Neuroprotection by Flaxseed Oil in a Model of Hippocampal Injury Induced by Trimethyltin Involves Purinergic System Modulation
Abstract
1. Introduction
2. Results
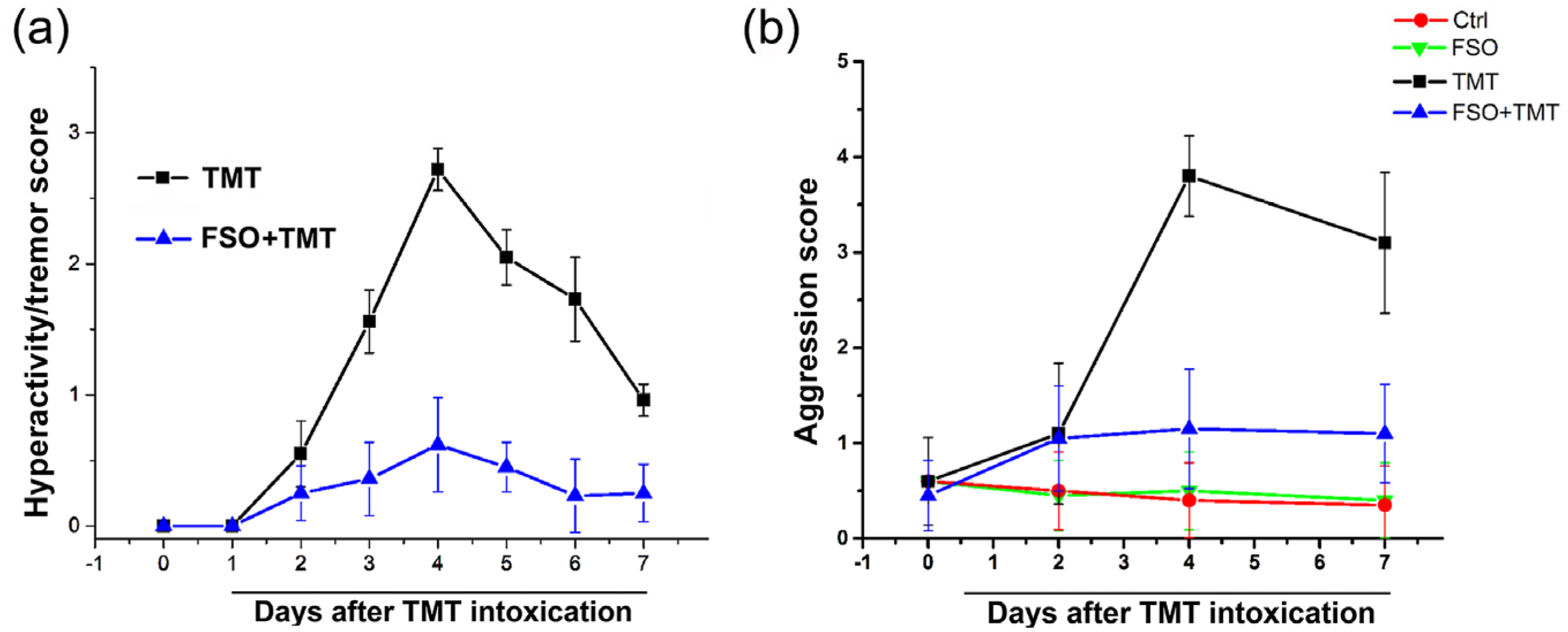
2.1. FSO Pretreatment Alleviated TMT-Induced Behavior Symptoms
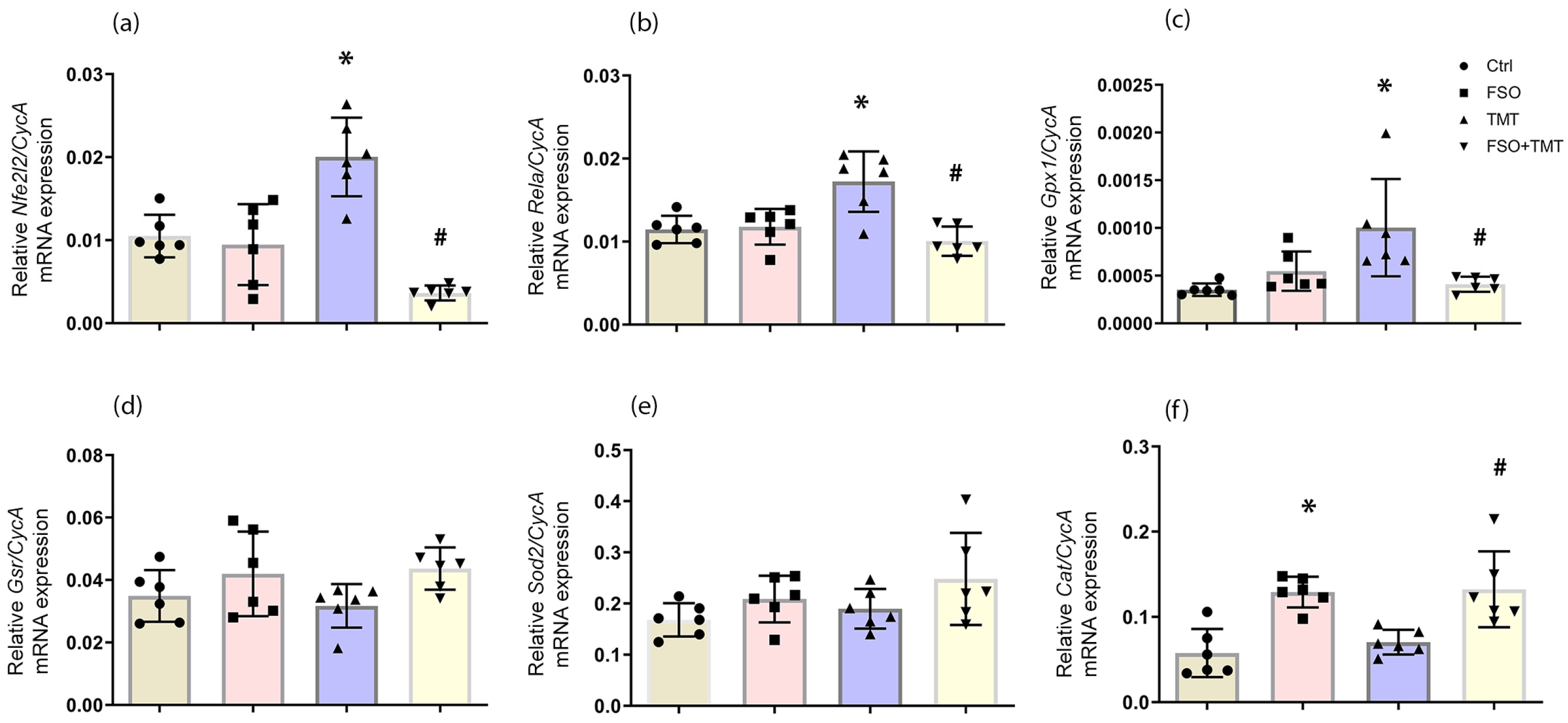
2.2. Effect of FSO on Antioxidant Genes Expression in the Hippocampus
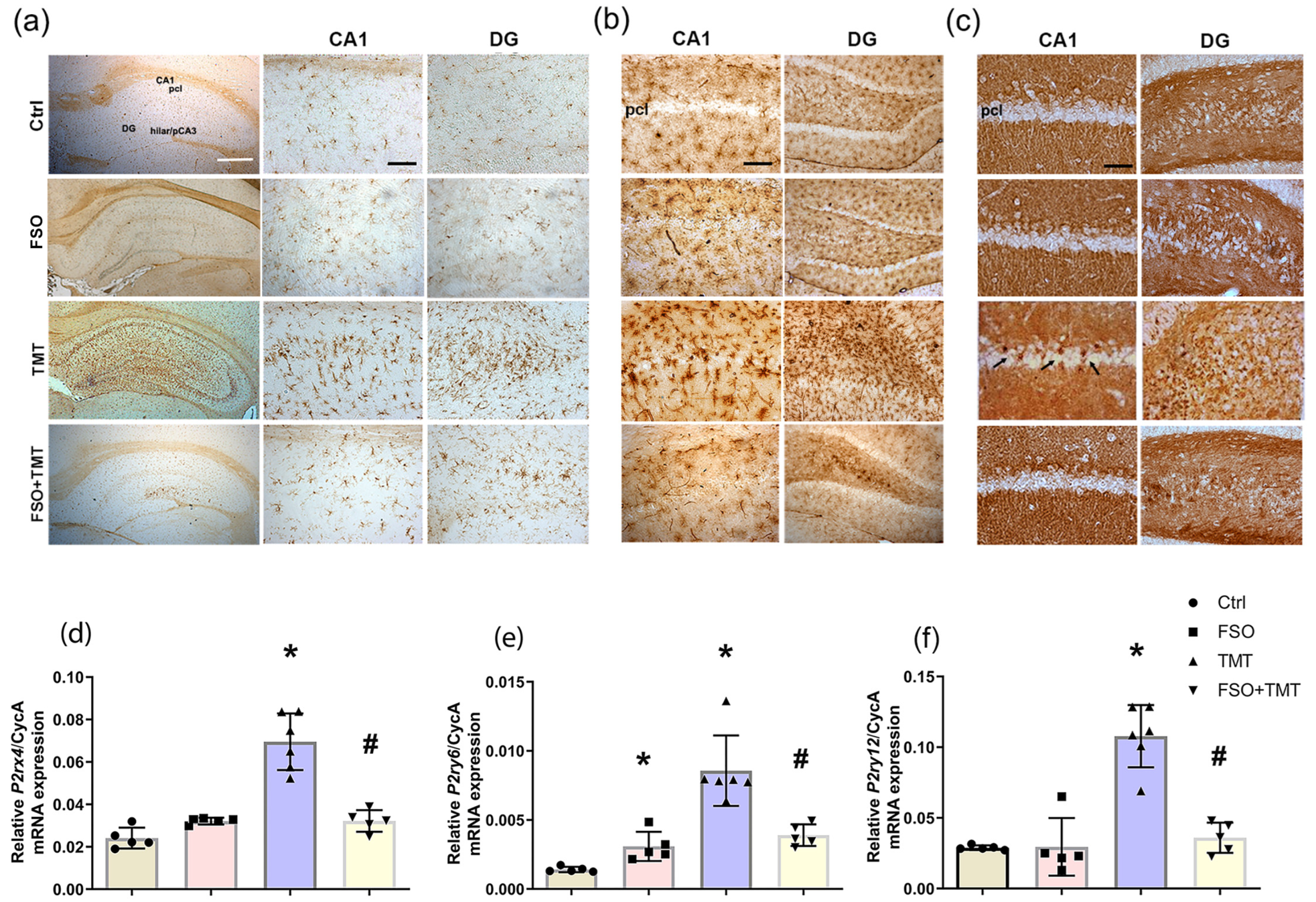
2.3. FSO Pretreatment Modulates NTPDase1/eN and Expression of Purinergic Receptors Involved in TMT-Induced Microgliosis
2.4. FSO Modulates Activity and Expressions of NTPDase1 and eN
2.5. FSO Modulates Expression of Adenosine Receptors
2.6. FSO Modulates Expression of ADA and ENT1
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatment
4.3. Hyperactivity/Tremor and Aggression Assessment
4.4. Tissue Processing for Immunohistochemistry and Enzyme Histochemistry
4.5. Gene Expression Analysis by RT-qPCR
4.6. Preparation of Membrane Fraction
4.7. SDS-PAGE and Immunoblotting
4.8. Ectonucleotidase Assays
4.9. Adenosine Deaminase (ADA) Assay
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| ATP | adenosine triphosphate |
| AMP | adenosine monophosphate |
| Cat | catalase |
| eN | ecto-5’-nucleotidase |
| ENT1s | equilibrative nucleoside transporter 1 |
| FSO | flaxseed oil |
| Gpx1 | glutathione peroxidase 1 |
| Gsr | glutathione reductase |
| n-3 PUFA | omega-3 polyunsaturated fatty acids |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | nerve growth factor |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NTPDase1 | ecto-nucleoside triphosphate diphosphohydrolase 1 |
| Sod2 | superoxide dismutase 2 |
| TMT | trimethyltin |
References
- Imran, S.; Munir, S.; Altemimi, A.B.; Fatima, I.; Rabail, R.; Batool, I.; Khalid, N.; Abdi, G.; Shabbir, M.A.; Aadil, R.M. Therapeutic Implications of Flaxseed Peptides and Bioactive Components against Various Diseases. J. Funct. Foods 2024, 119, 106324. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A Comprehensive Review of the Health Benefits of Flaxseed Oil in Relation to Its Chemical Composition and Comparison with Other Omega-3-Rich Oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef] [PubMed]
- Jangale, N.M.; Devarshi, P.P.; Dubal, A.A.; Ghule, A.E.; Koppikar, S.J.; Bodhankar, S.L.; Chougale, A.D.; Kulkarni, M.J.; Harsulkar, A.M. Dietary Flaxseed Oil and Fish Oil Modulates Expression of Antioxidant and Inflammatory Genes with Alleviation of Protein Glycation Status and Inflammation in Liver of Streptozotocin-Nicotinamide Induced Diabetic Rats. Food Chem. 2013, 141, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zao, F.; Fu, J. Cold-Pressed Flaxseed Oil Alleviates H2O2-Induced Oxidative Stress in HepG2 Cells by Regulating the Expression of the Genes Related to MTOR and MAPK Signaling Pathway. Agric. Prod. Process. Storage 2025, 1, 9. [Google Scholar] [CrossRef]
