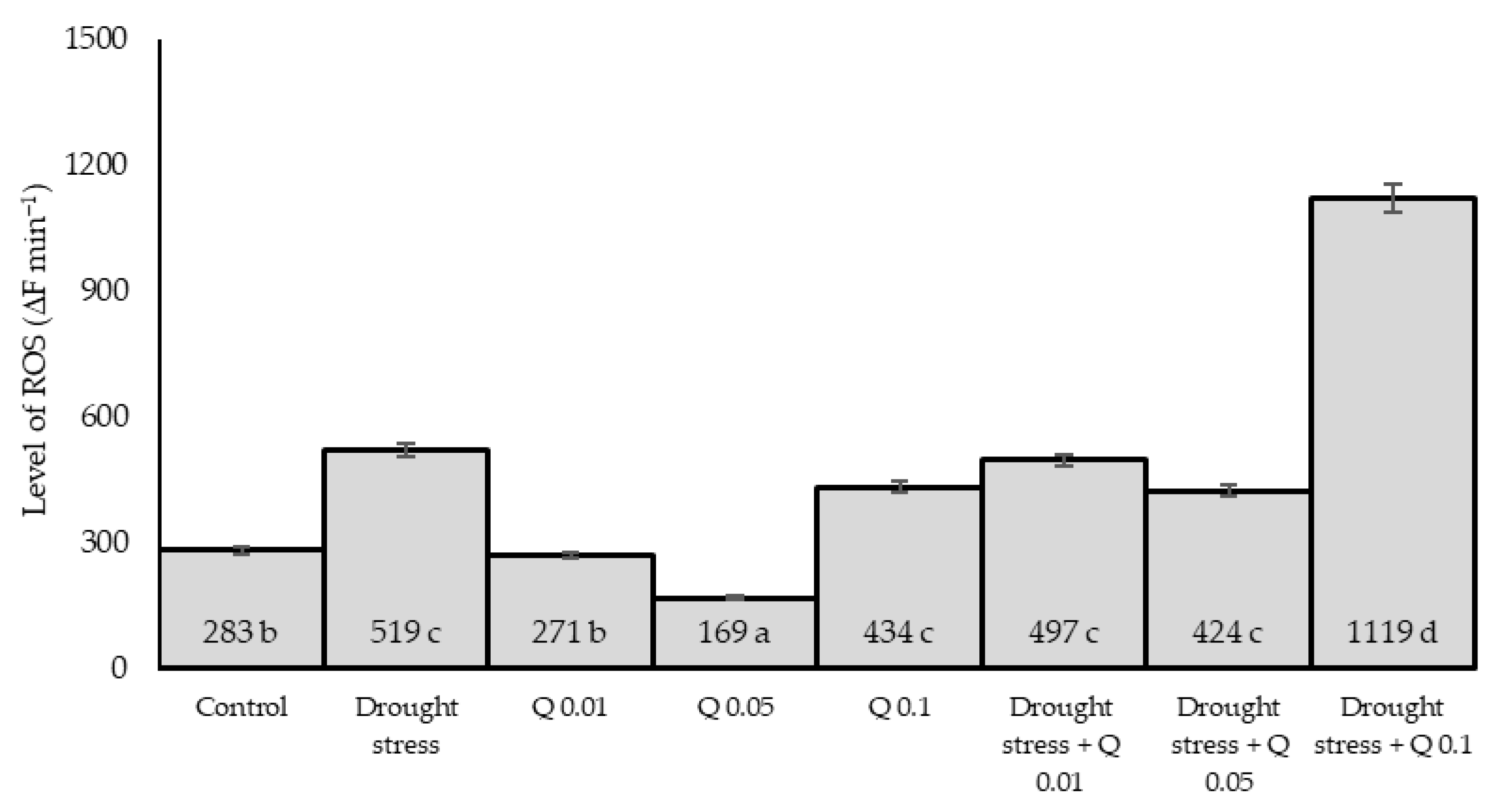
2.4. ROS Level
2.5. Activity of Enzymes
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Quercetin–copper (II) Complex (Q-Cu (II))
4.2. Experimental Design
4.3. Determination of Gas Exchange
4.4. Determination of Chlorophyll Content Index (CCI)
4.5. Determination of Chlorophyll Fluorescence
4.6. Measurement of Biochemical Parameters
4.6.1. Determination of ROS Level
4.6.2. Determination of Antioxidant Enzyme Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Q-Cu (II) | quercetin–copper complex |
| ROS | Reactive Oxygen Species |
| PSII | Photosystem II |
| Fv/Fm | The maximum quantum yield of PSII) photochemistry |
| Fv/Fo | the efficiency of the water-splitting complex on the donor side of PSI |
| PI | Photosynthetic efficiency index |
| PN | photosynthetic network intensity |
| gs | stomatal conductance |
| E | transpiration rate |
| Ci | intercellular CO2 concentration |
| CAT | catalase |
| SOD | peroxidase |
| GPOX | guaiacol peroxidase |
| ABA | abscisic acid |
| TFs | transcription factors |
| DREB | Dehydration Responsive Element Binding |
| LEA | late embryogenic abundance |
| HSP | heat shock proteins |
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 26 March 2025).
