Mitochondrial Function, Oxidative Stress, Inflammation and Thrombolytic Treatment in Ischemic Stroke
Abstract
1. Introduction
2. Results
2.1. Patients
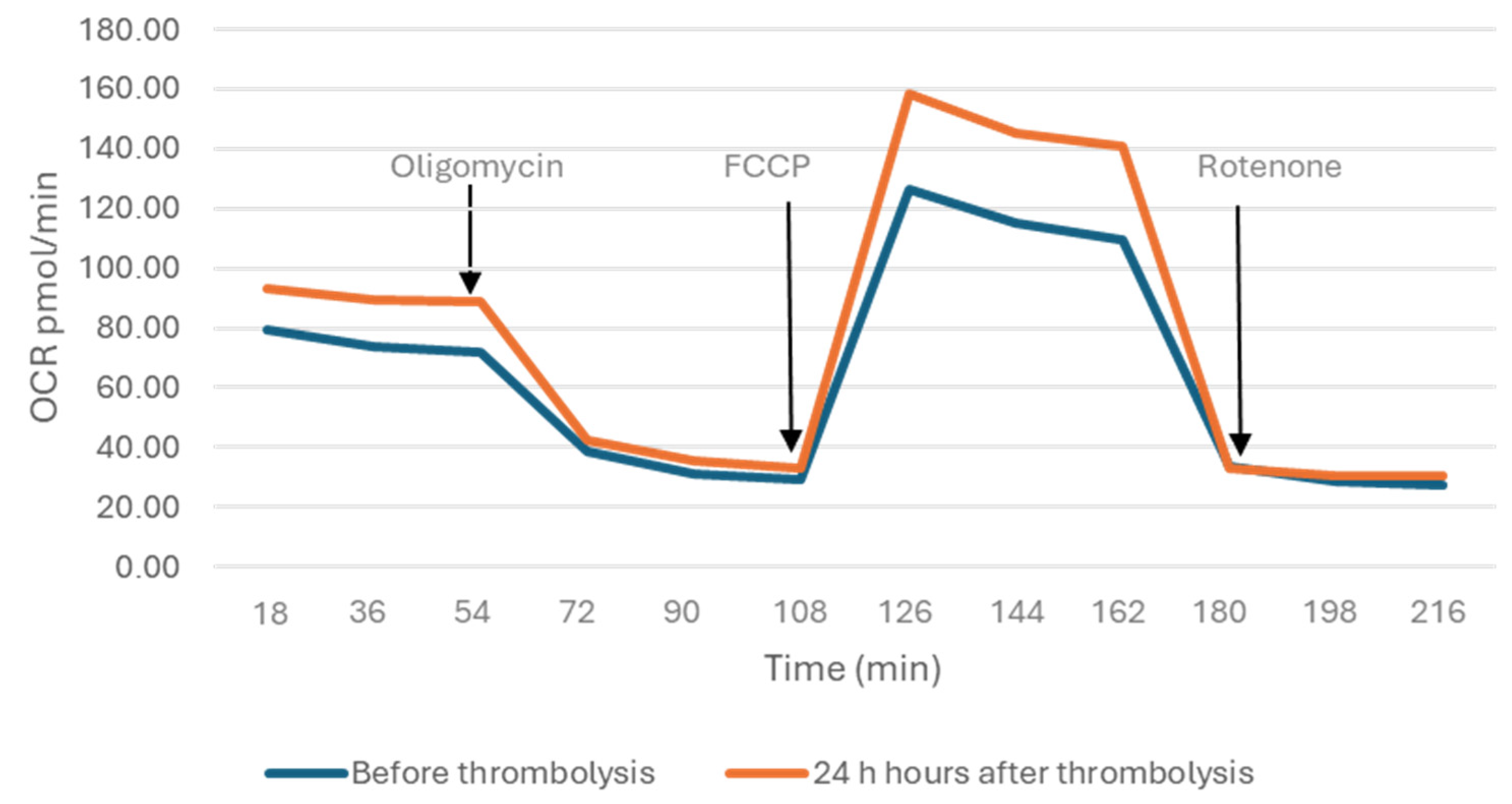
2.2. Mitochondrial Parameters
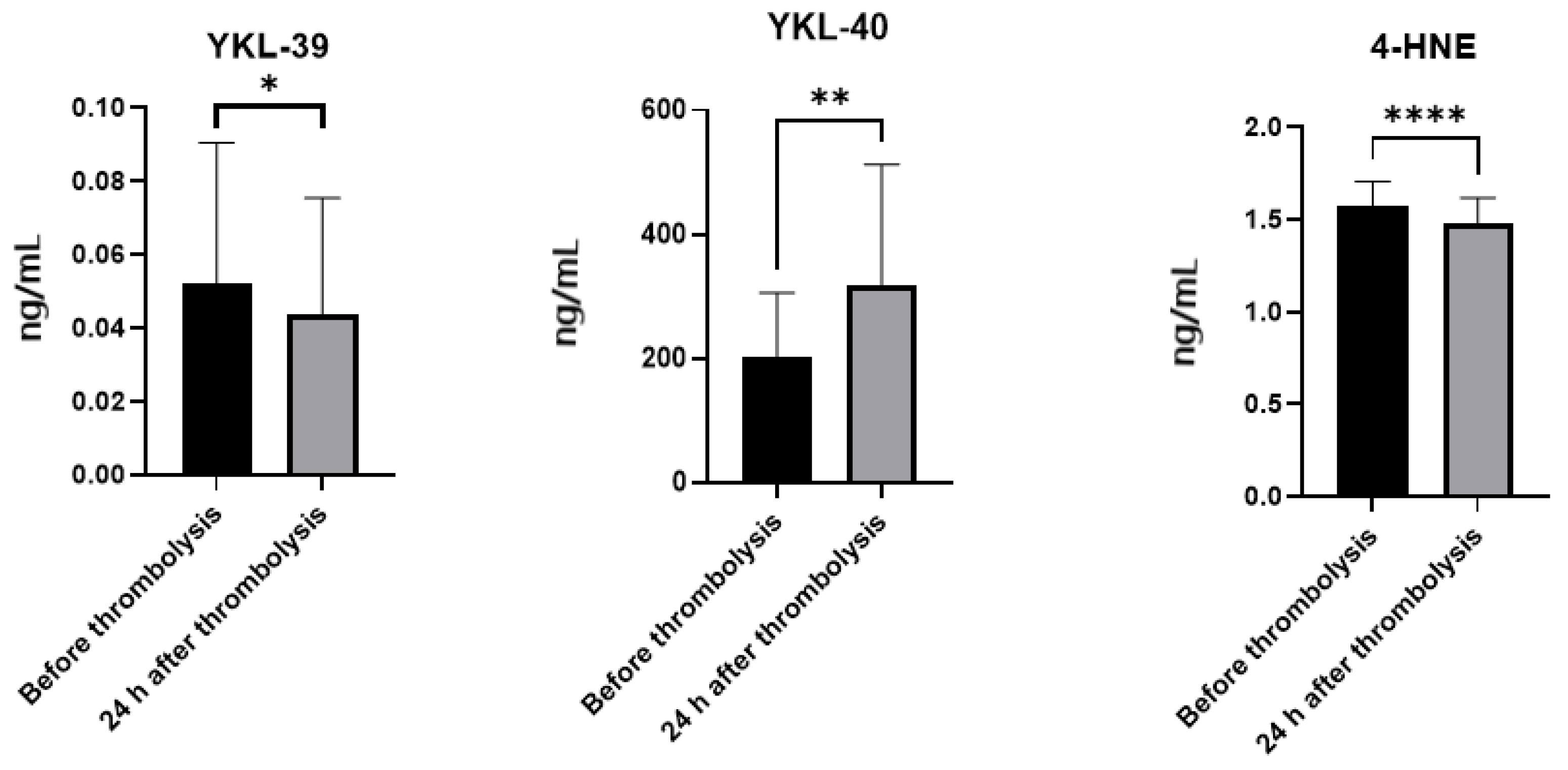
2.3. Plasma Levels of YKL-39, YKL-40 and 4-HNE
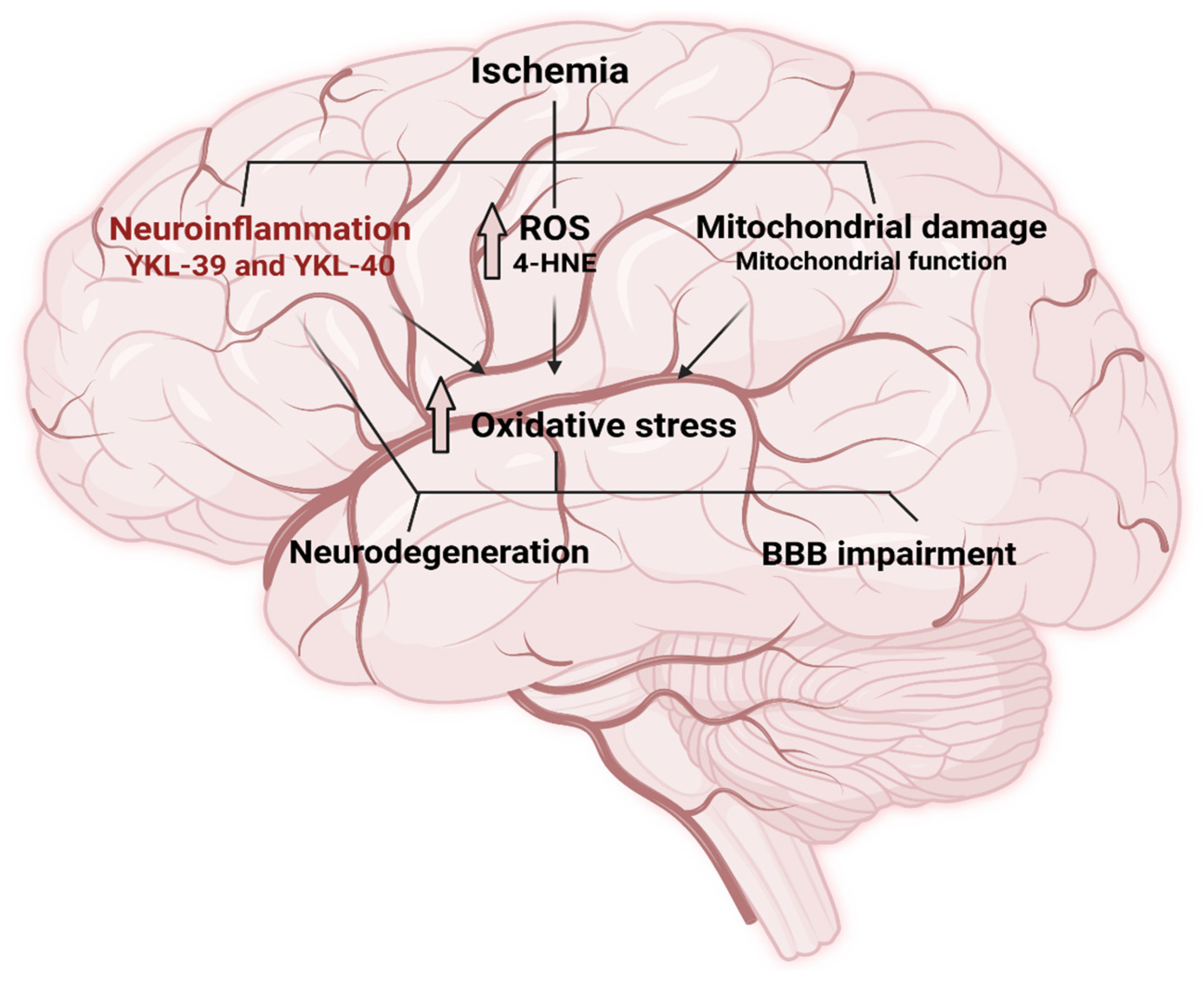
3. Discussion
3.1. Mitochondrial Spare Respiratory Capacity, Basal Respiration and Energetic Adaptation in Ischemic Stroke
3.2. Neuroinflammation and the Role of YKL-Proteins in Ischemic Stroke
3.3. 4-HNE as a Marker and Mediator of Oxidative Stress in Ischemic Stroke
4. Materials and Methods
4.1. Patients
4.2. Isolation of Plasma and Peripheral Blood Mononuclear Cells
4.3. Analysis of Bioenergetic Parameters of Mitochondria
4.4. Detection of YKL-39, YKL-40 and 4-HNE Plasma Levels by ELISA
4.5. Statistical Analyses
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IS | Ischemic stroke |
| ETC | Electron transport chain |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| CHI3L2 (YKL-39) | Chitinase-3-like protein 2 |
| CHI3L1 (YKL-40) | Chitinase-3-like protein 1 |
| 4-HNE | 4-hydroxy-2-nonenal |
| SRC | Spare respiratory capacity |
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
| NIHSS | National Institutes of Health Stroke Scale |
| GLCS | Glasgow-Liege Coma Scale |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| OCR | Oxygen consumption rate |
| FCCP | Carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone |
| ELISA | Enzyme-linked immunosorbent assay |
| SD | Standard deviation |
| TNF-α | Tumor necrosis factor-alpha |
| CT-scan | Computed tomography scan |
| AH | Arterial hypertension |
| FBS | Fetal bovine serum |
References
- Yan, F.; Tang, H.; Wang, L.; Huang, L.; Zhang, J. Editorial: Mitochondrial Dysfunction in Stroke. Front. Aging Neurosci. 2022, 14, 888952. [Google Scholar] [CrossRef]
- Posada-Duque, R.A.; Barreto, G.E.; Cardona-Gomez, G.P. Protection after stroke: Cellular effectors of neurovascular unit integrity. Front. Cell. Neurosci. 2014, 8, 231. [Google Scholar] [CrossRef]
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front. Physiol. 2020, 11, 605398. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Simel, D.L. Is this patient having a stroke? JAMA 2005, 293, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Berndt, N.; Papageorgiou, I.E.; Maurer, J.; Bulik, S.; Both, M.; Draguhn, A.; Holzhütter, H.G.; Kann, O. Local oxygen homeostasis during various neuronal network activity states in the mouse hippocampus. J. Cereb. Blood Flow Metab. 2019, 39, 859–873. [Google Scholar] [CrossRef]
