Mitochondrial DNA Replication and Disease: A Historical Perspective on Molecular Insights and Therapeutic Advances
Abstract
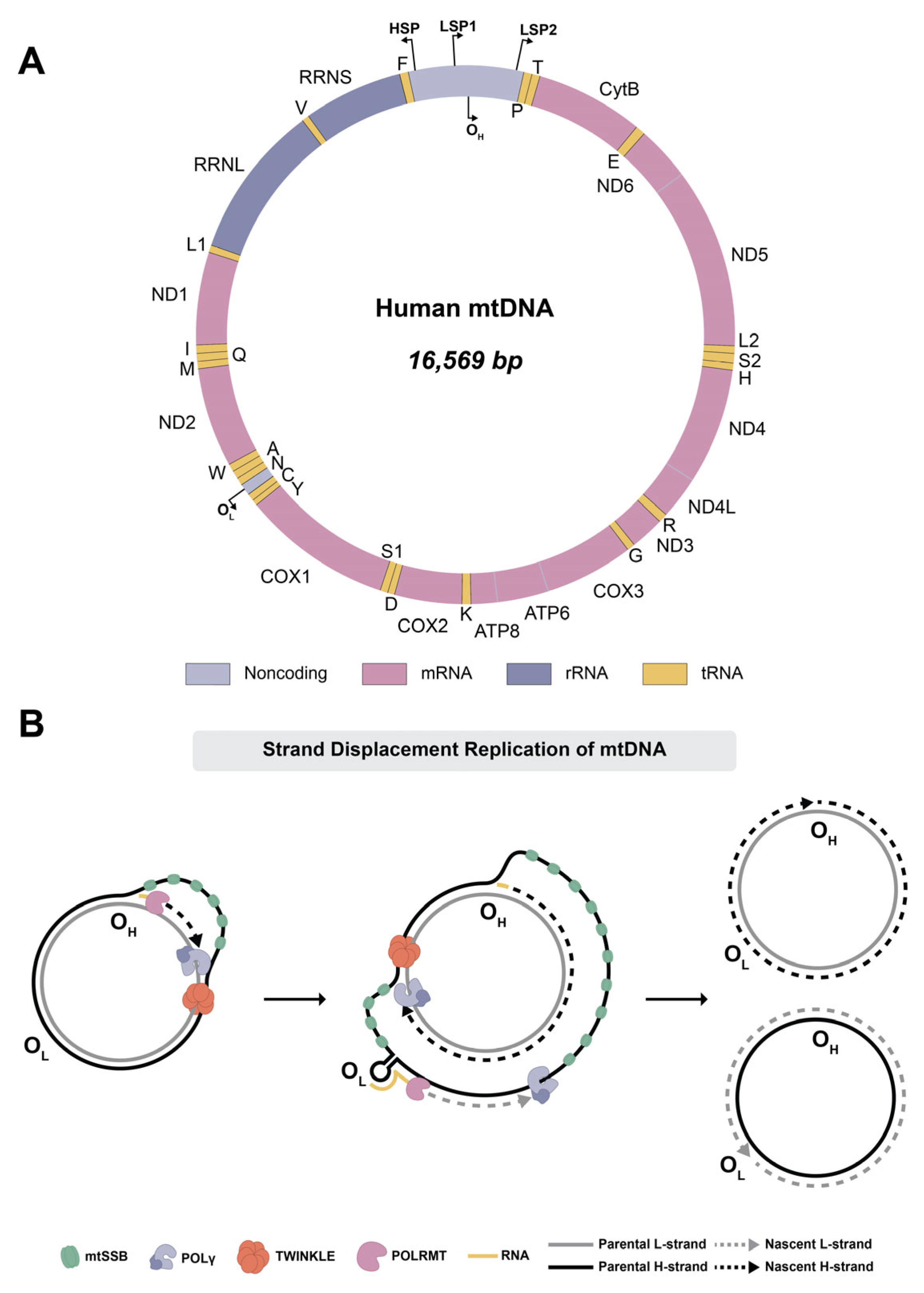
1. Introduction
2. DNA Polymerase γ
3. Catalytic Subunit, PolG
3.1. Fidelity of mtDNA Replication
3.2. POLG-Related Diseases
- Alpers–Huttenlocher syndrome: A severe childhood-onset encephalopathy marked by intractable epilepsy and progressive liver failure.
- Childhood myocerebrohepatopathy spectrum: Presents during the first months to three years of life with developmental delay or regression, lactic acidosis, and myopathy. Additional features may include liver failure, renal tubular acidosis, pancreatitis, cyclic vomiting, and sensorineural hearing loss.
- Myoclonic epilepsy myopathy sensory ataxia (MEMSA): A group of disorders involving epilepsy, myopathy, and ataxia without ophthalmoplegia. This category includes what was previously described as spinocerebellar ataxia with epilepsy.
- Ataxia–neuropathy spectrum: Encompasses mitochondrial recessive ataxia syndrome (MIRAS) and sensory ataxia with neuropathy, dysarthria, and ophthalmoplegia.
- Autosomal recessive progressive external ophthalmoplegia (arPEO): Characterized by progressive weakness of the extraocular muscles, leading to ptosis and ophthalmoparesis. Although initially isolated to eye movement, many individuals later develop additional systemic symptoms of POLG-related disease.
- Autosomal dominant progressive external ophthalmoplegia (adPEO): Typically involves generalized myopathy along with varying degrees of sensorineural hearing loss, axonal neuropathy, ataxia, depression, Parkinsonism, hypogonadism, and cataracts.
4. Accessory Subunit, PolG2
5. The Human Mitochondrial Single-Stranded DNA-Binding Protein, mtSSB
6. The Mitochondrial DNA Helicase, Twinkle
7. Other Proteins Involved in mtDNA Maintenance
8. Therapies for Mitochondrial Diseases
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| adPEO | autosomal dominant progressive external ophthalmoplegia |
| AID | accessory interacting determinant |
| arPEO | autosomal recessive progressive external ophthalmoplegia |
| ATP | adenosine 5′-triphosphate |
| bp | base pairs |
| CTD | C-terminal helicase domain |
| DGUOK | deoxyguanosine kinase |
| DNA2 | DNA replication helicase/nuclease 2 |
| dNTPs | deoxynucleotide triphosphates |
| dsDNA | double-stranded DNA |
| ETC | electron transport chain |
| gp4 | T7 gene protein 4 primase–helicase |
| H-strand | heavy strand |
| IOSCA | infantile-onset spinocerebellar ataxia |
| L-strand | light strand |
| LonP1 | Lon protease |
| MDS | mitochondrial DNA depletion syndrome |
| MEMSA | myoclonic epilepsy myopathy sensory ataxia |
| MGME1 | mitochondrial genome maintenance exonuclease 1 |
| MIRAS | mitochondrial recessive ataxia syndrome |
| mtDNA | mitochondrial DNA |
| MTS | mitochondrial targeting sequence |
| mtSSB | mitochondrial single-stranded DNA binding protein |
| NCR | noncoding region |
| NR | nicotinamide riboside |
| NTR | N-terminal region |
| OXPHOS | oxidative phosphorylation |
| p140 | catalytic subunit PolG |
| p55 | accessory subunit PolG2 |
| PEO | progressive external ophthalmoplegia |
| Pol γ | DNA polymerase γ |
| POLRMT | mitochondrial RNA polymerase |
| ROS | reactive oxygen species |
| RP-A | replication protein A |
| SEC-MALS | size-exclusion chromatography–multi-angle light scattering |
| SF4 | superfamily 4 |
| SLSMD | single large-scale mtDNA deletion |
| SNP | single nucleotide polymorph |
| SSBs | single-stranded DNA-binding proteins |
| ssDNA | single-stranded DNA |
| TCA | tricarboxylic acid |
| TFAM | transcription factor A, mitochondrial |
| TK2 | thymidine kinase 2 |
| TOP3A | topoisomerase 3α |
| WT | wild-type |
| ZBD | zinc finger binding domain |
References
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Casuso, R.A.; Huertas, J.R. Mitochondrial Functionality in Inflammatory Pathology-Modulatory Role of Physical Activity. Life 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Das, A.M. Control of mitochondrial ATP synthesis in the heart. Biochem. J. 1991, 280 Pt 3, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef]
- Hatefi, Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985, 54, 1015–1069. [Google Scholar] [CrossRef]
- Berg, O.G.; Kurland, C.G. Why Mitochondrial Genes are Most Often Found in Nuclei. Mol. Biol. Evol. 2000, 17, 951–961. [Google Scholar] [CrossRef]
- Larsson, N.G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef]
- Wiese, M.; Bannister, A.J. Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways. Mol. Metab. 2020, 38, 100942. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial diseases in man and mouse. Science 1999, 283, 1482–1488. [Google Scholar] [CrossRef]
- Tuppen, H.A.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta 2010, 1797, 113–128. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, J. Mitochondrial Nucleoid: Shield and Switch of the Mitochondrial Genome. Oxidative Med. Cell. Longev. 2017, 2017, 8060949. [Google Scholar] [CrossRef]
- Iborra, F.J.; Kimura, H.; Cook, P.R. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2004, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Vinograd, J.; Morris, J.; Davidson, N.; Dove, W.F., Jr. The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc. Natl. Acad. Sci. USA 1963, 49, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.D.; Larson, J.E. Buoyant density studies on natural and synthetic deoxyribonucleic acids in neutral and alkaline solutions. J. Biol. Chem. 1972, 247, 3405–3409. [Google Scholar] [CrossRef]
- Tan, B.G.; Mutti, C.D.; Shi, Y.; Xie, X.; Zhu, X.; Silva-Pinheiro, P.; Menger, K.E.; Díaz-Maldonado, H.; Wei, W.; Nicholls, T.J.; et al. The human mitochondrial genome contains a second light strand promoter. Mol. Cell 2022, 82, 3646–3660.e9. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, X.; Das, H.; Tan, B.G.; Shi, Y.; Al-Behadili, A.; Peter, B.; Motori, E.; Valenzuela, S.; Posse, V.; et al. Non-coding 7S RNA inhibits transcription via mitochondrial RNA polymerase dimerization. Cell 2022, 185, 2309–2323.e24. [Google Scholar] [CrossRef]
- Magnusson, J.; Orth, M.; Lestienne, P.; Taanman, J.W. Replication of mitochondrial DNA occurs throughout the mitochondria of cultured human cells. Exp. Cell Res. 2003, 289, 133–142. [Google Scholar] [CrossRef]
- Ylikallio, E.; Tyynismaa, H.; Tsutsui, H.; Ide, T.; Suomalainen, A. High mitochondrial DNA copy number has detrimental effects in mice. Hum. Mol. Genet. 2010, 19, 2695–2705. [Google Scholar] [CrossRef]
- Falkenberg, M. Mitochondrial DNA replication in mammalian cells: Overview of the pathway. Essays Biochem. 2018, 62, 287–296. [Google Scholar] [CrossRef]
- Robberson, D.L.; Kasamatsu, H.; Vinograd, J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc. Natl. Acad. Sci. USA 1972, 69, 737–741. [Google Scholar] [CrossRef]
- Fusté, J.M.; Wanrooij, S.; Jemt, E.; Granycome, C.E.; Cluett, T.J.; Shi, Y.; Atanassova, N.; Holt, I.J.; Gustafsson, C.M.; Falkenberg, M. Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol. Cell 2010, 37, 67–78. [Google Scholar] [CrossRef]
- Miralles Fusté, J.; Shi, Y.; Wanrooij, S.; Zhu, X.; Jemt, E.; Persson, Ö.; Sabouri, N.; Gustafsson, C.M.; Falkenberg, M. In vivo occupancy of mitochondrial single-stranded DNA binding protein supports the strand displacement mode of DNA replication. PLoS Genet. 2014, 10, e1004832. [Google Scholar] [CrossRef] [PubMed]
- Tapper, D.P.; Clayton, D.A. Mechanism of replication of human mitochondrial DNA. Localization of the 5′ ends of nascent daughter strands. J. Biol. Chem. 1981, 256, 5109–5115. [Google Scholar] [CrossRef]
- Phillips, A.F.; Millet, A.R.; Tigano, M.; Dubois, S.M.; Crimmins, H.; Babin, L.; Charpentier, M.; Piganeau, M.; Brunet, E.; Sfeir, A. Single-Molecule Analysis of mtDNA Replication Uncovers the Basis of the Common Deletion. Mol. Cell 2017, 65, 527–538.e6. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A. Replication and transcription of vertebrate mitochondrial DNA. Annu. Rev. Cell Biol. 1991, 7, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Wang, Z.H.; Delaney, K.M.; Tang, J.; Pirooznia, M.; Lee, D.Y.; Tunc, I.; Li, Y.; Xu, H. The PPR domain of mitochondrial RNA polymerase is an exoribonuclease required for mtDNA replication in Drosophila melanogaster. Nat. Cell Biol. 2022, 24, 757–765. [Google Scholar] [CrossRef]
- Wanrooij, S.; Fusté, J.M.; Farge, G.; Shi, Y.; Gustafsson, C.M.; Falkenberg, M. Human mitochondrial RNA polymerase primes lagging-strand DNA synthesis in vitro. Proc. Natl. Acad. Sci. USA 2008, 105, 11122–11127. [Google Scholar] [CrossRef]
- Tan, B.G.; Gustafsson, C.M.; Falkenberg, M. Mechanisms and regulation of human mitochondrial transcription. Nat. Rev. Mol. Cell Biol. 2024, 25, 119–132. [Google Scholar] [CrossRef]
