Chemical Structure of a Branched α-d-Glucan from the Eggs of Sea Urchin Tripneustes gratilla
Abstract
1. Introduction
2. Results
2.1. Polysaccharide Isolation
2.2. Methylation Analysis
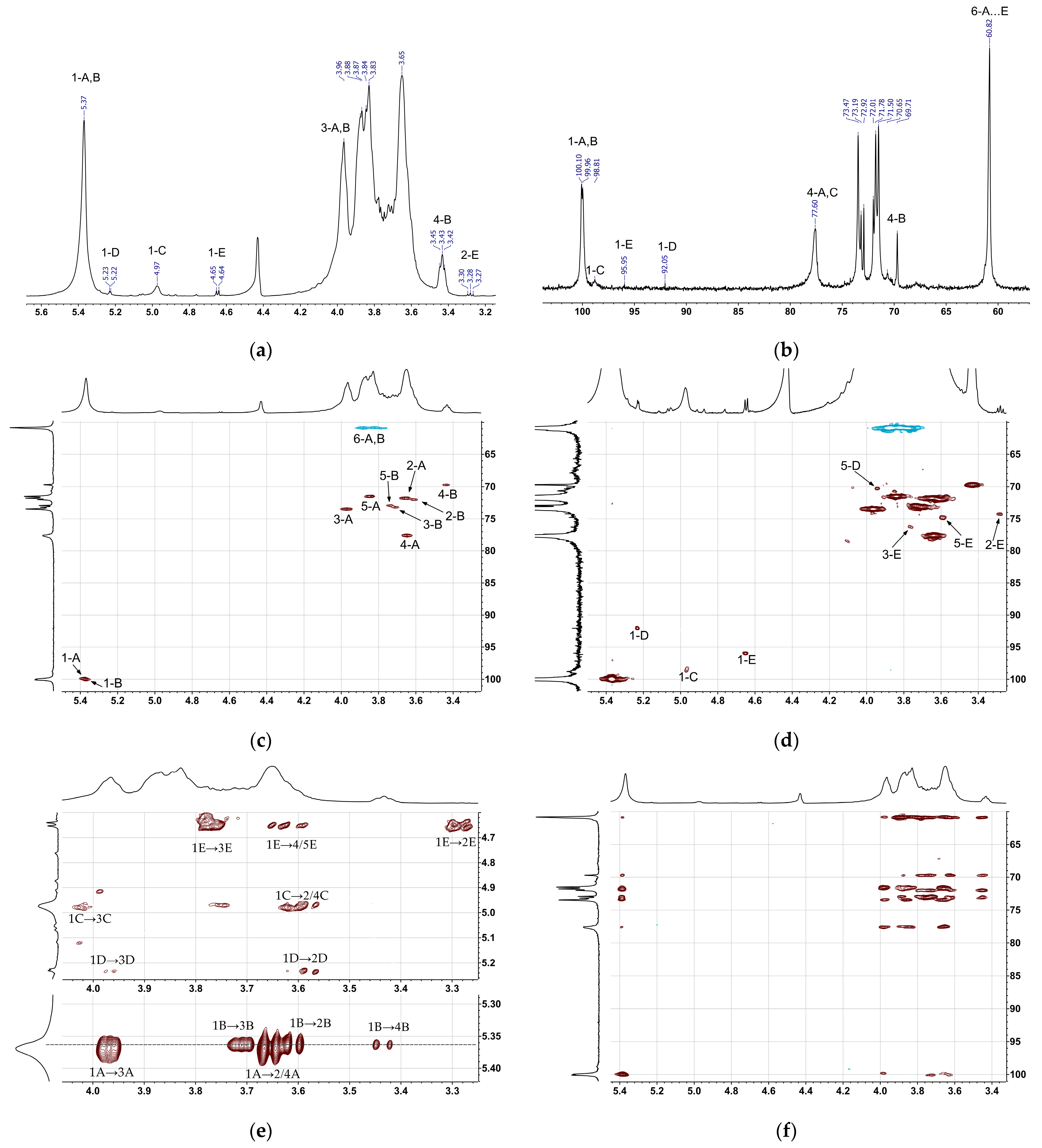
2.3. NMR Analysis
2.4. Analysis of Iodine Complexes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Methods
4.3. Isolation of NP
4.4. Partial Degradation of NP
4.5. Molecular Weight Determination
4.6. Methylation Analysis
4.7. NMR Spectroscopy
4.8. Absorption Spectra of Iodine Complexes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSDB | Carbohydrate Structure Database |
| ACL | Average chain length |
| PMAA | Partially methylated alditol acetate |
| TSP | 3-(Trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt |
| BSA | Bovine serum albumin |
| ROS | Reactive oxygen species |
References
- Pomin, V.H.; Mourão, P.A.S. Structure, Biology, Evolution, and Medical Importance of Sulfated Fucans and Galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Suzuki, N. Structure and Function of Sea Urchin Egg Jelly Molecules. Zoolog. Sci. 1990, 7, 355–370. [Google Scholar] [CrossRef]
- Mengerink, K.J.; Vacquier, V.D. Glycobiology of Sperm-Egg Interactions in Deuterostomes. Glycobiology 2001, 11, 37R–43R. [Google Scholar] [CrossRef]
- Pomin, V.H. Sulfated Glycans in Sea Urchin Fertilization. Glycoconj. J. 2015, 32, 9–15. [Google Scholar] [CrossRef]
- Mourão, P.A.; Vilanova, E.; Soares, P.A. Unveiling the Structure of Sulfated Fucose-Rich Polysaccharides via Nuclear Magnetic Resonance Spectroscopy. Curr. Opin. Struct. Biol. 2018, 50, 33–41. [Google Scholar] [CrossRef]
- Mourão, P.A.S. Perspective on the Use of Sulfated Polysaccharides from Marine Organisms as a Source of New Antithrombotic Drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. Marine Carbohydrate-Based Compounds with Medicinal Properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.I.S.P.; Pinto, M.M.M.; Sousa, E.; Correia-da-Silva, M. Antithrombotics from the Sea: Polysaccharides and Beyond. Mar. Drugs 2019, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Pomin, V.H. Marine Antithrombotics. Mar. Drugs 2020, 18, 514. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, C.; Liao, H.; Liu, Y.M.; Cheng, W.N.; Du, J.Y. Antitumor Activities of Extractive from Sea Urchin in Vitro. Chin. J. Cancer Prev. Treat. 2003, 10, 569–572. [Google Scholar]
- Bai, R.; Wang, C. Separation, Purification and Antitumor Activity Assay of a Polysaccharide from Shell Thorns of Sea Urchin Hemicentrotus pulcherrimus. J. Dalian Fish. Univ. 2010, 25, 183–186. [Google Scholar] [CrossRef]