- Poorbaferani, F.; Rouhani, M.H.; Heidari, Z.; Poorbaferani, M.; Safavi, S.M. Flaxseed Oil Supplementation on Severity of Depression and Brain-Derived Neurotrophic Factor: A Randomized, Double Blind Placebo Controlled Clinical Trial. Int. J. Food Prop. 2020, 23, 1518–1526. [Google Scholar] [CrossRef]
- Abdel Moneim, A.E. Flaxseed Oil as a Neuroprotective Agent on Lead Acetate-Induced Monoamineric Alterations and Neurotoxicity in Rats. Biol. Trace Elem. Res. 2012, 148, 363–370. [Google Scholar] [CrossRef]
- Lounis, W.; Kessas, K.; Chouari, Z.; Benyettou, I.; Boumechhour, A.; Chaib, F.; Aoul, S.H.; Lizard, G.; Kharoubi, O. Evaluating the Role of Flaxseed Oil in Improving Neurological Impairments in Rat Pups Intoxicated by Aluminum. J. Biol. Regul. Homeost. Agents 2023, 37, 4345–4359. [Google Scholar] [CrossRef]
- Bagheri, A.; Talei, S.; Hassanzadeh, N.; Mokhtari, T.; Akbari, M.; Malek, F.; Jameie, S.B.; Sadeghi, Y.; Hassanzadeh, G. The Neuroprotective Effects of Flaxseed Oil Supplementation on Functional Motor Recovery in a Model of Ischemic Brain Stroke: Upregulation of BDNF and GDNF. Acta Med. Iran. 2017, 55, 785–792. [Google Scholar]
- Uauy, R.; Dangour, A.D. Nutrition in Brain Development and Aging: Role of Essential Fatty Acids. Nutr. Rev. 2006, 64, S24–S33. [Google Scholar] [CrossRef]
- Itokazu, N.; Ikegaya, Y.; Nishikawa, M.; Matsuki, N. Bidirectional Actions of Docosahexaenoic Acid on Hippocampal Neurotransmissions in Vivo. Brain Res. 2000, 862, 211–216. [Google Scholar] [CrossRef]
- Wen, J.; Satyanarayanan, S.K.; Li, A.; Yan, L.; Zhao, Z.; Yuan, Q.; Su, K.P.; Su, H. Unraveling the Impact of Omega-3 Polyunsaturated Fatty Acids on Blood-Brain Barrier (BBB) Integrity and Glymphatic Function. Brain. Behav. Immun. 2024, 115, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [PubMed]
- Musazadeh, V.; Jafarzadeh, J.; Keramati, M.; Zarezadeh, M.; Ahmadi, M.; Farrokhian, Z.; Ostadrahimi, A. Flaxseed Oil Supplementation Augments Antioxidant Capacity and Alleviates Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. 2021, 2021, 4438613. [Google Scholar] [CrossRef]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef]
- du Bois, T.M.; Deng, C.; Bell, W.; Huang, X.F. Fatty Acids Differentially Affect Serotonin Receptor and Transporter Binding in the Rat Brain. Neuroscience 2006, 139, 1397–1403. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Vultaggio-Poma, V.; Falzoni, S.; Giuliani, A.L. Extracellular ATP: A Powerful Inflammatory Mediator in the Central Nervous System. Neuropharmacology 2023, 224, 109333. [Google Scholar] [CrossRef]