- Kheiralipour, K.; Brandão, M.; Holka, M.; Choryński, A. A Review of Environmental Impacts of Wheat Production in Different Agrotechnical Systems. Resources 2024, 13, 93. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.J. Global trends in wheat production, consumption and trade. In Wheat Improvement; Reynolds, M.P., Braun, H.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef] [PubMed]
- Qamer, Z.; Chaudhary, M.T.; Du, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Rane, J.; Singh, A.K.; Kumar, M.; Boraiah, K.M.; Meena, K.K.; Pradhan, A.; Prasad, P.V.V. The Adaptation and Tolerance of Major Cereals and Legumes to Important Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12970. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Pushpakaran, A.M.; Nayagam, J.R. Stressors in Agriculture: Challenges and Implications for Sustainable Crop Production. Int. J. Appl. Agric. Sci. 2025, 11, 146–156. [Google Scholar] [CrossRef]
- Oo, A.T.; van Huylenbroeck, G.; Speelman, S. Measuring the economic impact of climate change on crop production in the dry zone of Myanmar: A Ricardian Approach. Climate 2020, 8, 9. [Google Scholar] [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant tolerance to drought stress in the presence of supporting bacteria and fungi: An efficient strategy in horticulture. Horticulturae 2021, 7, 390. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar Patel, M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and Molecular Approaches Reveal Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Ayub, M.; Ashraf, M.Y.; Kausar, A.; Saleem, S.; Anwar, S.; Altay, V.; Ozturk, M. Growth and physio-biochemical responses of maize (Zea mays L.) to drought and heat stresses. Plant Biosyst. 2021, 155, 535–542. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayab, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, A.; Farooq, M.; Ashraf, M.; Cheema, M.A. Improving drought tolerance by exogenous application of glycine betaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.; Ristic, Z. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; Advances in Agricultural Systems Modeling; American Society of Agronomy, Inc.: Madison, WI, USA, 2008; Volume 1, pp. 301–355. [Google Scholar] [CrossRef]
- Verma, A.; Deepti, S. Abiotic stress and crop improvement: Current scenario. Adv. Plants Agric. Res. 2016, 4, 345–346. [Google Scholar] [CrossRef][Green Version]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Nabi, S.Z.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Pamungkas, S.S.T.; Suwarto; Suprayogi; Farid, N. Drought stress: Responses and mechanism in plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef]
- Hossain, M.A.; Mostofa, M.G.; Fujita, M. Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol. Plant Breed. 2013, 4, 50–70. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Chunduri, V.; Kaur, A.; Kaur, S.; Malhotra, N.; Kumar, A.; Kapoor, P.; Kumari, A.; Kaur, J.; et al. Genome- Wide Identification and Characterization of Heat Shock Protein Family Reveals Role in Development and Stress Conditions in Triticum aestivum L. Sci. Rep. 2020, 10, 7858. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Thakur, T.; Chirom, O.; Mandlik, R.; Deshmukh, R.; Salvi, P. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiol. Plant. 2021, 172, 847–868. [Google Scholar] [CrossRef]
- Wei, H.; Wang, X.; Wang, K.; Tang, X.; Zhang, N.; Si, H. Transcription factors as molecular switches regulating plant responses to drought stress. Physiol. Plant. 2024, 176, 14366. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xia, P. WRKY transcription factors: Key regulators in plant drought tolerance. Plant Sci. 2025, 359, 112647. [Google Scholar] [CrossRef]
- Xie, N.; Li, B.; Yu, J.; Shi, R.; Zeng, Q.; Jiang, Y.; Zhao, D. Transcriptomic and proteomic analyses uncover the drought adaption landscape of Phoebe zhennan. BMC Plant Biol. 2022, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xie, X.; Al Aboud, N.M.; Zhang, P.; Abou-Elwafa, S.F.; Ren, Z.; Deng, D. ZmNHL2 enhances drought tolerance by regulating the expression of stress-responsive genes and ABA signaling pathway in maize. Plant Growth Regul. 2024, 104, 523–533. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Mir, A.R.; Alam, P.; Hayat, S. Quercetin-mediated alteration in photosynthetic efficiency, sugar metabolism, elemental status, yield, and redox potential in two varieties of okra. Protoplasma 2024, 261, 125–142. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Update 2007, 2, 20–35. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Buczek, J.; Balawejder, M. The effect of exogenous application of quercetin derivative solutions on the course of physiological and biochemical processes in wheat seedlings. Int. J. Mol. Sci. 2021, 22, 6882. [Google Scholar] [CrossRef] [PubMed]
