Biologically Active Compounds of Plants of the Atraphaxis Genus: Chemical Composition and Immunomodulatory Evaluation
Abstract
1. Introduction
2. Results and Discussion
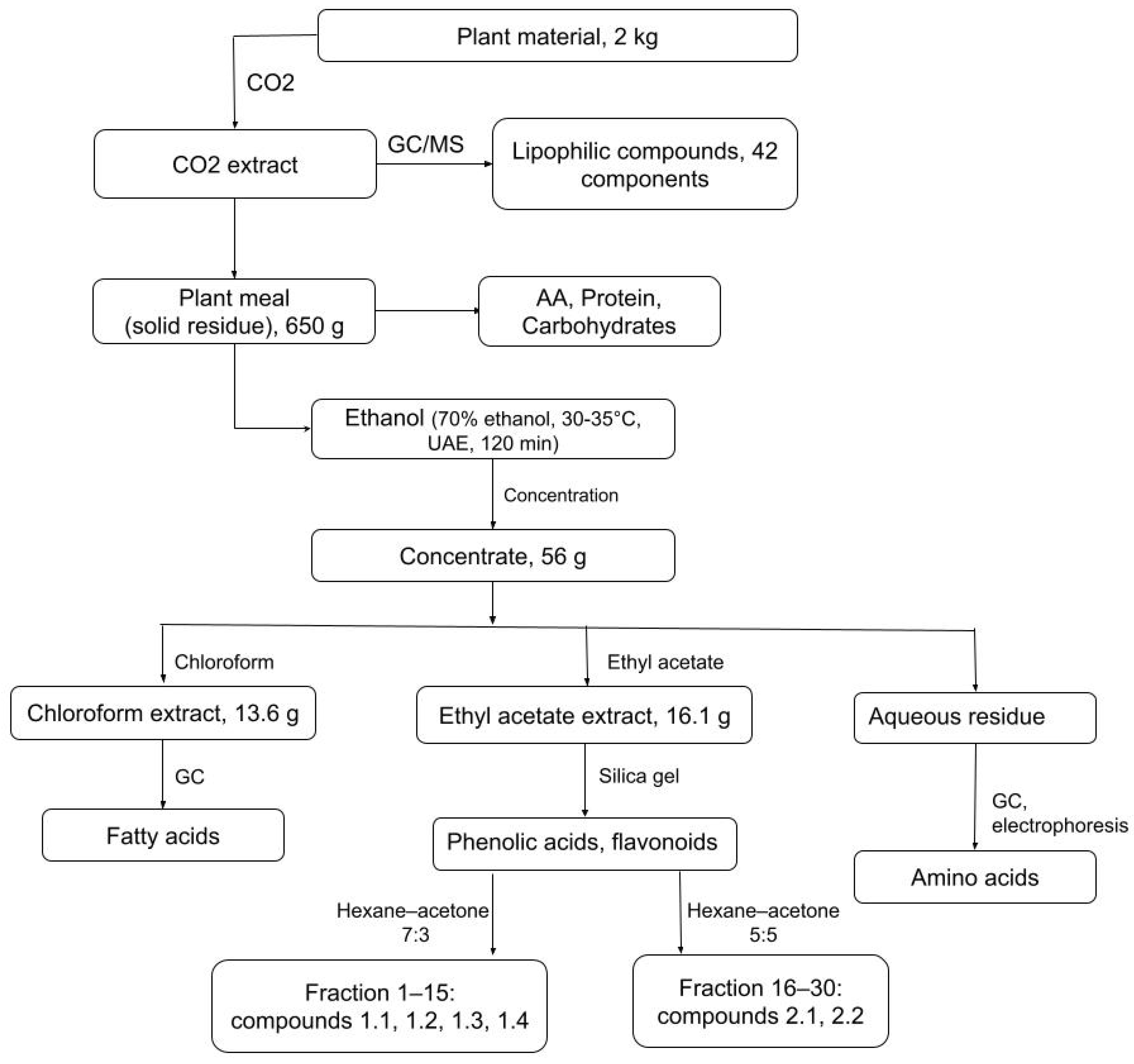
2.1. GC–MS Characterization of the CO2 Extract of Atraphaxis virgata (Lipophilic Constituents)
2.2. Polar Metabolites in the Ethanol–Water Extract: Amino Acids, Proteins, and Carbohydrates (Post-CO2 Plant Meal) and Aqueous Residue
2.3. Extraction Yield Assessment of A. virgata Polar Extracts
2.4. Fatty Acid Profiling of Chloroform Extracts from Atraphaxis virgata
2.5. Investigation of the Ethyl Acetate Extract Constituents (Structural Elucidation of Flavonoids and Phenolic Acids)
2.6. Determination of Antioxidant Content
2.7. Immunomodulatory Activity of A. virgata
3. Materials and Methods
3.1. Plant Material
3.2. General Analytical Procedures
3.3. Supercritical CO2 Extraction
3.4. Ultrasound-Assisted Ethanol–Water Extraction (UAE) of the Plant Meal
3.5. Fatty Acid Analysis
3.6. Amino Acid and Protein Determination
3.7. Phenolic and Flavonoid Analysis
3.8. Isolation and Structural Elucidation of Polyphenolic Compounds
3.9. Antioxidant Activity
3.10. Immunomodulatory Activity Assessment
- Leukocyte profile: WBC, lymphocytes (LYM, %, ×109/L), neutrophils (NEU, %, ×109/L), and monocytes (MON, %, ×109/L)
- Erythrocyte profile: RBC, HGB, HCT, MCV, MCH, MCHC, and RDW
- Platelet profile: PLT, PCT, MPV, and PDW
3.11. Ethical Compliance and Chronobiological Design for Immunomodulatory Activity Assessment
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC–MS | Gas Chromatography–Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| UV-Vis | Ultraviolet–Visible Spectroscopy (UV-Vis) |
| IR | Infrared Spectroscopy |
| NMR | Nuclear Magnetic Resonance Spectroscopy |
| MS | Mass Spectrometry |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode Array Detection |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| UAE | Ultrasound-Assisted Extraction |
| GLC | Gas-Liquid Chromatography |
| BA | Biochemical Assays |
| TLC | Thin-Layer Chromatography |
| PC | Paper Chromatography |
| 1H NMR | Proton Nuclear Magnetic Resonance Spectroscopy |
| 13C NMR | Carbon-13 Nuclear Magnetic Resonance Spectroscopy |
| 1H–1H COSY | Proton–Proton Correlation Spectroscopy |
| 1H–13C HMQC | Heteronuclear Multiple Quantum Coherence |
| 1H–13C HMBC | Heteronuclear Multiple Bond Correlation |