- Jiao, H.; Shang, X.; Dong, Q.; Wang, S.; Liu, X.; Zheng, H.; Lu, X. Polysaccharide Constituents of Three Types of Sea Urchin Shells and Their Anti-Inflammatory Activities. Mar. Drugs 2015, 13, 5882–5900. [Google Scholar] [CrossRef]
- Unuma, T.; Yamamoto, T.; Akiyama, T.; Shiraishi, M.; Ohta, H. Quantitative Changes in Yolk Protein and Other Components in the Ovary and Testis of the Sea Urchin Pseudocentrotus depressus. J. Exp. Biol. 2003, 206, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, Q.; Gao, Y.; Ye, L.; Xing, Y.; Xi, T. Characterization and Antitumor Activity of a Polysaccharide from Strongylocentrotus nudus Eggs. Carbohydr. Polym. 2007, 67, 313–318. [Google Scholar] [CrossRef]
- Liu, C.; Xi, T.; Lin, Q.; Xing, Y.; Ye, L.; Luo, X.; Wang, F. Immunomodulatory Activity of Polysaccharides Isolated from Strongylocentrotus nudus Eggs. Int. Immunopharmacol. 2008, 8, 1835–1841. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Tang, Y.; Kang, D.; Gao, Y.; Ke, M.; Dou, J.; Xi, T.; Zhou, C. Effective Inhibition of a Strongylocentrotus nudus Eggs Polysaccharide against Hepatocellular Carcinoma Is Mediated via Immunoregulation in Vivo. Immunol. Lett. 2011, 141, 74–82. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A Polysaccharide from Strongylocentrotus nudus Eggs Protects against Myelosuppression and Immunosuppression in Cyclophosphamide-Treated Mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef]
- Xie, X.; Ma, L.; Zhou, Y.; Shen, W.; Xu, D.; Dou, J.; Shen, B.; Zhou, C. Polysaccharide Enhanced NK Cell Cytotoxicity against Pancreatic Cancer via TLR4/MAPKs/NF-κB Pathway in Vitro/Vivo. Carbohydr. Polym. 2019, 225, 115223. [Google Scholar] [CrossRef]
- Zhou, M.; Zhi, J.; Zhi, J.; Xiong, Z.; Wu, F.; Lu, Y.; Hu, Q. Polysaccharide from Strongylocentrotus nudus Eggs Regulates Intestinal Epithelial Autophagy through CD36/PI3K-Akt Pathway to Ameliorate Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2023, 244, 125373. [Google Scholar] [CrossRef]
- Ma, Y.; Xing, Y.; Mi, H.; Guo, Z.; Lu, Y.; Xi, T. Extraction, Preliminary Characterization and Immunostimulatory Activity in Vitro of a Polysaccharide Isolated from Strongylocentrotus nudus Eggs. Carbohydr. Polym. 2014, 111, 576–583. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Wei, M.; Yue, Y.; Wu, N.; Wang, J.; Zhang, Q. Structural Characterization and Immunostimulatory Activity in Vitro of a Glycogen from Sea Urchin—Strongylocentyotus internedius. Carbohydr. Polym. 2021, 258, 117701. [Google Scholar] [CrossRef]
- Yang, J.; Yi, M.; Pan, J.; Zhao, J.; Sun, L.; Lin, X.; Cao, Y.; Huang, L.; Zhu, B.; Yu, C. Sea Urchin (Strongylocentrotus intermedius) Polysaccharide Enhanced BMP-2 Induced Osteogenic Differentiation and Its Structural Analysis. J. Funct. Foods 2015, 14, 519–528. [Google Scholar] [CrossRef]
