Simultaneous Determination of Reducing Sugars in Honey by Capillary Zone Electrophoresis with LIF Detection Using Low-Toxicity 2-Picoline Borane and APTS for Pre-Capillary Derivatization
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions for Sugar Derivatization
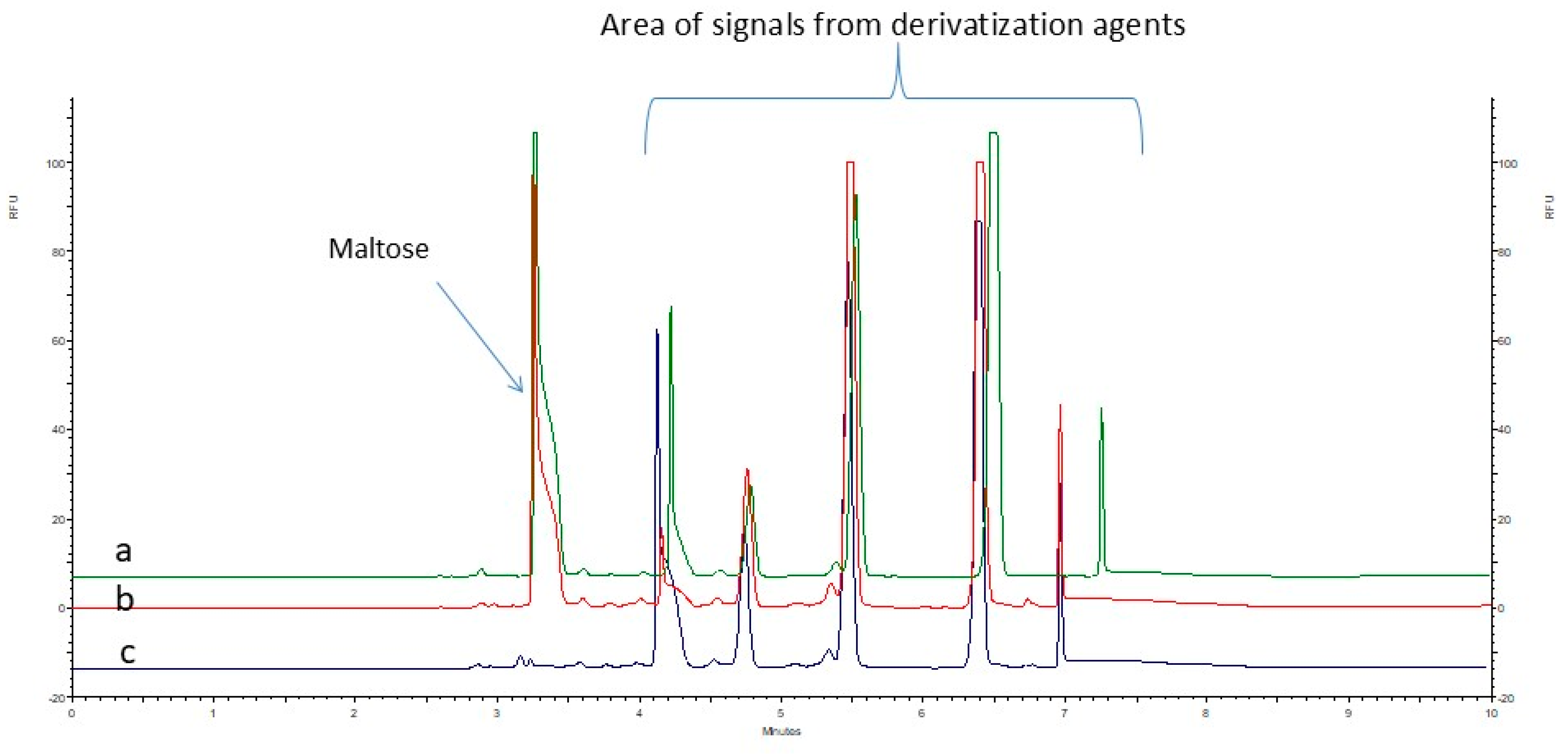
2.2. Assessment of Methods for Removing Excess Derivatization Reagents
2.2.1. A Liquid–Liquid Extraction (LLE)-Based Approach
2.2.2. A Solid-Phase Extraction (SPE)-Based Approach
2.2.3. A Solid-Phase Microextraction (SPME)-Based Approach
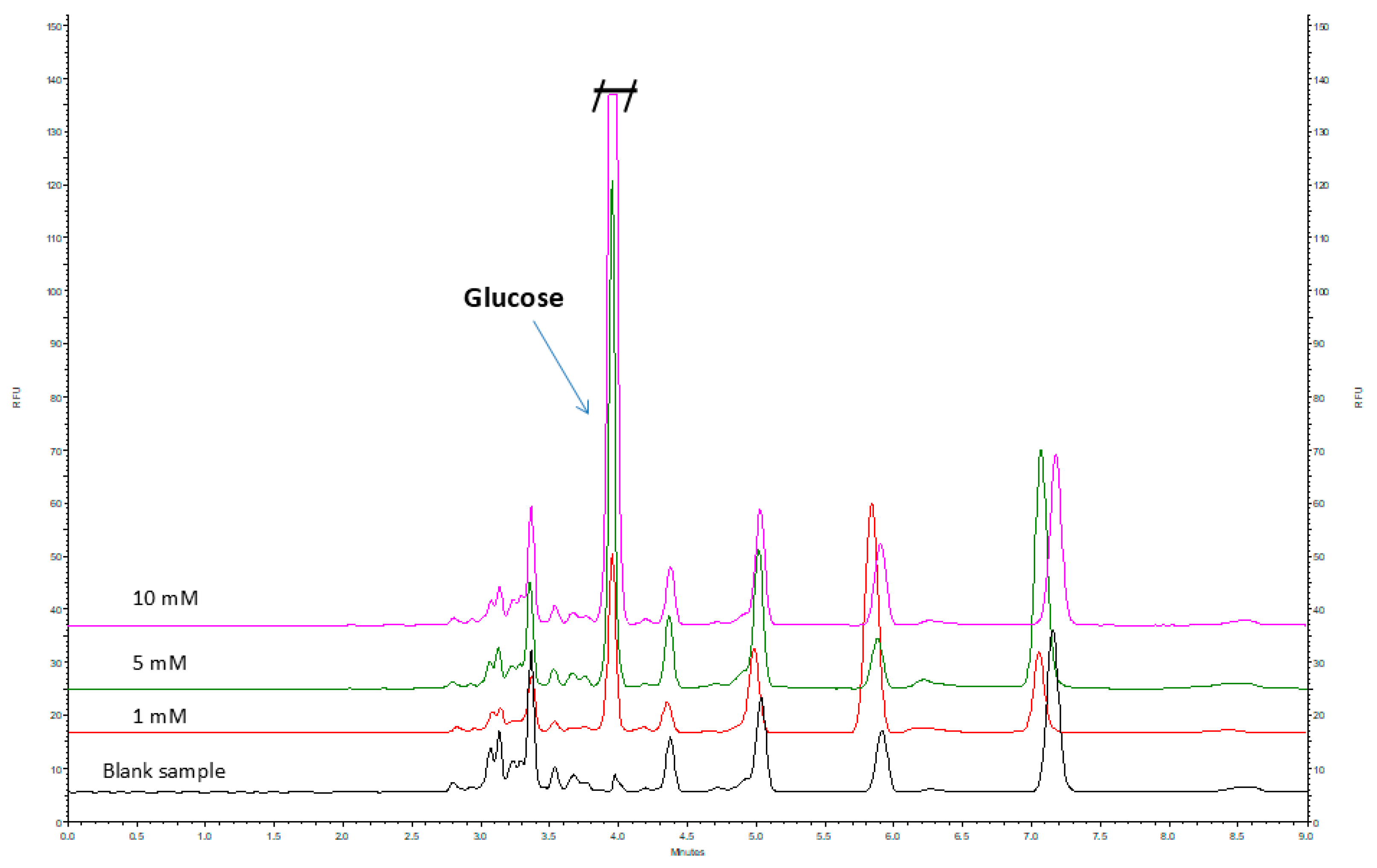
2.3. Method Validation
Dilution Strategy for Samples Exceeding the Validated Linear Range
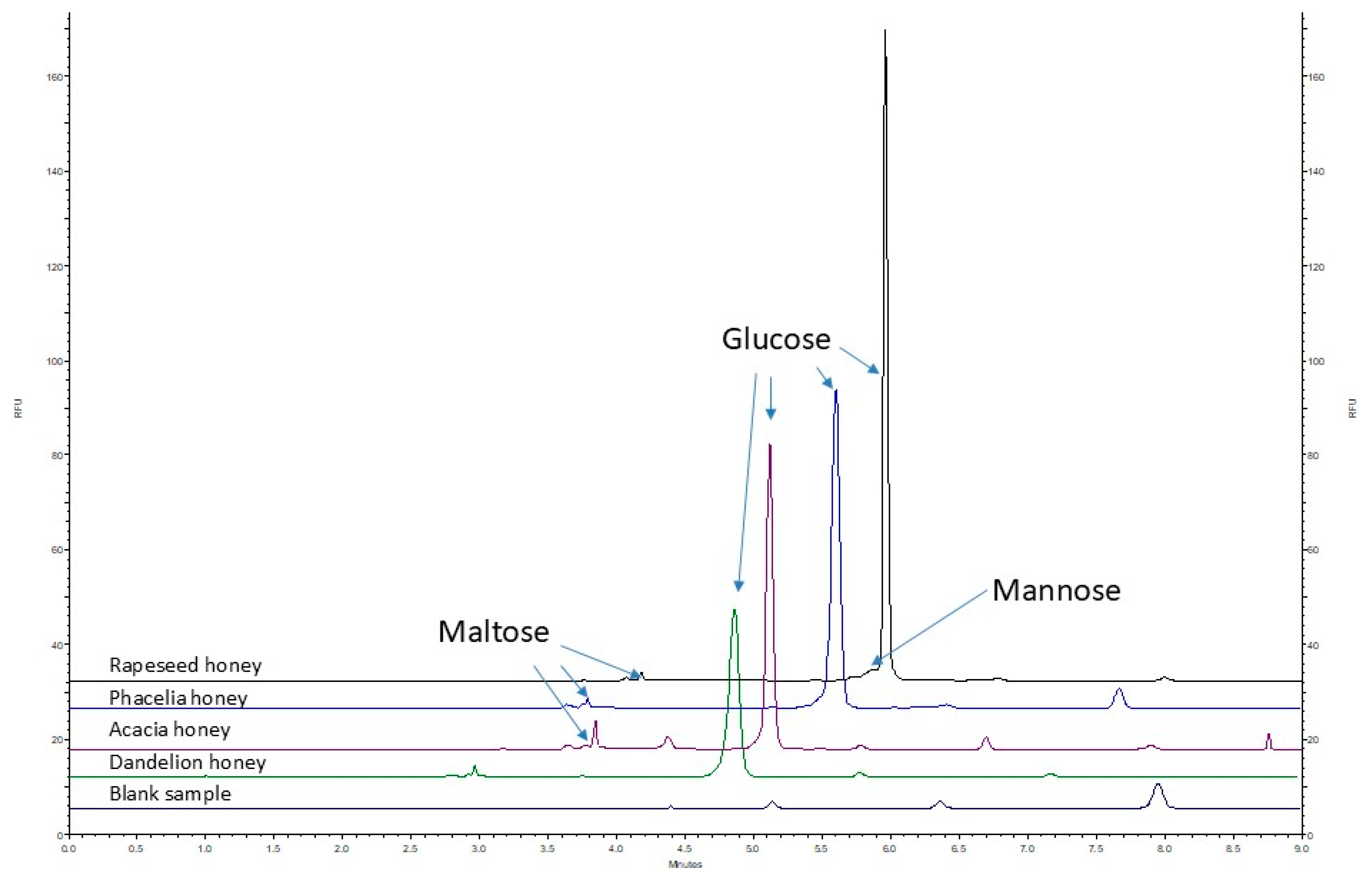
2.4. Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals
3.2. Stock Solution of Analyzed Sugars and Honey Samples
3.3. Instruments
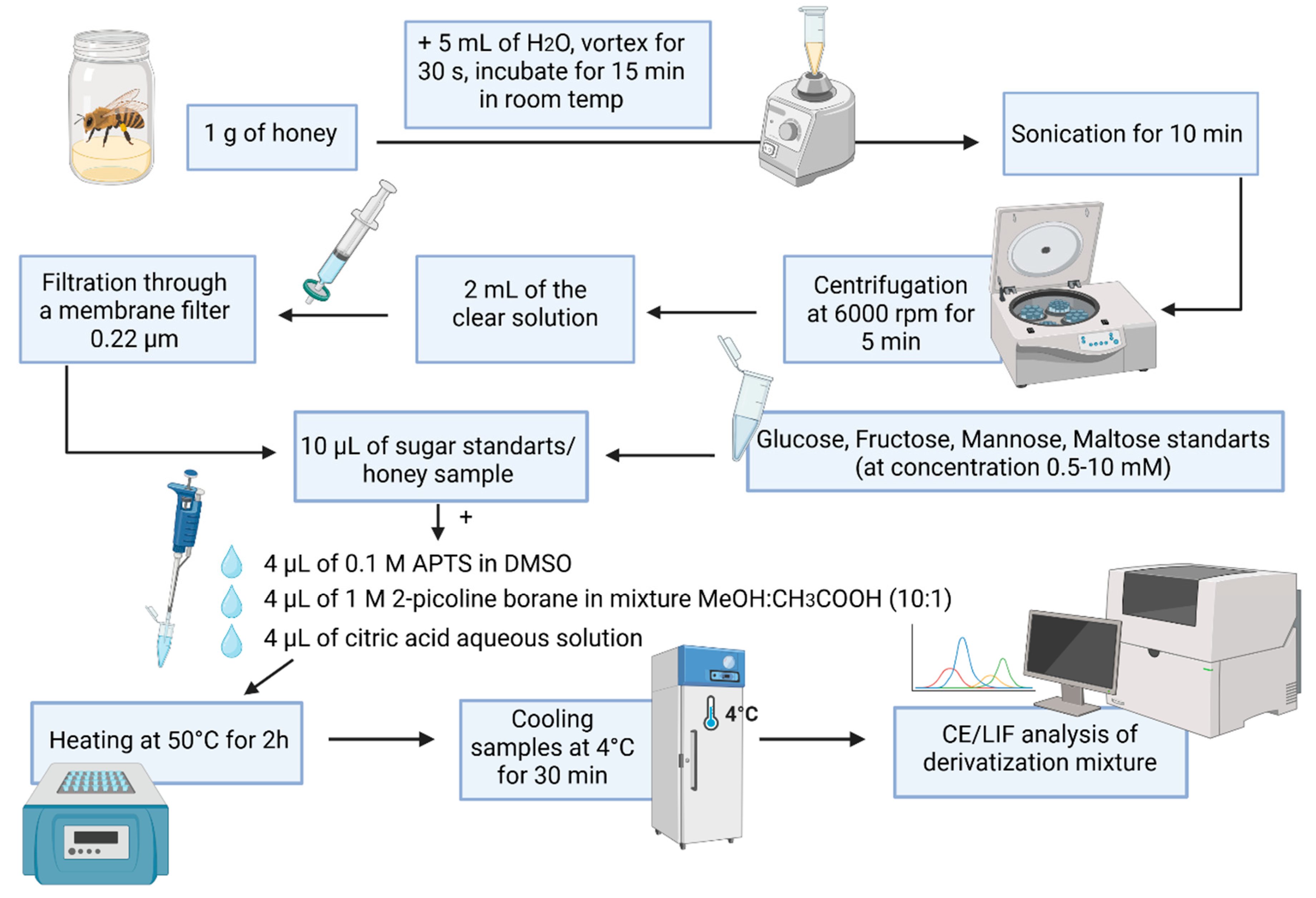
3.4. Sample Preparation Procedures
3.4.1. Sample Derivatization with APTS and 2-Picoline Borane
3.4.2. Procedures for the Removal of Excess Reagents from Samples
A Liquid–Liquid Extraction (LLE)-Based Approach
A Solid-Phase Extraction (SPE)-Based Approach
A Solid-Phase Microextraction (SPME)-Based Approach
3.5. Study of Analyte Stability Under Storage Conditions
3.6. Validation of the Method