- Wanrooij, S.; Miralles Fusté, J.; Stewart, J.B.; Wanrooij, P.H.; Samuelsson, T.; Larsson, N.G.; Gustafsson, C.M.; Falkenberg, M. In vivo mutagenesis reveals that OriL is essential for mitochondrial DNA replication. EMBO Rep. 2012, 13, 1130–1137. [Google Scholar] [CrossRef]
- Nicholls, T.J.; Nadalutti, C.A.; Motori, E.; Sommerville, E.W.; Gorman, G.S.; Basu, S.; Hoberg, E.; Turnbull, D.M.; Chinnery, P.F.; Larsson, N.-G.; et al. Topoisomerase 3α Is Required for Decatenation and Segregation of Human mtDNA. Mol. Cell 2018, 69, 9–23.e6. [Google Scholar] [CrossRef]
- Erdinc, D.; Rodríguez-Luis, A.; Fassad, M.R.; Mackenzie, S.; Watson, C.M.; Valenzuela, S.; Xie, X.; Menger, K.E.; Sergeant, K.; Craig, K.; et al. Pathological variants in TOP3A cause distinct disorders of mitochondrial and nuclear genome stability. EMBO Mol. Med. 2023, 15, e16775. [Google Scholar] [CrossRef]
- Miller, F.J.; Rosenfeldt, F.L.; Zhang, C.; Linnane, A.W.; Nagley, P. Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: Lack of change of copy number with age. Nucleic Acids Res. 2003, 31, e61. [Google Scholar] [CrossRef]
- Kotrys, A.V.; Durham, T.J.; Guo, X.A.; Vantaku, V.R.; Parangi, S.; Mootha, V.K. Single-cell analysis reveals context-dependent, cell-level selection of mtDNA. Nature 2024, 629, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Greaves, L.C.; Nooteboom, M.; Elson, J.L.; Tuppen, H.A.; Taylor, G.A.; Commane, D.M.; Arasaradnam, R.P.; Khrapko, K.; Taylor, R.W.; Kirkwood, T.B.; et al. Clonal expansion of early to mid-life mitochondrial DNA point mutations drives mitochondrial dysfunction during human ageing. PLoS Genet. 2014, 10, e1004620. [Google Scholar] [CrossRef]
- Picard, M.; Zhang, J.; Hancock, S.; Derbeneva, O.; Golhar, R.; Golik, P.; O’Hearn, S.; Levy, S.; Potluri, P.; Lvova, M.; et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl. Acad. Sci. USA 2014, 111, E4033–E4042. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.B.; Chinnery, P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef]
- Pickett, S.J.; Taylor, R.W.; McFarland, R. Fit for purpose: Selecting the best mitochondrial DNA for the job. Cell Metab. 2024, 36, 1436–1438. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kanai, M.; Durham, T.J.; Tsuo, K.; McCoy, J.G.; Kotrys, A.V.; Zhou, W.; Chinnery, P.F.; Karczewski, K.J.; Calvo, S.E.; et al. Nuclear genetic control of mtDNA copy number and heteroplasmy in humans. Nature 2023, 620, 839–848, Erratum in Nature 2024, 630, E10. [Google Scholar] [CrossRef]
- Medeiros, T.C.; Thomas, R.L.; Ghillebert, R.; Graef, M. Autophagy balances mtDNA synthesis and degradation by DNA polymerase POLG during starvation. J. Cell Biol. 2018, 217, 1601–1611. [Google Scholar] [CrossRef]
- Copeland, W.C. Defects in mitochondrial DNA replication and human disease. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 64–74. [Google Scholar] [CrossRef]
- Taylor, S.D.; Zhang, H.; Eaton, J.S.; Rodeheffer, M.S.; Lebedeva, M.A.; O’Rourke, T.W.; Siede, W.; Shadel, G.S. The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol. Biol. Cell 2005, 16, 3010–3018. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Copeland, W.C. Human mitochondrial DNA replication machinery and disease. Curr. Opin. Genet. Dev. 2016, 38, 52–62. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Scaglia, F. Mitochondrial DNA depletion syndromes: Review and updates of genetic basis, manifestations, and therapeutic options. Neurotherapeutics 2013, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.A.; McCormick, E.M.; Perera, L.; Longley, M.J.; Bai, R.; Kong, J.; Dulik, M.; Shen, L.; Goldstein, A.C.; McCormack, S.E.; et al. Mitochondrial single-stranded DNA binding protein novel de novo SSBP1 mutation in a child with single large-scale mtDNA deletion (SLSMD) clinically manifesting as Pearson, Kearns-Sayre, and Leigh syndromes. PLoS ONE 2019, 14, e0221829. [Google Scholar] [CrossRef]
- Longley, M.J.; Clark, S.; Yu Wai Man, C.; Hudson, G.; Durham, S.E.; Taylor, R.W.; Nightingale, S.; Turnbull, D.M.; Copeland, W.C.; Chinnery, P.F. Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia. Am. J. Hum. Genet. 2006, 78, 1026–1034. [Google Scholar] [CrossRef]
- Van Dyck, E.; Foury, F.; Stillman, B.; Brill, S.J. A single-stranded DNA binding protein required for mitochondrial DNA replication in S. cerevisiae is homologous to E. coli SSB. EMBO J. 1992, 11, 3421–3430. [Google Scholar] [CrossRef]
- Van Goethem, G.; Dermaut, B.; Löfgren, A.; Martin, J.J.; Van Broeckhoven, C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat. Genet. 2001, 28, 211–212. [Google Scholar] [CrossRef]
- Khan, N.A.; Govindaraj, P.; Meena, A.K.; Thangaraj, K. Mitochondrial disorders: Challenges in diagnosis & treatment. Indian J. Med. Res. 2015, 141, 13–26. [Google Scholar] [CrossRef]
- Copeland, W.C. Inherited mitochondrial diseases of DNA replication. Annu. Rev. Med. 2008, 59, 131–146. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Zweers-van Essen, H.; Rodenburg, R.J.; van den Heuvel, L.P.; de Vries, M.C.; Rasmussen-Conrad, E.; Smeitink, J.A.; Morava, E. Mitochondrial energy production correlates with the age-related BMI. Pediatr. Res. 2009, 65, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Morava, E.; Rodenburg, R.; van Essen, H.Z.; De Vries, M.; Smeitink, J. Dietary intervention and oxidative phosphorylation capacity. J. Inherit. Metab. Dis. 2006, 29, 589. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, A.M.; Tawfik, E.A.; Alomary, M.N.; Alsudir, S.A.; Alfahad, A.J.; Alshehri, A.A.; Almughem, F.A.; Mohammed, R.Y.; Alzaydi, M.M. Recent advances in mitochondrial diseases: From molecular insights to therapeutic perspectives. Saudi Pharm. J. 2022, 30, 1065–1078. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Schon, K.R.; Horvath, R.; Wei, W.; Calabrese, C.; Tucci, A.; Ibañez, K.; Ratnaike, T.; Pitceathly, R.D.S.; Bugiardini, E.; Quinlivan, R.; et al. Use of whole genome sequencing to determine genetic basis of suspected mitochondrial disorders: Cohort study. BMJ 2021, 375, e066288. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A. Renal manifestations of primary mitochondrial disorders. Biomed. Rep. 2017, 6, 487–494. [Google Scholar] [CrossRef]
- Muraresku, C.C.; McCormick, E.M.; Falk, M.J. Mitochondrial Disease: Advances in clinical diagnosis, management, therapeutic development, and preventative strategies. Curr. Genet. Med. Rep. 2018, 6, 62–72. [Google Scholar] [CrossRef]
- Graziewicz, M.A.; Longley, M.J.; Copeland, W.C. DNA polymerase gamma in mitochondrial DNA replication and repair. Chem. Rev. 2006, 106, 383–405. [Google Scholar] [CrossRef]
- Ropp, P.A.; Copeland, W.C. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics 1996, 36, 449–458. [Google Scholar] [CrossRef]
- Young, M.J.; Copeland, W.C. Mitochondrial Disorders Associated with the Mitochondrial DNA Polymerase g: A Focus on Intersubunit Interactions. In Mitochondrial Disorders Caused by Nuclear Genes; Wong, L.-J.C., Ed.; Springer: New York, NY, USA, 2013; pp. 49–72. [Google Scholar]
- Wang, Y.; Kaguni, L.S. Baculovirus expression reconstitutes Drosophila mitochondrial DNA polymerase. J. Biol. Chem. 1999, 274, 28972–28977. [Google Scholar] [CrossRef] [PubMed]
- Hance, N.; Ekstrand, M.I.; Trifunovic, A. Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum. Mol. Genet. 2005, 14, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Humble, M.M.; Young, M.J.; Foley, J.F.; Pandiri, A.R.; Travlos, G.S.; Copeland, W.C. Polg2 is essential for mammalian embryogenesis and is required for mtDNA maintenance. Hum. Mol. Genet. 2013, 22, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Longley, M.J.; Prasad, R.; Srivastava, D.K.; Wilson, S.H.; Copeland, W.C. Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase gamma and its role in mitochondrial base excision repair in vitro. Proc. Natl. Acad. Sci. USA 1998, 95, 12244–12248. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kennedy, W.D.; Yin, Y.W. Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell 2009, 139, 312–324. [Google Scholar] [CrossRef]
- Szymanski, M.R.; Kuznetsov, V.B.; Shumate, C.; Meng, Q.; Lee, Y.S.; Patel, G.; Patel, S.; Yin, Y.W. Structural basis for processivity and antiviral drug toxicity in human mitochondrial DNA replicase. EMBO J. 2015, 34, 1959–1970. [Google Scholar] [CrossRef]
- Longley, M.J.; Nguyen, D.; Kunkel, T.A.; Copeland, W.C. The fidelity of human DNA polymerase gamma with and without exonucleolytic proofreading and the p55 accessory subunit. J. Biol. Chem. 2001, 276, 38555–38562. [Google Scholar] [CrossRef]
- Zheng, W.; Khrapko, K.; Coller, H.A.; Thilly, W.G.; Copeland, W.C. Origins of human mitochondrial point mutations as DNA polymerase gamma-mediated errors. Mutat. Res. 2006, 599, 11–20. [Google Scholar] [CrossRef]
- Ju, Y.S.; Alexandrov, L.B.; Gerstung, M.; Martincorena, I.; Nik-Zainal, S.; Ramakrishna, M.; Davies, H.R.; Papaemmanuil, E.; Gundem, G.; Shlien, A.; et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife 2014, 3, e02935. [Google Scholar] [CrossRef]
