Gender and Age-Related Trends in Inhalant Allergen Sensitization in Lithuania: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Sensitization Profile of the Sample Group and Sex-Dependent Differences
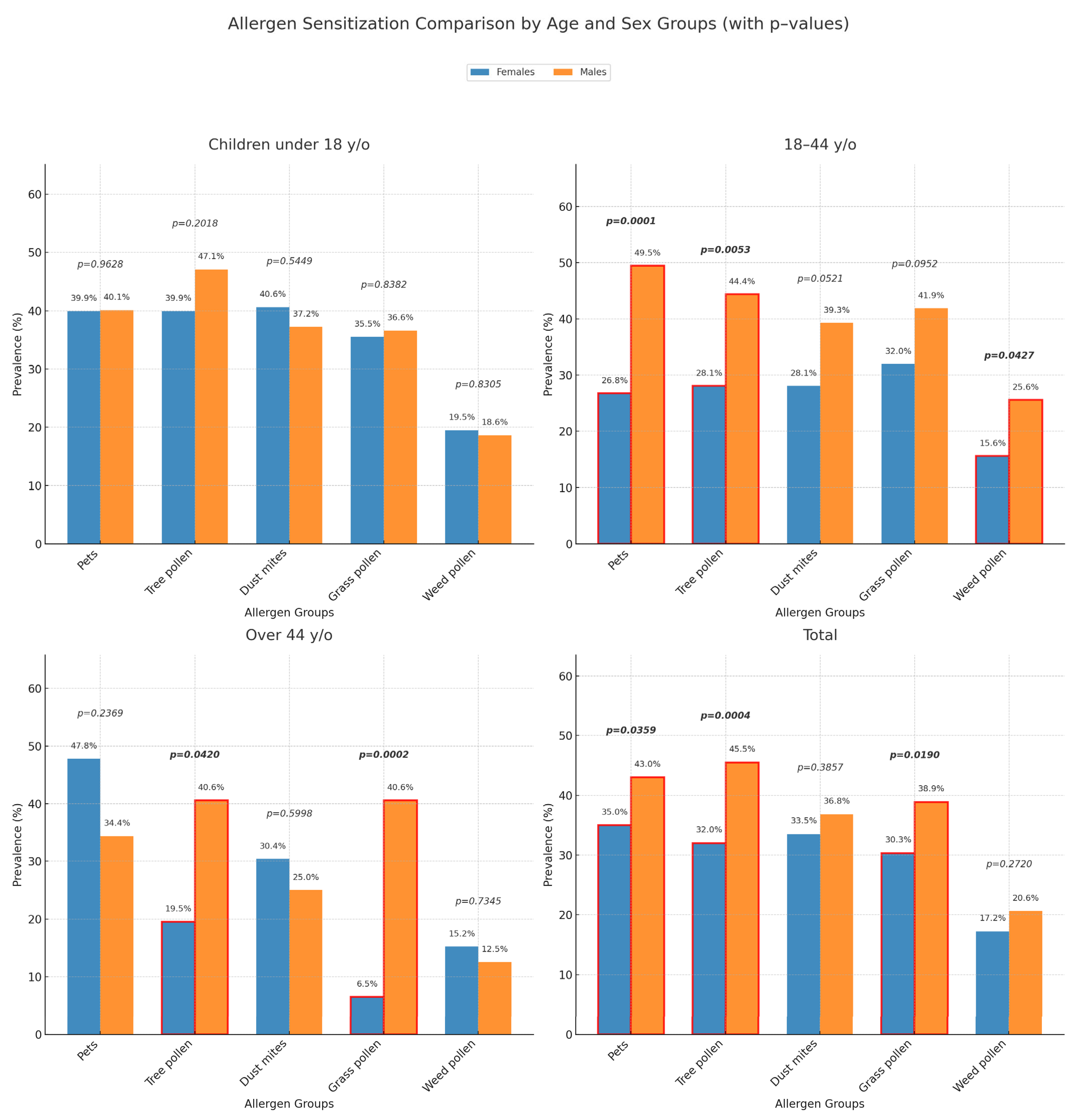
2.2. Sensitization Differences for the Most Common Inhalant Allergen Groups Between Subgroups of Different Age and Sex
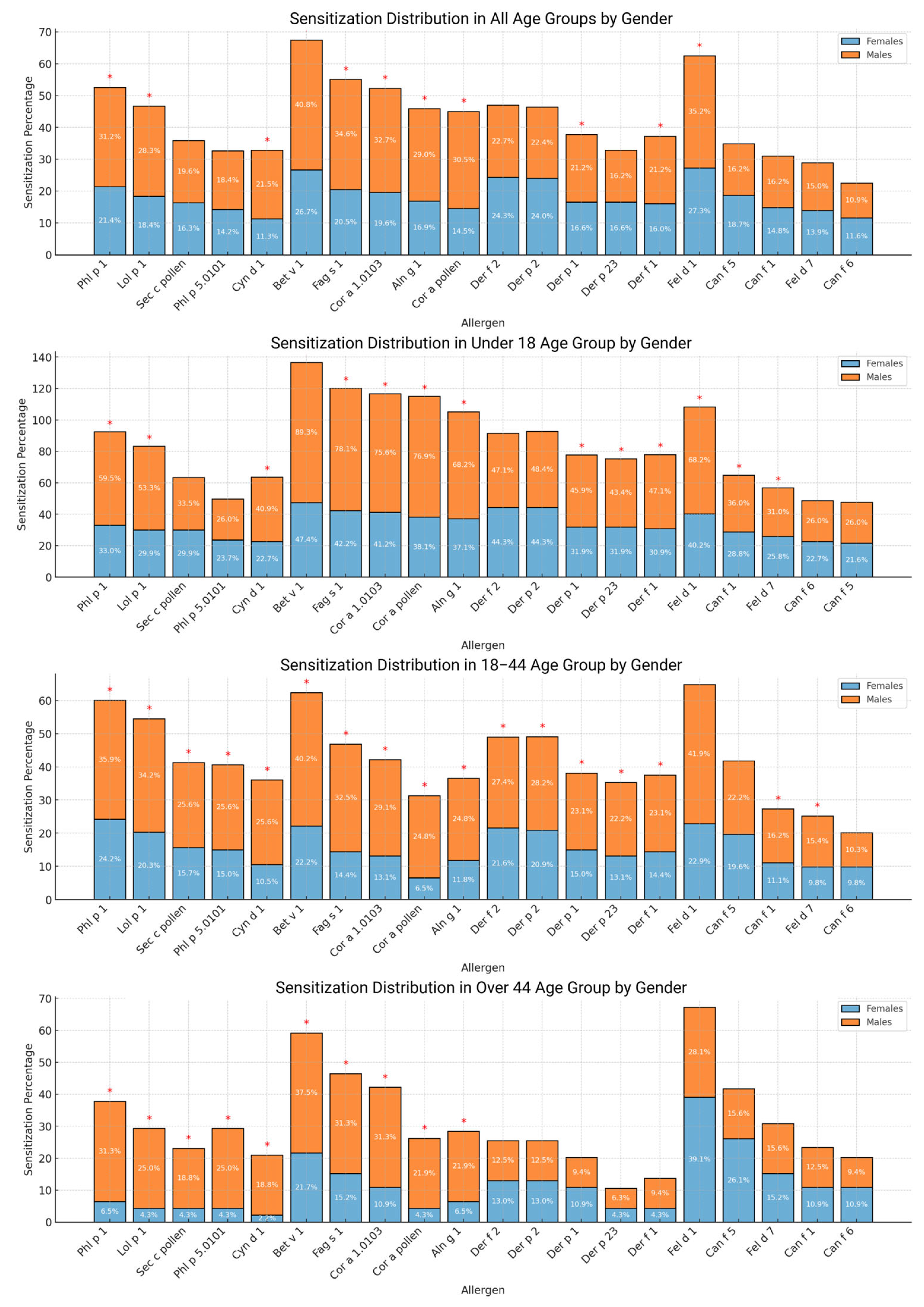
2.3. Sensitizations to the Most Common Sensitizing Allergen Components in Allergen Groups
2.4. Sensitization Differences for Allergen Components Between Subgroups According to Sex
2.5. Sensitization Profiles of the Age Subgroup Under 18 Years Old
2.6. The Most Common Sensitizing Allergen Components for Age Subgroup 18–44 Years Old and Sensitization Differences According to Sex
2.7. The Differences in Sensitization Patterns to Inhalant Allergens of Males and Females Between All Adults of the Sample
2.8. The Most Common Sensitizing Allergen Components for Age Subgroup over 44 Years Old
2.9. Differences in Sensitization to Inhalant Allergen Components Between Children and Adults
2.10. Sensitization Differences Between Children and Adults According to Sex
3. Discussion
3.1. Prevalence of Sensitization to Inhalant Allergens in Different Populations
3.2. Most Common Allergen Groups in Different Populations
3.3. Geographical Differences as a Factor Influencing Different Sensitization Patterns to Inhalant Allergens in Different Populations
3.4. Age-Related Differences
3.5. Sex-Related Differences
3.6. Hormonal Influence on Allergic Diseases
3.7. Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, D.K.; Grammer, L.C. An overview of allergens. Allergy Asthma Proc. 2019, 40, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K. Environmental exposures and mechanisms in allergy and asthma development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.; Dahlin, A.; Wang, A.L. The Role of Environmental Risk Factors on the Development of Childhood Allergic Rhinitis. Children 2021, 8, 708. [Google Scholar] [CrossRef]
- Seastedt, H.; Nadeau, K. Factors by which global warming worsens allergic disease. Ann. Allergy Asthma Immunol. 2023, 131, 694–702. [Google Scholar] [CrossRef]
- Klimek, L.; Mullol, J.; Ellis, A.K.; Izquierdo-Domínguez, A.; Hagemann, J.; Casper, I.; Davis, A.; Becker, S. Current Management of Allergic Rhinitis. J. Allergy Clin. Immunol. Pr. 2024, 12, 1399–1412. [Google Scholar] [CrossRef]
- Hoyte, F.C.L.; Nelson, H.S. Recent advances in allergic rhinitis. F1000Research 2018, 7, 1333. [Google Scholar] [CrossRef]