References
- Wang, X.; Khutsishvili, M.; Fayvush, G.; Tamanyan, K.; Atha, D.; Borris, R.P. Phytochemical investigations of Atraphaxis spinosa L (Polygonaceae). Biochem. Syst. Ecol. 2018, 77, 44–47. [Google Scholar] [CrossRef]
- Zhang, M.L.; Sanderson, S.C.; Sun, Y.X.; Byalt, V.V.; Hao, X.L. Tertiary montane origin of the Central Asian flora, evidence inferred from cpDNA sequences of Atraphaxis (Polygonaceae). J. Integr. Plant Biol. 2014, 56, 1125–1135. [Google Scholar] [CrossRef]
- Odonbayar, B.; Murata, T.; Batkhuu, J.; Yasunaga, K.; Goto, R.; Sasaki, K. Antioxidant flavonols and phenolic compounds from Atraphaxis frutescens and their inhibitory activities against insect phenoloxidase and mushroom tyrosinase. J. Nat. Prod. 2016, 79, 3065–3071. [Google Scholar] [CrossRef]
- Umbetova, A.K.; Slan, G.O.; Omarova, A.T.; Burasheva, G.S.; Abidkulova, K.T. The study of chemical composition of Atraphaxis virgata from the Almaty region. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Chem. Technol. 2018, 1, 42–45. [Google Scholar]
- Kuzmin, E.V.; Egegbaeva, R.A.; Tugelbayev, S.U.; Gemedzhieva, N.G. Development of Phytochemistry and Prospects of Creation of New Medicines; Science: Almaty, Kazakhstan, 2003; pp. 22–30. [Google Scholar]
- Mamonov, L.K.; Muzychkina, R.A. Introduction to Phytochemical Studies and Identification of Biologically Active Substances of Plants; School XXI Century: Almaty, Kazakhstan, 2008; 216p. [Google Scholar]
- Reddy, D.N. Essential oils extracted from medicinal plants and their applications. In Natural Bio-Active Compounds; Akhtar, M., Swamy, M., Sinniah, U., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Omarova, B.A.; Sermukhamedova, O.V.; Sakipova, Z.B.; Esikova, A.A.; Yevtushenko, E.N.; Datkhaev, U.M. Overview of the Kazakhstani market of herbal medicines. Pharm. Kazakhstan 2015, 6, 7–12. [Google Scholar]
- Shin, S.; Kim, S.; Lee, J.; Son, H.; Paik, J.-H.; Gemejiyeva, N.G.; Karzhaubekova, Z.Z.; Lee, T.; Yoo, H.Y. Investigation of Atraphaxis virgata, an Unexplored Medicinal Plant Rich in Flavonoids, as a Functional Material. Horticulturae 2025, 11, 70. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2019, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Dauletova, M.D.; Umbetova, A.K.; Litvinenko, Y.A.; Burasheva, G.S.; Yelibaeva, N.S. Development of a Method for Obtaining a Biologically Active Composition Based on Plants of the Polygonaceae Family. News Natl. Acad. Sci. Ser. Chem. Technol. 