- King, D.E.; Copeland, W.C. DNA Repair Pathways in the Mitochondria. DNA Repair 2025, 146, 103814. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.R.; Salk, J.J.; Schmitt, M.W.; Loeb, L.A. Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet. 2013, 9, e1003794. [Google Scholar] [CrossRef] [PubMed]
- Itsara, L.S.; Kennedy, S.R.; Fox, E.J.; Yu, S.; Hewitt, J.J.; Sanchez-Contreras, M.; Cardozo-Pelaez, F.; Pallanck, L.J. Oxidative stress is not a major contributor to somatic mitochondrial DNA mutations. PLoS Genet. 2014, 10, e1003974. [Google Scholar] [CrossRef] [PubMed]
- Valente, W.J.; Ericson, N.G.; Long, A.S.; White, P.A.; Marchetti, F.; Bielas, J.H. Mitochondrial DNA exhibits resistance to induced point and deletion mutations. Nucleic Acids Res. 2016, 44, 8513–8524. [Google Scholar] [CrossRef]
- Leuthner, T.C.; Meyer, J.N. Mitochondrial DNA Mutagenesis: Feature of and Biomarker for Environmental Exposures and Aging. Curr. Environ. Health Rep. 2021, 8, 294–308. [Google Scholar] [CrossRef]
- Longley, M.J.; Ropp, P.A.; Lim, S.E.; Copeland, W.C. Characterization of the native and recombinant catalytic subunit of human DNA polymerase gamma: Identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry 1998, 37, 10529–10539. [Google Scholar] [CrossRef]
- Spelbrink, J.N.; Toivonen, J.M.; Hakkaart, G.A.; Kurkela, J.M.; Cooper, H.M.; Lehtinen, S.K.; Lecrenier, N.; Back, J.W.; Speijer, D.; Foury, F.; et al. In vivo functional analysis of the human mitochondrial DNA polymerase POLG expressed in cultured human cells. J. Biol. Chem. 2000, 275, 24818–24828. [Google Scholar] [CrossRef]
- Bensch, K.G.; Degraaf, W.; Hansen, P.A.; Zassenhaus, H.P.; Corbett, J.A. A transgenic model to study the pathogenesis of somatic mtDNA mutation accumulation in beta-cells. Diabetes Obes. Metab. 2007, 9 (Suppl. S2), 74–80. [Google Scholar] [CrossRef]
- Bensch, K.G.; Mott, J.L.; Chang, S.W.; Hansen, P.A.; Moxley, M.A.; Chambers, K.T.; de Graaf, W.; Zassenhaus, H.P.; Corbett, J.A. Selective mtDNA mutation accumulation results in beta-cell apoptosis and diabetes development. Am. J. Physiol. 2009, 296, E672–E680. [Google Scholar] [CrossRef]
- Zhang, D.; Mott, J.L.; Chang, S.W.; Denniger, G.; Feng, Z.; Zassenhaus, H.P. Construction of transgenic mice with tissue-specific acceleration of mitochondrial DNA mutagenesis. Genomics 2000, 69, 151–161. [Google Scholar] [CrossRef]
- Zhang, D.; Mott, J.L.; Farrar, P.; Ryerse, J.S.; Chang, S.W.; Stevens, M.; Denniger, G.; Zassenhaus, H.P. Mitochondrial DNA mutations activate the mitochondrial apoptotic pathway and cause dilated cardiomyopathy. Cardiovasc. Res. 2003, 57, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mott, J.L.; Chang, S.W.; Stevens, M.; Mikolajczak, P.; Zassenhaus, H.P. Mitochondrial DNA mutations activate programmed cell survival in the mouse heart. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2476–H2483. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly, Y.M.; Gidlof, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Vermulst, M.; Bielas, J.H.; Kujoth, G.C.; Ladiges, W.C.; Rabinovitch, P.S.; Prolla, T.A.; Loeb, L.A. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 2007, 39, 540–543. [Google Scholar] [CrossRef]
- Williams, S.L.; Huang, J.; Edwards, Y.J.; Ulloa, R.H.; Dillon, L.M.; Prolla, T.A.; Vance, J.M.; Moraes, C.T.; Zuchner, S. The mtDNA mutation spectrum of the progeroid Polg mutator mouse includes abundant control region multimers. Cell Metab. 2010, 12, 675–682. [Google Scholar] [CrossRef]
- Vermulst, M.; Bielas, J.H.; Loeb, L.A. Quantification of random mutations in the mitochondrial genome. Methods 2008, 46, 263–268. [Google Scholar] [CrossRef]
- Hämäläinen, R.H.; Landoni, J.C.; Ahlqvist, K.J.; Goffart, S.; Ryytty, S.; Rahman, M.O.; Brilhante, V.; Icay, K.; Hautaniemi, S.; Wang, L.; et al. Defects in mtDNA replication challenge nuclear genome stability through nucleotide depletion and provide a unifying mechanism for mouse progerias. Nat. Metab. 2019, 1, 958–965, Erratum in Nat. Metab. 2020, 2, 793. [Google Scholar] [CrossRef]
- Gorman, E.; Dai, H.; Feng, Y.; Craigen, W.J.; Chen, D.C.Y.; Xia, F.; Meng, L.; Liu, P.; Rigobello, R.; Neogi, A.; et al. Experiences from dual genome next-generation sequencing panel testing for mitochondrial disorders: A comprehensive molecular diagnosis. Front. Genet. 2025, 16, 1488956. [Google Scholar] [CrossRef]
- Barca, E.; Long, Y.; Cooley, V.; Schoenaker, R.; Emmanuele, V.; DiMauro, S.; Cohen, B.H.; Karaa, A.; Vladutiu, G.D.; Haas, R.; et al. Mitochondrial diseases in North America: An analysis of the NAMDC Registry. Neurol. Genet. 2020, 6, e402. [Google Scholar] [CrossRef]
- Rahman, S.; Copeland, W.C. POLG-related disorders and their neurological manifestations. Nat. Rev. Neurol. 2019, 15, 40–52. [Google Scholar] [CrossRef]
- Cohen, B.H.; Chinnery, P.F.; Copeland, W.C. POLG-Related Disorders. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2024. [Google Scholar]
- Lujan, S.A.; Longley, M.J.; Humble, M.H.; Lavender, C.A.; Burkholder, A.; Blakely, E.L.; Alston, C.L.; Gorman, G.S.; Turnbull, D.M.; McFarland, R.; et al. Ultrasensitive deletion detection links mitochondrial DNA replication, disease, and aging. Genome Biol. 2020, 21, 248. [Google Scholar] [CrossRef]
- Chan, S.S.; Longley, M.J.; Copeland, W.C. The common A467T mutation in the human mitochondrial DNA polymerase (POLG) compromises catalytic efficiency and interaction with the accessory subunit. J. Biol. Chem. 2005, 280, 31341–31346. [Google Scholar] [CrossRef]
- Van Goethem, G.; Martin, J.J.; Dermaut, B.; Lofgren, A.; Wibail, A.; Ververken, D.; Tack, P.; Dehaene, I.; Van Zandijcke, M.; Moonen, M.; et al. Recessive POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul. Disord. 2003, 13, 133–142. [Google Scholar] [CrossRef]
- Chan, S.S.; Longley, M.J.; Copeland, W.C. Modulation of the W748S mutation in DNA polymerase gamma by the E1143G polymorphismin mitochondrial disorders. Hum. Mol. Genet. 2006, 15, 3473–3483. [Google Scholar] [CrossRef]
- Kasiviswanathan, R.; Longley, M.J.; Chan, S.S.; Copeland, W.C. Disease mutations in the human mitochondrial DNA polymerase thumb subdomain impart severe defects in mitochondrial DNA replication. J. Biol. Chem. 2009, 284, 19501–19510. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Lamantea, E.; Donati, A.; Filosto, M.; Briem, E.; Carrara, F.; Parini, R.; Simonati, A.; Santer, R.; Zeviani, M. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-gammaA. Brain 2005, 128, 723–731. [Google Scholar] [CrossRef] [PubMed]
- DeBalsi, K.L.; Longley, M.J.; Hoff, K.E.; Copeland, W.C. Synergistic Effects of the in cis T251I and P587L Mitochondrial DNA Polymerase gamma Disease Mutations. J. Biol. Chem. 2017, 292, 4198–4209. [Google Scholar] [CrossRef] [PubMed]
- Graziewicz, M.A.; Longley, M.J.; Bienstock, R.J.; Zeviani, M.; Copeland, W.C. Structure-function defects of human mitochondrial DNA polymerase in autosomal dominant progressive external ophthalmoplegia. Nat. Struct. Mol. Biol. 2004, 11, 770–776. [Google Scholar] [CrossRef]
- Ponamarev, M.V.; Longley, M.J.; Nguyen, D.; Kunkel, T.A.; Copeland, W.C. Active site mutation in DNA polymerase gamma associated with progressive external ophthalmoplegia causes error-prone DNA synthesis. J. Biol. Chem. 2002, 277, 15225–15228. [Google Scholar] [CrossRef]
- Silva-Pinheiro, P.; Pardo-Hernández, C.; Reyes, A.; Tilokani, L.; Mishra, A.; Cerutti, R.; Li, S.; Rozsivalova, D.H.; Valenzuela, S.; Dogan, S.A.; et al. DNA polymerase gamma mutations that impair holoenzyme stability cause catalytic subunit depletion. Nucleic Acids Res. 2021, 49, 5230–5248, Erratum in Nucleic Acids Res. 2021, 49, 10803. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.A.; Brannon, A.J.; Krahn, J.M.; Bouvette, J.; Williams, J.G.; Borgnia, M.J.; Copeland, W.C. Coordinated DNA polymerization by Polgamma and the region of LonP1 regulated proteolysis. Nucleic Acids Res. 2024, 52, 7863–7875. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.E.; Longley, M.J.; Copeland, W.C. The Mitochondrial p55 Accessory Subunit of Human DNA Polymerase γ Enhances DNA Binding, Promotes Processive DNA Synthesis, and Confers N-Ethylmaleimide Resistance. J. Biol. Chem. 1999, 274, 38197–38203. [Google Scholar] [CrossRef]
- Olson, M.W.; Wang, Y.; Elder, R.H.; Kaguni, L.S. Subunit structure of mitochondrial DNA polymerase from Drosophila embryos. Physical and immunological studies. J. Biol. Chem. 1995, 270, 28932–28937. [Google Scholar] [CrossRef]
- Wang, Y.; Farr, C.L.; Kaguni, L.S. Accessory subunit of mitochondrial DNA polymerase from Drosophila embryos. Cloning, molecular analysis, and association in the native enzyme. J. Biol. Chem. 1997, 272, 13640–13646. [Google Scholar] [CrossRef]
- Fan, L.; Sanschagrin, P.C.; Kaguni, L.S.; Kuhn, L.A. The accessory subunit of mtDNA polymerase shares structural homology with aminoacyl-tRNA synthetases: Implications for a dual role as a primer recognition factor and processivity clamp. Proc. Natl. Acad. Sci. USA 1999, 96, 9527–9532. [Google Scholar] [CrossRef]