- Widuri, A.; Hidayat VAzahra, N. Differences in the Prevalence of Adults with Allergic Rhinitis by Gender. In Proceedings of the International Conference on Sustainable Innovation on Health Sciences and Nursing (ICOSI-HSN 2022); Permana, I., Rochmawati, E., Eds.; Atlantis Press International BV: Dordrecht, The Netherlands, 2022; pp. 15–20. Available online: https://www.atlantis-press.com/doi/10.2991/978-94-6463-070-1_4 (accessed on 7 May 2025).
- Urrutia-Pereira, M.; Mocelin, L.P.; Ellwood, P.; Garcia-Marcos, L.; Simon, L.; Rinelli, P.; Chong-Neto, H.J.; Solé, D. Prevalence of rhinitis and associated factors in adolescents and adults: A Global Asthma Network study. Rev. Paul. Pediatr. 2023, 41, e2021400. [Google Scholar] [CrossRef]
- Fröhlich, M.; Pinart, M.; Keller, T.; Reich, A.; Cabieses, B.; Hohmann, C.; Postma, D.S.; Bousquet, J.; Antó, J.M.; Keil, T.; et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clin. Transl. Allergy 2017, 7, 44. [Google Scholar] [CrossRef]
- Nissen, S.P.; Kjær, H.F.; Høst, A.; Nielsen, J.; Halken, S. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr. Allergy Immunol. 2013, 24, 549–555. [Google Scholar] [CrossRef]
- Haarala, A.K.; Sinikumpu, S.-P.; Vaaramo, E.; Jokelainen, J.; Timonen, M.; Auvinen, J.; Pekkanen, J.; Huilaja, L. A Childhood Farm Environment Protects from Allergic Sensitization until Middle Age but Not from New-Onset Sensitization in Adulthood: A 15 Year Longitudinal Study. Int. J. Environ. Res. Public Health 2021, 18, 7078. [Google Scholar] [CrossRef]
- Owora, A.; Tepper, R.; Ramsey, C.; Watson, W.; Krupp, N.; Kloepfer, K.; Becker, A. Transitions Between Alternating Childhood Allergy Sensitization-Asthma States—A Retrospective Cohort Analysis. In Proceedings of the American Thoracic Society 2022 International Conference, San Francisco, CA, USA, 13–18 May 2022; p. A2504. [Google Scholar]
- Salo, P.M.; Arbes, S.J.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J. Allergy Clin. Immunol. 2014, 134, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, J.; Wang, Q.; Shi, G.; Yang, J.; Ming, L. Prevalence of allergen sensitization among 15,534 patients with suspected allergic diseases in Henan Province, China. Asian Pac. J. Allergy Immunol. 2018, 37, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-G.; Kim, M.-Y.; Song, W.-J.; Kim, S.; Jo, E.-J.; Lee, S.-E.; Kwon, J.-W.; Lee, S.-M.; Park, C.-S.; Park, H.