- Erb, L.; Woods, L.T.; Khalafalla, M.G.; Weisman, G.A. Purinergic Signaling in Alzheimer’s Disease. Brain Res. Bull. 2019, 151, 25–37. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular Function and Molecular Structure of Ecto-Nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Sakai, K.; Koizumi, S. Extracellular ATP/Adenosine Dynamics in the Brain and Its Role in Health and Disease. Front. Cell Dev. Biol. 2024, 11, 1343653. [Google Scholar] [CrossRef] [PubMed]
- Weltha, L.; Reemmer, J.; Boison, D. The Role of Adenosine in Epilepsy. Brain Res. Bull. 2019, 151, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Wu, K.C.; Lin, C.Y.; Chern, Y. Emerging Roles of Dysregulated Adenosine Homeostasis in Brain Disorders with a Specific Focus on Neurodegenerative Diseases. J. Biomed. Sci. 2021, 28, 70. [Google Scholar] [CrossRef]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P.; Vizi, E.S.; Illes, P. Adenosine Receptor Signaling in the Brain Immune System. Trends Pharmacol. Sci. 2005, 26, 511–516. [Google Scholar] [CrossRef]
- Jang, M.H.; Song, J. Adenosine and Adenosine Receptors in Metabolic Imbalance-Related Neurological Issues. Biomed. Pharmacother. 2024, 177, 116996. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Camici, M.; Allegrini, S.; Pesi, R.; Tozzi, M.G. Metabolic Aspects of Adenosine Functions in the Brain. Front. Pharmacol. 2021, 12, 672182. [Google Scholar] [CrossRef]
- Chang, C.P.; Chang, Y.G.; Chuang, P.Y.; Nguyen, T.N.A.; Wu, K.C.; Chou, F.Y.; Cheng, S.J.; Chen, H.M.; Jin, L.W.; Carvalho, K.; et al. Equilibrative Nucleoside Transporter 1 Inhibition Rescues Energy Dysfunction and Pathology in a Model of Tauopathy. Acta Neuropathol. Commun. 2021, 9, 112. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.M.; Chu, S.F.; Peng, Y.; Chen, N.H. Research Progress on Adenosine in Central Nervous System Diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef]
- Ye, M.; Han, B.H.; Kim, J.S.; Kim, K.; Shim, I. Neuroprotective Effect of Bean Phosphatidylserine on TMT-Induced Memory Deficits in a Rat Model. Int. J. Mol. Sci. 2020, 21, 4901. [Google Scholar] [CrossRef] [PubMed]
- Chvojkova, M.; Kubova, H.; Vales, K. Effects of Dizocilpine, Midazolam and Their Co-Application on the Trimethyltin (TMT)-Induced Rat Model of Cognitive Deficit. Brain Sci. 2021, 11, 400. [Google Scholar] [CrossRef]
- Ye, M.; Rhie, S.J.; Jeong, W.; Yu, H.J.; Kim, Y.; Kim, J.; Shim, I. Anti-Inflammatory Effect of Grounding Mat on Trimethyltin-Induced Neurotoxicity Rats. J. Exerc. Rehabil. 2025, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Kang, S.; Kim, J.; Kim, J.C.; Jung, C.; Shin, T.; Kim, S.H.; Moon, C. Trimethyltin-Induced Hippocampal Neurodegeneration: A Mechanism-Based Review. Brain Res. Bull. 2016, 125, 187–199. [Google Scholar] [CrossRef]