- Carrodeguas, J.A.; Theis, K.; Bogenhagen, D.F.; Kisker, C. Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase gamma, Pol gamma B, functions as a homodimer. Mol. Cell 2001, 7, 43–54. [Google Scholar] [CrossRef]
- Carrodeguas, J.A.; Kobayashi, R.; Lim, S.E.; Copeland, W.C.; Bogenhagen, D.F. The accessory subunit of Xenopus laevis mitochondrial DNA polymerase gamma increases processivity of the catalytic subunit of human DNA polymerase gamma and is related to class II aminoacyl-tRNA synthetases. Mol. Cell Biol. 1999, 19, 4039–4046. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.; Demeler, B.; Molineux, I.J.; Johnson, K.A.; Yin, Y.W. Each monomer of the dimeric accessory protein for human mitochondrial DNA polymerase has a distinct role in conferring processivity. J. Biol. Chem. 2010, 285, 1490–1499. [Google Scholar] [CrossRef]
- Wojtaszek, J.L.; Hoff, K.E.; Longley, M.J.; Kaur, P.; Andres, S.N.; Wang, H.; Williams, R.S.; Copeland, W.C. Structure-specific roles for PolG2–DNA complexes in maintenance and replication of mitochondrial DNA. Nucleic Acids Res. 2023, 51, 9716–9732. [Google Scholar] [CrossRef]
- Farge, G.; Pham, X.H.; Holmlund, T.; Khorostov, I.; Falkenberg, M. The accessory subunit B of DNA polymerase gamma is required for mitochondrial replisome function. Nucleic Acids Res. 2007, 35, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.C.; Czermin, B.; Muller-Ziermann, S.; Bulst, S.; Stewart, J.D.; Hudson, G.; Schneiderat, P.; Abicht, A.; Holinski-Feder, E.; Lochmuller, H.; et al. Late-onset ptosis and myopathy in a patient with a heterozygous insertion in POLG2. J. Neurol. 2010, 257, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Lehmann Urban, D.; Motlagh Scholle, L.; Alt, K.; Ludolph, A.C.; Rosenbohm, A. Camptocormia as a Novel Phenotype in a Heterozygous POLG2 Mutation. Diagnostics 2020, 10, 68. [Google Scholar] [CrossRef]
- Young, M.J.; Longley, M.J.; Li, F.Y.; Kasiviswanathan, R.; Wong, L.J.; Copeland, W.C. Biochemical analysis of human POLG2 variants associated with mitochondrial disease. Hum. Mol. Genet. 2011, 20, 3052–3066. [Google Scholar] [CrossRef]
- Craig, K.; Young, M.J.; Blakely, E.L.; Longley, M.J.; Turnbull, D.M.; Copeland, W.C.; Taylor, R.W. A p.R369G POLG2 mutation associated with adPEO and multiple mtDNA deletions causes decreased affinity between polymerase γ subunits. Mitochondrion 2012, 12, 313–319. [Google Scholar] [CrossRef]
- Young, M.J.; Humble, M.M.; DeBalsi, K.L.; Sun, K.Y.; Copeland, W.C. POLG2 disease variants: Analyses reveal a dominant negative heterodimer, altered mitochondrial localization and impaired respiratory capacity. Hum. Mol. Genet. 2015, 24, 5184–5197. [Google Scholar] [CrossRef]
- Varma, H.; Faust, P.L.; Iglesias, A.D.; Lagana, S.M.; Wou, K.; Hirano, M.; DiMauro, S.; Mansukani, M.M.; Hoff, K.E.; Nagy, P.L.; et al. Whole exome sequencing identifies a homozygous POLG2 missense variant in an infant with fulminant hepatic failure and mitochondrial DNA depletion. Eur. J. Med. Genet. 2016, 59, 540–545. [Google Scholar] [CrossRef]
- Hoff, K.E.; DeBalsi, K.L.; Sanchez-Quintero, M.J.; Longley, M.J.; Hirano, M.; Naini, A.B.; Copeland, W.C. Characterization of the human homozygous R182W POLG2 mutation in mitochondrial DNA depletion syndrome. PLoS ONE 2018, 13, e0203198. [Google Scholar] [CrossRef]
- Lee, S.J.; Kanwal, S.; Yoo, D.H.; Park, H.R.; Choi, B.O.; Chung, K.W. A POLG2 Homozygous Mutation in an Autosomal Recessive Epilepsy Family Without Ophthalmoplegia. J. Clin. Neurol. 2019, 15, 418–420. [Google Scholar] [CrossRef]
- Dosekova, P.; Dubiel, A.; Karlowicz, A.; Zietkiewicz, S.; Rydzanicz, M.; Habalova, V.; Pienkowski, V.M.; Skirkova, M.; Han, V.; Mosejova, A.; et al. Whole exome sequencing identifies a homozygous POLG2 missense variant in an adult patient presenting with optic atrophy, movement disorders, premature ovarian failure and mitochondrial DNA depletion. Eur. J. Med. Genet. 2020, 63, 103821. [Google Scholar] [CrossRef]
- Van Maldergem, L.; Besse, A.; De Paepe, B.; Blakely, E.L.; Appadurai, V.; Humble, M.M.; Piard, J.; Craig, K.; He, L.; Hella, P.; et al. POLG2 deficiency causes adult-onset syndromic sensory neuropathy, ataxia and parkinsonism. Ann. Clin. Transl. Neurol. 2017, 4, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.A.; Wang, X.; Wang, J.; Peters, A.; Simmons, J.R.; Moran, M.C.; Mathur, A.; Husami, A.; Qian, Y.; Sheridan, R.; et al. Deep Sequencing Reveals Novel Genetic Variants in Children with Acute Liver Failure and Tissue Evidence of Impaired Energy Metabolism. PLoS ONE 2016, 11, e0156738. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhao, X.; Xie, Z.; Yu, M.; Lv, H.; Zhang, W.; Yuan, Y.; Wang, Z. Novel and recurrent nuclear gene variations in a cohort of Chinese progressive external ophthalmoplegia patients with multiple mtDNA deletions. Mol. Genet. Genom. Med. 2022, 10, e1921. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; Dulovic-Mahlow, M.; Baumann, H.; Tunc, S.; Lüth, T.; Schaake, S.; Özcakir, S.; Westenberger, A.; Münchau, A.; Knappe, E.; et al. POLG2-Linked Mitochondrial Disease: Functional Insights from New Mutation Carriers and Review of the Literature. Cerebellum 2024, 23, 479–488. [Google Scholar] [CrossRef]
- Siegert, S.; Mindler, G.T.; Brücke, C.; Kranzl, A.; Patsch, J.; Ritter, M.; Janecke, A.R.; Vodopiutz, J. Expanding the Phenotype of the FAM149B1-Related Ciliopathy and Identification of Three Neurogenetic Disorders in a Single Family. Genes 2021, 12, 1648. [Google Scholar] [CrossRef]
- Kim, M.; Kim, A.R.; Kim, J.S.; Park, J.; Youn, J.; Ahn, J.H.; Mun, J.K.; Lee, C.; Kim, N.S.; Kim, N.K.D.; et al. Clarification of undiagnosed ataxia using whole-exome sequencing with clinical implications. Park. Relat. Disord. 2020, 80, 58–64. [Google Scholar] [CrossRef]
- Do, Y.; Matsuda, S.; Inatomi, T.; Nakada, K.; Yasukawa, T.; Kang, D. The accessory subunit of human DNA polymerase gamma is required for mitochondrial DNA maintenance and is able to stabilize the catalytic subunit. Mitochondrion 2020, 53, 133–139. [Google Scholar] [CrossRef]
- Johnson, A.A.; Tsai, Y.-c.; Graves, S.W.; Johnson, K.A. Human Mitochondrial DNA Polymerase Holoenzyme: Reconstitution and Characterization. Biochemistry 2000, 39, 1702–1708. [Google Scholar] [CrossRef]
- Iyengar, B.; Luo, N.; Farr, C.L.; Kaguni, L.S.; Campos, A.R. The accessory subunit of DNA polymerase gamma is essential for mitochondrial DNA maintenance and development in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2002, 99, 4483–4488. [Google Scholar] [CrossRef]
- Lee, S.K.; Zhao, M.H.; Zheng, Z.; Kwon, J.W.; Liang, S.; Kim, S.H.; Kim, N.H.; Cui, X.S. Polymerase subunit gamma 2 affects porcine oocyte maturation and subsequent embryonic development. Theriogenology 2015, 83, 121–130. [Google Scholar] [CrossRef]
- Di Re, M.; Sembongi, H.; He, J.; Reyes, A.; Yasukawa, T.; Martinsson, P.; Bailey, L.J.; Goffart, S.; Boyd-Kirkup, J.D.; Wong, T.S.; et al. The accessory subunit of mitochondrial DNA polymerase gamma determines the DNA content of mitochondrial nucleoids in human cultured cells. Nucleic Acids Res. 2009, 37, 5701–5713. [Google Scholar] [CrossRef]
- Sigal, N.; Delius, H.; Kornberg, T.; Gefter, M.L.; Alberts, B. A DNA-unwinding protein isolated from Escherichia coli: Its interaction with DNA and with DNA polymerases. Proc. Natl. Acad. Sci. USA 1972, 69, 3537–3541. [Google Scholar] [CrossRef]
- Wold, M.S. Replication protein A: A heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 1997, 66, 61–92. [Google Scholar] [CrossRef]
- Lohman, T.M.; Ferrari, M.E. Escherichia coli single-stranded DNA-binding protein: Multiple DNA-binding modes and cooperativities. Annu. Rev. Biochem. 1994, 63, 527–570. [Google Scholar] [CrossRef]
- Pestryakov, P.E.; Lavrik, O.I. Mechanisms of single-stranded DNA-binding protein functioning in cellular DNA metabolism. Biochemistry 2008, 73, 1388–1404. [Google Scholar] [CrossRef] [PubMed]
- Perales, C.; Cava, F.; Meijer, W.J.; Berenguer, J. Enhancement of DNA, cDNA synthesis and fidelity at high temperatures by a dimeric single-stranded DNA-binding protein. Nucleic Acids Res. 2003, 31, 6473–6480. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.W.; Williams, K.R. Single-stranded DNA binding proteins required for DNA replication. Annu. Rev. Biochem. 1986, 55, 103–136. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Wu, X.; Shell, S.M. Functions of human replication protein A (RPA): From DNA replication to DNA damage and stress responses. J. Cell Physiol. 2006, 208, 267–273. [Google Scholar] [CrossRef]
- Shereda, R.D.; Kozlov, A.G.; Lohman, T.M.; Cox, M.M.; Keck, J.L. SSB as an organizer/mobilizer of genome maintenance complexes. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 289–318. [Google Scholar] [CrossRef]
- Clayton, D.A. Replication of animal mitochondrial DNA. Cell 1982, 28, 693–705. [Google Scholar] [CrossRef]
