Utility of the Ribosomal Gene 18S rRNA in the Classification of the Main House Dust Mites Involved in Hypersensitivity
Abstract
1. Introduction
2. Results
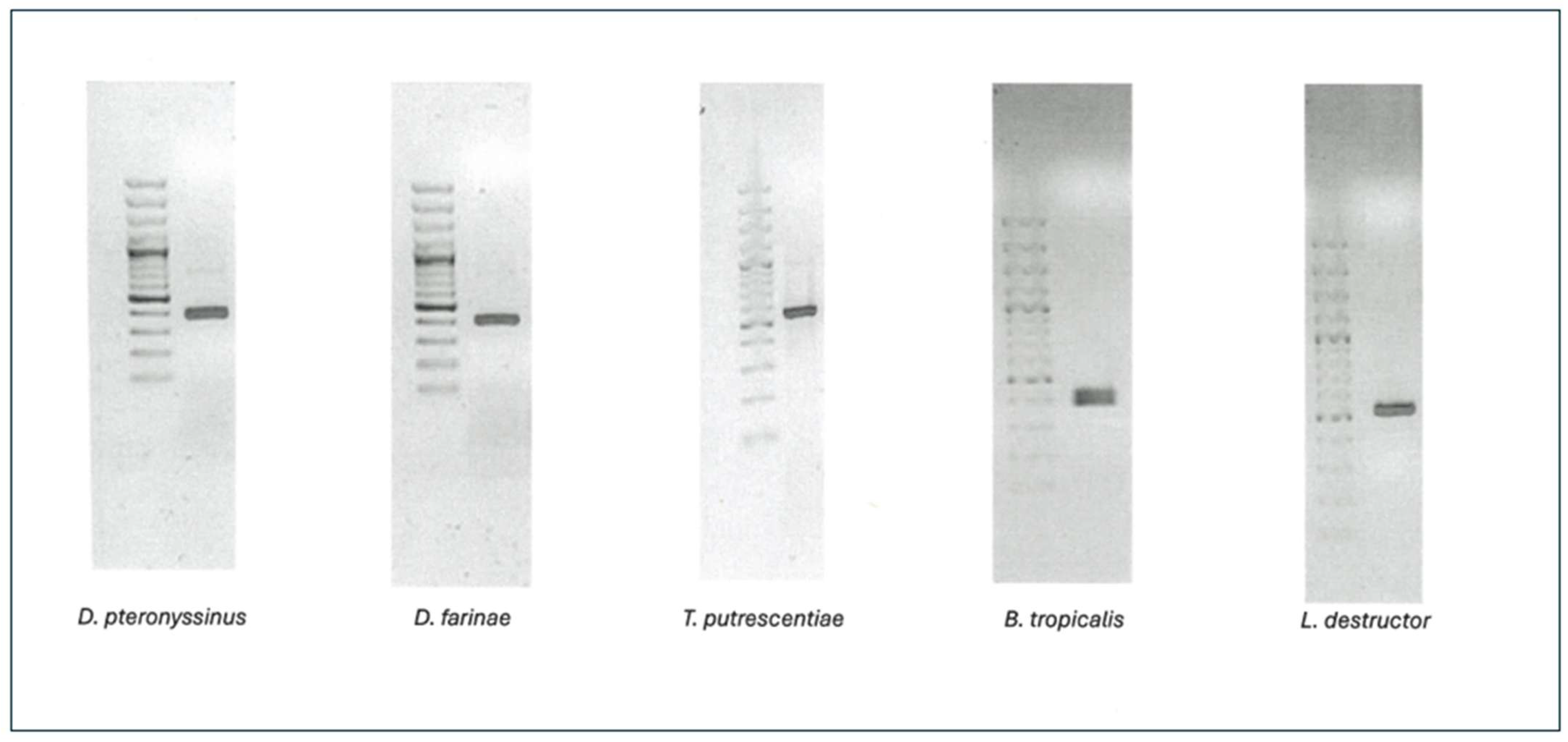
2.1. Amplification of the 18S rRNA Gene of the Different Species
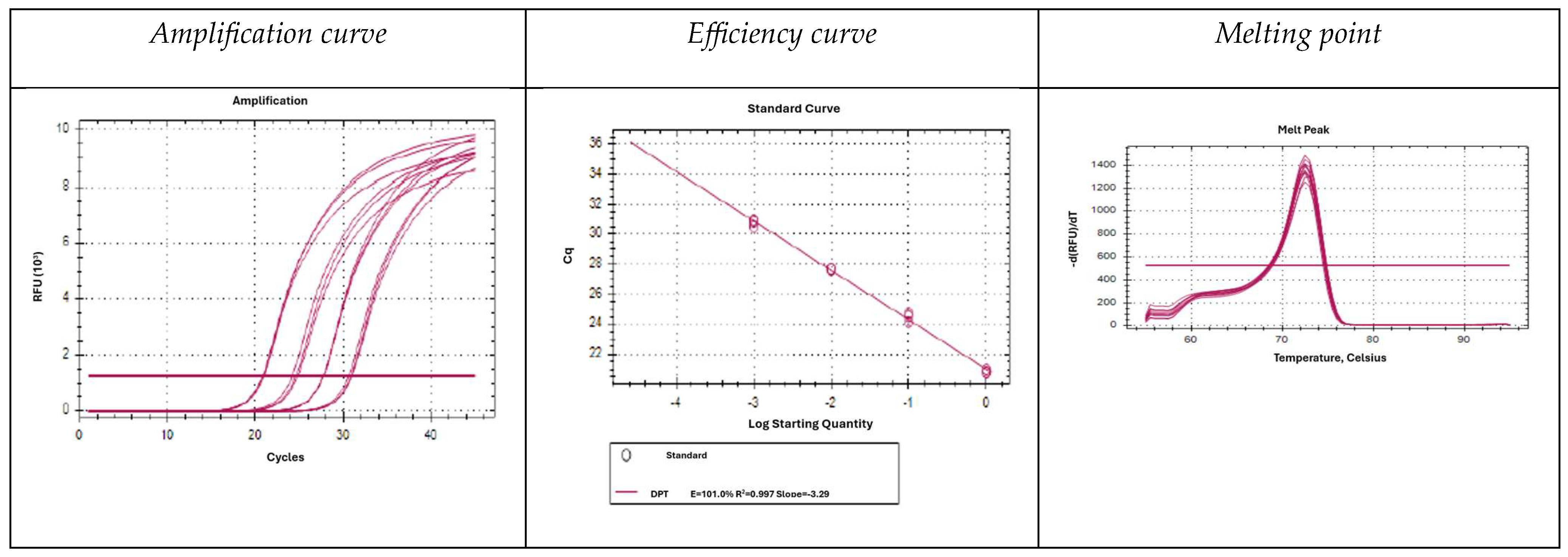
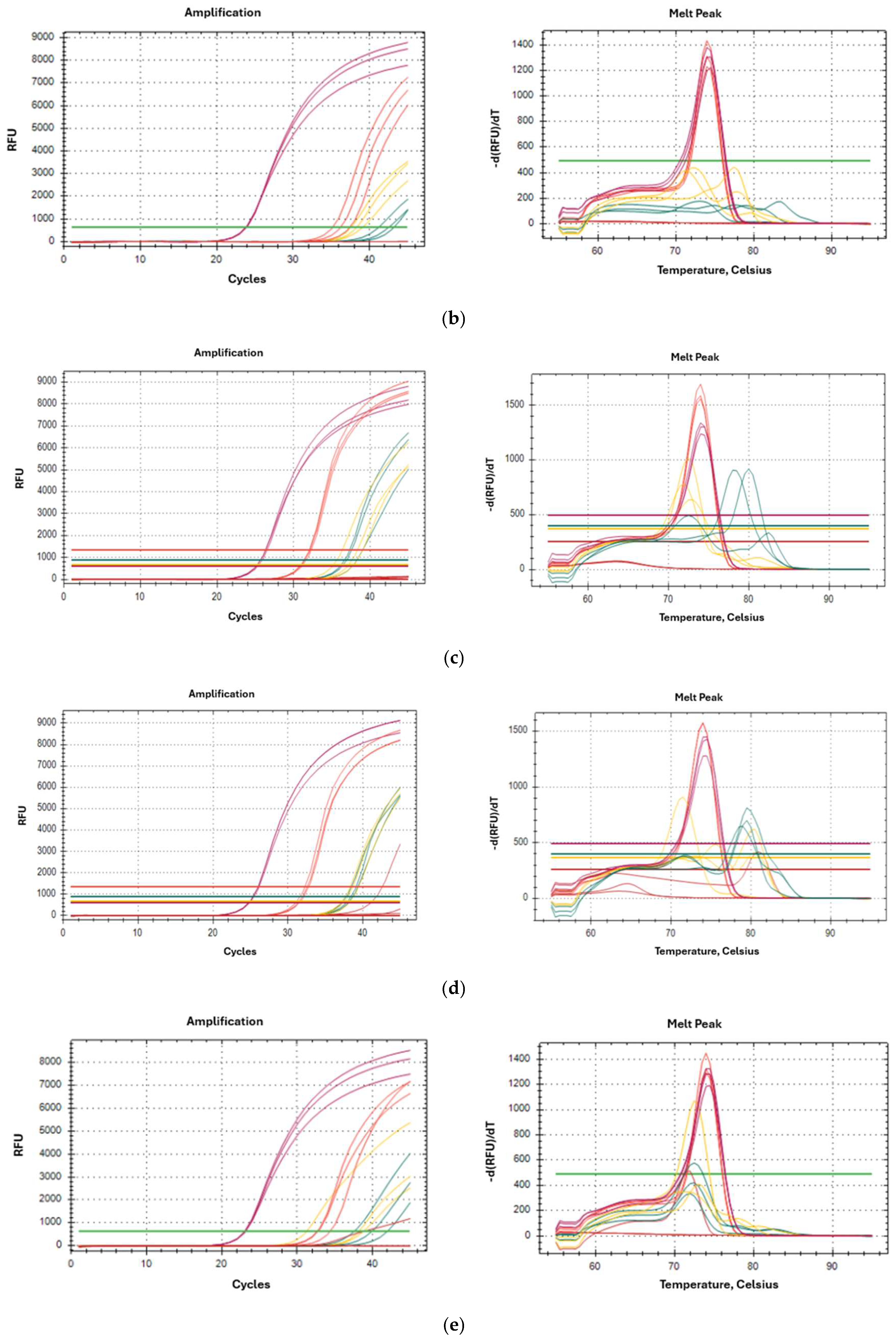
2.2. 18S Amplification with Specific Oligonucleotides by Real-Time PCR
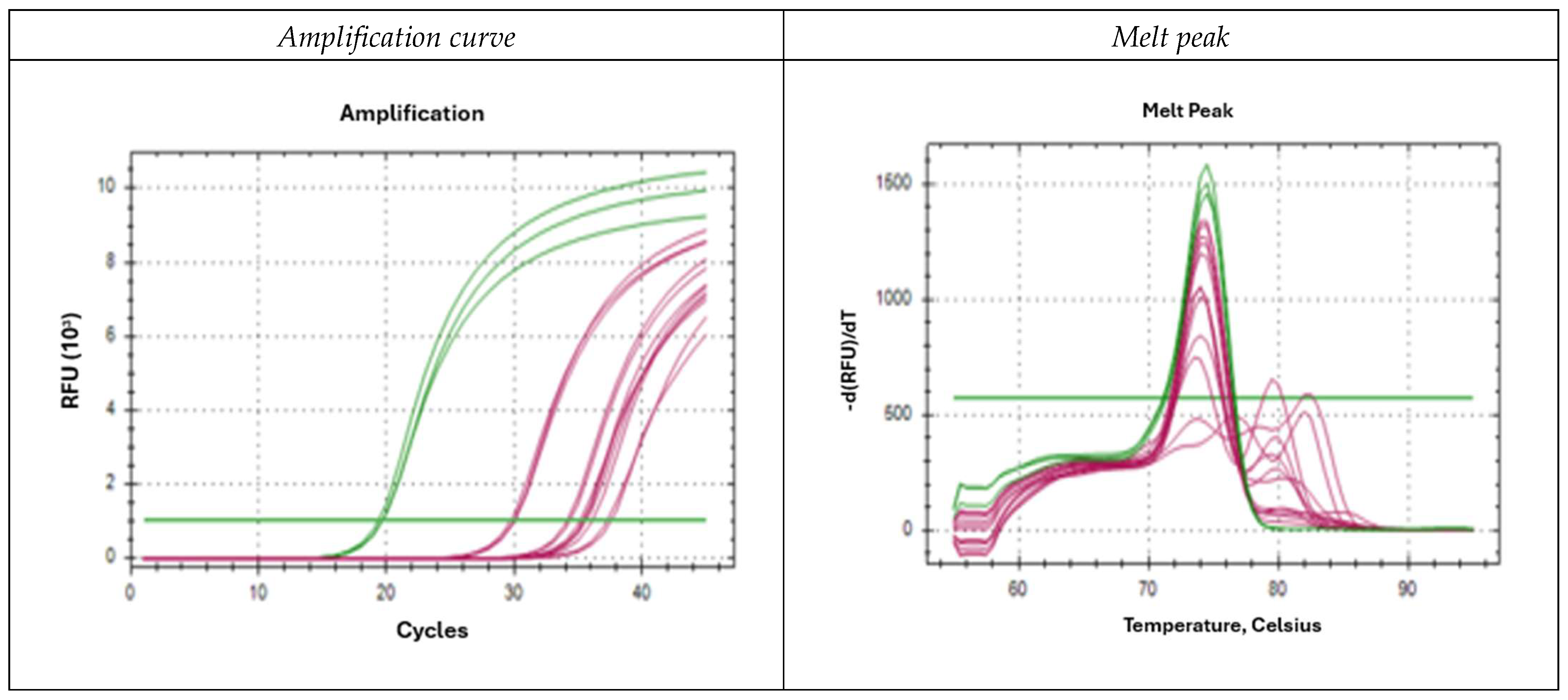
2.3. Specificity of Oligonucleotides
2.4. Oligonucleotides Species-Specificity (Results for D. pteronyssinus)
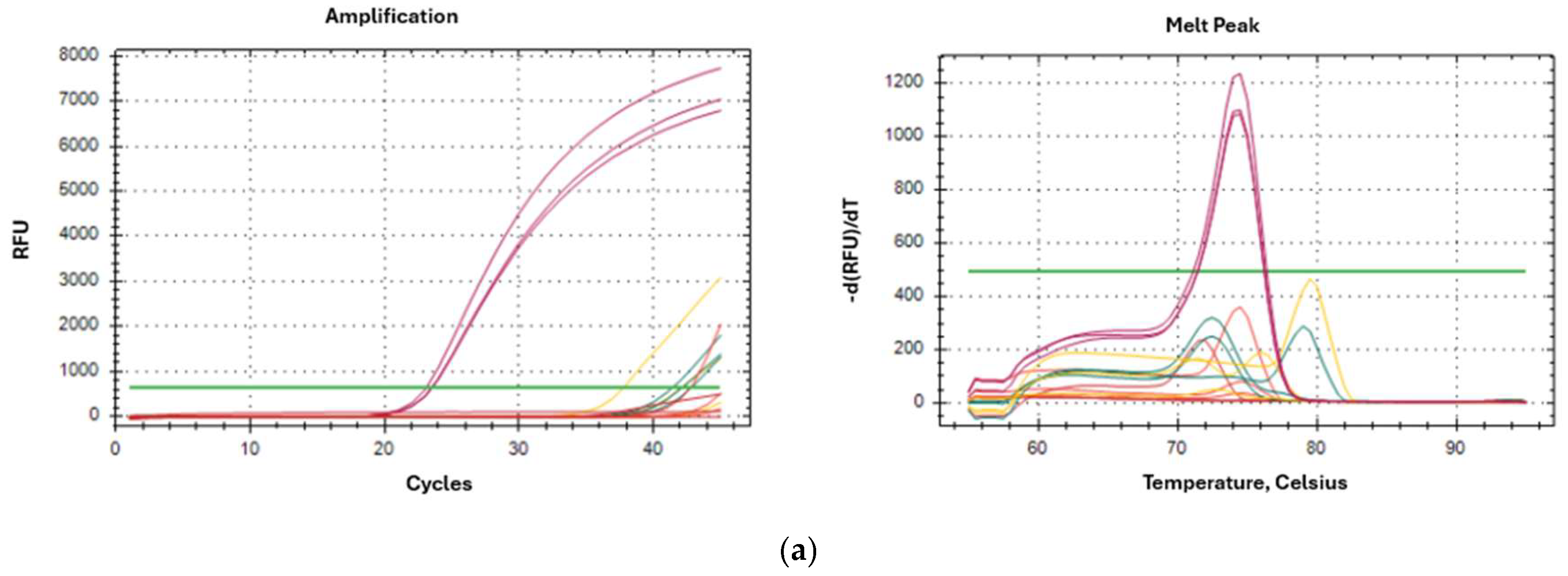
2.5. Specificity of Oligonucleotides in Artificially Contaminated Cultures
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Absorbance |
| bp | base pairs |