- Rutkai, I.; Merdzo, I.; Wunnava, S.V.; Curtin, G.T.; Katakam, P.V.; Busija, D.W. Cerebrovascular function and mitochondrial bioenergetics after ischemia-reperfusion in male rats. J. Cereb. Blood Flow Metab. 2019, 39, 1056–1068. [Google Scholar] [CrossRef]
- Lee, M.J.; Jang, Y.; Han, J.; Kim, S.J.; Ju, X.; Lee, Y.L.; Cui, J.; Zhu, J.; Ryu, M.J.; Choi, S.Y.; et al. Endothelial-specific Crif1 deletion induces BBB maturation and disruption via the alteration of actin dynamics by impaired mitochondrial respiration. J. Cereb. Blood Flow Metab. 2020, 40, 1546–1561, Erratum in J. Cereb. Blood Flow Metab. 2020, 40, 1562. [Google Scholar] [CrossRef]
- Shu, L.; Chen, B.; Chen, B.; Xu, H.; Wang, G.; Huang, Y.; Zhao, Y.; Gong, H.; Jiang, M.; Chen, L.; et al. Brain ischemic insult induces cofilin rod formation leading to synaptic dysfunction in neurons. J. Cereb. Blood Flow Metab. 2019, 39, 2181–2195. [Google Scholar] [CrossRef]
- Kuroda, S.; Katsura, K.I.; Tsuchidate, R.; Siesjö, B.K. Secondary bioenergetic failure after transient focal ischaemia is due to mitochondrial injury. Acta Physiol. Scand. 1996, 156, 149–150. [Google Scholar] [CrossRef]
- Anderson, M.F.; Sims, N.R. Mitochondrial respiratory function and cell death in focal cerebral ischemia. J. Neurochem. 1999, 73, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Shaafi, S.; Hadisi, F.; Mahmoudinezhad, M.; Razmi, H.; Nejadghaderi, S.A.; Khalili, M. The significance of the oxidative stress markers in the one-year prognosis of patients with acute ischemic stroke: A case-control study. BMC Neurol. 2021, 21, 258. [Google Scholar] [CrossRef]
- Syafrita, Y.; Amir, D.; Susanti, R.; Fadhilah, I. Relationship of brain-derived neurotrophic factor, malondialdehyde, and 8-Hydroxy 2-Deoxyguanosine with post-ischemic stroke depression. Dement. Neuropsychol. 2020, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, Y.; He, J. High serum levels of 8-OHdG are an independent predictor of post-stroke depression in Chinese stroke survivors. Neuropsychiatr. Dis. Treat. 2018, 14, 587–596. [Google Scholar] [CrossRef]