- Farr, C.L.; Wang, Y.; Kaguni, L.S. Functional interactions of mitochondrial DNA polymerase and single-stranded DNA-binding protein. Template-primer DNA binding and initiation and elongation of DNA strand synthesis. J. Biol. Chem. 1999, 274, 14779–14785. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.A.; Gaspari, M.; Falkenberg, M. TWINKLE Has 5′→3′ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J. Biol. Chem. 2003, 278, 48627–48632. [Google Scholar] [CrossRef] [PubMed]
- Shutt, T.E.; Gray, M.W. Bacteriophage origins of mitochondrial replication and transcription proteins. Trends Genet. 2006, 22, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Tiranti, V.; Rocchi, M.; DiDonato, S.; Zeviani, M. Cloning of human and rat cDNAs encoding the mitochondrial single-stranded DNA-binding protein (SSB). Gene 1993, 126, 219–225. [Google Scholar] [CrossRef]
- Riccio, A.A.; Bouvette, J.; Pedersen, L.C.; Somai, S.; Dutcher, R.C.; Borgnia, M.J.; Copeland, W.C. Structures of the mitochondrial single-stranded DNA binding protein with DNA and DNA polymerase γ. Nucleic Acids Res. 2024, 52, 10329–10340. [Google Scholar] [CrossRef]
- Yang, C.; Curth, U.; Urbanke, C.; Kang, C. Crystal structure of human mitochondrial single-stranded DNA binding protein at 2.4 A resolution. Nat. Struct. Biol. 1997, 4, 153–157. [Google Scholar] [CrossRef]
- Qian, Y.; Johnson, K.A. The human mitochondrial single-stranded DNA-binding protein displays distinct kinetics and thermodynamics of DNA binding and exchange. J. Biol. Chem. 2017, 292, 13068–13084. [Google Scholar] [CrossRef]
- Chrysogelos, S.; Griffith, J. Escherichia coli single-strand binding protein organizes single-stranded DNA in nucleosome-like units. Proc. Natl. Acad. Sci. USA 1982, 79, 5803–5807. [Google Scholar] [CrossRef]
- Lohman, T.M.; Bujalowski, W. Negative cooperativity within individual tetramers of Escherichia coli single strand binding protein is responsible for the transition between the (SSB)35 and (SSB)56 DNA binding modes. Biochemistry 1988, 27, 2260–2265. [Google Scholar] [CrossRef]
- Lohman, T.M.; Bujalowski, W.; Overman, L.B.; Wei, T.F. Interactions of the E. coli single strand binding (SSB) protein with ss nucleic acids. Binding mode transitions and equilibrium binding studies. Biochem. Pharmacol. 1988, 37, 1781–1782. [Google Scholar] [CrossRef]
- Kaur, P.; Longley, M.J.; Pan, H.; Wang, H.; Copeland, W.C. Single-molecule DREEM imaging reveals DNA wrapping around human mitochondrial single-stranded DNA binding protein. Nucleic Acids Res. 2018, 46, 11287–11302. [Google Scholar] [CrossRef] [PubMed]
- Curth, U.; Genschel, J.; Urbanke, C.; Greipel, J. In Vitro and in Vivo Function of the C-Terminus of Escherichia coli Single-Stranded DNA Binding Protein. Nucleic Acids Res. 1996, 24, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.R.; Spicer, E.K.; LoPresti, M.B.; Guggenheimer, R.A.; Chase, J.W. Limited proteolysis studies on the Escherichia coli single-stranded DNA binding protein. Evidence for a functionally homologous domain in both the Escherichia coli and T4 DNA binding proteins. J. Biol. Chem. 1983, 258, 3346–3355. [Google Scholar] [CrossRef]
- Kaur, P.; Longley, M.J.; Pan, H.; Wang, W.; Countryman, P.; Wang, H.; Copeland, W.C. Single-molecule level structural dynamics of DNA unwinding by human mitochondrial Twinkle helicase. J. Biol. Chem. 2020, 295, 5564–5576. [Google Scholar] [CrossRef]
- Ciesielski, G.L.; Bermek, O.; Rosado-Ruiz, F.A.; Hovde, S.L.; Neitzke, O.J.; Griffith, J.D.; Kaguni, L.S. Mitochondrial Single-stranded DNA-binding Proteins Stimulate the Activity of DNA Polymerase γ by Organization of the Template DNA. J. Biol. Chem. 2015, 290, 28697–28707. [Google Scholar] [CrossRef]
- Korhonen, J.A.; Pham, X.H.; Pellegrini, M.; Falkenberg, M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004, 23, 2423–2429. [Google Scholar] [CrossRef]
- Gustafson, M.A.; Perera, L.; Shi, M.; Copeland, W.C. Mechanisms of SSBP1 variants in mitochondrial disease: Molecular dynamics simulations reveal stable tetramers with altered DNA binding surfaces. DNA Repair 2021, 107, 103212. [Google Scholar] [CrossRef]
- Maier, D.; Farr, C.L.; Poeck, B.; Alahari, A.; Vogel, M.; Fischer, S.; Kaguni, L.S.; Schneuwly, S. Mitochondrial single-stranded DNA-binding protein is required for mitochondrial DNA replication and development in Drosophila melanogaster. Mol. Biol. Cell 2001, 12, 821–830. [Google Scholar] [CrossRef]
- Ruhanen, H.; Borrie, S.; Szabadkai, G.; Tyynismaa, H.; Jones, A.W.; Kang, D.; Taanman, J.W.; Yasukawa, T. Mitochondrial single-stranded DNA binding protein is required for maintenance of mitochondrial DNA and 7S DNA but is not required for mitochondrial nucleoid organisation. Biochim. Biophys. Acta 2010, 1803, 931–939. [Google Scholar] [CrossRef]
- Kullar, P.J.; Gomez-Duran, A.; Gammage, P.A.; Garone, C.; Minczuk, M.; Golder, Z.; Wilson, J.; Montoya, J.; Häkli, S.; Kärppä, M.; et al. Heterozygous SSBP1 start loss mutation co-segregates with hearing loss and the m.1555A>G mtDNA variant in a large multigenerational family. Brain 2018, 141, 55–62. [Google Scholar] [CrossRef]
- Jurkute, N.; Leu, C.; Pogoda, H.M.; Arno, G.; Robson, A.G.; Nürnberg, G.; Altmüller, J.; Thiele, H.; Motameny, S.; Toliat, M.R.; et al. SSBP1 mutations in dominant optic atrophy with variable retinal degeneration. Ann. Neurol. 2019, 86, 368–383. [Google Scholar] [CrossRef]
- Del Dotto, V.; Ullah, F.; Di Meo, I.; Magini, P.; Gusic, M.; Maresca, A.; Caporali, L.; Palombo, F.; Tagliavini, F.; Baugh, E.H.; et al. SSBP1 mutations cause mtDNA depletion underlying a complex optic atrophy disorder. J. Clin. Investig. 2020, 130, 108–125. [Google Scholar] [CrossRef]
- Piro-Mégy, C.; Sarzi, E.; Tarrés-Solé, A.; Péquignot, M.; Hensen, F.; Quilès, M.; Manes, G.; Chakraborty, A.; Sénéchal, A.; Bocquet, B.; et al. Dominant mutations in mtDNA maintenance gene SSBP1 cause optic atrophy and foveopathy. J. Clin. Investig. 2020, 130, 143–156. [Google Scholar] [CrossRef]
- Goffart, S.; Cooper, H.M.; Tyynismaa, H.; Wanrooij, S.; Suomalainen, A.; Spelbrink, J.N. Twinkle mutations associated with autosomal dominant progressive external ophthalmoplegia lead to impaired helicase function and in vivo mtDNA replication stalling. Hum. Mol. Genet. 2009, 18, 328–340. [Google Scholar] [CrossRef]
- Wanrooij, S.; Goffart, S.; Pohjoismäki, J.L.; Yasukawa, T.; Spelbrink, J.N. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 2007, 35, 3238–3251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xie, X.; Zhu, X.; Jiang, S.; Milenkovic, D.; Misic, J.; Shi, Y.; Tandukar, N.; Li, X.; Atanassov, I.; et al. The mitochondrial single-stranded DNA binding protein is essential for initiation of mtDNA replication. Sci. Adv. 2021, 7, eabf8631. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V. Helicases: Amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 1993, 3, 419–429. [Google Scholar] [CrossRef]
- Fairman-Williams, M.E.; Guenther, U.-P.; Jankowsky, E. SF1 and SF2 helicases: Family matters. Curr. Opin. Struct. Biol. 2010, 20, 313–324. [Google Scholar] [CrossRef]
- Peter, B.; Falkenberg, M. TWINKLE and Other Human Mitochondrial DNA Helicases: Structure, Function and Disease. Genes 2020, 11, 408. [Google Scholar] [CrossRef]
- Singleton, M.R.; Dillingham, M.S.; Wigley, D.B. Structure and Mechanism of Helicases and Nucleic Acid Translocases. Annu. Rev. Biochem. 2007, 76, 23–50. [Google Scholar] [CrossRef]
- Spelbrink, J.N.; Li, F.-Y.; Tiranti, V.; Nikali, K.; Yuan, Q.-P.; Tariq, M.; Wanrooij, S.; Garrido, N.; Comi, G.; Morandi, L.; et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 2001, 28, 223–231, Erratum in Nat. Genet. 2001, 29, 100. [Google Scholar] [CrossRef]
- Korhonen, J.A.; Pande, V.; Holmlund, T.; Farge, G.; Pham, X.H.; Nilsson, L.; Falkenberg, M. Structure–Function Defects of the TWINKLE Linker Region in Progressive External Ophthalmoplegia. J. Mol. Biol. 2008, 377, 691–705. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial inheritance in yeast. Biochim. Biophys. Acta 2014, 1837, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, Y. Borrowing nuclear DNA helicases to protect mitochondrial DNA. Int. J. Mol. Sci. 2015, 16, 10870–10887. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, T.; Farge, G.; Pande, V.; Korhonen, J.; Nilsson, L.; Falkenberg, M. Structure-function defects of the twinkle amino-terminal region in progressive external ophthalmoplegia. Biochim. Biophys. Acta 2009, 1792, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Matic, S.; Kühl, I.; Ruzzenente, B.; Freyer, C.; Jemt, E.; Park, C.B.; Falkenberg, M.; Larsson, N.G. TWINKLE is an essential mitochondrial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Hum. Mol. Genet. 2013, 22, 1983–1993. [Google Scholar] [CrossRef]
- Farge, G.; Holmlund, T.; Khvorostova, J.; Rofougaran, R.; Hofer, A.; Falkenberg, M. The N-terminal domain of TWINKLE contributes to single-stranded DNA binding and DNA helicase activities. Nucleic Acids Res. 2008, 36, 393–403. [Google Scholar] [CrossRef]