3.7. Verification of Method Applicability Using Real Honey Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizelio, V.M.; Tenfen, L.; Da Silveira, R.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Development of a fast capillary electrophoresis method for determination of carbohydrates in honey samples. Talanta 2012, 93, 62–66. [Google Scholar] [CrossRef]
- Andrasi, M.; Gyemant, G.; Sajtos, Z.; Nagy, C. Analysis of Sugars in Honey Samples by Capillary Zone Electrophoresis Using Fluorescence Detection. Separations 2023, 10, 150. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Luo, Y.; Zhang, C.; Li, S.; Zheng, H.; Jiang, X.; Hu, F. Comparison of the Physicochemical Properties, Microbial Communities, and Hydrocarbon Composition of Honeys Produced by Different Apis Species. Foods 2024, 13, 3753. [Google Scholar] [CrossRef] [PubMed]
- Trifković, J.; Andrić, F.; Ristivojević, P.; Guzelmeric, E.; Yesilada, E. Analytical Methods in Tracing Honey Authenticity. J. AOAC Int. 2017, 100, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qin, G.; Ma, J.; She, Y.M. Quantification of plant cell wall monosaccharides by reversed-phase liquid chromatography with 2-aminobenzamide pre-column derivatization and a non-toxic reducing reagent 2-picoline borane. J. Chromatogr. A 2015, 1414, 122–128. [Google Scholar] [CrossRef]
