The Role of Gene Therapy and RNA-Based Therapeutic Strategies in Diabetes
Abstract
1. Introduction
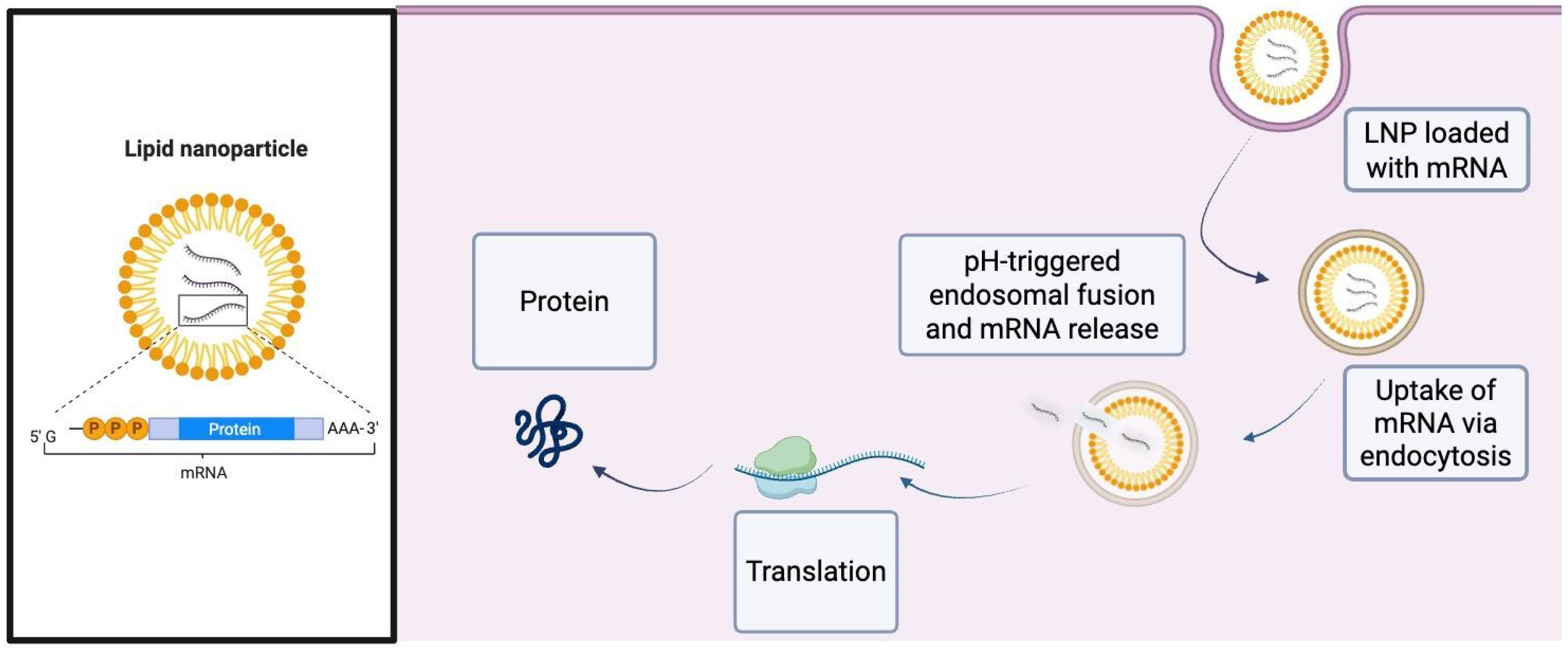
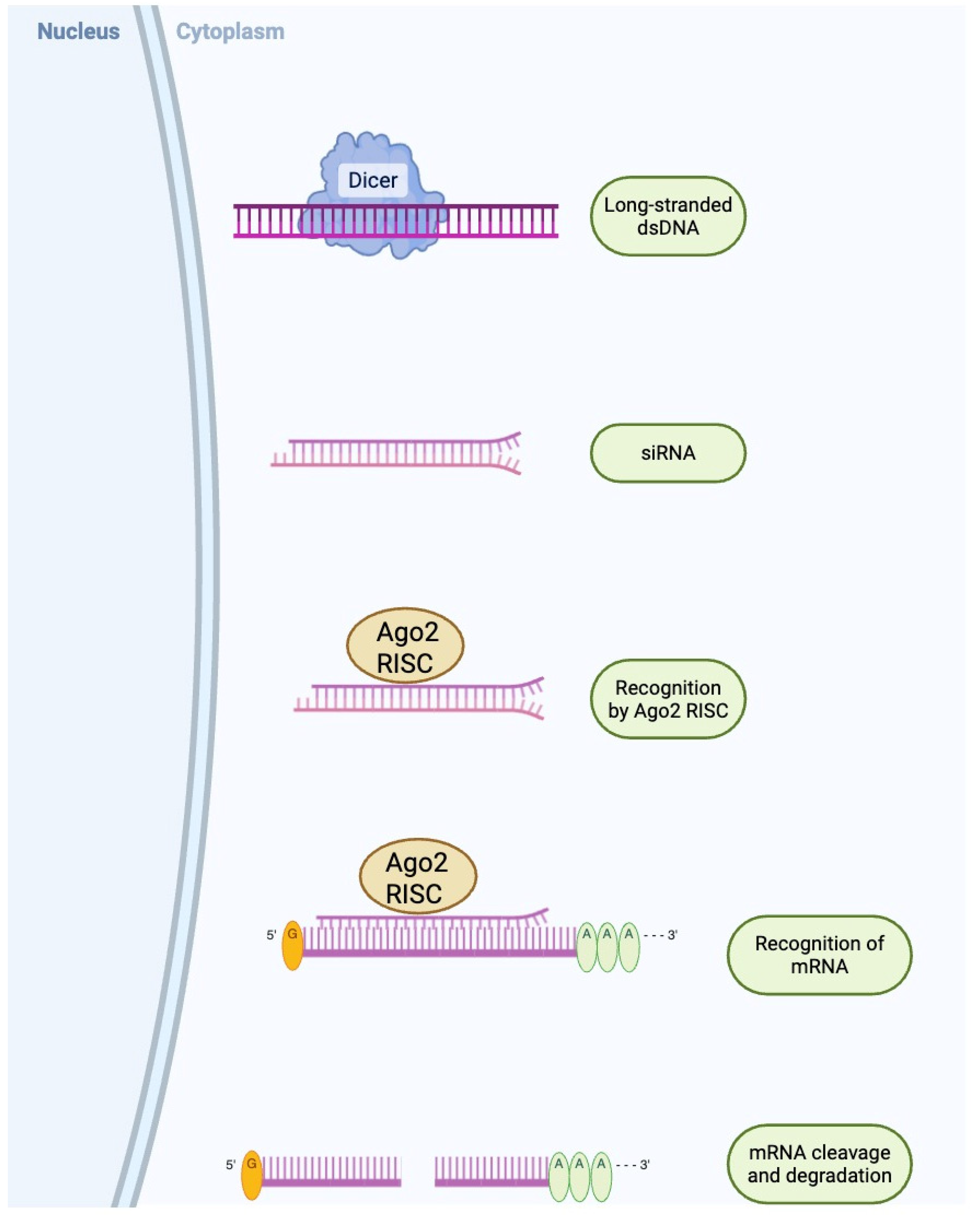
2. Fundamental Concepts and Mechanisms
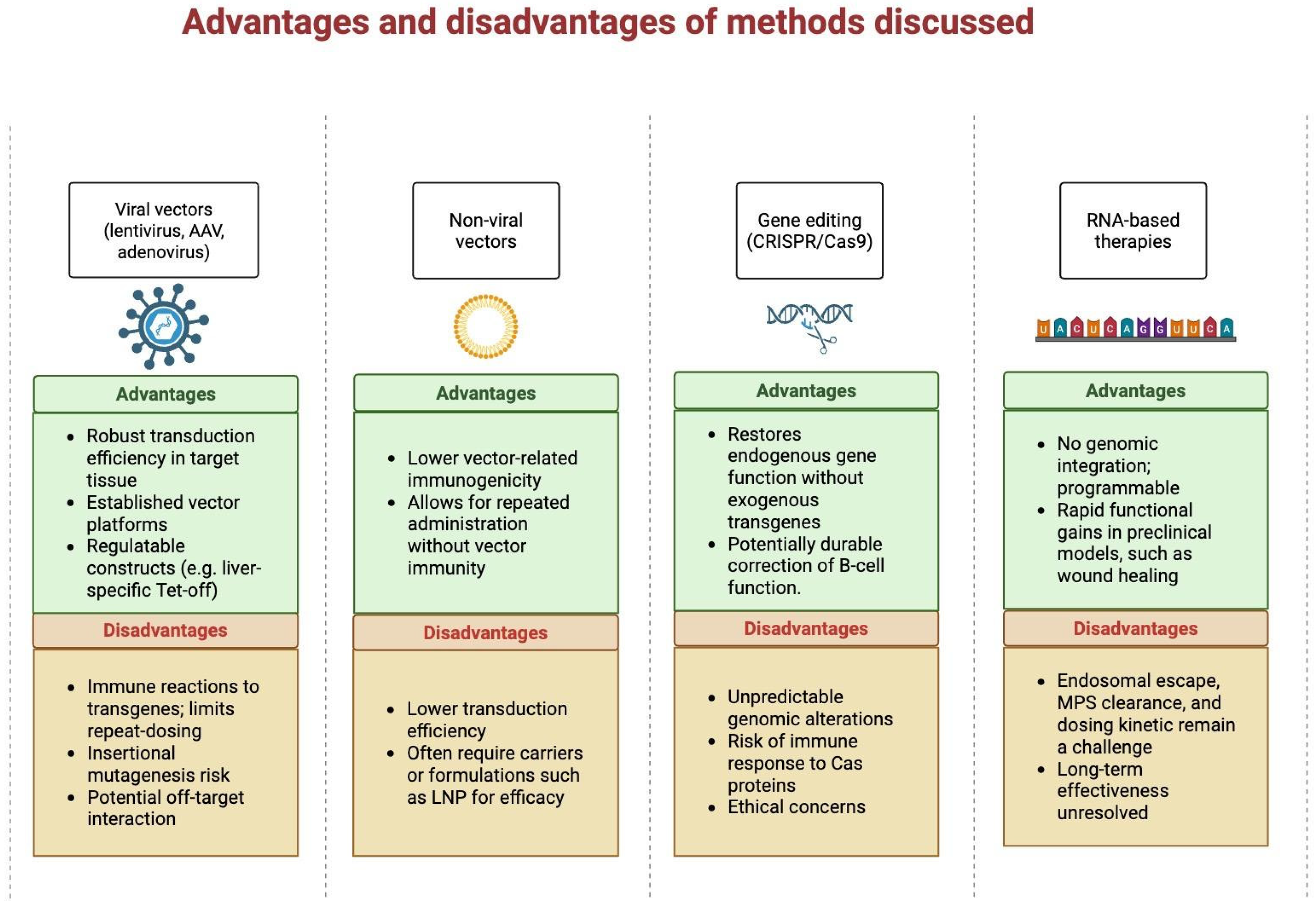
3. Gene Delivery Vectors
| Species | Therapy | Outcome |
|---|---|---|
| NOD mice | Tolerogenic glutamic acid decarboxylase 65 (GAD 65) autoantigen mRNA vaccine | Prevention/delay of T1D onset; improved glucose tolerance [42]. |
| Diabetic mice | Interleukin (IL-4) mRNA to enhance wound healing | Accelerated wound closure, reduced inflammation in diabetic mice [41]. |
| STZ diabetic rats | GLP-1 gene therapy | Lower blood glucose, better insulin sensitivity & glucose tolerance, β-cell regeneration [24]. |
| Autoimmune & chemically induced diabetic mice | PDX1 + MAFA to reprogram α-cells to insulin-producing cells | α → β-like cell reprogramming and correction of hyperglycemia [51]. |
| Dogs | Insulin + Glucokinase via AAV1 in skeletal muscle | Normoglycemia, improved weight, normalized fructosamine, no hypoglycemia during exercise, sustained survival [25]. |
| Adult with T2D | PTP1B inhibition (gene-silencing class) | Improved insulin sensitivity and HbA1c reduction [52]. |
| Therapeutic Strategy | Mechanism/Target | Applications & Outcomes | Limitations/Disadvantages |
|---|---|---|---|
| Gene Addition (e.g., AAV1, AAV8 vectors) | Delivery of insulin, glucokinase, or transcription factors (PDX1, MAFA) to non-dividing cells. | Achieved long-term normoglycemia in diabetic dogs (8 years); α-cell reprogramming restored insulin production in rats. | Immunogenicity risk, limited cargo capacity, insertional mutagenesis (rare), potential for antibody-mediated inactivation. |
| Gene Editing (CRISPR/Cas9, Base & Prime Editing) | Correction of insulin receptor, PDX1, or β-cell regulatory loci. | Restores native glucose responsiveness without exogenous transgenes. | Off-target mutations, low HDR efficiency in quiescent β-cells, unpredictable genome alterations, ethical concerns. |
| Gene Silencing (siRNA, shRNA) | Knockdown of PTP1B, TXNIP, or other insulin resistance/apoptosis mediators. | Improved β-cell viability and reduced fasting glucose; synergy with insulin transgenes enhances control. | Transient effect, delivery inefficiency, potential hepatotoxicity, immune response if viral vector used. |
| Non-Viral & Hybrid Delivery (Lipid nanoparticles, electroporation, RNA complexes) | Non-integrative transfer of DNA/RNA cargo; co-delivery of siRNA and mRNA (e.g., insulin mRNA + PTP1B siRNA). | Enhanced β-cell viability and insulin secretion in T2D models; allows repeat dosing with low immunogenicity. | Lower transduction efficiency, transient expression, manufacturing complexity. |