| BT | Blomia tropicalis |
| Ct | cycle threshold |
| DF | Dermatophagoides farinae |
| DNA | deoxyribonucleic acid |
| DPT | Dermatophagoides pteronyssinus |
| HDM | House Dust Mites |
| IUIS | International Union of Immunological Societies |
| LD | Lepidoglyphus destructor |
| PCR | polymerase chain reaction |
| qPCR | quantitative PCR |
| RNA | ribonucleic acid |
| rRNA | ribosomal ribonucleic acid |
| RT-qPCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| TP | Tyrophagus putrescentiae |
| WHO | World Health Organization |
References
- Floyer, J. Abhandkungen von der Engbrüstigkeit: A Treatise of the Asthma. London 1698; Deutsche Übersetzung; von Johann Christian Friedrich Scherf: Leipzig, Germany, 1782; p. 106. [Google Scholar]
- Voorhorst, R.; Spieksma-Boezeman, M.I.; Spieksma, F.T. Is a mite (Dermatophagoides spp.) the producer of the house dust mite allergen? Allerg Asthma 1964, 10, 329–334. [Google Scholar] [PubMed]
- Platts-Mills, T.A.E.; Erwin, E.A.; Heymann, P.W.; Woodfolk, J.A. Pro: The evidence for a causal role of dust mites in asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Borges, M.; Fernández-Caldas, E.; Thomas, W.R.; Chapman, M.D.; Lee, B.W.; Caraballo, L.; Acevedo, N.; Chew, F.T.; Ansotegui, I.J.; Behrooz, L.; et al. International consensus (ICON) on: Clinical consequences of mite hypersensitivity, a global problem. World Allergy Org. J. 2017, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.A.; Linneberg, A.; Kleine-Tebbe, J.; De Blay, F.; de Rojas, D.H.F.; Wirchow, J.; Demoly, P. Respiratory allergy caussed by house dust mite. What do we really know? J. Allergy Clin. Immunol. 