- Nakajima, H.; Unoda, K.; Ito, T.; Kitaoka, H.; Kimura, F.; Hanafusa, T. The Relation of Urinary 8-OHdG, A Marker of Oxidative Stress to DNA, and Clinical Outcomes for Ischemic Stroke. Open Neurol. J. 2012, 6, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Niizuma, K.; Yoshioka, H.; Chen, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Okami, N.; Chan, P.H. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta 2010, 1802, 92–99. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, H.Y.; Yang, D.I.; Lee, S.Y.; Shaw, F.Z.; Lin, T.K.; Liou, C.W.; Chuang, Y.C. Effects of rosiglitazone on global ischemia-induced hippocampal injury and expression of mitochondrial uncoupling protein 2. Biochem. Biophys. Res. Commun. 2006, 351, 198–203. [Google Scholar] [CrossRef]
- Bayir, H.; Kagan, V.E. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis--there is nothing more practical than a good theory. Crit. Care 2008, 12, 206. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Kunz, A.; Park, L.; Abe, T.; Gallo, E.F.; Anrather, J.; Zhou, P.; Iadecola, C. Neurovascular protection by ischemic tolerance: Role of nitric oxide and reactive oxygen species. J. Neurosci. 2007, 27, 7083–7093. [Google Scholar] [CrossRef]
- Mo, Y.; Sun, Y.Y.; Liu, K.Y. Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 2020, 15, 1388–1396. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Renkema, G.H.; Boot, R.G.; Au, F.L.; Donker-Koopman, W.E.; Strijland, A.; Muijsers, A.O.; Hrebicek, M.; Aerts, J.M. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998, 251, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Brasso, K.; Christensen, I.J.; Johansen, J.S.; Teisner, B.; Garnero, P.; Price, P.A.; Iversen, P. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate 2006, 66, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Shao, R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front. Physiol. 2013, 4, 122. [Google Scholar] [CrossRef]
- Hall, S.; Janelidze, S.; Surova, Y.; Widner, H.; Zetterberg, H.; Hansson, O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci. Rep. 2018, 8, 13276. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Bissel, S.J.; Kofler, J.; Starkey, A.; Wang, G.; Wiley, C.A. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012, 22, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Liu, G.; Zhang, Y.; Zhang, J.; Wang, A.; Yu, M.; Zhang, R.; Lin, J.; Liang, X.; Liu, L.; et al. Independent Predictive Value of Elevated YKL-40 in Ischemic Stroke Prognosis: Findings from a Nationwide Stroke Registry. Cerebrovasc. Dis. 2023, 52, 460–470. [Google Scholar] [CrossRef]