- Jiang, Y.; Shang, Z.; Lv, X.; Du, M.; Ma, L.; Hou, G.; Chen, J.; Wang, C.; Zhao, F. Structure Elucidation and Antitumor Activity of a Water Soluble Polysaccharide from Hemicentrotus pulcherrimus. Carbohydr. Polym. 2022, 292, 119718. [Google Scholar] [CrossRef]
- Shang, Z.; Jiang, Y.; Yang, F.; Wu, K.; Zheng, G.; Lin, Y.; Wang, C.; Xin, W.; Zhao, F. A Homologous Series of α-Glucans from Hemicentrotus pulcherrimus and Their Immunomodulatory Activity. Int. J. Biol. Macromol. 2024, 260, 129657. [Google Scholar] [CrossRef]
- Jodelet, A.; Rigby, N.M.; Colquhoun, I.J. Separation and NMR Structural Characterisation of Singly Branched α-Dextrins Which Differ in the Location of the Branch Point. Carbohydr. Res. 1998, 312, 139–151. [Google Scholar] [CrossRef]
- Petersen, B.O.; Motawie, M.S.; Møller, B.L.; Hindsgaul, O.; Meier, S. NMR Characterization of Chemically Synthesized Branched α-Dextrin Model Compounds. Carbohydr. Res. 2015, 403, 149–156. [Google Scholar] [CrossRef]
- Kapaev, R.R.; Toukach, P.V. GRASS: Semi-Automated NMR-Based Structure Elucidation of Saccharides. Bioinforma. Oxf. Engl. 2018, 34, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Kapaev, R.R.; Egorova, K.S.; Toukach, P.V. Carbohydrate Structure Generalization Scheme for Database-Driven Simulation of Experimental Observables, Such as NMR Chemical Shifts. J. Chem. Inf. Model. 2014, 54, 2594–2611. [Google Scholar] [CrossRef] [PubMed]
- Kapaev, R.R.; Toukach, P.V. Improved Carbohydrate Structure Generalization Scheme for 1H and 13C NMR Simulations. Anal. Chem. 2015, 87, 7006–7010. [Google Scholar] [CrossRef]
- Kapaev, R.R.; Toukach, P.V. Simulation of 2D NMR Spectra of Carbohydrates Using GODESS Software. J. Chem. Inf. Model. 2016, 56, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.; Reuhs, B.L.; Ovando Martinez, M.; Simsek, S. Analysis of Octenylsuccinate Rice and Tapioca Starches: Distribution of Octenylsuccinic Anhydride Groups in Starch Granules. Food Chem. 2016, 211, 608–615. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Cui, S.W.; Wang, Z.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. Structural Characterization and Immunostimulatory Activity of a Glucan from Natural Cordyceps sinensis. Food Hydrocoll. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Shi, X.; Li, O.; Yin, J.; Nie, S. Structure Identification of α-Glucans from Dictyophora echinovolvata by Methylation and 1D/2D NMR Spectroscopy. Food Chem. 2019, 271, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Bi, S.; Hu, X.; Yang, J.; Li, C.; Li, H.; Yu, D.B.; Zhu, J.; Song, L.; Yu, R. Structural Characterization and Immunomodulatory Mechanisms of Two Novel Glucans from Morchella importuna Fruiting Bodies. Int. J. Biol. Macromol. 