2015, 136, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. The role of dust mite in allergy. Clin. Rev. Allerg. Immunol. 2019, 57, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.R.; Jeong, K.Y.; Yi, M.H.; Kim, H.P.; Shin, H.J.; Yong, T.S. Cross-reactivity between group-5 and 21 mite allergens from Dermatophagoides farinae, Tyrophagus putrescentiae and Blomia tropicalis. Mol. Med. Resp. 2015, 12, 5467–5474. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Sarzsinzky, E.; Vrtala, S. House dust mite allergy: The importance of house dust mite allergens for diagnosis and immunotherapy. Mol. Immunol. 2023, 158, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sub-Committee WIAN: WHO/IUIS Allergen Tree View. Available online: http://www.allergen.org/treeview.php (accessed on 23 May 2019).
- Weghofer, M.; Grote, M.; Resch, Y.; Casset, A.; Kneidinger, M.; Kopec, J.; Thomas, W.R.; Fernández-Caldas, E.; Kabesch, M.; Ferrara, R.; et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J. Immunol. 2013, 190, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution and Behaviour; CABI Publishing: Wallingford, CT, USA, 1999. [Google Scholar]
- Krantz, G.W. A Manual of Acarology; Oregon State University Bookstores: Corvallis, OR, USA, 1978. [Google Scholar]
- EMEA/CHMP/BWP/304831/2007; Guideline on Allergen Products: Production and Quality Issues. European Medicines Agency: London, UK, 2007.