- Hu, B.; Trinh, K.; Figueira, W.F.; Price, P.A. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J. Biol. Chem. 1996, 271, 19415–19420. [Google Scholar] [CrossRef]
- Yeo, I.J.; Lee, C.K.; Han, S.B.; Yun, J.; Hong, J.T. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol. Ther. 2019, 203, 107394. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Longo, A.; Lazzara, F.; Cambria, D.; Distefano, G.; Palumbo, M.; Cantarella, A.; Malaguarnera, L.; Di Rosa, M. CHI3L1 and CHI3L2 overexpression in motor cortex and spinal cord of sALS patients. Mol. Cell. Neurosci. 2017, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Møllgaard, M.; Degn, M.; Sellebjerg, F.; Frederiksen, J.L.; Modvig, S. Cerebrospinal fluid chitinase-3-like 2 and chitotriosidase are potential prognostic biomarkers in early multiple sclerosis. Eur. J. Neurol. 2016, 23, 898–905. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Malaguarnera, L.; Di Rosa, M. Chitinase expression in Alzheimer’s disease and non-demented brains regions. J. Neurol. Sci. 2016, 369, 242–249. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Rašić, I.; Rašić, A.; Akšamija, G.; Radović, S. The Relationship Between Serum Level of Malondialdehyde and Progression of Colorectal Cancer. Acta Clin. Croat. 2018, 57, 411–416. [Google Scholar] [CrossRef]
- Shrivastava, A.; Mishra, S.P.; Pradhan, S.; Choudhary, S.; Singla, S.; Zahra, K.; Aggarwal, L.M. An assessment of serum oxidative stress and antioxidant parameters in patients undergoing treatment for cervical cancer. Free Radic. Biol. Med. 2021, 167, 29–35. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, B.; Yadav, S.K.; Mahdi, A.A. Novel biomarker for neurodegenerative diseases- motor neuron disease (MND), cerebellar ataxia (CA) and Parkinson’s disease (PD). Clin. Chim. Acta 2018, 485, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Yoo, H.J.; Sun, Y.; Lee, S.H.; Lee, J.H. PPARD rs7770619 polymorphism in a Korean population: Association with plasma malondialdehyde and impaired fasting glucose or newly diagnosed type 2 diabetes. Diabetes Vasc. Dis. Res. 2018, 15, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Kato, Y.; Inose, Y.; Hiroi, A.; Yamamoto, T.; Morikawa, S.; Sawada, M.; Kobayashi, M. 4-Hydroxy-2-nonenal upregulates and phosphorylates cytosolic phospholipase A2 in cultured Ra2 microglial cells via MAPK pathways. Neuropathology 2011, 31, 122–128. [Google Scholar] [CrossRef]