| Cell-Based Therapy (iPSC/hESC-derived β-like cells, encapsulation) | Stem cell differentiation and ex vivo gene correction; immune-evasive β-cell generation. | Functional glucose-responsive β-cells; teratoma-free populations via GP2 sorting; proof-of-concept for neonatal diabetes correction. | Risk of teratoma from undifferentiated cells, alloimmune rejection, limited long-term graft survival, ethical issues. |
4. Current Strategies and Applications
5. Challenges and Considerations
6. Efficacy Considerations
7. Ethical Debates
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J.; Committee IDFDAtes. IDF Diabetes Atlas. In Idf Diabetes Atlas; © International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Rohban, R.; Martins, C.P.; Esni, F. Advanced therapy to cure diabetes: Mission impossible is now possible? Front. Cell Dev. Biol. 2024, 12, 1484859. [Google Scholar] [CrossRef]
- Khartabil, N.; Avoundjian, A. Gene Therapy and Diabetes: A Narrative Review of Recent Advances and the Role of Multidisciplinary Healthcare Teams. Genes 2025, 16, 107. [Google Scholar] [CrossRef]
- Nguyen Thi, Y.V.; Ho, T.T.; Caglayan, S.; Ramasamy, T.S.; Chu, D.T. RNA therapeutics for treatment of diabetes. Prog. Mol. Biol. Transl. Sci. 2024, 203, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Dave, U.P.; Akagi, K.; Tripathi, R.; Cleveland, S.; Thompson, M.; Yi, M.; Stephens, R.; Downing, J.R.; Jenkins, N.; Copeland, N.G. Murine Leukemias with Insertional Mutations at Lmo2 Are Highly Predicitive of Leukemias Induced Following Gene Therapy in SCID-X1 Patients. Blood 2008, 112, 4629. [Google Scholar] [CrossRef]
- Schulz, M.; Levy, D.I.; Petropoulos, C.J.; Bashirians, G.; Winburn, I.; Mahn, M.; Somanathan, S.; Cheng, S.H.; Byrne, B.J. Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy. Mol. Ther. 2023, 31, 616–630. [Google Scholar] [CrossRef]
- Mallol, C.; Casana, E.; Jimenez, V.; Casellas, A.; Haurigot, V.; Jambrina, C.; Sacristan, V.; Morró, M.; Agudo, J.; Vilà, L.; et al. AAV-mediated pancreatic overexpression of Igf1 counteracts progression to autoimmune diabetes in mice. Mol. Metab. 2017, 6, 664–680. [Google Scholar] [CrossRef]
- Chen, W.; Xie, A.; Chan, L. Mechanistic basis of immunotherapies for type 1 diabetes mellitus. Transl. Res. 2013, 161, 217–229. [Google Scholar] [CrossRef]
- So, W.Y.; Han, W. Gene therapy targeting key beta cell regulators as a potential intervention for diabetes. EMBO Mol. Med. 2024, 16, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Krook, A.; Björnholm, M.; Galuska, D.; Jiang, X.J.; Fahlman, R.; Myers, M.G., Jr.; Wallberg-Henriksson, H.; Zierath, J.R. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 2000, 49, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Sending the signal: Molecular mechanisms regulating glucose uptake. Med. Sci. Sports Exerc. 2004, 36, 1212–1217. [Google Scholar] [CrossRef]