-K.; et al. Patterns of Inhalant Allergen Sensitization and Geographical Variation in Korean Adults: A Multicenter Retrospective Study. Allergy Asthma Immunol. Res. 2017, 9, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Ellert, U.; Kalcklösch, M.; Dahm, S.; Thamm, M. Patterns of Sensitization to Inhalant and Food Allergens - Findings from the German Health Interview and Examination Survey for Children and Adolescents. Int. Arch. Allergy Immunol. 2013, 162, 263–270. [Google Scholar] [CrossRef]
- Dor-Wojnarowska, A.; Liebhart, J.; Miecielica, J.; Rabski, M.; Fal, A.; Samoliński, B.; Nittner-Marszalska, M. The Impact of Sex and Age on the Prevalence of Clinically Relevant Sensitization and Asymptomatic Sensitization in the General Population. Arch. Immunol. Ther. Exp. 2016, 65, 253–261. [Google Scholar] [CrossRef]
- Plaschke, P.; Janson, C.; Norrman, E.; Björnsson, E.; Lundbäck, B.; Lindholm, N.; Rosenhall, L.; Järvholm, B.; Boman, G. Skin prick tests and specific IgE in adults from three different areas of Sweden. Allergy 1996, 51, 461–472. [Google Scholar] [CrossRef]
- Bergmann, K.C.; Heinrich, J.; Niemann, H. Current status of allergy prevalence in Germany: Position paper of the Environmental Medicine Commission of the Robert Koch Institute. Allergo J. Int. 2016, 25, 6–10. [Google Scholar] [CrossRef]
- Baratawidjaja, I.R.; Baratawidjaja, P.P.; Darwis, A.; Soo-Hwee, L.; Fook-Tim, C.; Bee-Wah, L.; Baratawidjaja, K.G. Prevalence of allergic sensitization to regional inhalants among allergic patients in Jakarta, Indonesia. Asian Pac. J. Allergy Immunol. 1999, 17, 9–12. [Google Scholar]
- Song, W.-J.; Sohn, K.-H.; Kang, M.-G.; Park, H.-K.; Kim, M.-Y.; Kim, S.-H.; Lim, M.K.; Choi, M.-H.; Kim, K.W.; Cho, S.-H.; et al. Urban–rural differences in the prevalence of allergen sensitization and self-reported rhinitis in the elderly population. Ann. Allergy Asthma Immunol. 2015, 114, 455–461. [Google Scholar] [CrossRef]
- Toth, I.; Peternel, R.; Gajnik, D.; Vojniković, B. Micro-regional hypersensitivity variations to inhalant allergens in the city of Zagreb and Zagreb County. Coll. Antropol. 2011, 35, 31–37. [Google Scholar] [PubMed]
- Charpin, D.; Ramadour, M.; Lavaud, F.; Raherison, C.; Caillaud, D.; de Blay, F.; Pauli, G.; Annesi-Maesano, I. Climate and Allergic Sensitization to Airborne Allergens in the General Population: Data from the French Six Cities Study. Int. Arch. Allergy Immunol. 2017, 172, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Zakzuk, J.; Caraballo, L. House Dust Mite Allergy Under Changing Environments. Allergy Asthma Immunol. Res. 2019, 11, 450–469. [Google Scholar] [CrossRef] [PubMed]