- Trabucco, A.; Di Pietro, P.; Nori, S.L.; Fulceri, F.; Fumagalli, L.; Paparelli, A.; Fornai, F. Methylated Tin Toxicity a Reappraisal Using Rodents Models. Arch. Ital. Biol. 2009, 147, 141–153. [Google Scholar] [PubMed]
- Geloso, M.C.; Corvino, V.; Michetti, F. Trimethyltin-Induced Hippocampal Degeneration as a Tool to Investigate Neurodegenerative Processes. Neurochem. Int. 2011, 58, 729–738. [Google Scholar] [CrossRef]
- Corvino, V.; Marchese, E.; Michetti, F.; Geloso, M.C. Neuroprotective Strategies in Hippocampal Neurodegeneration Induced by the Neurotoxicant Trimethyltin. Neurochem. Res. 2013, 38, 240–253. [Google Scholar] [CrossRef]
- Little, A.R.; Miller, D.B.; Li, S.; Kashon, M.L.; O’Callaghan, J.P. Trimethyltin-Induced Neurotoxicity: Gene Expression Pathway Analysis, q-RT-PCR and Immunoblotting Reveal Early Effects Associated with Hippocampal Damage and Gliosis. Neurotoxicol. Teratol. 2012, 34, 72–82. [Google Scholar] [CrossRef]
- Mitrović, N.; Adžić Bukvić, M.; Zarić Kontić, M.; Dragić, M.; Petrović, S.; Paunović, M.; Vučić, V.; Grković, I. Flaxseed Oil Alleviates Trimethyltin-Induced Cell Injury and Inhibits the Pro-Inflammatory Activation of Astrocytes in the Hippocampus of Female Rats. Cells 2024, 13, 1184. [Google Scholar] [CrossRef]
- Pershina, E.V.; Chernomorets, I.Y.; Fedorov, D.A.; Arkhipov, V.I. Pharmacological Modulation of Excitotoxicity through the Combined Use of NMDA Receptor Inhibition and Group III MGlu Activation Reduces TMT-Induced Neurodegeneration in the Rat Hippocampus. Int. J. Mol. Sci. 2023, 24, 8249. [Google Scholar] [CrossRef]
- Kaur, S.; Nehru, B. Alteration in Glutathione Homeostasis and Oxidative Stress during the Sequelae of Trimethyltin Syndrome in Rat Brain. Biol. Trace Elem. Res. 2013, 153, 299–308. [Google Scholar] [CrossRef]
- Dragić, M.; Mitrović, N.; Adžić, M.; Nedeljković, N.; Grković, I. Microglial- and Astrocyte-Specific Expression of Purinergic Signaling Components and Inflammatory Mediators in the Rat Hippocampus During Trimethyltin-Induced Neurodegeneration. ASN Neuro 2021, 13, 17590914211044882. [Google Scholar] [CrossRef]
- Dragić, M.; Zarić, M.; Mitrović, N.; Nedeljković, N.; Grković, I. Two Distinct Hippocampal Astrocyte Morphotypes Reveal Subfield-Different Fate during Neurodegeneration Induced by Trimethyltin Intoxication. Neuroscience 2019, 423, 38–54. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Cho, S.G.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Wogonin Attenuates Hippocampal Neuronal Loss and Cognitive Dysfunction in Trimethyltin-Intoxicated Rats. Biomol. Ther. 2016, 24, 328. [Google Scholar] [CrossRef] [PubMed]