2021, 183, 145–157. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, R.; Ho, C.-T.; Li, B.; Chen, S.; Xiao, C.; Hu, H.; Cai, M.; Chen, Z.; Xie, Y.; et al. Preparation, Chemical Structure, and Immunostimulatory Activity of a Water-Soluble Heteropolysaccharide from Suillus granulatus Fruiting Bodies. Food Chem. X 2022, 13, 100211. [Google Scholar] [CrossRef]
- Luo, L.; Song, X.; Chang, X.; Huang, S.; Zhou, Y.; Yang, S.; Zhu, Y.; Zhang, L.; Wu, Y.; Zhang, J.; et al. Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium bovis BCG. Molecules 2022, 27, 5691. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Lin, Y.; Xu, B.; Yang, J.; Mo, L.; Huang, R.; Zhang, X. Structural Characterization and Immunomodulatory Activity of an Exopolysaccharide from Marine-Derived Aspergillus versicolor SCAU141. Int. J. Biol. Macromol. 2023, 227, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Dinadayala, P.; Lemassu, A.; Granovski, P.; Cérantola, S.; Winter, N.; Daffé, M. Revisiting the Structure of the Anti-Neoplastic Glucans of Mycobacterium bovis Bacille Calmette-Guerin. Structural Analysis of the Extracellular and Boiling Water Extract-Derived Glucans of the Vaccine Substrains. J. Biol. Chem. 2004, 279, 12369–12378. [Google Scholar] [CrossRef]
- Ma, X.; Wang, G.; Yang, L.; Liu, H.; Zou, Y.; Zhang, D.; An, Y.; Liu, X.; Li, Z.; Guo, D. Structural Characterization of Two α-Glucans with Different Molecular Weights from Flemingia philippinensis and Their Immune-Enhancing Activities on Cyclophosphamide-Treated Mice through Regulation of Gut Microbiota. Int. J. Biol. Macromol. 2025, 321, 146555. [Google Scholar] [CrossRef]
- Bittencourt, V.C.B.; Figueiredo, R.T.; da Silva, R.B.; Mourão-Sá, D.S.; Fernandez, P.L.; Sassaki, G.L.; Mulloy, B.; Bozza, M.T.; Barreto-Bergter, E. An α-Glucan of Pseudallescheria boydii Is Involved in Fungal Phagocytosis and Toll-like Receptor Activation. J. Biol. Chem. 2006, 281, 22614–22623. [Google Scholar] [CrossRef]
- Archibald, A.R.; Fleming, I.D.; Liddle, A.M.; Manners, D.J.; Mercer, G.A.; Wright, A. α-1,4-Glucosans. Part XI. The Absorption Spectra of Glycogen– and Amylopectin–Iodine Complexes. J. Chem. Soc. 1961, 1183–1190. [Google Scholar] [CrossRef]
- Manners, D.J.; Stark, J.R.; Thambyrajah, V. Iodine Staining Properties of Branched α-(1,4)-D-Glucans. J. Jpn. Soc. Starch Sci. 1983, 30, 13–18. [Google Scholar] [CrossRef]
- Stadnichuk, I.N.; Semenova, L.R.; Smirnova, G.P.; Usov, A.I. A Highly Branched Storage Polyglucan in the Thermoacidophilic Red Microalga Galdieria maxima Cells. Appl. Biochem. Microbiol. 2007, 43, 78–83. [Google Scholar] [CrossRef]