- Jin, W.; Goldfine, A.B.; Boes, T.; Henry, R.R.; Ciaraldi, T.P.; Kim, E.Y.; Emecan, M.; Fitzpatrick, C.; Sen, A.; Shah, A.; et al. Increased SRF transcriptional activity in human and mouse skeletal muscle is a signature of insulin resistance. J. Clin. Investig. 2011, 121, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Kai, Y.; Ono, M.; Ezaki, O. Overexpression of peroxisome proliferator-activated receptor gamma coactivator-1alpha down-regulates GLUT4 mRNA in skeletal muscles. J. Biol. Chem. 2003, 278, 31385–31390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Park, K.G.; Morishita, R.; Kaneda, Y.; Kim, S.Y.; Song, D.K.; Kim, H.S.; Nam, C.W.; Lee, H.C.; Lee, K.U.; et al. Liver-directed gene therapy of diabetic rats using an HVJ-E vector containing EBV plasmids expressing insulin and GLUT 2 transporter. Gene Ther. 2006, 13, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Nett, P.C.; Sollinger, H.W.; Alam, T. Hepatic insulin gene therapy in insulin-dependent diabetes mellitus. Am. J. Transplant. 2003, 3, 1197–1203. [Google Scholar] [CrossRef]
- O’Neill, S.M.; Hinkle, C.; Chen, S.J.; Sandhu, A.; Hovhannisyan, R.; Stephan, S.; Lagor, W.R.; Ahima, R.S.; Johnston, J.C.; Reilly, M.P. Targeting adipose tissue via systemic gene therapy. Gene Ther. 2014, 21, 653–661. [Google Scholar] [CrossRef]
- Safwat, Y.; Yassin, N.; Gamal El Din, M.; Kassem, L. Modulation of skeletal muscle performance and SERCA by exercise and adiponectin gene therapy in insulin-resistant rat. DNA Cell Biol. 2013, 32, 378–385. [Google Scholar] [CrossRef]
- Hashimoto, H.; Mizushima, T.; Ogura, T.; Kagawa, T.; Tomiyama, K.; Takahashi, R.; Yagoto, M.; Kawai, K.; Chijiwa, T.; Nakamura, M.; et al. Study on AAV-mediated gene therapy for diabetes in humanized liver mouse to predict efficacy in humans. Biochem. Biophys. Res. Commun. 2016, 478, 1254–1260. [Google Scholar] [CrossRef]
- Sia, K.C.; Fu, Z.Y.; Calne, R.Y.; Nathwani, A.C.; Lee, K.O.; Gan, S.U. Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice. Cells 2020, 9, 2474. [Google Scholar] [CrossRef]
- Erendor, F.; Eksi, Y.E.; Sahin, E.O.; Balci, M.K.; Griffith, T.S.; Sanlioglu, S. Lentivirus Mediated Pancreatic Beta-Cell-Specific Insulin Gene Therapy for STZ-Induced Diabetes. Mol. Ther. 2021, 29, 149–161. [Google Scholar] [CrossRef]
- Zaia, J.A. The status of gene vectors for the treatment of diabetes. Cell Biochem. Biophys. 2007, 48, 183–190. [Google Scholar] [CrossRef]