- Lee-Wong, M.; Collins, J.S.; Nozad, C.; Resnick, D.J. Diagnosis and Treatment of Human Seminal Plasma Hypersensitivity. Obstet. Gynecol. 2008, 111, 538–539. [Google Scholar] [CrossRef]
- Didziokaite, G.; Kuznecovaite, A.; Biliute, G.; Kvedariene, V. Case report: Human seminal plasma allergy diagnosis for a woman with unexplained infertility. Front. Med. 2024, 11, 1–6. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Borres, M.P.; Åberg, M.K.; Yang, L.; Fukuie, T.; Narita, M.; Saito, H.; Ohya, Y. IgE responses to multiple allergen components among school-aged children in a general population birth cohort in Tokyo. World Allergy Organ. J. 2020, 13, 100105. [Google Scholar] [CrossRef]
- Su, Y.-T.; Yang, Y.-N.; Li, Y.-C.; Tsai, C.-C.; Chen, L.-M.; Lin, Y.-C.; Niu, C.-K.; Tsai, Y.-C. Age-dependent distribution of the atopic phenotype and allergen sensitization among asthmatic children in southern Taiwan. Asian Pac. J. Allergy Immunol. 2016, 34, 206–211. [Google Scholar] [CrossRef]
- Niemeijer, N.R.; de Monchy, J.G. Age-dependency of sensitization to aero-allergens in asthmatics. Allergy 1992, 47, 431–435. [Google Scholar] [CrossRef]
- Emin, O.; Nermin, G.; Ulker, O.; Gökçay, G. Skin sensitization to common allergens in Turkish wheezy children less than 3 years of age. Asian Pac. J. Allergy Immunol. 2004, 22, 97–101. [Google Scholar]
- Sporik, R.; Holgate, S.T.; Platts-Mills, T.A.; Cogswell, J.J. Exposure to House-Dust Mite Allergen (Der pI) and the Development of Asthma in Childhood. N. Engl. J. Med. 1990, 323, 502–507. [Google Scholar] [CrossRef]
- Silvestsri, M.; Oddera, S.; A Rossi, G.; Crimi, P. Sensitization to Airborne Allergens in Children with Respiratory Symptoms. Ann. Allergy Asthma Immunol. 1996, 76, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Newcomb, D.C. Sex Bias in Asthma Prevalence and Pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-N.; Won, J.Y.; Nam, E.-C.; Kim, T.S.; Ryu, Y.-J.; Kwon, J.-W.; Lee, W.H. Clinical Manifestations of Allergic Rhinitis by Age and Gender: A 12-Year Single-Center Study. Ann. Otol. Rhinol. Laryngol. 2020, 129, 910–917. [Google Scholar] [CrossRef]
- A Barbee, R.; Brown, W.G.; Kaltenborn, W.; Halonen, M. Allergen skin-test reactivity in a community population sample: Correlation with age, histamine skin reactions, and total serum immunoglobulin E. J. Allergy Clin. Immunol. 1981, 68, 15–19. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, J.; Ji, Y.; Zhang, Y.; Zhou, X.; Yin, J.; Zhang, M.; Bao, W. K--means cluster analysis of characteristic patterns of allergen in different ages: Real life study. Clin. Transl. Allergy 2023, 13, e12281. [Google Scholar] [CrossRef]