2024, 2, 46–61. [Google Scholar] [CrossRef]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Zhumagul, M.Z.; Kurmanbayeva, M.S.; Kudrina, N.O.; Tolenova, K.D.; Seilkhan, A.S.; Höhn, M. GC-MS analysis of the lipophilic compounds of medicinal plant Rhodiola rosea L. Int. J. Biol. Chem. 2019, 12, 103–109. [Google Scholar] [CrossRef]
- Uminska, K.; Gudžinskas, Z.; Ivanauskas, L.; Georgiyants, V.; Kozurak, A.; Skibytska, M.; Mykhailenko, O. Amino acid profiling in wild Chamaenerion angustifolium populations applying chemometric analysis. J. Appl. Pharm. Sci. 2023, 13, 171–180. [Google Scholar] [CrossRef]
- Kaachra, A.; Tamang, A.; Hallan, V. An expedited qualitative profiling of free amino acids in plant tissues using liquid chromatography-mass spectrometry (LC–MS) in conjunction with MS-DIAL. J. Mass Spectrom. 2024, 59, e5094. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Bhardwaj, R.; Radhamani, J.; Yadav, R.; Gautam, R.K.; Kalia, S.; Kumar, A. A quick analysis method for protein quantification in oilseed crops: A comparison with standard protocol. Front. Nutr. 2022, 9, 892695. [Google Scholar] [CrossRef] [PubMed]
- Petkova, T.; Nadezhda, P.; Brabant, A.P.; Masson, A.; Denev, P.P. HPLC analysis of mono- and disaccharides in food products. In Scientific Works; Food Science, Engineering and Technology; University of Food Technology: Plovdiv, Bulgaria, 2013; Volume LX, pp. 761–765. [Google Scholar]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Karimzadeh, H.R.; Farhang, H.R.; Rahimmalek, M.; Esfahani, M.T. Spatio-temporal variations of extract produced and fatty acid compounds identified of Gundelia tournefortii L. seeds in central Zagros, Iran. Sci. Rep. 2023, 13, 7665. [Google Scholar] [CrossRef]
- Nengroo, Z.R.; Rauf, A. Fatty acid composition and antioxidant activities of five medicinal plants from Kashmir. Ind. Crops Prod. 2019, 140, 111596. [Google Scholar] [CrossRef]
- Jacuńska, W.; Biel, W.; Tokarczyk, G.; Biernacka, P.; Bienkiewicz, G.; Janda-Milczarek, K. Fatty acid composition and bioactive profiles in the aerial parts of Cannabis sativa. Molecules 2025, 30, 1947. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Patil, J.R.; Mhatre, K.J.; Yadav, K.; Yadav, L.S.; Srivastava, S.; Nikalje, G.C. Flavonoids in plant–environment interactions and stress responses. Discov. Plants 2024, 1, 68. [Google Scholar] [CrossRef]