- Gan, S.U.; Fu, Z.; Sia, K.C.; Kon, O.L.; Calne, R.; Lee, K.O. Development of a liver-specific Tet-off AAV8 vector for improved safety of insulin gene therapy for diabetes. J. Gene Med. 2019, 21, e3067. [Google Scholar] [CrossRef] [PubMed]
- Michael, L.F.; Wu, Z.; Cheatham, R.B.; Puigserver, P.; Adelmant, G.; Lehman, J.J.; Kelly, D.P.; Spiegelman, B.M. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3820–3825. [Google Scholar] [CrossRef]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Griffith, T.S.; Sanlioglu, S. Therapeutic Potential of Lentivirus-Mediated Glucagon-Like Peptide-1 Gene Therapy for Diabetes. Hum. Gene Ther. 2018, 29, 802–815. [Google Scholar] [CrossRef]
- Jaén, M.L.; Vilà, L.; Elias, I.; Jimenez, V.; Rodó, J.; Maggioni, L.; Ruiz-de Gopegui, R.; Garcia, M.; Muñoz, S.; Callejas, D.; et al. Long-Term Efficacy and Safety of Insulin and Glucokinase Gene Therapy for Diabetes: 8-Year Follow-Up in Dogs. Mol. Ther. Methods Clin. Dev. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Draghia-Akli, R.; Wang, Q. Plasmid-based gene therapy of diabetes mellitus. Gene Ther. 2007, 14, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, H.C. Long-term, antidiabetogenic effects of GLP-1 gene therapy using a double-stranded, adeno-associated viral vector. Gene Ther. 2011, 18, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Jun, H.S. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol. Med. 2002, 8, 62–68. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Tse, L.Y.; Kung, H.F.; Lu, H.; Lam, K.S. Diabetes gene therapy: Potential and challenges. Curr. Gene Ther. 2003, 3, 65–82. [Google Scholar] [CrossRef]
- Lotfi, M.; Butler, A.E.; Sukhorukov, V.N.; Sahebkar, A. Application of CRISPR-Cas9 technology in diabetes research. Diabet. Med. 2024, 41, e15240. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Li, M. The promise of CRISPR/Cas9 technology in diabetes mellitus therapy: How gene editing is revolutionizing diabetes research and treatment. J. Diabetes Complicat. 2023, 37, 108524. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Palyha, O.; Mu, J. Restoration of insulin receptor improves diabetic phenotype in T2DM mice. JCI Insight 2019, 4, e124945. [Google Scholar] [CrossRef]
- Israr, J.; Kumar, A. Current progress in CRISPR-Cas systems for autoimmune diseases. Prog. Mol. Biol. Transl. Sci. 2024, 208, 231–259. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; Zakharova, E.N.; Goncharenko, A.O.; Vorontsova, M.V.; Rumyantsev, S.A.; Mokrysheva, N.G.; Loguinova, M.Y.; Chekhonin, V.P. Current views on etiology, diagnosis, epidemiology and gene therapy of maturity onset diabetes in the young. Front. Endocrinol. 2024, 15, 1497298. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Yerneni, S.S.; Arral, M.L.; LoPresti, S.T.; Chaudhary, N.; Sehrawat, A.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Weissman, D.; et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci. Adv. 2023, 9, eade1444. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094, Correction in: Nat. Rev. Mater. 2022, 7, 65.. [Google Scholar] [CrossRef]