- Di Domenico, F.; Tramutola, A.; Butterfield, D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017, 111, 253–261. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Villamil Giraldo, A.M.; Öllinger, K.; Hallbeck, M.; Civitelli, L. Lipid vesicles affect the aggregation of 4-hydroxy-2-nonenal-modified α-synuclein oligomers. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Ciniglio Appiani, M.; de Vincentiis, M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.J., Jr.; Probstel, A.K.; Zamvil, S.S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019, 20, 728–745, Erratum in Nat. Rev. Neurosci. 2019, 21, 56. [Google Scholar] [CrossRef]
- Nicholls, D.G. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem. Soc. Trans. 2009, 37, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R., Jr.; Ballinger, S.; Dell’Italia, L.; Jianhua, Z.; Darley-Usmar, V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef]
- James, A.D.; Patel, W.; Butt, Z.; Adiamah, M.; Dakhel, R.; Latif, A.; Uggenti, C.; Swanton, E.; Imamura, H.; Siriwardena, A.K.; et al. The Plasma Membrane Calcium Pump in Pancreatic Cancer Cells Exhibiting the Warburg Effect Relies on Glycolytic ATP. J. Biol. Chem. 2015, 290, 24760–24771. [Google Scholar] [CrossRef]
- Gevezova, M.; Ivanov, Z.; Pacheva, I.; Timova, E.; Kazakova, M.; Kovacheva, E.; Ivanov, I.; Sarafian, V. Bioenergetic and Inflammatory Alterations in Regressed and Non-Regressed Patients with Autism Spectrum Disorder. Int. J. Mol. Sci. 2024, 25, 8211. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Morino, K.; Mengistu, L.; Ishibashi, T.; Kiriyama, K.; Ikami, T.; Maegawa, H. Amla Enhances Mitochondrial Spare Respiratory Capacity by Increasing Mitochondrial Biogenesis and Antioxidant Systems in a Murine Skeletal Muscle Cell Line. Oxid. Med. Cell. Longev. 2016, 2016, 1735841. [Google Scholar] [CrossRef]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Gu, X.; Ma, Y.; Liu, Y.; Wan, Q. Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protoc. 2020, 2, 100245. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef]
- Chiba, T.; Umegaki, K. Pivotal roles of monocytes/macrophages in stroke. Mediators Inflamm. 2013, 2013, 759103. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Nilupul Perera, M.; Ma, H.K.; Arakawa, S.; Howells, D.W.; Markus, R.; Rowe, C.C.; Donnan, G.A. Inflammation following stroke. J. Clin. Neurosci. 2006, 13, 1–8. [Google Scholar] [CrossRef]