- Warm, K.; Hedman, L.; Lindberg, A.; Lötvall, J.; Lundbäck, B.; Rönmark, E. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J. Allergy Clin. Immunol. 2015, 136, 1559–1565.e2. [Google Scholar] [CrossRef]
- Melén, E.; Bergström, A.; Kull, I.; Almqvist, C.; Andersson, N.; Asarnoj, A.; Borres, M.P.; Georgellis, A.; Pershagen, G.; Westman, M.; et al. Male sex is strongly associated with IgE-sensitization to airborne but not food allergens: Results up to age 24 years from the BAMSE birth cohort. Clin. Transl. Allergy 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Goldhahn, K.; Bockelbrink, A.; Nocon, M.; Almqvist, C.; DunnGalvin, A.; Willich, S.N.; Keil, T. Sex-specific differences in allergic sensitization to house dust mites: A meta-analysis. Ann. Allergy Asthma Immunol. 2009, 102, 487–494. [Google Scholar] [CrossRef]
- Ballardini, N.; Bergström, A.; Kull, I.; Almqvist, C.; Andersson, N.; Asarnoj, A.; Borres, M.P.; Georgellis, A.; Pershagen, G.; Westman, M.; et al. Resolved allergen--specific IgE sensitization among females and early poly--sensitization among males impact IgE sensitization up to age 24 years. Clin. Exp. Allergy 2021, 51, 849–852. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Instanes, C.; Granum, B.; Carlsen, K.C.L.; Hetland, G.; Carlsen, K.; Mowinckel, P.; Løvik, M. Gender differences in indoor allergen exposure and association with current rhinitis. Clin. Exp. Allergy 2010, 40, 1388–1397. [Google Scholar] [CrossRef]
- Uekert, S.J.; Akan, G.; Evans, M.D.; Li, Z.; Roberg, K.; Tisler, C.; DaSilva, D.; Anderson, E.; Gangnon, R.; Allen, D.B.; et al. Sex-related differences in immune development and the expression of atopy in early childhood. J. Allergy Clin. Immunol. 2006, 118, 1375–1381. [Google Scholar] [CrossRef]
- Hohmann, C.; Keller, T.; Gehring, U.; Wijga, A.; Standl, M.; Kull, I.; Bergstrom, A.; Lehmann, I.; von Berg, A.; Heinrich, J.; et al. Sex-specific incidence of asthma, rhinitis and respiratory multimorbidity before and after puberty onset: Individual participant meta-analysis of five birth cohorts collaborating in MeDALL. BMJ Open Respir. Res. 2019, 6, e000460. [Google Scholar] [CrossRef] [PubMed]