- Jemt, E.; Farge, G.; Bäckström, S.; Holmlund, T.; Gustafsson, C.M.; Falkenberg, M. The mitochondrial DNA helicase TWINKLE can assemble on a closed circular template and support initiation of DNA synthesis. Nucleic Acids Res. 2011, 39, 9238–9249. [Google Scholar] [CrossRef]
- Ziebarth, T.D.; Farr, C.L.; Kaguni, L.S. Modular Architecture of the Hexameric Human Mitochondrial DNA Helicase. J. Mol. Biol. 2007, 367, 1382–1391. [Google Scholar] [CrossRef]
- Ziebarth, T.D.; Gonzalez-Soltero, R.; Makowska-Grzyska, M.M.; Núñez-Ramírez, R.; Carazo, J.M.; Kaguni, L.S. Dynamic effects of cofactors and DNA on the oligomeric state of human mitochondrial DNA helicase. J. Biol. Chem. 2010, 285, 14639–14647. [Google Scholar] [CrossRef]
- Fernández-Millán, P.; Lázaro, M.; Cansız-Arda, Ş.; Gerhold, J.M.; Rajala, N.; Schmitz, C.A.; Silva-Espiña, C.; Gil, D.; Bernadó, P.; Valle, M.; et al. The hexameric structure of the human mitochondrial replicative helicase Twinkle. Nucleic Acids Res. 2015, 43, 4284–4295. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.A.; Bouvette, J.; Perera, L.; Longley, M.J.; Krahn, J.M.; Williams, J.G.; Dutcher, R.; Borgnia, M.J.; Copeland, W.C. Structural insight and characterization of human Twinkle helicase in mitochondrial disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2207459119. [Google Scholar] [CrossRef] [PubMed]
- Peeva, V.; Blei, D.; Trombly, G.; Corsi, S.; Szukszto, M.J.; Rebelo-Guiomar, P.; Gammage, P.A.; Kudin, A.P.; Becker, C.; Altmüller, J.; et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018, 9, 1727. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Nandakumar, D.; Tang, G.Q.; Patel, S.S. Human mitochondrial DNA helicase TWINKLE is both an unwinding and annealing helicase. J. Biol. Chem. 2012, 287, 14545–14556. [Google Scholar] [CrossRef]
- Khan, I.; Crouch, J.D.; Bharti, S.K.; Sommers, J.A.; Carney, S.M.; Yakubovskaya, E.; Garcia-Diaz, M.; Trakselis, M.A.; Brosh, R.M., Jr. Biochemical Characterization of the Human Mitochondrial Replicative Twinkle Helicase: SUBSTRATE SPECIFICITY, DNA BRANCH MIGRATION, AND ABILITY TO OVERCOME BLOCKADES TO DNA UNWINDING. J. Biol. Chem. 2016, 291, 14324–14339. [Google Scholar] [CrossRef]
- Sen, D.; Patel, G.; Patel, S.S. Homologous DNA strand exchange activity of the human mitochondrial DNA helicase TWINKLE. Nucleic Acids Res. 2016, 44, 4200–4210. [Google Scholar] [CrossRef]
- Singh, A.; Patel, G.; Patel, S.S. Twinkle-Catalyzed Toehold-Mediated DNA Strand Displacement Reaction. J. Am. Chem. Soc. 2023, 145, 24522–24534. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Kaguni, L.S. Functional roles of the N- and C-terminal regions of the human mitochondrial single-stranded DNA-binding protein. PLoS ONE 2010, 5, e15379. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Kaguni, L.S. Reduced stimulation of recombinant DNA polymerase γ and mitochondrial DNA (mtDNA) helicase by variants of mitochondrial single-stranded DNA-binding protein (mtSSB) correlates with defects in mtDNA replication in animal cells. J. Biol. Chem. 2011, 286, 40649–40658. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lee, Y.Y.; Chien, P.J.; Tsai, W.A.; Sun, P.J.; Wang, L.T.; Wu, C.C.; Fan, H.F. Single-molecule fluorescence reveals the DNA unwinding mechanism of mitochondrial helicase TWINKLE and its interplay with single-stranded DNA-binding proteins. Nucleic Acids Res. 2025, 53, gkaf803. [Google Scholar] [CrossRef]
- Garrido, N.; Griparic, L.; Jokitalo, E.; Wartiovaara, J.; van der Bliek, A.M.; Spelbrink, J.N. Composition and dynamics of human mitochondrial nucleoids. Mol. Biol. Cell 2003, 14, 1583–1596. [Google Scholar] [CrossRef]
- Tyynismaa, H.; Sembongi, H.; Bokori-Brown, M.; Granycome, C.; Ashley, N.; Poulton, J.; Jalanko, A.; Spelbrink, J.N.; Holt, I.J.; Suomalainen, A. Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum. Mol. Genet. 2004, 13, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, Y.; Kaguni, L.S. Differential phenotypes of active site and human autosomal dominant progressive external ophthalmoplegia mutations in Drosophila mitochondrial DNA helicase expressed in Schneider cells. J. Biol. Chem. 2007, 282, 9436–9444. [Google Scholar] [CrossRef] [PubMed]
- Lönnqvist, T.; Paetau, A.; Valanne, L.; Pihko, H. Recessive twinkle mutations cause severe epileptic encephalopathy. Brain 2009, 132, 1553–1562. [Google Scholar] [CrossRef]
- Hakonen, A.H.; Goffart, S.; Marjavaara, S.; Paetau, A.; Cooper, H.; Mattila, K.; Lampinen, M.; Sajantila, A.; Lönnqvist, T.; Spelbrink, J.N.; et al. Infantile-onset spinocerebellar ataxia and mitochondrial recessive ataxia syndrome are associated with neuronal complex I defect and mtDNA depletion. Hum. Mol. Genet. 2008, 17, 3822–3835. [Google Scholar] [CrossRef]
- Sarzi, E.; Goffart, S.; Serre, V.; Chrétien, D.; Slama, A.; Munnich, A.; Spelbrink, J.N.; Rötig, A. Twinkle helicase (PEO1) gene mutation causes mitochondrial DNA depletion. Ann. Neurol. 2007, 62, 579–587. [Google Scholar] [CrossRef]
- Nikali, K.; Suomalainen, A.; Saharinen, J.; Kuokkanen, M.; Spelbrink, J.N.; Lönnqvist, T.; Peltonen, L. Infantile onset spinocerebellar ataxia is caused by recessive mutations in mitochondrial proteins Twinkle and Twinky. Hum. Mol. Genet. 2005, 14, 2981–2990. [Google Scholar] [CrossRef]
- Longley, M.J.; Humble, M.M.; Sharief, F.S.; Copeland, W.C. Disease variants of the human mitochondrial DNA helicase encoded by C10orf2 differentially alter protein stability, nucleotide hydrolysis, and helicase activity. J. Biol. Chem. 2010, 285, 29690–29702. [Google Scholar] [CrossRef]
- Hakonen, A.H.; Isohanni, P.; Paetau, A.; Herva, R.; Suomalainen, A.; Lönnqvist, T. Recessive Twinkle mutations in early onset encephalopathy with mtDNA depletion. Brain 2007, 130, 3032–3040. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Calleja, M.; Peralta, S.; Matsushima, Y.; Hernandez-Sierra, R.; Whitworth, A.J.; Kaguni, L.S.; Garesse, R. Modeling pathogenic mutations of human twinkle in Drosophila suggests an apoptosis role in response to mitochondrial defects. PLoS ONE 2012, 7, e43954. [Google Scholar] [CrossRef]
- Tyynismaa, H.; Mjosund, K.P.; Wanrooij, S.; Lappalainen, I.; Ylikallio, E.; Jalanko, A.; Spelbrink, J.N.; Paetau, A.; Suomalainen, A. Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 17687–17692. [Google Scholar] [CrossRef] [PubMed]
- Tyynismaa, H.; Carroll, C.J.; Raimundo, N.; Ahola-Erkkilä, S.; Wenz, T.; Ruhanen, H.; Guse, K.; Hemminki, A.; Peltola-Mjøsund, K.E.; Tulkki, V.; et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010, 19, 3948–3958. [Google Scholar] [CrossRef] [PubMed]
- Zeviani, M.; Servidei, S.; Gellera, C.; Bertini, E.; DiMauro, S.; DiDonato, S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature 1989, 339, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Majander, A.; Haltia, M.; Somer, H.; Lönnqvist, J.; Savontaus, M.L.; Peltonen, L. Multiple deletions of mitochondrial DNA in several tissues of a patient with severe retarded depression and familial progressive external ophthalmoplegia. J. Clin. Investig. 1992, 90, 61–66. [Google Scholar] [CrossRef]
- Baloh, R.H.; Salavaggione, E.; Milbrandt, J.; Pestronk, A. Familial parkinsonism and ophthalmoplegia from a mutation in the mitochondrial DNA helicase twinkle. Arch. Neurol. 2007, 64, 998–1000. [Google Scholar] [CrossRef]
- Cerritelli, S.M.; Frolova, E.G.; Feng, C.; Grinberg, A.; Love, P.E.; Crouch, R.J. Failure to Produce Mitochondrial DNA Results in Embryonic Lethality in Rnaseh1 Null Mice. Mol. Cell 2003, 11, 807–815. [Google Scholar] [CrossRef]
- Holmes, J.B.; Akman, G.; Wood, S.R.; Sakhuja, K.; Cerritelli, S.M.; Moss, C.; Bowmaker, M.R.; Jacobs, H.T.; Crouch, R.J.; Holt, I.J. Primer retention owing to the absence of RNase H1 is catastrophic for mitochondrial DNA replication. Proc. Natl. Acad. Sci. USA 2015, 112, 9334–9339. [Google Scholar] [CrossRef]
- Al-Behadili, A.; Uhler, J.P.; Berglund, A.-K.; Peter, B.; Doimo, M.; Reyes, A.; Wanrooij, S.; Zeviani, M.; Falkenberg, M. A two-nuclease pathway involving RNase H1 is required for primer removal at human mitochondrial OriL. Nucleic Acids Res. 2018, 46, 9471–9483. [Google Scholar] [CrossRef]
- Posse, V.; Al-Behadili, A.; Uhler, J.P.; Clausen, A.R.; Reyes, A.; Zeviani, M.; Falkenberg, M.; Gustafsson, C.M. RNase H1 directs origin-specific initiation of DNA replication in human mitochondria. PLoS Genet. 2019, 15, e1007781. [Google Scholar] [CrossRef]
- Akman, G.; Desai, R.; Bailey, L.J.; Yasukawa, T.; Dalla Rosa, I.; Durigon, R.; Holmes, J.B.; Moss, C.F.; Mennuni, M.; Houlden, H.; et al. Pathological ribonuclease H1 causes R-loop depletion and aberrant DNA segregation in mitochondria. Proc. Natl. Acad. Sci. USA 2016, 113, E4276–E4285. [Google Scholar] [CrossRef]