- Grković, I.; Mitrović, N.; Dragić, M.; Kontić, M.Z. Enzyme Histochemistry: A Useful Tool for Examining the Spatial Distribution of Brain Ectonucleotidases in (Patho)Physiological Conditions. Histol. Histopathol. 2022, 37, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.X.; Ying, R.F.; Lv, B.F.; Yang, L.H.; Xu, Z.; Yan, L.Q.; Bu, J.Z.; Wei, Y.S. Flaxseed Oil: Extraction, Health Benefits and Products. Qual. Assur. Saf. Crop. Foods 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Taheri, M.; Roghani, M.; Sedaghat, R. Metformin Mitigates Trimethyltin-Induced Cognition Impairment and Hippocampal Neurodegeneration. Cell. Mol. Neurobiol. 2024, 44, 70. [Google Scholar] [CrossRef]
- Catino, S.; Paciello, F.; Miceli, F.; Rolesi, R.; Troiani, D.; Calabrese, V.; Santangelo, R.; Mancuso, C. Ferulic Acid Regulates the Nrf2/Heme Oxygenase-1 System and Counteracts Trimethyltin-Induced Neuronal Damage in the Human Neuroblastoma Cell Line SH-SY5Y. Front. Pharmacol. 2016, 6, 305. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-ΚB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef]
- Mercado-Gómez, O.F.; Arriaga-Ávila, V.S.; Vega-García, A.; Orozco-Suarez, S.; Pérez-Koldenkova, V.; Camarillo-Sánchez, J.J.; Álvarez-Herrera, M.; Guevara-Guzmán, R. Daytime-Restricted Feeding Ameliorates Oxidative Stress by Increasing NRF2 Transcriptional Factor in the Rat Hippocampus in the Pilocarpine-Induced Acute Seizure Model. Brain Sci. 2023, 13, 1442. [Google Scholar] [CrossRef]
- Yuliani, S.; Mustofa; Partadiredja, G. The Neuroprotective Effects of an Ethanolic Turmeric (Curcuma longa L.) Extract against Trimethyltin-Induced Oxidative Stress in Rats. Nutr. Neurosci. 2019, 22, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z. Therapeutic Potentials of Catalase: Mechanisms, Applications, and Future Perspectives. Int. J. Health Sci. 2024, 18, 1. [Google Scholar]
- Borroto-Escuela, D.O.; Hinz, S.; Navarro, G.; Franco, R.; Müller, C.E.; Fuxe, K. Understanding the Role of Adenosine A2AR Heteroreceptor Complexes in Neurodegeneration and Neuroinflammation. Front. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Huang, Y.; Zhao, H.; Gui, L. Adenosine Receptor A1-A2a Heteromers Regulate EAAT2 Expression and Glutamate Uptake via YY1-Induced Repression of PPAR γ Transcription. PPAR Res. 2020, 2020, 2410264. [Google Scholar] [CrossRef]
- Udo, M.S.B.; Zaccarelli-Magalhães, J.; Clemons, G.A.; Citadin, C.T.; Langman, J.; Smith, D.J.; Matuguma, L.H.; Tesic, V.; Lin, H.W. Blockade of A2AR Improved Brain Perfusion and Cognitive Function in a Mouse Model of Alzheimer’s Disease. GeroScience 2025, 47, 4153–4167. [Google Scholar] [CrossRef]