- Rosário, C.S.; Cardozo, C.A.; Neto, H.J.C.; Filho, N.A.R. Do gender and puberty influence allergic diseases? Allergol. Immunopathol. 2021, 49, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Pali-Schöll, I.; Jensen-Jarolim, E. Gender aspects in food allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef]
- Farage, M.; Berardesca, E.; Maibach, H. The effect of sex hormones on irritant and allergic response: Possible relevance for skin testing. Br. J. Dermatol. 2009, 160, 450–451. [Google Scholar] [CrossRef]
- Kirmaz, C.; Yuksel, H.; Mete, N.; Bayrak, P.; Baytur, Y.B. Is the menstrual cycle affecting the skin prick test reactivity? Asian Pac. J. Allergy Immunol. 2004, 22, 197–203. [Google Scholar]
- Kalogeromitros, D.; Katsarou, A.; Armenaka, M.; Rigopoulos, D.; Zapanti, M.; Stratigos, I. Influence of the menstrual cycle on skin--prick test reactions to histamine, morphine and allergen. Clin. Exp. Allergy 1995, 25, 461–466. [Google Scholar] [CrossRef]
- Eliasson, O.; Scherzer, H.H.; DeGraff, A.C. Morbidity in asthma in relation to the menstrual cycle. J. Allergy Clin. Immunol. 1986, 77, 87–94. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Shah, A. Menstrual-Linked Asthma. J. Asthma 1997, 34, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Shames, R.S.; Heilbron, D.C.; Janson, S.L.; Kishiyama, J.L.; Au, D.S.; Adelman, D.C. Clinical Differences Among Women with and Without Self-Reported Perimenstrual Asthma. Ann. Allergy Asthma Immunol. 1998, 81, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.Á.; Muñoz-Valle, J.F.; Oregon-Romero, E.; Corona-Angeles, J.A.; Hernández-Bello, J. Sex hormones and allergies: Exploring the gender differences in immune responses. Front. Allergy 2025, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pakkasela, J.; Ilmarinen, P.; Honkamäki, J.; Tuomisto, L.E.; Andersén, H.; Piirilä, P.; Hisinger-Mölkänen, H.; Sovijärvi, A.; Backman, H.; Lundbäck, B.; et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm. Med. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Warm, K.; Backman, H.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Low incidence and high remission of allergic sensitization among adults. J. Allergy Clin. Immunol. 2012, 129, 136–142. [Google Scholar] [CrossRef]
- Hourvitz, A.; Machtinger, R.; Maman, E.; Baum, M.; Dor, J.; Levron, J. Assisted reproduction in women over 40years of age: How old is too old? Reprod. Biomed. Online 2009, 19, 599–603. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Drakopoulos, P.; Rosetti, J.; Uvin, V.; Mackens, S.; Bardhi, E.; De Vos, M.; Camus, M.; Tournaye, H.; De Brucker, M. IVF in women aged 43 years and older: A 20-year experience. Reprod. Biomed. Online 2021, 42, 768–773. [Google Scholar] [CrossRef]