- Molnár-Gábor, D.; Lengyel, M.; Krongaard, T. Rapid method for quantitation of seven human milk oligosaccharides in infant formula and premix. Carbohydr. Res. 2024, 541, 109149. [Google Scholar] [CrossRef]
- Shah, M.; Patel, N.; Tripathi, N.; Vyas, V.K. Capillary electrophoresis methods for impurity profiling of drugs: A review of the past decade. J. Pharm. Anal. 2022, 12, 15–28. [Google Scholar] [CrossRef]
- Tagliaro, F.; Manetto, G.; Crivellente, F.; Smith, F.P. A brief introduction to capillary electrophoresis. Forensic Sci. Int. 1998, 92, 75–88. [Google Scholar] [CrossRef]
- Lu, G.; Crihfield, C.L.; Gattu, S.; Veltri, L.M.; Holland, L.A. Capillary Electrophoresis Separations of Glycans. Chem. Rev. 2018, 118, 7867–7885. [Google Scholar] [CrossRef]
- Mantovani, V.; Galeotti, F.; Maccari, F.; Volpi, N. Recent advances in capillary electrophoresis separation of monosaccharides, oligosaccharides, and polysaccharides. Electrophoresis 2018, 39, 179–189. [Google Scholar] [CrossRef]
- Szigeti, M.; Guttman, A. Sample Preparation Scale-Up for Deep N-glycomic Analysis of Human Serum by Capillary Electrophoresis and CE-ESI-MS. Mol. Cell. Proteom. 2019, 18, 2524–2531. [Google Scholar] [CrossRef]