- Kristijarti, A.P.; Jurak, E.; van der Maarel, M.J.E.C. The Production and Characteristics of Glycogen Synthesized by Various Strains of the Thermoacidophilic Red Microalgae Galdieria Grown Heterotrophically. J. Appl. Phycol. 2024, 36, 3199–3207. [Google Scholar] [CrossRef]
- Neoh, G.K.S.; Tan, X.; Chen, S.; Roura, E.; Dong, X.; Gilbert, R.G. Glycogen Metabolism and Structure: A Review. Carbohydr. Polym. 2024, 346, 122631. [Google Scholar] [CrossRef]
- Hu, X.; Gilbert, R.G. Molecular Structural Insights into Starch and Glycogen: Impact on Health. Carbohydr. Polym. 2025, 367, 123958. [Google Scholar] [CrossRef]
- Wang, L.; Wise, M.J. Glycogen with Short Average Chain Length Enhances Bacterial Durability. Naturwissenschaften 2011, 98, 719. [Google Scholar] [CrossRef] [PubMed]
- Komarova, B.S.; Wong, S.S.W.; Orekhova, M.V.; Tsvetkov, Y.E.; Krylov, V.B.; Beauvais, A.; Bouchara, J.-P.; Kearney, J.; Aimanianda, V.; Latgé, J.-P.; et al. Chemical synthesis and application of biotinylated oligo-α-(1→3)-D-glucosides to study the antibody and cytokine response against the cell wall α-(1→3)-D-glucan of Aspergillus fumigatus. J. Org. Chem. 2018, 83, 12965–12976. [Google Scholar] [CrossRef]
- Clark, A.M.; Rowe, F.W.E. Monograph of Shallow-Water Indo-West Pacific Echinoderms; Trustees of the British Museum (Natural History): London, UK, 1971; p. 238. [Google Scholar]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a Fucoidan from the Brown Seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Zakharova, A.N.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Polysaccharides of Algae: 60. Fucoidan from the Pacific Brown Alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorgan. Chem. 2007, 33, 38–46. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A Note on the Determination of the Ester Sulphate Content of Sulphated Polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of Algae: 48. Polysaccharide Composition of Several Calcareous Red Algae: Isolation of Alginate from Corallina pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.N.C.; Southcott, B.A. An extraction procedure for plants: Extracts from the red alga Rhodomela larix. Phytochemistry 1970, 9, 1159–1161. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mulloy, B.; Mourão, P.A.S. Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-β-D-glucuronosyl residues. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Bilan, M.I.; Shashkov, A.S. Polysaccharides of Algae. 53. Brown Alga Laminaria saccharina as the Source of Fucoidan. Bioorgan. Khim. 1998, 24, 437–445. [Google Scholar]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A Study of Fucoidan from the Brown Seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef] [PubMed]