- Dong, X.; Nao, J.; Gao, Y. Peripheral Monocyte Count Predicts Outcomes in Patients with Acute Ischemic Stroke Treated with rtPA Thrombolysis. Randomized Control. Trial 2020, 37, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Larionova, I.; Liu, T. YKL-39 as a Potential New Target for Anti-Angiogenic Therapy in Cancer. Front. Immunol. 2019, 10, 2930. [Google Scholar] [CrossRef] [PubMed]
- Angajala, A.; Lim, S.; Phillips, J.B.; Kim, J.H.; Yates, C.; You, Z.; Tan, M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.; et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.H.; Lambertsen, K.L.; Babcock, A.A.; Holm, T.H.; Dagnaes-Hansen, F.; Finsen, B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflammation 2008, 5, 46. [Google Scholar] [CrossRef]
- Thomsen, S.B.; Rathcke, C.N.; Skaaby, T.; Linneberg, A.; Vestergaard, H. The Association between genetic variations of CHI3L1, levels of the encoded glycoprotein YKL-40 and the lipid profile in a Danish population. PLoS ONE 2012, 7, 47094. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jun, C.D.; Jeon, S.J.; Choi, S.S.; Kim, H.R.; Choi, D.B.; Kwak, S.; Lee, H.S.; Cheong, J.S.; So, H.S.; et al. Serum YKL-40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS ONE 2012, 7, 51722. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Amantea, D.; Nappi, G.; Bernardi, G.; Bagetta, G.; Corasaniti, M.T. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2009, 276, 13–26. [Google Scholar] [CrossRef]
- Kriz, J. Inflammation in ischemic brain injury: Timing is important. Crit. Rev. Neurobiol. 2006, 18, 145–157. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Florang, V.R.; Rees, J.N.; Anderson, D.G.; Strack, S.; Doorn, J.A. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem. Res. Toxicol. 2009, 22, 835–841. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef]
- Shinmura, K.; Bolli, R.; Liu, S.Q.; Tang, X.L.; Kodani, E.; Xuan, Y.T.; Srivastava, S.; Bhatnagar, A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ. Res. 2002, 91, 240–246. [Google Scholar] [CrossRef]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic. Biol. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Galam, L.; Failla, A.; Soundararajan, R.; Lockey, R.F.; Kolliputi, N. 4-hydroxynonenal regulates mitochondrial function in human small airway epithelial cells. Oncotarget 2015, 6, 41508–41521. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Hood, D.A. Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am. J. Physiol. Cell Physiol. 2014, 306, 1176–1183. [Google Scholar] [CrossRef]