- Makrydaki, E.; Kotidis, P.; Polizzi, K.M.; Kontoravdi, C. Hitting the sweet spot with capillary electrophoresis: Advances in N-glycomics and glycoproteomics. Curr. Opin. Biotechnol. 2021, 71, 182–190. [Google Scholar] [CrossRef]
- Ban, E.; Kwon, H.; Song, E.J. A rapid and reliable CE-LIF method for the quantitative analysis of miRNA-497 in plasma and organs and its application to a pharmacokinetic and biodistribution study. RSC Adv. 2020, 10, 18648–18654. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Sato, S.; Sakamoto, T.; Miyazawa, E.; Kikugawa, Y. One-pot reductive amination of aldehydes and ketones with α-picoline-borane in methanol, in water, and in neat conditions. Tetrahedron 2004, 60, 7899–7906. [Google Scholar] [CrossRef]
- Váradi, C.; Lew, C.; Guttman, A. Rapid magnetic bead based sample preparation for automated and high throughput N-glycan analysis of therapeutic antibodies. Anal. Chem. 2014, 86, 5682–5687. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Steenvoorden, E.; Koeleman, C.A.M.; Deelder, A.M.; Wuhrer, M. 2-Picoline-borane: A non-toxic reducing agent for oligosaccharide labeling by reductive amination. Proteomics 2010, 10, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Krenkova, J.; Dusa, F.; Cmelik, R. Characterization of multi-cationic aminopyrene-based tag for oligosaccharide labeling by capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 2021, 42, 1333–1339. [Google Scholar] [CrossRef]