- Cheng, Z.; Fobian, S.F.; Gurrieri, E.; Amin, M.; D’Agostino, V.G.; Falahati, M.; Zalba, S.; Debets, R.; Garrido, M.J.; Saeed, M.; et al. Lipid-based nanosystems: The next generation of cancer immune therapy. J. Hematol. Oncol. 2024, 17, 53. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhong, Y.; Xue, Y.; Liu, Z.; Wang, C.; Kang, D.D.; Li, H.; Hou, X.; Tian, M.; et al. Accelerating diabetic wound healing by ROS-scavenging lipid nanoparticle-mRNA formulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2322935121. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Chen, Y.; Zhou, Z.; Shen, Y.; Wang, Y.; Liu, Z.; Li, X.; Su, Z.; Wu, J. LNP-mRNA vaccine prevents type 1 diabetes in non-obese diabetes mice. J. Control. Release 2024, 375, 513–523. [Google Scholar] [CrossRef]
- Xu, F.; Wu, K.; Zhang, L.; Dai, Y.; Xu, Y. 1860-LB: Preliminary Study of Efficacy and Duration of an mRNA-Based GLP-1R Agonist in Diabetic Monkeys. Diabetes 2024, 73, 1860-LB. [Google Scholar] [CrossRef]
- El-Araby, R.E.; Tu, Q.; Xie, Y.; Aboushousha, T.; Li, Z.; Xu, X.; Zhu, Z.X.; Dong, L.Q.; Chen, J. Adiponectin mRNA Conjugated with Lipid Nanoparticles Specifically Targets the Pathogenesis of Type 2 Diabetes. Aging Dis. 2024, 16, 1059–1079. [Google Scholar] [CrossRef]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef]
- Kasiewicz, L.N.; Whitehead, K.A. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng. Transl. Med. 2019, 4, 75–82. [Google Scholar] [CrossRef]
- Vakili, S.; Ebrahimi, S.S.; Sadeghi, A.; Gorgani-Firuzjaee, S.; Beigy, M.; Pasalar, P.; Meshkani, R. Hydrodynamic-based delivery of PTP1B shRNA reduces plasma glucose levels in diabetic mice. Mol. Med. Rep. 2013, 7, 211–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zinker, B.A.; Rondinone, C.M.; Trevillyan, J.M.; Gum, R.J.; Clampit, J.E.; Waring, J.F.; Xie, N.; Wilcox, D.; Jacobson, P.; Frost, L.; et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 11357–11362. [Google Scholar] [CrossRef] [PubMed]
- Thulé, P.M.; Campbell, A.G.; Jia, D.; Lin, Y.; You, S.; Paveglio, S.; Olson, D.E.; Kozlowski, M. Long-term glycemic control with hepatic insulin gene therapy in streptozotocin-diabetic mice. J. Gene Med. 2015, 17, 141–152. [Google Scholar] [CrossRef]