- Mali, V.R.; Ning, R.; Chen, J.; Yang, X.P.; Xu, J.; Palaniyandi, S.S. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp. Biol. Med. 2014, 239, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Rodriguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef]

| Characteristic | Patients (n = 16) |
|---|---|
| Demographic | |
| Age (mean ± SD) | 70 ± 9.16 |
| Male sex, n (%) | 12 (75%) |
| Female sex, n (%) | 4 (25%) |
| Risk factors | |
| Hypertension (AH), n (%) | 16 (100%) |
| Diabetes mellitus, n (%) | 10 (62.5%) |
| Hyperlipidemia, n (%) | 15 (93.75%) |
| Obesity, n (%) | 6 (37.5%) |
| Prior stroke, n (%) | 3 (18.75%) |
| Clinical presentation | |
| NIHSS score at admission (mean ± SD) | 7.69 ± 3.50 |
| GLCS score at admission (mean ± SD) | 19.50 ± 1.55 |
| Glucose, mmol/L (mean ± SD) | 7.85 ± 3.19 |
| Total cholesterol, mmol/L (mean ± SD) | 6.03 ± 1.23 |
| Triglycerides, mmol/L (mean ± SD) | 1.75 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacheva, E.; Gevezova, M.; Koeva, M.; Mihaylova, V.; Kormova, V.; Kostadinova, E.; Kostadinova, Y.; Kazakova, M.; Sarafian, V. Mitochondrial Function, Oxidative Stress, Inflammation and Thrombolytic Treatment in Ischemic Stroke. Int. J. Mol. Sci. 2025, 26, 10289. https://doi.org/10.3390/ijms262110289
Kovacheva E, Gevezova M, Koeva M, Mihaylova V, Kormova V, Kostadinova E, Kostadinova Y, Kazakova M, Sarafian V. Mitochondrial Function, Oxidative Stress, Inflammation and Thrombolytic Treatment in Ischemic Stroke. International Journal of Molecular Sciences. 2025; 26(21):10289. https://doi.org/10.3390/ijms262110289
Chicago/Turabian StyleKovacheva, Eleonora, Maria Gevezova, Margarita Koeva, Valentina Mihaylova, Vasilka Kormova, Emanuela Kostadinova, Yulia Kostadinova, Maria Kazakova, and Victoria Sarafian. 2025. "Mitochondrial Function, Oxidative Stress, Inflammation and Thrombolytic Treatment in Ischemic Stroke" International Journal of Molecular Sciences 26, no. 21: 10289. https://doi.org/10.3390/ijms262110289
APA StyleKovacheva, E., Gevezova, M., Koeva, M., Mihaylova, V., Kormova, V., Kostadinova, E., Kostadinova, Y., Kazakova, M., & Sarafian, V. (2025). Mitochondrial Function, Oxidative Stress, Inflammation and Thrombolytic Treatment in Ischemic Stroke. International Journal of Molecular Sciences, 26(21), 10289. https://doi.org/10.3390/ijms262110289