- Umbetova, A.K.; Beyatli, A.; Seitimova, G.A.; Yeskaliyeva, B.K.; Burasheva, G.S. Flavonoids from the Plant Atraphaxis virgata. Chem. Nat. Compd. 2021, 57, 531–533. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Miftakhova, A.F. Phytochemical Study of Certain Types of Semey Marevyks: Abstract of Dissertation, Candidate of Chemical Sciences. Ph.D. Thesis, Al-Farabi Kazakh National University, Almaty, Kazakhstan, 2003; 25p. (In Russian). [Google Scholar]
- Zhumakova, S.; Ten, A.; Zharkynbek, T.; Yu, V.; Seilkhanov, T.; Basharimova, A.; Aydemir, M.; Zazybin, A. NMR study of the inclusion complexes of β-cyclodextrin with diphenhydramine, clonidine and tolperisone. SN Appl. Sci. 2022, 4, 75. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, S.; Lu, X.; Xiang, W.; Zhou, X.; Bu, Y.; Li, L.; Huang, R. Variation in flavonoid and antioxidant activities of Pyrrosia petiolosa (Christ) Ching from different geographic origins. Front. Plant Sci. 2023, 4, 1173489. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef]
- Waris, M.; Koçak, E.; Gonulalan, E.M.; Demirezer, L.O.; Kır, S.; Nemutlu, E. Metabolomics analysis insight into medicinal plant science. TrAC Trends Anal. Chem. 2022, 157, 116795. [Google Scholar] [CrossRef]
- Raskar, S.; Purkar, V.; Sardesai, M.; Mitra, S. Assessing the impact of geographical distribution and genetic diversity on metabolic profiles of a medicinal plant, Embelia ribes Burm. f. Plants 2022, 11, 2861. [Google Scholar] [CrossRef]
- Long, A.; Kleiner, A.; Looney, R.J. Immune dysregulation. J. Allergy Clin. Immunol. 2023, 151, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, P.; Pang, X.; Zhang, L.; Qian, L.; Jia, X.; Chen, W.; Ruan, S.; Sun, L. The new exploration of pure total flavonoids extracted from Citrus maxima (Burm.) Merr. as a new therapeutic agent to bring health benefits for people. Front. Nutr. 2022, 9, 958329. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Ma, X.-N.; Wang, Y.; Wei, Y.-X.; Hu, P.; Pan, Y. Validated herbal extracts for alleviating chemotherapy-induced myelosuppression: A systematic review and meta-analysis. Phytomedicine 2025, 141, 156659. [Google Scholar] [CrossRef] [PubMed]

| No. | Compound | Molecular Weight | Retention Time, min | Identification Probability, % | Relative Content, % |
|---|---|---|---|---|---|
| 1 | Heneicosane | 296.58 | 15.00 | 71 | 0.32 |