| Sample | Glc | Man | Uronic Acids | Sulfate | Protein |
|---|---|---|---|---|---|
| NP | 95.7 | 1.0 | n.d. 1 | n.d. 1 | n.d. 1 |
| Residue | PMAA | Relative Percentages | |
|---|---|---|---|
| NP | Amylopectin | ||
| α-Glcp-(1→ | 1,5-Ac2-2,3,4,6-Me4-Glc | 10 | 4 |
| →4)-α-Glcp-(1→ | 1,4,5-Ac3-2,3,6-Me3-Glc | 80 | 91 |
| →6]→4)-α-Glcp-(1→ | 1,4,5,6-Ac4-2,3-Me2-Glc | 10 | 5 |
| Residue | H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5 C-5 | H-6 C-6 | |
|---|---|---|---|---|---|---|---|
| A | →4)-α-Glcp-(1→4) | 5.37 | 3.65 | 3.96 | 3.64 | 3.84 | 3.88, 3.80 |
| 99.96 | 71.77 | 73.46 | 77.60 | 71.5 | 60.82 | ||
| B | α-Glcp-(1→4) | 5.36 | 3.60 | 3.70 | 3.43 | 3.73 | 3.87, 3.76 |
| 100.10 | 72.01 | 73.19 | 69.71 | 72.92 | 60.91 | ||
| C | →4)-α-Glcp-(1→6) | 4.96 | 3.57 | 4.01 | 3.64 | n.d. 1 | n.d. 1 |
| 98.81 | 72.01 | 73.46 | 77.60 | n.d. 1 | n.d. 1 | ||
| D 2 | →4)-α-Glcp-(1→OH | 5.23 | 3.57 | 3.97 | 3.64 | 3.94 | n.d. 1 |
| 92.05 | 71.60 | 73.30 | 77.82 | 70.28 | n.d. 1 | ||
| E 2 | →4)-β-Glcp-(1→OH | 4.65 | 3.28 | 3.76 | 3.64 | 3.59 | n.d. 1 |
| 95.95 | 74.22 | 76.28 | 77.60 | 74.77 | 61.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilan, M.I.; Argunov, D.A.; Torgov, V.I.; Dmitrenok, A.S.; Trung, D.T.; Pham, T.D.; Cao, H.T.T.; Usov, A.I.; Nifantiev, N.E. Chemical Structure of a Branched α-d-Glucan from the Eggs of Sea Urchin Tripneustes gratilla. Int. J. Mol. Sci. 2025, 26, 10326. https://doi.org/10.3390/ijms262110326
Bilan MI, Argunov DA, Torgov VI, Dmitrenok AS, Trung DT, Pham TD, Cao HTT, Usov AI, Nifantiev NE. Chemical Structure of a Branched α-d-Glucan from the Eggs of Sea Urchin Tripneustes gratilla. International Journal of Molecular Sciences. 2025; 26(21):10326. https://doi.org/10.3390/ijms262110326
Chicago/Turabian StyleBilan, Maria I., Dmitry A. Argunov, Vladimir I. Torgov, Andrey S. Dmitrenok, Dinh Thanh Trung, Thinh Duc Pham, Hang Thi Thuy Cao, Anatolii I. Usov, and Nikolay E. Nifantiev. 2025. "Chemical Structure of a Branched α-d-Glucan from the Eggs of Sea Urchin Tripneustes gratilla" International Journal of Molecular Sciences 26, no. 21: 10326. https://doi.org/10.3390/ijms262110326
APA StyleBilan, M. I., Argunov, D. A., Torgov, V. I., Dmitrenok, A. S., Trung, D. T., Pham, T. D., Cao, H. T. T., Usov, A. I., & Nifantiev, N. E. (2025). Chemical Structure of a Branched α-d-Glucan from the Eggs of Sea Urchin Tripneustes gratilla. International Journal of Molecular Sciences, 26(21), 10326. https://doi.org/10.3390/ijms262110326