- Martí Navia, A.; Dal Ben, D.; Lambertucci, C.; Spinaci, A.; Volpini, R.; Marques-Morgado, I.; Coelho, J.E.; Lopes, L.V.; Marucci, G.; Buccioni, M. Adenosine Receptors as Neuroinflammation Modulators: Role of A1 Agonists and A2A Antagonists. Cells 2020, 9, 1739. [Google Scholar] [CrossRef]
- Nunes, A.C.L.; Carmo, M.; Behrenswerth, A.; Canas, P.M.; Agostinho, P.; Cunha, R.A. Adenosine A2A Receptor Blockade Provides More Effective Benefits at the Onset Rather than after Overt Neurodegeneration in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 4903. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.; Nguyen, H.G.; Koupenova, M.; Chauhan, A.K.; Makitalo, M.; Jones, M.R.; St. Hilaire, C.; Seldin, D.C.; Toselli, P.; et al. The A2B Adenosine Receptor Protects against Inflammation and Excessive Vascular Adhesion. J. Clin. Investig. 2006, 116, 1913–1923. [Google Scholar] [CrossRef]
- Coppi, E.; Dettori, I.; Cherchi, F.; Bulli, I.; Venturini, M.; Lana, D.; Giovannini, M.G.; Pedata, F.; Pugliese, A.M. A2B Adenosine Receptors: When Outsiders May Become an Attractive Target to Treat Brain Ischemia or Demyelination. Int. J. Mol. Sci. 2020, 21, 9697. [Google Scholar] [CrossRef] [PubMed]
- Fusco, I.; Cherchi, F.; Catarzi, D.; Colotta, V.; Varano, F.; Pedata, F.; Pugliese, A.M.; Coppi, E. Functional Characterization of a Novel Adenosine A2B Receptor Agonist on Short-Term Plasticity and Synaptic Inhibition during Oxygen and Glucose Deprivation in the Rat CA1 Hippocampus. Brain Res. Bull. 2019, 151, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.; Deng, P.; Wang, D.; Bai, X.; Li, Y.; Luo, C.; Belguise, K.; Wang, X.; Wei, X.; et al. Activation of Adenosine A3 Receptor Reduces Early Brain Injury by Alleviating Neuroinflammation after Subarachnoid Hemorrhage in Elderly Rats. Aging 2020, 13, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.B.; Julius, D. The P2Y12 Receptor Regulates Microglial Activation by Extracellular Nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef]
- Ohsawa, K.; Sanagi, T.; Nakamura, Y.; Suzuki, E.; Inoue, K.; Kohsaka, S. Adenosine A3 Receptor Is Involved in ADP-Induced Microglial Process Extension and Migration. J. Neurochem. 2012, 121, 217–227. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, J.C.; Ju, C.; Hwang, S.; Cho, G.S.; Lee, H.W.; Choi, W.J.; Jeong, L.S.; Kim, W.K. A3 Adenosine Receptor Agonist Reduces Brain Ischemic Injury and Inhibits Inflammatory Cell Migration in Rats. Am. J. Pathol. 2011, 179, 2042–2052. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Gracia, E.; Pérez-Capote, K.; Moreno, E.; Barkešová, J.; Mallol, J.; Lluís, C.; Franco, R.; Cortés, A.; Casadó, V.; Canela, E.I. A2A Adenosine Receptor Ligand Binding and Signalling Is Allosterically Modulated by Adenosine Deaminase. Biochem. J. 2011, 435, 701–709. [Google Scholar] [CrossRef]