| 2 | Tetradecane | 198.39 | 12.75 | 78 | 0.33 |
| 3 | 2,6,10-Trimethyl-tetradecane | 240.5 | 21.39 | 63 | 0.26 |
| 4 | 5,9-undecadien-2-one, 6,10-Dimethyl-, (Z) | 190.31 | 21.65 | 62 | 0.56 |
| 5 | Hexadecane | 226.44 | 22.49 | 83 | 0.43 |
| 6 | 3-Hydroxy-4-methoxybenzaldehyde | 152.15 | 23.33 | 61 | 0.73 |
| 7 | Vanillin | 152.15 | 23.41 | 65 | 1.06 |
| 8 | 5-Pentyl-1,3-benzenediol | 104 | 24.85 | 61 | 0.85 |
| 9 | (R)-2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 213.37 | 26.44 | 72 | 1.45 |
| 10 | Fumaric acid, 2-chlorophenylethyl ester | 116.07 | 26.52 | 71 | 0.85 |
| 11 | Octadecane | 254.49 | 27.05 | 82 | 0.81 |
| 12 | 3,4,5-Trimethoxybenzaldehyde | 196.20 | 28.23 | 72 | 0.40 |
| 13 | 6,10,14-Trimethyl-2-pentadecanone | 268.5 | 29.49 | 71 | 1.53 |
| 14 | Heneicosane | 296.57 | 29.73 | 77 | 1.21 |
| 15 | 3-(1-Hydroxy-1-methylethyl)-5-methoxy-1,2,3,4-tetrahydro-2-naphthalenol | 222.28 | 30.59 | 70 | 0.63 |
| 16 | 2-Heptadecanone | 254.44 | 31.05 | 74 | 0.95 |
| 17 | Eicosane | 282.55 | 31.19 | 82 | 0.83 |
| 18 | 2-Methyl-1-hexadecanol | 258.47 | 31.36 | 63 | 0.85 |
| 19 | (E,E)-6,10,14-Trimethyl-5,9,13-pentadecatrien-2-one | 290.50 | 32.09 | 73 | 0.41 |
| 20 | Palmitic acid | 256.42 | 32.43 | 78 | 7.67 |
| 21 | Ethyl hexadecanoate | 256.43 | 32.55 | 86 | 4.35 |
| 22 | 3-Ethyl-5-(2-ethylbutyl)octadecane | 284.48 | 32.76 | 63 | 1.27 |
| 23 | Heneicosane | 354.68 | 33.13 | 87 | 1.93 |
| 24 | Dibutyl phthalate | 282.54 | 34.55 | 91 | 1.71 |
| 25 | Phytol | 278.34 | 34.83 | 82 | 2.95 |
| 26 | 2-Nonadecanone | 296.53 | 34.93 | 88 | 5.14 |
| 27 | Ethyl oleate | 389.50 | 36.08 | 84 | 4.06 |
| 28 | Ethyl 9,12-octadecadienoate | 310.5 | 36.26 | 79 | 10.66 |
| 29 | Ethyl 9,12,15-octadecatrienoate | 308.5 | 36.62 | 71 | 2.18 |
| 30 | 2-Nonadecanone | 278.44 | 38.51 | 79 | 7.40 |
| 31 | 4,8,12,16-Tetramethylheptadecan-4-olide | 268.52 | 40.29 | 72 | 1.43 |
| 32 | Octacosanol | 410.73 | 41.47 | 80 | 4.44 |
| 33 | 2-Nonadecanone | 410.75 | 41.81 | 68 | 1.86 |
| 34 | Heptacosane | 268.52 | 43.25 | 91 | 7.17 |
| 35 | Tetratetracontane | 380 | 43.53 | 60 | 0.40 |
| 36 | Bis(2-ethylhexyl) phthalate | 478 | 43.78 | 74 | 1.26 |
| 37 | Octacosane | 390.55 | 41.47 | 78 | 1.47 |
| 38 | 17-Pentatriacontene | 394 | 44.90 | 69 | 1.33 |
| 39 | Nonacosane | 492.96 | 46.16 | 91 | 7.99 |
| 40 | Squalene | 408.79 | 46.36 | 92 | 4.73 |
| 41 | Hentriacontane | 410.73 | 48.94 | 80 | 2.89 |