- Yang, B.; Mai, T.D.; Tran, N.T.; Taverna, M. In capillary labeling and online electrophoretic separation of N-glycans from glycoproteins. J. Sep. Sci. 2022, 45, 3594–3603. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.; Rejzek, M.; Nepogodiev, S.A.; Field, R.A. The impact of aminopyrene trisulfonate (APTS) label in acceptor glycan substrates for profiling plant pectin β-galactosyltransferase activities. Carbohydr. Res. 2016, 433, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Vlčková, N.; Šimonová, A.; Ďuriš, M.; Čokrtová, K.; Almquist, S.; Křížek, T. Detection techniques for carbohydrates in capillary electrophoresis—A comparative study. Monatsh Chem. 2023, 154, 967–975. [Google Scholar] [CrossRef]
- Lamari, F.N.; Kuhn, R.; Karamanos, N.K. Derivatization of carbohydrates for chromatographic, electrophoretic and mass spectrometric structure analysis. J. Chromatogr. B 2003, 793, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Senn, J.P. Quantification of polysaccharides in water using capillary electrophoresis. Int. J. Environ. Anal. Chem. 2005, 85, 177–198. [Google Scholar] [CrossRef]
- Evangelista, R.A.; Guttman, A.; Chen, F.T.A. Acid-catalyzed reductive amination of aldoses with 8-ammopyrene-1,3,6-trisulfonate. Electrophoresis 1996, 17, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.T.A.; Evangelista, R.A. Analysis of Mono- and Oligosaccharide Isomers Derivatized with 9-Aminopyrene-1,4,6-trisulfonate by Capillary Electrophoresis with Laser-Induced Fluorescence. Anal. Biochem. 1995, 230, 273–280. [Google Scholar] [CrossRef]
- Chen, F.T.A.; Dobashi, T.S.; Evangelista, R.A. Quantitative analysis of sugar constituents of glycoproteins by capillary electrophoresis. Glycobiology 1998, 8, 1045–1052. [Google Scholar] [CrossRef]
- Čokrtová, K.; Mareš, V.; Křížek, T. On-capillary fluorescent labeling of saccharides for capillary electrophoresis. Electrophoresis 2023, 44, 35–43. [Google Scholar] [CrossRef]
- Boyaci, E.; Rodríguez-Lafuente, Á.; Gorynski, K.; Mirnaghi, F.; Souza-Silva, É.A.; Hein, D.; Pawliszyn, J. Sample preparation with solid phase microextraction and exhaustive extraction approaches: Comparison for challenging cases. Anal. Chim. Acta 2015, 873, 14–30. [Google Scholar] [CrossRef] [PubMed]
- FDA. CDER, Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations. 2018. Available online: http://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htm (accessed on 28 October 2024).