- Al-Noshokaty, T.M.; Abdelhamid, R.; Abdelmaksoud, N.M.; Khaled, A.; Hossam, M.; Ahmed, R.; Saber, T.; Khaled, S.; Elshaer, S.S.; Abulsoud, A.I. Unlocking the multifaceted roles of GLP-1: Physiological functions and therapeutic potential. Toxicol. Rep. 2025, 14, 101895. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, T.; Lu, A.; Shiota, C.; Huard, M.; Whitney, K.E.; Huard, J. Specific reprogramming of alpha cells to insulin-producing cells by short glucagon promoter-driven Pdx1 and MafA. Mol. Ther. Methods Clin. Dev. 2023, 28, 355–365. [Google Scholar] [CrossRef]
- Digenio, A.; Pham, N.C.; Watts, L.M.; Morgan, E.S.; Jung, S.W.; Baker, B.F.; Geary, R.S.; Bhanot, S. Antisense Inhibition of Protein Tyrosine Phosphatase 1B With IONIS-PTP-1BRx Improves Insulin Sensitivity and Reduces Weight in Overweight Patients with Type 2 Diabetes. Diabetes Care 2018, 41, 807–814. [Google Scholar] [CrossRef]
- Hughes, A.; Jessup, C.; Drogemuller, C.; Mohanasundaram, D.; Milner, C.; Rojas, D.; Russ, G.R.; Coates, P.T. Gene therapy to improve pancreatic islet transplantation for Type 1 diabetes mellitus. Curr. Diabetes Rev. 2010, 6, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Dixena, B.; Jain, N.; Jain, A.K. Lipid-based Non-viral Vector: Promising Approach for Gene Delivery. Curr. Pharm. Des. 2025, 31, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Taghdiri, M.; Mussolino, C. Viral and Non-Viral Systems to Deliver Gene Therapeutics to Clinical Targets. Int. J. Mol. Sci. 2024, 25, 7333. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, S.M.; MacDougald, O.A. Viral and Nonviral Transfer of Genetic Materials to Adipose Tissues: Toward a Gold Standard Approach. Diabetes 2020, 69, 2581–2588. [Google Scholar] [CrossRef]
- Lu, S.L.; Pan, Z.H.; Cui, Z.; Wang, J.L.; Yang, J.L.; Lv, Y.F.; Cao, C.Y.; Huang, X.F. AAV2-mediated ABD-FGF21 gene delivery produces a sustained anti-hyperglycemic effect in type 2 diabetic mouse. Life Sci. 2025, 362, 123344. [Google Scholar] [CrossRef]
- Andretto, V.; Repellin, M.; Pujol, M.; Almouazen, E.; Sidi-Boumedine, J.; Granjon, T.; Zhang, H.; Remaut, K.; Jordheim, L.P.; Briançon, S.; et al. Hybrid core-shell particles for mRNA systemic delivery. J. Control. Release 2023, 353, 1037–1049. [Google Scholar] [CrossRef]
- Manturthi, S.; El-Sahli, S.; Bo, Y.; Durocher, E.; Kirkby, M.; Popatia, A.; Mediratta, K.; Daniel, R.; Lee, S.-H.; Iqbal, U.; et al. Nanoparticles Codelivering mRNA and SiRNA for Simultaneous Restoration and Silencing of Gene/Protein Expression In Vitro and In Vivo. ACS Nanosci. Au 2024, 4, 416–425. [Google Scholar] [CrossRef]
- Karpov, D.S.; Sosnovtseva, A.O.; Pylina, S.V.; Bastrich, A.N.; Petrova, D.A.; Kovalev, M.A.; Shuvalova, A.I.; Eremkina, A.K.; Mokrysheva, N.G. Challenges of CRISPR/Cas-Based Cell Therapy for Type 1 Diabetes: How Not to Engineer a “Trojan Horse”. Int. J. Mol. Sci. 2023, 24, 17320. [Google Scholar] [CrossRef]