| 42 | Triacontanoic acid | 436.85 | 52.11 | 60 | 1.26 |
| Total | 98.25 | ||||
| No. | Amino Acid | Conc., mg/100 g |
|---|---|---|
| 1 | Arginine (Arg) | 87.0 |
| 2 | Lysine (Lys) | 20.0 |
| 3 | Tyrosine (Tyr) | 24.0 |
| 4 | Phenylalanine (Phe) | 50.0 |
| 5 | Histidine (His) | 18.0 |
| 6 | Leucine + Isoleucine (Leu + Ile) | 59.0 |
| 7 | Methionine (Met) | 22.0 |
| 8 | Valine (Val) | 43.0 |
| 9 | Proline (Pro) | 150.0 |
| 10 | Threonine (Thr) | 42.0 |
| 11 | Serine (Ser) | 45.0 |
| 12 | Alanine (Ala) | 30.0 |
| 13 | Glycine (Gly) | 33.0 |
| No. | Amino Acid | Abbreviation | Content, % |
|---|---|---|---|
| 1 | Alanine | Ala | 0.292 |
| 2 | Glycine | Gly | 0.380 |
| 3 | Leucine | Leu | 0.320 |
| 4 | Isoleucine | Ile | 0.280 |
| 5 | Valine | Val | 0.274 |
| 6 | Glutamic acid | Glu | 2.510 |
| 7 | Threonine | Thr | 0.263 |
| 8 | Proline | Pro | 0.506 |
| 9 | Methionine | Met | 0.074 |
| 10 | Serine | Ser | 0.345 |
| 11 | Aspartic acid | Asp | 1.348 |
| 12 | Cystine | Cys | 0.020 |
| 13 | Hydroxyproline | O-prp | 0.001 |
| 14 | Phenylalanine | Phe | 0.292 |
| 15 | Tyrosine | Tyr | 0.334 |
| 16 | Histidine | His | 0.200 |
| 17 | Ornithine | Orn | 0.002 |
| 18 | Arginine | Arg | 0.542 |
| 19 | Lysine | Lys | 0.305 |
| 20 | Tryptophan | Trp | 0.080 |
| No. | Fatty Acid Index | Fatty Acid Name | Content (%) |
|---|---|---|---|
| 1 | C14:0 | Myristic acid | 1.0 |
| 2 | C15:0 | Pentadecanoic acid | 0.7 |
| 3 | C16:0 | Palmitic acid | 11.2 |
| 4 | C16:1 | Palmitoleic acid | 0.1 |
| 5 | C18:0 | Stearic acid | 5.4 |
| 6 | C18:1 | Oleic acid | 50.5 |
| 7 | C18:2 | Linoleic acid | 30.5 |
| 8 | C18:3 | Linolenic acid | 0.5 |
| No. | Parameter | Percentage (%) |
|---|---|---|
| 1 | Lipid-soluble antioxidant content | 1.78 ± 0.03 |
| 2 | Water-soluble antioxidant content | 3.15 ± 0.04 |
| A. virgata Extract Group | Control (Cyclophosphamide) Group | Placebo Group | Intact (Untreated) Group | |
|---|---|---|---|---|
| WBC, ×109/L | 12.32 ± 2.22 ** | 6.20 ± 0.47 | 4.72 ± 0.35 | 11.08 ± 0.32 |
| LYM, % | 92.5 ± 4.24 * | 60.04 ± 3.93 | 57.01 ± 1.65 | 69.72 ± 1.1 |
| NEU, % | 3.5 ± 0.03 | 32.68 ± 1.6 | 36.05 ± 9.3 | 30 ± 0.8 |
| MI, % | 3.9 ± 0.01 | 7.28 ± 0.4 | 7.03 ± 5.3 | 0.28 ± 0.1 |
| LYM, ×109/L | 0.44 ± 0.00 | 3.73 ± 0.30 | 2.69 ± 0.87 | 7.72 ± 1.03 |
| NEU, ×109/L | 11.39 ± 0.84 ** | 2.02 ± 0.91 | 1.70 ± 0.6 | 3.32 ± 0.72 |
| MON, ×109/L | 0.49 ± 0.01 | 0.45 ± 0.00 | 0.33 ± 0.00 | 0.03 ± 0.00 |