- Cortés, A.; Gracia, E.; Moreno, E.; Mallol, J.; Lluís, C.; Canela, E.I.; Casadó, V. Moonlighting Adenosine Deaminase: A Target Protein for Drug Development. Med. Res. Rev. 2015, 35, 85–125. [Google Scholar] [CrossRef]
- Ho, S.Y.; Chen, I.C.; Chang, K.C.; Lin, H.R.; Tsai, C.W.; Lin, C.J.; Liou, H.H. Equilibrative Nucleoside Transporters-1 Inhibitors Act as Anti-Epileptic Agents by Inhibiting Glutamatergic Transmission. Front. Neurosci. 2020, 14, 610898. [Google Scholar] [CrossRef]
- Kao, Y.H.; Lin, M.S.; Chen, C.M.; Wu, Y.R.; Chen, H.M.; Lai, H.L.; Chern, Y.; Lin, C.J. Targeting ENT1 and Adenosine Tone for the Treatment of Huntington’s Disease. Hum. Mol. Genet. 2017, 26, 467–478. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, P.; Chen, Y.; Liu, J.; Zhang, Y.; Lv, Y.; Luo, J.; Fang, M.; Zhang, J.; Wang, J.; et al. ENT1 Inhibition Attenuates Epileptic Seizure Severity via Regulation of Glutamatergic Neurotransmission. Neuromol. Med. 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Dolores Constantino, M.; Fonseca, E.; Ribeiro, J.A. Age-Dependent Decrease in Adenosine A1 Receptor Binding Sites in the Rat Brain. Effect of Cis Unsaturated Free Fatty Acids. Eur. J. Biochem. 2001, 268, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Pinel, J.P.J.; Treit, D.; Rovner, L.I. Temporal Lobe Aggression in Rats. Science 1977, 197, 1088–1089. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates—The New Coronal Set; Elsevier: Amsterdam, The Netherlands, 2004. (In English) [Google Scholar]
- Dragic, M.; Stekic, A.; Zeljkovic, M.; Zaric Kontic, M.; Mihajlovic, K.; Adzic, M.; Grkovic, I.; Nedeljkovic, N. Altered Topographic Distribution and Enhanced Neuronal Expression of Adenosine-Metabolizing Enzymes in Rat Hippocampus and Cortex from Early to Late Adulthood. Neurochem. Res. 2022, 47, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Grković, I.; Mitrović, N.; Dragić, M.; Adžić, M.; Drakulić, D.; Nedeljković, N. Spatial Distribution and Expression of Ectonucleotidases in Rat Hippocampus After Removal of Ovaries and Estradiol Replacement. Mol. Neurobiol. 2019, 56, 1933–1945. [Google Scholar] [CrossRef] [PubMed]



| Fatty Acids | % |
|---|---|
| C16:0 | 7.96 ± 0.52 |
| C16:1 | 0.18 ± 0.04 |
| C18:0 | 4.29 ± 0.26 |
| C18:1 (n-9) | 22.52 ± 0.88 |
| C18:1 (n-7) | 0.00 ± 0.00 |
| C18:2 | 25.34 ± 3.10 |
| C18:3 (n-6) | 0.32 ± 0.09 |
| C18:3 (n-3) | 39.16 ± 1.32 |
| C20:3 | 0.22 ± 0.08 |
| C20:4 | 0.00 ± 0.00 |