- Lima, W.F.; Murray, H.M.; Damle, S.S.; Hart, C.E.; Hung, G.; De Hoyos, C.L.; Liang, X.-H.; Crooke, S.T. Viable RNaseH1 knockout mice show RNaseH1 is essential for R loop processing, mitochondrial and liver function. Nucleic Acids Res. 2016, 44, 5299–5312. [Google Scholar] [CrossRef]
- Reyes, A.; Rusecka, J.; Tońska, K.; Zeviani, M. RNase H1 Regulates Mitochondrial Transcription and Translation via the Degradation of 7S RNA. Front. Genet. 2020, 10, 1393. [Google Scholar] [CrossRef]
- Wu, H.; Sun, H.; Liang, X.; Lima, W.F.; Crooke, S.T. Human RNase H1 Is Associated with Protein P32 and Is Involved in Mitochondrial Pre-rRNA Processing. PLoS ONE 2013, 8, e71006. [Google Scholar] [CrossRef]
- Misic, J.; Milenkovic, D.; Al-Behadili, A.; Xie, X.; Jiang, M.; Jiang, S.; Filograna, R.; Koolmeister, C.; Siira, S.J.; Jenninger, L.; et al. Mammalian RNase H1 directs RNA primer formation for mtDNA replication initiation and is also necessary for mtDNA replication completion. Nucleic Acids Res. 2022, 50, 8749–8766. [Google Scholar] [CrossRef]
- Carreño-Gago, L.; Blázquez-Bermejo, C.; Díaz-Manera, J.; Cámara, Y.; Gallardo, E.; Martí, R.; Torres-Torronteras, J.; García-Arumí, E. Identification and Characterization of New RNASEH1 Mutations Associated With PEO Syndrome and Multiple Mitochondrial DNA Deletions. Front. Genet. 2019, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Fratter, C.; McMullan, T.F.W. Novel mutation in the RNASEH1 gene in a chronic progressive external ophthalmoplegia patient. Can. J. Ophthalmol. 2018, 53, e203–e205. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Melchionda, L.; Nasca, A.; Carrara, F.; Lamantea, E.; Zanolini, A.; Lamperti, C.; Fang, M.; Zhang, J.; Ronchi, D.; et al. RNASEH1 Mutations Impair mtDNA Replication and Cause Adult-Onset Mitochondrial Encephalomyopathy. Am. J. Hum. Genet. 2015, 97, 186–193. [Google Scholar] [CrossRef]
- Bugiardini, E.; Poole, O.V.; Manole, A.; Pittman, A.M.; Horga, A.; Hargreaves, I.; Woodward, C.E.; Sweeney, M.G.; Holton, J.L.; Taanman, J.-W.; et al. Clinicopathologic and molecular spectrum of RNASEH1-related mitochondrial disease. Neurol. Genet. 2017, 3, e149. [Google Scholar] [CrossRef]
- Duxin, J.P.; Dao, B.; Martinsson, P.; Rajala, N.; Guittat, L.; Campbell, J.L.; Spelbrink, J.N.; Stewart, S.A. Human Dna2 is a nuclear and mitochondrial DNA maintenance protein. Mol. Cell Biol. 2009, 29, 4274–4282. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, M.; Guo, Z.; Lu, H.; Qian, L.; Dai, H.; Qiu, J.; Yakubovskaya, E.; Bogenhagen, D.F.; Demple, B.; et al. Human DNA2 Is a Mitochondrial Nuclease/Helicase for Efficient Processing of DNA Replication and Repair Intermediates. Mol. Cell 2008, 32, 325–336. [Google Scholar] [CrossRef]
- Zheng, L.; Meng, Y.; Campbell, J.L.; Shen, B. Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases. Nucleic Acids Res. 2019, 48, 16–35. [Google Scholar] [CrossRef]
- Copeland, W.C.; Longley, M.J. Mitochondrial genome maintenance in health and disease. DNA Repair 2014, 19, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, D.; Di Fonzo, A.; Lin, W.; Bordoni, A.; Liu, C.; Fassone, E.; Pagliarani, S.; Rizzuti, M.; Zheng, L.; Filosto, M.; et al. Mutations in DNA2 Link Progressive Myopathy to Mitochondrial DNA Instability. Am. J. Hum. Genet. 2013, 92, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, D.; Liu, C.; Caporali, L.; Piga, D.; Li, H.; Tagliavini, F.; Valentino, M.L.; Ferrò, M.T.; Bini, P.; Zheng, L.; et al. Novel mutations in DNA2 associated with myopathy and mtDNA instability. Ann. Clin. Transl. Neurol. 2019, 6, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.H.; Vasta, V.; Cho, A.; Lim, B.C.; Zhang, Q.; Eun, S.H.; Hahn, S.H. Utility of next generation sequencing in genetic diagnosis of early onset neuromuscular disorders. J. Med. Genet. 2015, 52, 208–216. [Google Scholar] [CrossRef]
- González-del Angel, A.; Bisciglia, M.; Vargas-Cañas, S.; Fernandez-Valverde, F.; Kazakova, E.; Escobar, R.E.; Romero, N.B.; Jardel, C.; Rucheton, B.; Stojkovic, T.; et al. Novel Phenotypes and Cardiac Involvement Associated with DNA2 Genetic Variants. Front. Neurol. 2019, 10, 1049. [Google Scholar] [CrossRef]
- Phowthongkum, P.; Sun, A. Novel truncating variant in DNA2-related congenital onset myopathy and ptosis suggests genotype–phenotype correlation. Neuromuscul. Disord. 2017, 27, 616–618. [Google Scholar] [CrossRef]
- Tarnauskaitė, Ž.; Bicknell, L.S.; Marsh, J.A.; Murray, J.E.; Parry, D.A.; Logan, C.V.; Bober, M.B.; de Silva, D.C.; Duker, A.L.; Sillence, D.; et al. Biallelic variants in DNA2 cause microcephalic primordial dwarfism. Hum. Mutat. 2019, 40, 1063–1070. [Google Scholar] [CrossRef]
- Shaheen, R.; Faqeih, E.; Ansari, S.; Abdel-Salam, G.; Al-Hassnan, Z.N.; Al-Shidi, T.; Alomar, R.; Sogaty, S.; Alkuraya, F.S. Genomic analysis of primordial dwarfism reveals novel disease genes. Genome Res. 2014, 24, 291–299. [Google Scholar] [CrossRef]
- Sun, J.; Su, W.; Deng, J.; Qin, Y.; Wang, Z.; Liu, Y. DNA2 mutation causing multisystemic disorder with impaired mitochondrial DNA maintenance. J. Hum. Genet. 2022, 67, 691–699. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Gan, S.; He, L.; Zeng, R.; Xiao, T.; Wu, L. A novel mutation of DNA2 regulates neuronal cell membrane potential and epileptogenesis. Cell Death Discov. 2024, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Manousakis, G.; Chompoopong, P. Novel DNA2 Mutation Leading to Mitochondrial DNA Deletions with MELAS-like Phenotype (P2-11.031). Neurology 2025, 104, 3261. [Google Scholar] [CrossRef]
- Kornblum, C.; Nicholls, T.J.; Haack, T.B.; Schöler, S.; Peeva, V.; Danhauser, K.; Hallmann, K.; Zsurka, G.; Rorbach, J.; Iuso, A.; et al. Loss-of-function mutations in MGME1 impair mtDNA replication and cause multisystemic mitochondrial disease. Nat. Genet. 2013, 45, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, R.J.; Hejnowicz, M.S.; Steczkiewicz, K.; Muszewska, A.; Borowski, L.S.; Ginalski, K.; Dziembowski, A. Identification of a novel human mitochondrial endo-/exonuclease Ddk1/c20orf72 necessary for maintenance of proper 7S DNA levels. Nucleic Acids Res. 2013, 41, 3144–3161. [Google Scholar] [CrossRef]
- Nicholls, T.J.; Zsurka, G.; Peeva, V.; Schöler, S.; Szczesny, R.J.; Cysewski, D.; Reyes, A.; Kornblum, C.; Sciacco, M.; Moggio, M.; et al. Linear mtDNA fragments and unusual mtDNA rearrangements associated with pathological deficiency of MGME1 exonuclease. Hum. Mol. Genet. 2014, 23, 6147–6162. [Google Scholar] [CrossRef]
- Hebbar, M.; Girisha, K.M.; Srivastava, A.; Bielas, S.; Shukla, A. Homozygous c.359del variant in MGME1 is associated with early onset cerebellar ataxia. Eur. J. Med. Genet. 2017, 60, 533–535. [Google Scholar] [CrossRef]
- Hangas, A.; Kekäläinen, N.J.; Potter, A.; Michell, C.; Aho, K.J.; Rutanen, C.; Spelbrink, J.N.; Pohjoismäki, J.L.; Goffart, S. Top3α is the replicative topoisomerase in mitochondrial DNA replication. Nucleic Acids Res. 2022, 50, 8733–8748. [Google Scholar] [CrossRef]
- Jiang, W.; Jia, N.; Guo, C.; Wen, J.; Wu, L.; Ogi, T.; Zhang, H. Predominant cellular mitochondrial dysfunction in the TOP3A gene-caused Bloom syndrome-like disorder. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166106. [Google Scholar] [CrossRef]
- Martin, C.A.; Sarlós, K.; Logan, C.V.; Thakur, R.S.; Parry, D.A.; Bizard, A.H.; Leitch, A.; Cleal, L.; Ali, N.S.; Al-Owain, M.A.; et al. Mutations in TOP3A Cause a Bloom Syndrome-like Disorder. Am. J. Hum. Genet. 2018, 103, 456. [Google Scholar] [CrossRef]
- Primiano, G.; Torraco, A.; Verrigni, D.; Sabino, A.; Bertini, E.; Carrozzo, R.; Silvestri, G.; Servidei, S. Novel TOP3A Variant Associated With Mitochondrial Disease. Neurol. Genet. 2022, 8, e200007. [Google Scholar] [CrossRef]
- Llauradó, A.; Rovira-Moreno, E.; Codina-Solà, M.; Martínez-Saez, E.; Salvadó, M.; Sanchez-Tejerina, D.; Sotoca, J.; López-Diego, V.; Restrepo-Vera, J.L.; Garcia-Arumi, E.; et al. Chronic progressive external ophthalmoplegia plus syndrome due to homozygous missense variant in TOP3A gene. Clin. Genet. 2023, 103, 492–494. [Google Scholar] [CrossRef]
- Kozhukhar, N.; Alexeyev, M.F. 35 Years of TFAM Research: Old Protein, New Puzzles. Biology 2023, 12, 823. [Google Scholar] [CrossRef]
- Stiles, A.R.; Simon, M.T.; Stover, A.; Eftekharian, S.; Khanlou, N.; Wang, H.L.; Magaki, S.; Lee, H.; Partynski, K.; Dorrani, N.; et al. Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol. Genet. Metab. 2016, 119, 91–99. [Google Scholar] [CrossRef]
- Mehmedović, M.; Martucci, M.; Spåhr, H.; Ishak, L.; Mishra, A.; Sanchez-Sandoval, M.E.; Pardo-Hernández, C.; Peter, B.; van den Wildenberg, S.M.; Falkenberg, M.; et al. Disease causing mutation (P178L) in mitochondrial transcription factor A results in impaired mitochondrial transcription initiation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166467. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Rauf, W.; Khan, K.; Khan, S.; Bell, K.M.; de Oliveira, V.C.; Tariq, M.; Bakhshalizadeh, S.; Touraine, P.; Katsanis, N.; et al. A recessive variant in TFAM causes mtDNA depletion associated with primary ovarian insufficiency, seizures, intellectual disability and hearing loss. Hum. Genet. 2021, 140, 1733–1751. [Google Scholar] [CrossRef]