| RBC, ×1012/L | 6.92 ± 0.02 ** | 6.06 ± 0.06 | 3.59 ± 0.20 | 7.02 ± 0.23 |
| HGB, g/L | 122 ± 21.22 * | 125 ± 4.00 | 96 ± 2.67 | 147 ± 6.00 |
| HCT, % | 32.09 ± 3.25 * | 23.35 ± 0.70 | 20.75 ± 0.30 | 37.3 ± 0.27 |
| MCV, fL | 46 ± 4.54 * | 54.45 ± 0.43 | 41.8 ± 0.07 | 82.6 ± 0.23 |
| MCH, pg | 17.6 ± 4.24 | 12.75 ± 0.43 | 12.25 ± 0.30 | 18.4 ± 0.17 |
| MCHC, g/L | 379 ± 122.24 | 428 ± 9.33 | 363.6 ± 5.00 | 406 ± 4.00 |
| RDWC, % | 23.6 ± 2.88 | 25.35 ± 0.57 | 23 ± 0.40 | 23.6 ± 0.20 |
| PLT, ×109/L | 382 ± 45.87 | 521 ± 135.33 | 422 ± 41.33 | 690 ± 166.33 |
| PCT, % | 0.27 ± 0.01 | 0.2815 ± 0.07 | 0.23 ± 0.02 | 0.372 ± 0.08 |
| MPV, fL | 7.1 ± 0.02 | 6.1 ± 0.47 | 5.5 ± 0.13 | 7.4 ± 0.30 |
| PDWS, % | 10.1 ± 0.51 | 12.25 ± 0.57 | 11.25 ± 0.23 | 11.4 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauletova, M.D.; Umbetova, A.K.; Yelibayeva, N.S.; Burasheva, G.S.; Kabdraisova, A.Z.; Karzhaubekova, Z.Z.; Litvinenko, Y.A.; Assylkhanov, Z.S.; Korul’kin, D.Y. Biologically Active Compounds of Plants of the Atraphaxis Genus: Chemical Composition and Immunomodulatory Evaluation. Int. J. Mol. Sci. 2025, 26, 10301. https://doi.org/10.3390/ijms262110301
Dauletova MD, Umbetova AK, Yelibayeva NS, Burasheva GS, Kabdraisova AZ, Karzhaubekova ZZ, Litvinenko YA, Assylkhanov ZS, Korul’kin DY. Biologically Active Compounds of Plants of the Atraphaxis Genus: Chemical Composition and Immunomodulatory Evaluation. International Journal of Molecular Sciences. 2025; 26(21):10301. https://doi.org/10.3390/ijms262110301
Chicago/Turabian StyleDauletova, Meruyert D., Almagul K. Umbetova, Nazym S. Yelibayeva, Gauhar Sh. Burasheva, Aisulu Zh. Kabdraisova, Zhanat Zh. Karzhaubekova, Yuliya A. Litvinenko, Zhanibek S. Assylkhanov, and Dmitriy Yu. Korul’kin. 2025. "Biologically Active Compounds of Plants of the Atraphaxis Genus: Chemical Composition and Immunomodulatory Evaluation" International Journal of Molecular Sciences 26, no. 21: 10301. https://doi.org/10.3390/ijms262110301
APA StyleDauletova, M. D., Umbetova, A. K., Yelibayeva, N. S., Burasheva, G. S., Kabdraisova, A. Z., Karzhaubekova, Z. Z., Litvinenko, Y. A., Assylkhanov, Z. S., & Korul’kin, D. Y. (2025). Biologically Active Compounds of Plants of the Atraphaxis Genus: Chemical Composition and Immunomodulatory Evaluation. International Journal of Molecular Sciences, 26(21), 10301. https://doi.org/10.3390/ijms262110301