- Arivarasan, V.K.; Diwakar, D.; Kamarudheen, N.; Loganathan, K. Current approaches in CRISPR-Cas systems for diabetes. Prog. Mol. Biol. Transl. Sci. 2025, 210, 95–125. [Google Scholar] [CrossRef]
- Bevacqua, R.J.; Dai, X.; Lam, J.Y.; Gu, X.; Friedlander, M.S.H.; Tellez, K.; Miguel-Escalada, I.; Bonàs-Guarch, S.; Atla, G.; Zhao, W.; et al. CRISPR-based genome editing in primary human pancreatic islet cells. Nat. Commun. 2021, 12, 2397. [Google Scholar] [CrossRef]
- Peterson, K.A.; Khalouei, S.; Hanafi, N.; Wood, J.A.; Lanza, D.G.; Lintott, L.G.; Willis, B.J.; Seavitt, J.R.; Braun, R.E.; Dickinson, M.E.; et al. Whole genome analysis for 163 gRNAs in Cas9-edited mice reveals minimal off-target activity. Commun. Biol. 2023, 6, 626. [Google Scholar] [CrossRef]
- Través, P.G.; Pardo, V.; Pimentel-Santillana, M.; González-Rodríguez, Á.; Mojena, M.; Rico, D.; Montenegro, Y.; Calés, C.; Martín-Sanz, P.; Valverde, A.M.; et al. Pivotal role of protein tyrosine phosphatase 1B (PTP1B) in the macrophage response to pro-inflammatory and anti-inflammatory challenge. Cell Death Dis. 2014, 5, e1125. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Ma, S.; Viola, R.; Sui, L.; Cherubini, V.; Barbetti, F.; Egli, D. β Cell Replacement after Gene Editing of a Neonatal Diabetes-Causing Mutation at the Insulin Locus. Stem Cell Rep. 2018, 11, 1407–1415. [Google Scholar] [CrossRef]
- Migliorini, A.; Nostro, M.C.; Sneddon, J.B. Human pluripotent stem cell-derived insulin-producing cells: A regenerative medicine perspective. Cell Metab. 2021, 33, 721–731. [Google Scholar] [CrossRef]
- Karimova, M.V.; Gvazava, I.G.; Vorotelyak, E.A. Overcoming the Limitations of Stem Cell-Derived Beta Cells. Biomolecules 2022, 12, 810. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, Y.; Sarangi, F.; Poon, F.; Nkennor, B.; McGaugh, E.C.; Nunes, S.S.; Nostro, M.C. GP2-enriched pancreatic progenitors give rise to functional beta cells in vivo and eliminate the risk of teratoma formation. Stem Cell Rep. 2022, 17, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Fowler, J.L.; Cromer, M.K.; Lesch, B.J.; Ponce, E.; Uchida, N.; Nishimura, T.; Porteus, M.H.; Loh, K.M. Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. Nat. Commun. 2020, 11, 2713. [Google Scholar] [CrossRef] [PubMed]
- van der Torren, C.R.; Zaldumbide, A.; Duinkerken, G.; Brand-Schaaf, S.H.; Peakman, M.; Stangé, G.; Martinson, L.; Kroon, E.; Brandon, E.P.; Pipeleers, D.; et al. Immunogenicity of human embryonic stem cell-derived beta cells. Diabetologia 2017, 60, 126–133. [Google Scholar] [CrossRef]