| Analyte | Linear Range [mM] | Regression a | R2 | LOD [mM] | LOQ [mM] |
|---|---|---|---|---|---|
| Maltose | 0.5–10 | y = 42,274x − 7749.7 | 0.9940 | 0.16 | 0.5 |
| Mannose | 0.5–10 | y = 173,542x − 71,114 | 0.9951 | 0.16 | 0.5 |
| Glucose | 0.5–10 | y = 48,804x − 14,430 | 0.9941 | 0.16 | 0.5 |
| Fructose | No data | No data | No data | No data | No data |
| Concentration Added [mM] | AVG Concentration Found [mM] | SD | RSD [%] | Accuracy [%] |
|---|---|---|---|---|
| Maltose | ||||
| 0.5 | 0.60 | 0.06 | 10.44 | 119.75 |
| 5 | 4.87 | 0.07 | 1.34 | 97.47 |
| 10 | 10.39 | 0.08 | 0.81 | 103.94 |
| Mannose | ||||
| 0.5 | 0.56 | 0.09 | 12.97 | 112.96 |
| 5 | 4.76 | 0.25 | 5.32 | 95.29 |
| 10 | 9.48 | 0.73 | 7.68 | 94.80 |
| Glucose | ||||
| 0.5 | 0.55 | 0.09 | 13.73 | 110.13 |
| 5 | 4.67 | 0.08 | 1.77 | 93.47 |
| 10 | 10.44 | 0.15 | 1.42 | 104.41 |
| Honey Type | Concentration in Honey Samples [mM] | ||
|---|---|---|---|
| Maltose | Mannose | Glucose | |
| Facelia honey | 7.94 | - | 197.4 a |
| Rapeseed honey | 3.84 | 1.98 | 121.75 a |
| Acacia honey | 13.4 a | - | 133.02 a |
| Dandelion honey | 5.74 | - | 72.84 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulesowska, J.; Pieckowski, M.; Kowalski, P.; Bączek, T.; Olędzka, I. Simultaneous Determination of Reducing Sugars in Honey by Capillary Zone Electrophoresis with LIF Detection Using Low-Toxicity 2-Picoline Borane and APTS for Pre-Capillary Derivatization. Int. J. Mol. Sci. 2025, 26, 7569. https://doi.org/10.3390/ijms26157569
Bulesowska J, Pieckowski M, Kowalski P, Bączek T, Olędzka I. Simultaneous Determination of Reducing Sugars in Honey by Capillary Zone Electrophoresis with LIF Detection Using Low-Toxicity 2-Picoline Borane and APTS for Pre-Capillary Derivatization. International Journal of Molecular Sciences. 2025; 26(15):7569. https://doi.org/10.3390/ijms26157569
Chicago/Turabian StyleBulesowska, Joanna, Michał Pieckowski, Piotr Kowalski, Tomasz Bączek, and Ilona Olędzka. 2025. "Simultaneous Determination of Reducing Sugars in Honey by Capillary Zone Electrophoresis with LIF Detection Using Low-Toxicity 2-Picoline Borane and APTS for Pre-Capillary Derivatization" International Journal of Molecular Sciences 26, no. 15: 7569. https://doi.org/10.3390/ijms26157569
APA StyleBulesowska, J., Pieckowski, M., Kowalski, P., Bączek, T., & Olędzka, I. (2025). Simultaneous Determination of Reducing Sugars in Honey by Capillary Zone Electrophoresis with LIF Detection Using Low-Toxicity 2-Picoline Borane and APTS for Pre-Capillary Derivatization. International Journal of Molecular Sciences, 26(15), 7569. https://doi.org/10.3390/ijms26157569