- Migut, D.; Jańczak-Pieniążek, M.; Piechowiak, T.; Buczek, J.; Balawejder, M. Physiological response of maize plants (Zea mays L.) to the use of the potassium quercetin derivative. Int. J. Mol. Sci. 2021, 22, 7384. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Balawejder, M. Assessment of the impact of the application of a quercetin—Copper complex on the course of physiological and biochemical processes in wheat plants (Triticum aestivum L.) growing under saline conditions. Cells 2022, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.S.; Westgate, M.E. Reproductive development in graincrops during drought. Adv. Agron. 2000, 68, 59–96. [Google Scholar] [CrossRef]
- Khan, R.; Gilani, H. Global drought monitoring with big geospatial datasets using Google Earth Engine. Environ. Sci. Pollut. Res. 2021, 28, 17244–17264. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–167. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Faisal, S.; Mujtaba, S.M.; Asma; Mahboob, W. Polyethylene Glycol Mediated Osmotic Stress Impacts on Growth and Biochemical Aspects of Wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 2019, 22, 213–223. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Ashaf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis drought stress response using kinetic chlorophyll fluorescence and multicolor fluorescence imaging. Front. Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll Fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Barboričová, M.; Filaček, A.; Vysoká, D.M.; Gašparovič, K.; Živčák, M.; Brestič, M. Sensitivity of fast chlorophyll fluorescence parameters to combined heat and drought stress in wheat genotypes. Plant Soil Environ. 2022, 68, 309–316. [Google Scholar] [CrossRef]
- Mladenov, P.; Aziz, S.; Topalova, E.; Renaut, J.; Planchon, S.; Raina, A.; Tomlekova, N. Physiological Responses of Common Bean Genotypes to Drought Stress. Agronomy 2023, 13, 1022. [Google Scholar] [CrossRef]
- Turyagyenda, L.F.; Kizito, E.B.; Ferguson, M.; Baguma, Y.; Agaba, M.; Harvey, J.J.; Osiru, D.S. Physiological and molecular characterization of drought responses and identification of candidate tolerance genes in cassava. AoB Plants 2013, 5, plt007. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Devadasu, E.; Saini, D.; Dhokne, K.; Marriboina, S.; Raghavendra, A.S.; Subramanyam, R. Reversible changes in structure and function of photosynthetic apparatus of Pea (Pisum sativum) leaves under drought stress. Plant J. 2023, 113, 60–74. [Google Scholar] [CrossRef]
- Souza, R.; Machado, E.; Silva, J.; Lagôa, A.; Silveira, J. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Shirke, P.A.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef]
- Islam, M.T. Effect of drought stress on photosynthesis, stomatal conductance, transpiration and yield of mungbean genotypes under high temperature. Bangladesh J. Nucl. Agric. 2022, 36, 83–91. [Google Scholar]
- Helm, L.T.; Shi, H.; Lerdau, M.T.; Yang, X. Solar-induced chlorophyll fluorescence and short-term photosynthetic response to drought. Ecol. Appl. 2020, 30, e02101. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Hossain, P.Z.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Saeidi, M.; Abdoli, M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J. Agric. Sci. Technol. 2018, 17, 885–898. [Google Scholar]
- Dastborhan, S.; Ghassemi-Golezani, K. Influence of seed priming and water stress on selected physiological traits of borage. Folia Hortic. 2015, 27, 151–159. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Stress-Triggered Redox Signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef]
- Moustakas, M. Plant Photochemistry, Reactive Oxygen Species, and Photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. ROS Generation in the Light Reactions of Photosynthesis Triggers Acclimation Signaling to Environmental Stress. Photochem 2025, 5, 28. [Google Scholar] [CrossRef]