- Belin, A.C.; Bjork, B.F.; Westerlund, M.; Galter, D.; Sydow, O.; Lind, C.; Pernold, K.; Rosvall, L.; Hakansson, A.; Winblad, B.; et al. Association study of two genetic variants in mitochondrial transcription factor A (TFAM) in Alzheimer’s and Parkinson’s disease. Neurosci. Lett. 2007, 420, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gaweda-Walerych, K.; Safranow, K.; Maruszak, A.; Bialecka, M.; Klodowska-Duda, G.; Czyzewski, K.; Slawek, J.; Rudzinska, M.; Styczynska, M.; Opala, G.; et al. Mitochondrial transcription factor A variants and the risk of Parkinson’s disease. Neurosci. Lett. 2010, 469, 24–29. [Google Scholar] [CrossRef]
- Golubickaite, I.; Ugenskiene, R.; Bartnykaite, A.; Poskiene, L.; Vegiene, A.; Padervinskis, E.; Rudzianskas, V.; Juozaityte, E. Mitochondria-Related TFAM and POLG Gene Variants and Associations with Tumor Characteristics and Patient Survival in Head and Neck Cancer. Genes 2023, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zheng, L.; Liu, W.; Wang, X.; Wang, Z.; Wang, Z.; French, A.J.; Kang, D.; Chen, L.; Thibodeau, S.N.; et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011, 71, 2978–2987. [Google Scholar] [CrossRef]
- Oláhová, M.; Peter, B.; Szilagyi, Z.; Diaz-Maldonado, H.; Singh, M.; Sommerville, E.W.; Blakely, E.L.; Collier, J.J.; Hoberg, E.; Stránecký, V.; et al. POLRMT mutations impair mitochondrial transcription causing neurological disease. Nat. Commun. 2021, 12, 1135. [Google Scholar] [CrossRef]
- Fassad, M.R.; Valenzuela, S.; Oláhová, M.; Collier, J.J.; Knowles, C.V.Y.; Mavraki, E.; Elbracht, M.; Güzel, N.; Herberhold, T.; Kurth, I.; et al. Expanding the Genetic and Phenotypic Spectrum of POLRMT-Related Mitochondrial Disease. Clin. Genet. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garone, C.; Garcia-Diaz, B.; Emmanuele, V.; Lopez, L.C.; Tadesse, S.; Akman, H.O.; Tanji, K.; Quinzii, C.M.; Hirano, M. Deoxypyrimidine monophosphate bypass therapy for thymidine kinase 2 deficiency. EMBO Mol. Med. 2014, 6, 1016–1027. [Google Scholar] [CrossRef]
- Lopez-Gomez, C.; Levy, R.J.; Sanchez-Quintero, M.J.; Juanola-Falgarona, M.; Barca, E.; Garcia-Diaz, B.; Tadesse, S.; Garone, C.; Hirano, M. Deoxycytidine and Deoxythymidine Treatment for Thymidine Kinase 2 Deficiency. Ann. Neurol. 2017, 81, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Blazquez-Bermejo, C.; Carreno-Gago, L.; Molina-Granada, D.; Aguirre, J.; Ramon, J.; Torres-Torronteras, J.; Cabrera-Perez, R.; Martin, M.A.; Dominguez-Gonzalez, C.; de la Cruz, X.; et al. Increased dNTP pools rescue mtDNA depletion in human POLG-deficient fibroblasts. FASEB J. 2019, 33, 7168–7179. [Google Scholar] [CrossRef]
- Dombi, E.; Marinaki, T.; Spingardi, P.; Millar, V.; Hadjichristou, N.; Carver, J.; Johnston, I.G.; Fratter, C.; Poulton, J. Nucleoside supplements as treatments for mitochondrial DNA depletion syndrome. Front. Cell Dev. Biol. 2024, 12, 1260496. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, C.K.; Furriol, J.; Chen, A.; Sullivan, G.J.; Bindoff, L.A.; Liang, K.X. Deoxyribonucleoside treatment rescues EtBr-induced mtDNA depletion in iPSC-derived neural stem cells with POLG mutations. FASEB J. 2023, 37, e23139. [Google Scholar] [CrossRef]
- Berrahmoune, S.; Dassi, C.; Pekeles, H.; Cheung, A.C.T.; Gagnon, T.; Waters, P.J.; Buhas, D.; Myers, K.A. Investigating the safety and efficacy of deoxycytidine/deoxythymidine in mitochondrial DNA depletion disorders: Phase 2 open-label trial. J. Neurol. 2025, 272, 307. [Google Scholar] [CrossRef]
- Pekeles, H.; Berrahmoune, S.; Dassi, C.; Cheung, A.C.T.; Gagnon, T.; Waters, P.J.; Eberhard, R.; Buhas, D.; Myers, K.A. Safety and efficacy of deoxycytidine/deoxythymidine combination therapy in POLG-related disorders: 6-month interim results of an open-label, single arm, phase 2 trial. eClinicalMedicine 2024, 74, 102740. [Google Scholar] [CrossRef]
- Chen, A.; Kristiansen, C.K.; Hong, Y.; Kianian, A.; Fang, E.F.; Sullivan, G.J.; Wang, J.; Li, X.; Bindoff, L.A.; Liang, K.X. Nicotinamide Riboside and Metformin Ameliorate Mitophagy Defect in Induced Pluripotent Stem Cell-Derived Astrocytes with POLG Mutations. Front. Cell Dev. Biol. 2021, 9, 737304. [Google Scholar] [CrossRef]
- Valenzuela, S.; Zhu, X.; Macao, B.; Stamgren, M.; Geukens, C.; Charifson, P.S.; Kern, G.; Hoberg, E.; Jenninger, L.; Gruszczyk, A.V.; et al. Small molecules restore mutant mitochondrial DNA polymerase activity. Nature 2025, 642, 501–507. [Google Scholar] [CrossRef]
- Gustafson, M.A.; Sullivan, E.D.; Copeland, W.C. Consequences of compromised mitochondrial genome integrity. DNA Repair 2020, 93, 102916. [Google Scholar] [CrossRef]

| Gene | Disorder | Locus | Function |
|---|---|---|---|
| mtDNA replication and repair | |||
| APTX | ataxia | 9p21.1 | DNA repair |
| DNA2 | mtDNA deletions, PEO, epilepsy | 10q21.3 | Mito/nuclear helicase–nuclease |
| MGME1 | PEO, mtDNA depletion | 20p11.23 | Single-stranded DNA nuclease |
| POLG | PEO, Alpers, ataxia, epilepsy, mtDNA depletion | 15q26.1 | Pol γ catalytic subunit |
| POLG2 | PEO, ataxia, mtDNA depletion | 17q23.3 | Pol γ accessory subunit |
| POLRMT | PEO | 19p13.3 | RNA polymerase |
| RNASEH1 | PEO, encephalopathy, ataxia, mtDNA deletions | 2p25.3 | RNA/DNA hybrid endoribonuclease |
| SSBP1 | Optic atrophy, mtDNA depletion/deletions | 7q34 | Single-stranded DNA-binding protein |
| TFAM | mtDNA depletion | 10q21.1 | DNA compaction, transcription factor |
| TOP3A | Bloom-syndrome-like disorders, PEO | 17p11.2 | Topoisomerase |
| TWNK | PEO, ataxia, mtDNA depletion | 10q24.31 | Replicative helicase |
| nucleotide pool metabolism and maintenance | |||
| ABAT | mtDNA deletions, depletion | 16p13.2 | 4-Aminobutyrate aminotransferase |
| DGUOK | mtDNA depletion, PEO | 2p13.1 | Deoxyguanosine kinase |
| RRM2B | PEO, mtDNA deletions, depletion | 8q22.3 | p53-Ribonucleotide reductase subunit |
| SAMHD1 | mtDNA deletions | 20q11.23 | dNTP triphosphohydrolase |
| SLC25A4 | PEO | 4q34.1 | Adenine nucleotide translocator |
| SUCLA2 | mtDNA depletion | 13q14.2 | ATP-dep Succinate-CoA ligase |
| SUCLG1 | mtDNA depletion | 2p11.2 | GTP-dep Succinate-CoA ligase |
| TK2 | PEO, mtDNA depletion | 16q21 | Mitochondrial thymidine kinase |
| TYMP | MNGIE, mtDNA deletions/depletion | 22q13.33 | Thymidine phosphorylase |
| mitochondrial homeostasis and dynamics | |||
| AFG3L2 | Spinocerebellar ataxia, mtDNA deletions | 18p11.21 | Mitochondrial metalloprotease |
| DNM1L | Encephalopathy, neurological disorders, epilepsy | 12p11.21 | GTPase involved in mitochondrial fission |
| FBXL4 | mtDNA depletion, encephalopathy | 6q16.1–16.2 | Mitochondrial LLR F-Box protein |
| GDAP1 | CMT disease | 8q21.11 | Mitochondrial fission protein |
| GFER | mtDNA deletions, myopathy | 16p13.3 | Protein import to inner membrane |
| MFF | Encephalopathy, hypotonia, neurological disorders | 2q36.3 | Mitochondrial fission protein |
| MFN2 | CMT disease, dominant optic atrophy, mtDNA deletions | 1p36.22 | Mitochondrial fusion protein |
| MPV17 | mtDNA depletion, CMT disease | 2p23.3 | Unknown, inner membrane protein |
| NME3 | Neurodegeneration, hypotonia | 16p13.3 | Nucleoside diphosphate kinase, fusion |
| OPA1 | Dominant optic atrophy, mtDNA deletions, ataxia | 3q29 | Dynamin-related GTPase |
| SLC25A46 | Leigh syndrome, optic atrophy, ataxia, CMT disease | 5q22.1 | Mitochondrial fission protein |
| SPG7 | ataxia, spastic paraplegia | 16q24.3 | Mitochondrial metalloprotease |
| STAT2 | Immunodeficiency | 12q13.3 | Mitochondrial fission protein |
| TMEM65 | mtDNA depletion | 8q24.13 | Na+/Ca2+ exchange |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somai, S.; Aloh, C.H.; King, D.E.; Copeland, W.C. Mitochondrial DNA Replication and Disease: A Historical Perspective on Molecular Insights and Therapeutic Advances. Int. J. Mol. Sci. 2025, 26, 10275. https://doi.org/10.3390/ijms262110275
Somai S, Aloh CH, King DE, Copeland WC. Mitochondrial DNA Replication and Disease: A Historical Perspective on Molecular Insights and Therapeutic Advances. International Journal of Molecular Sciences. 2025; 26(21):10275. https://doi.org/10.3390/ijms262110275
Chicago/Turabian StyleSomai, Shruti, Chioma H. Aloh, Dillon E. King, and William C. Copeland. 2025. "Mitochondrial DNA Replication and Disease: A Historical Perspective on Molecular Insights and Therapeutic Advances" International Journal of Molecular Sciences 26, no. 21: 10275. https://doi.org/10.3390/ijms262110275
APA StyleSomai, S., Aloh, C. H., King, D. E., & Copeland, W. C. (2025). Mitochondrial DNA Replication and Disease: A Historical Perspective on Molecular Insights and Therapeutic Advances. International Journal of Molecular Sciences, 26(21), 10275. https://doi.org/10.3390/ijms262110275