- Wang, X.; Brown, N.K.; Wang, B.; Shariati, K.; Wang, K.; Fuchs, S.; Melero-Martin, J.M.; Ma, M. Local Immunomodulatory Strategies to Prevent Allo-Rejection in Transplantation of Insulin-Producing Cells. Adv. Sci. 2021, 8, e2003708. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Yang, L.; Bai, H.H.; Liu, J.P.; Suo, Z.W.; Yang, X.; Hu, X.D. Inhibition of protein tyrosine phosphatase 1B in spinal cord dorsal horn of rats attenuated diabetic neuropathic pain. Eur. J. Pharmacol. 2018, 827, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.H.F.; O’Donnell, B.T.; Shergill, B.; Pham, B.Q.; Tahmouresie, S.; Sanchez, C.N.; Juat, D.J.; Hatch, M.M.S.; Shirure, V.S.; Wortham, M.; et al. A vascularized 3D model of the human pancreatic islet forex vivostudy of immune cell-islet interaction. Biofabrication 2024, 16, 025001. [Google Scholar] [CrossRef] [PubMed]
- Bora, J.; Dey, A.; Lyngdoh, A.R.; Dhasmana, A.; Ranjan, A.; Kishore, S.; Rustagi, S.; Tuli, H.S.; Chauhan, A.; Rath, P.; et al. A critical review on therapeutic approaches of CRISPR-Cas9 in diabetes mellitus. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3459–3481. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.A.A.; Patni, M.A.M.F.; Ba-Hutair, S.N.M.; Wadid, N.A.; Akikwala, M.S. Exploring Novel Treatment Modalities for Type 1 Diabetes Mellitus: Potential and Prospects. Healthcare 2024, 12, 1485. [Google Scholar] [CrossRef]
| Type 1 Diabetes | Type 2 Diabetes |
|---|---|
| Autoimmune destruction of beta cells of the pancreas | Insulin resistance (in muscle, fat, liver) |
| ↓ insulin production → absolute insulin deficiency | ↓ cellular response to insulin → relative deficiency |
| Rapid onset, usually in young | Gradual onset, usually adults |
| Requires insulin therapy | Lifestyle ± oral meds ± insulin therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.T.; Al-Dhaleai, R.E.; Alayadhi, S.M.; Alhalwachi, Z.; Butler, A.E. The Role of Gene Therapy and RNA-Based Therapeutic Strategies in Diabetes. Int. J. Mol. Sci. 2025, 26, 10264. https://doi.org/10.3390/ijms262110264
Khan MT, Al-Dhaleai RE, Alayadhi SM, Alhalwachi Z, Butler AE. The Role of Gene Therapy and RNA-Based Therapeutic Strategies in Diabetes. International Journal of Molecular Sciences. 2025; 26(21):10264. https://doi.org/10.3390/ijms262110264
Chicago/Turabian StyleKhan, Mustafa Tariq, Reem Emad Al-Dhaleai, Sarah M. Alayadhi, Zainab Alhalwachi, and Alexandra E. Butler. 2025. "The Role of Gene Therapy and RNA-Based Therapeutic Strategies in Diabetes" International Journal of Molecular Sciences 26, no. 21: 10264. https://doi.org/10.3390/ijms262110264
APA StyleKhan, M. T., Al-Dhaleai, R. E., Alayadhi, S. M., Alhalwachi, Z., & Butler, A. E. (2025). The Role of Gene Therapy and RNA-Based Therapeutic Strategies in Diabetes. International Journal of Molecular Sciences, 26(21), 10264. https://doi.org/10.3390/ijms262110264