- Mangold, A.J.; Bargues, M.D.; Mas Coma, S. 18S rRNA gene sequences and phylogenetic relationships of European hard-tick species (Acari: Ixodidae). Parasitol. Res. 1998, 84, 31–37. [Google Scholar] [CrossRef]
- Dobson, S.J.; Barker, S.C. Phylogeny of the hard ticks (Ixodidae) inferred 18S rRNA indicates that genus Aponomma is paraphyletic. Mol. Phylohenet. 1999, 11, 285–295. [Google Scholar]
- Zhao, Y.; Zhang, W.-Y.; Wang, R.-L.; Niu, D.-L. Divergent domains of 28S ribosomal RNA gene: DNA barcodes for molecular classification and identification of mites. Parasites Vectors 2020, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T. An Introduction to Biotechnology; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-907568-28-2. [Google Scholar]
- Heid, C.A.; Stevens, J.; Livak, J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.H.; Kent, J.O.; Wittwer, C.T. High-resolution DNA melting analysis for simple and efficient molecular diagnosis. Pharmacogenomics 2007, 8, 597–608. [Google Scholar] [CrossRef] [PubMed]
| Oligonucleotide Sequence Forward/Reverse | Tm °C | PCR Efficiency % | R2 | Ct °C | Ct (Other) °C | |
|---|---|---|---|---|---|---|
| DPT | 5′-TGACTTTTGTGGTGAAGAAG-3′ 5′-CTCACAAGCGGTATTTAGCGT-3′ | 74.5 | 98.8 | 0.997 | 19.5 | >30 |
| DF | 5′-ATTCATTTCGGTGTCGTGAG-3′ 5′-CATTAAAAAATCACCAAAGG-3′ | 72.5 | 101.0 | 0.997 | 21.0 | >30 |
| TP | 5′-CTGCCCATACGAGCGTAGG-3′ 5′-TGAATCGAGTACCAGCAAAC-3′ | 76.5 | 95.8 | 0.999 | 18.7 | >30 |
| BT | 5′-AAAGGAGACTTAAATAAGTTGTC-3′ 5′-GTTTTTCTCAATAAGTGAACA-3′ | 74.0 | 116.9 | 0.943 | 21.1 | >30 |
| LD | 5′-TTTGAAATTTGGTGGTATTTG-3′ 5′-CAATATCAATTGTAATCACAA-3′ | 71.5 | 91.1 | 15.5 | >30 |
| Species | Forward | Reverse |
|---|---|---|
| D. pteronyssinus | 5′-TGACTTTTGTGGTGAAGAAG-3′ | 5′-CTCACAAGCGGTATTTAGCGT-3′ |
| D. farinae | 5′-ATTCATTTCGGTGTCGTGAG-3′ | 5′-CATTAAAAAATCACCAAAGG-3′ |
| T. putrescentiae | 5′-CTGCCCATACGAGCGTAGG-3′ | 5′-TGAATCGAGTACCAGCAAAC-3′ |
| B. tropicalis | 5′-AAAGGAGACTTAAATAAGTTGTC-3′ | 5′-GTTTTTCTCAATAAGTGAACA-3′ |
| L. destructor | 5′-TTTGAAATTTGGTGGTATTTG-3′ | 5′-CAATATCAATTGTAATCACAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Dumpierrez, A.; Rodriguez Gil, D.; Gallego Segovia, M.D.; Alcover, J.; Martínez-Gomariz, M.; Gómez, A.; Palacios, R. Utility of the Ribosomal Gene 18S rRNA in the Classification of the Main House Dust Mites Involved in Hypersensitivity. Int. J. Mol. Sci. 2025, 26, 10308. https://doi.org/10.3390/ijms262110308
García-Dumpierrez A, Rodriguez Gil D, Gallego Segovia MD, Alcover J, Martínez-Gomariz M, Gómez A, Palacios R. Utility of the Ribosomal Gene 18S rRNA in the Classification of the Main House Dust Mites Involved in Hypersensitivity. International Journal of Molecular Sciences. 2025; 26(21):10308. https://doi.org/10.3390/ijms262110308
Chicago/Turabian StyleGarcía-Dumpierrez, Antonio, David Rodriguez Gil, M. Dolores Gallego Segovia, Javier Alcover, Montserrat Martínez-Gomariz, Aida Gómez, and Ricardo Palacios. 2025. "Utility of the Ribosomal Gene 18S rRNA in the Classification of the Main House Dust Mites Involved in Hypersensitivity" International Journal of Molecular Sciences 26, no. 21: 10308. https://doi.org/10.3390/ijms262110308
APA StyleGarcía-Dumpierrez, A., Rodriguez Gil, D., Gallego Segovia, M. D., Alcover, J., Martínez-Gomariz, M., Gómez, A., & Palacios, R. (2025). Utility of the Ribosomal Gene 18S rRNA in the Classification of the Main House Dust Mites Involved in Hypersensitivity. International Journal of Molecular Sciences, 26(21), 10308. https://doi.org/10.3390/ijms262110308