| C20:5 | 0.00 ± 0.00 |
| C22:4 | 0.00 ± 0.00 |
| C22:5 | 0.00 ± 0.00 |
| C22:6 | 0.00 ± 0.00 |
| SFA * | 12.25 ± 0.77 |
| MUFA * | 22.71 ± 0.84 |
| PUFA * | 65.04 ± 1.61 |
| Gene | Sequence (5′-3′) | Length (bp) |
|---|---|---|
| Nrf2 (Nfe2l2) | GACTTGGAATTGCCACCGC CCTGTTCCTTCTGGAGTTGCT | 193 |
| NF-kB (Rela) | AGCATGTACAGATTCTGGGGAG AGAGCCGACTATCGTACAGGG | 195 |
| GPx1 (Gpx1) | AGCGACCAGATGAAGCAGTG TCCGCTCTCTGTCAAAGTGTG | 181 |
| Gsr (Gsr) | CCCACATCGAAGTCATCCAC GATCAGGATGTGTGGAGCAG | 101 |
| SOD2 (Sod2) | TGACCTGCCTTACGACTATGG CTCGTGGTACTTCTCCTCGG | 127 |
| CAT (Cat) | TAATATCATGACTGCGGGGC TCTCTCAGGAATCCGCTCTC | 100 |
| NTPDase1 (Entpd1) | TCAAGGACCCGTGCTTTTAC TCTGGTGGCACTGTTCGTAG | 150 |
| eN (Nt5e) | CAAATCTGCCTCTGGAAAGC ACCTTCCAGAAGGACCCTGT | 160 |
| P2X4R (P2rx4) | ACCAGGAAACGGACTCTGTG TCACGGTGACGATCATGTTGG | 168 |
| P2Y6R (P2ry6) | CAGTTATGGAGCGGGACAAT GTAAACTGGGGGTAGCAGCA | 104 |
| P2Y12R (P2ry12) | TCACCCGCACCCTCTATTAC GCCAGGAAGTAGAGCACAGG | 139 |
| A1R (Adora1) | GTGATTTGGGCTGTGAAGGT GAGCTCTGGGTGAGGATGAG | 194 |
| A2AR (Adora2a) | TGCAGAACGTCACCAACTTC CAAAACAGGCGAAGAAGAGG | 141 |
| A2BR (Adora2b) | CGTCCCGCTCAGGTATAAAG CCAGGAAAGGAGTCAGTCCA | 104 |
| A3R (Adora3) | TTCTTGTTTGCCTTGTGCTG AGGGTTCATCATGGAGTTCG | 129 |
| NGF (Ngf) | CTGGAGCCGAAGGGGAG ACTGAGGTGAGCTTGGGTCC | 103 |
| ADA (Ada) | GAGCCTCATCCTGTGAATGG ATGCCCATGATTGTCAAGGT | 143 |
| ENT1 (Slc29a1) | CACTTCCTTCGCTGTTAGGG TGTCCCCCTACCACTCTGAC | 144 |
| CycA (Ppia) | GGCAAATGCTGGACCAAACAC TTAGAGTTGTCCACAGTCGGAGATG | 196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrović, N.; Zarić Kontić, M.; Grković, I. Neuroprotection by Flaxseed Oil in a Model of Hippocampal Injury Induced by Trimethyltin Involves Purinergic System Modulation. Int. J. Mol. Sci. 2025, 26, 10283. https://doi.org/10.3390/ijms262110283
Mitrović N, Zarić Kontić M, Grković I. Neuroprotection by Flaxseed Oil in a Model of Hippocampal Injury Induced by Trimethyltin Involves Purinergic System Modulation. International Journal of Molecular Sciences. 2025; 26(21):10283. https://doi.org/10.3390/ijms262110283
Chicago/Turabian StyleMitrović, Nataša, Marina Zarić Kontić, and Ivana Grković. 2025. "Neuroprotection by Flaxseed Oil in a Model of Hippocampal Injury Induced by Trimethyltin Involves Purinergic System Modulation" International Journal of Molecular Sciences 26, no. 21: 10283. https://doi.org/10.3390/ijms262110283
APA StyleMitrović, N., Zarić Kontić, M., & Grković, I. (2025). Neuroprotection by Flaxseed Oil in a Model of Hippocampal Injury Induced by Trimethyltin Involves Purinergic System Modulation. International Journal of Molecular Sciences, 26(21), 10283. https://doi.org/10.3390/ijms262110283