- Carvalho, M.D. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Awad, D.; Samarah, N. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J. Plant Interact. 2015, 10, 109–116. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Luna, C.M. Drought Controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2004, 56, 417–423. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Barutçular, C.; Gormus, O.; Hussain, S.; Islam, M.S.; Alharaby, H.; Bamagoos, A.; Kumar, N.; Akdeniz, A.; et al. Effects of drought stress on the quality of major oilseed crops: Implications and possible mitigation strategies—A review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Arikan, B.; Yildiztugay, E.; Ozfidan-Konakci, C. Protective role of quercetin and kaempferol against oxidative damage and photosynthesis inhibition in wheat chloroplasts under arsenic stress. Physiol. Plant. 2023, 175, e13964. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, L.; Zhang, L.; Guo, Y.; Qi, X.; He, L. Effects of quercetin on postharvest blue mold control in kiwi fruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, A.M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M. Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: Complexation, oxidation and reactivity towards radicals. BioMetals 2010, 24, 41–49. [Google Scholar] [CrossRef]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 Confers Drought and Cold Tolerance through Up-Regulating Antioxidant Capacity and Stress-Resistant Genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, Z.; Li, L.; Li, Q.; Geng, Z.; Liu, W.; Hou, R.; Zhang, L.; Han, D. MbWRKY50 confers cold and drought tolerance through upregulating antioxidant capacity associated with ROS scavenging. J. Plant Physiol. 2025, 310, 154526. [Google Scholar] [CrossRef]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a Strawberry R2R3-MYB Transcription Factor, Improved Salt and Cold Stress Tolerance in Transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Ren, C.; Li, Z.; Song, P.; Wang, Y.; Liu, W.; Zhang, L.; Li, X.; Li, W.; Han, D. Overexpression of a Grape MYB Transcription Factor Gene VhMYB2 Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ni, Y.; Xie, K.; Li, Y.; Wu, W.; Shan, H.; Cheng, B.; Li, X. Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi. Int. J. Mol. Sci. 2024, 25, 4205. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperaturę. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Ali, S. New spectrophotometric assay for assessments of catalase activity in biological samples. Anal. Biochem. 2018, 542, 29–33. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Moresco, R.; Schmidt, E.C.; Bouzon, Z.L.; Nunes, E.C.; Neubert, E.O.; Peruch, L.A.M.; Rocha, M.; Maraschin, M. The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem. 2016, 197 Pt A, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. In Basic Protein and Peptide Protocols; Walker, J.M., Ed.; Methods in Molecular Biology™; Humana Press: Totowa, NJ, USA, 1994; Volume 32. [Google Scholar] [CrossRef]



| Parameter | Typical Value | Unit |
|---|---|---|
| Soil Texture | Peat-based mix | – |
| pH | 5.5–6.5 | – |
| Water Holding Capacity | 30–70 | % (w/w) |
| Field Capacity | ~30–40 | % (w/w) |
| Permanent Wilting Point | ~15–20 | % (w/w) |
| Bulk Density | 0.2–0.4 | g/cm3 |
| Organic Matter Content | 30–60 | % |
| Electrical Conductivity (EC) | <1.5 | mS/cm |
| Macronutrients (N, P, K) | N: 50–150, P: 10–50, K: 100–300 | mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Balawejder, M. Enhancement of Photosynthetic Efficiency and Antioxidant Response in Wheat Under Drought Stress by Quercetin–Copper Complex. Int. J. Mol. Sci. 2025, 26, 10365. https://doi.org/10.3390/ijms262110365
Jańczak-Pieniążek M, Migut D, Piechowiak T, Balawejder M. Enhancement of Photosynthetic Efficiency and Antioxidant Response in Wheat Under Drought Stress by Quercetin–Copper Complex. International Journal of Molecular Sciences. 2025; 26(21):10365. https://doi.org/10.3390/ijms262110365
Chicago/Turabian StyleJańczak-Pieniążek, Marta, Dagmara Migut, Tomasz Piechowiak, and Maciej Balawejder. 2025. "Enhancement of Photosynthetic Efficiency and Antioxidant Response in Wheat Under Drought Stress by Quercetin–Copper Complex" International Journal of Molecular Sciences 26, no. 21: 10365. https://doi.org/10.3390/ijms262110365
APA StyleJańczak-Pieniążek, M., Migut, D., Piechowiak, T., & Balawejder, M. (2025). Enhancement of Photosynthetic Efficiency and Antioxidant Response in Wheat Under Drought Stress by Quercetin–Copper Complex. International Journal of Molecular Sciences, 26(21), 10365. https://doi.org/10.3390/ijms262110365