- Aldemir, O.; Ozelci, R.; Dilbaz, S.; Dilbaz, B.; Baser, E.; Ustun, Y. Intracytoplasmic Sperm Injection Cycle Outcomes in Women Aged 40 Years and Over. Gynecol. Obstet. Reprod. Med. 2022, 28, 1–6. [Google Scholar] [CrossRef]
| Group | Group Size | Median | IQR | Q1 | Q3 | |
|---|---|---|---|---|---|---|
| Female | In total | 337 (51.22% of total sample size) | 26 | 29 | 8 | 37 |
| Under 18 y/o | 138 (40.95% of all females) | 6 | 7 | 3 | 10 | |
| 18–44 y/o | 153 (45.40% of all females) | 33 | 10 | 27 | 37 | |
| Over 44 y/o | 46 (13.65% of all females) | 48 | 10.25 | 46 | 56.25 | |
| Male | In total | 321 (48.78% of total sample size) | 15.5 | 28 | 5 | 33 |
| Under 18 y/o | 172 (53.58% of all males) | 5 | 7 | 3 | 10 | |
| 18–44 y/o | 117 (36.45% of all males) | 32 | 10 | 26 | 36 | |
| Over 44 y/o | 32 (9.97% of all males) | 53 | 11 | 48 | 59 |
| Groups | Total | Sensitization to at Least One Inhalant Allergen | p-Value |
|---|---|---|---|
| Children | 310 | 197 (63.55%) | |
| Females | 138 | 86 (62.32%) | 0.6870 |
| Males | 172 | 111 (64.53%) | |
| Adults 18–44 y/o | 270 | 165 (61.11%) | |
| Females | 153 | 84 (54.90%) | 0.0167 |
| Males | 117 | 81 (69.23%) | |
| Older than 44 y/o | 78 | 47 (60.26%) | |
| Females | 46 | 25 (54.35%) | 0.2011 |
| Males | 32 | 22 (66.75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didžiokaitė, G.; Kuznecovaitė, A.; Biliūtė, G.; Kvedarienė, V. Gender and Age-Related Trends in Inhalant Allergen Sensitization in Lithuania: A Cross-Sectional Study. Int. J. Mol. Sci. 2025, 26, 9719. https://doi.org/10.3390/ijms26199719
Didžiokaitė G, Kuznecovaitė A, Biliūtė G, Kvedarienė V. Gender and Age-Related Trends in Inhalant Allergen Sensitization in Lithuania: A Cross-Sectional Study. International Journal of Molecular Sciences. 2025; 26(19):9719. https://doi.org/10.3390/ijms26199719
Chicago/Turabian StyleDidžiokaitė, Gabija, Aida Kuznecovaitė, Gabija Biliūtė, and Violeta Kvedarienė. 2025. "Gender and Age-Related Trends in Inhalant Allergen Sensitization in Lithuania: A Cross-Sectional Study" International Journal of Molecular Sciences 26, no. 19: 9719. https://doi.org/10.3390/ijms26199719
APA StyleDidžiokaitė, G., Kuznecovaitė, A., Biliūtė, G., & Kvedarienė, V. (2025). Gender and Age-Related Trends in Inhalant Allergen Sensitization in Lithuania: A Cross-Sectional Study. International Journal of Molecular Sciences, 26(19), 9719. https://doi.org/10.3390/ijms26199719







