Genetic Variations in the P2X7 Receptor: Opportunities and Challenges for Drug Development
Abstract
1. Introduction
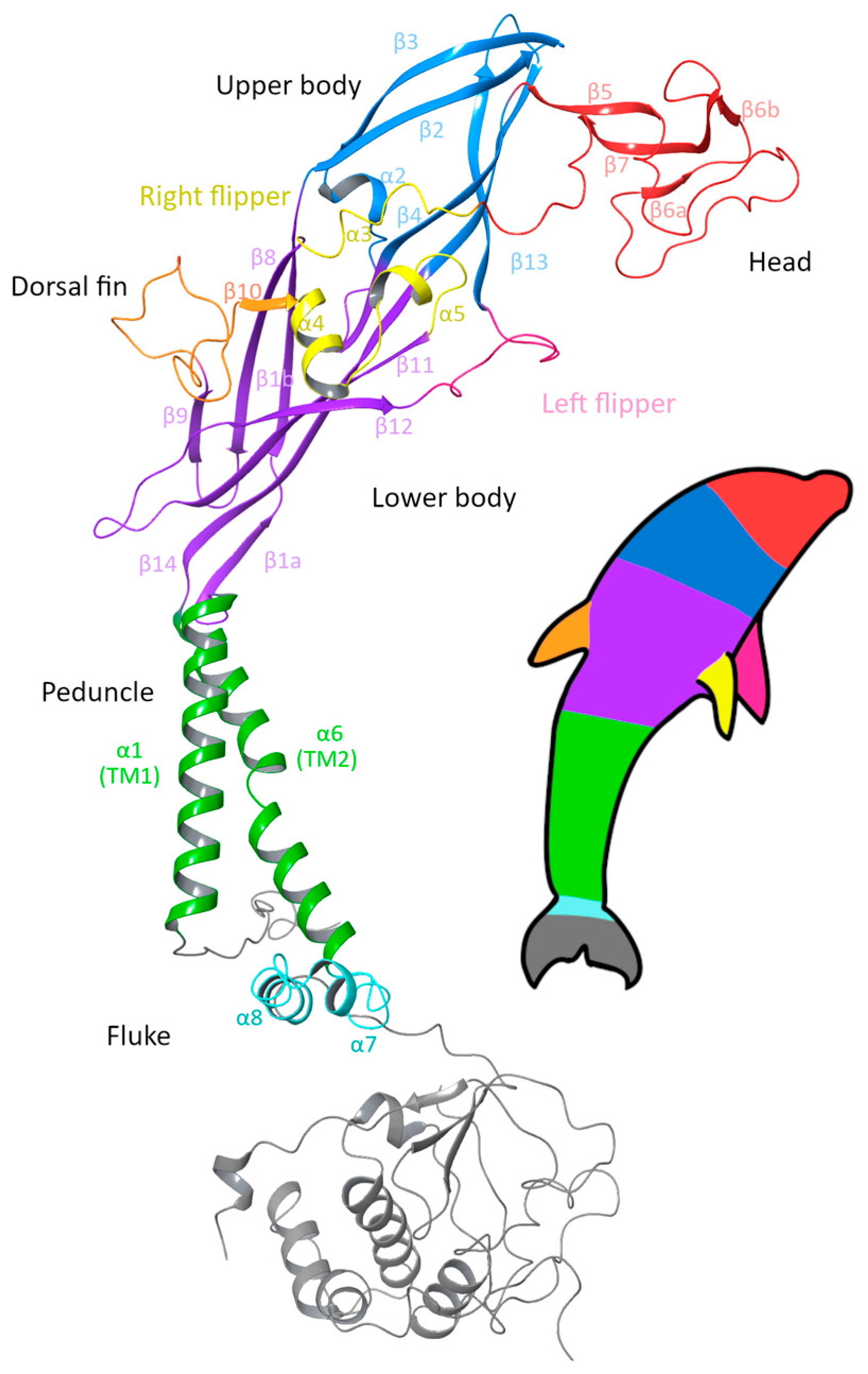
2. Structural Insights and Gating Mechanisms
2.1. Subunit Structure
2.2. Gating Mechanism
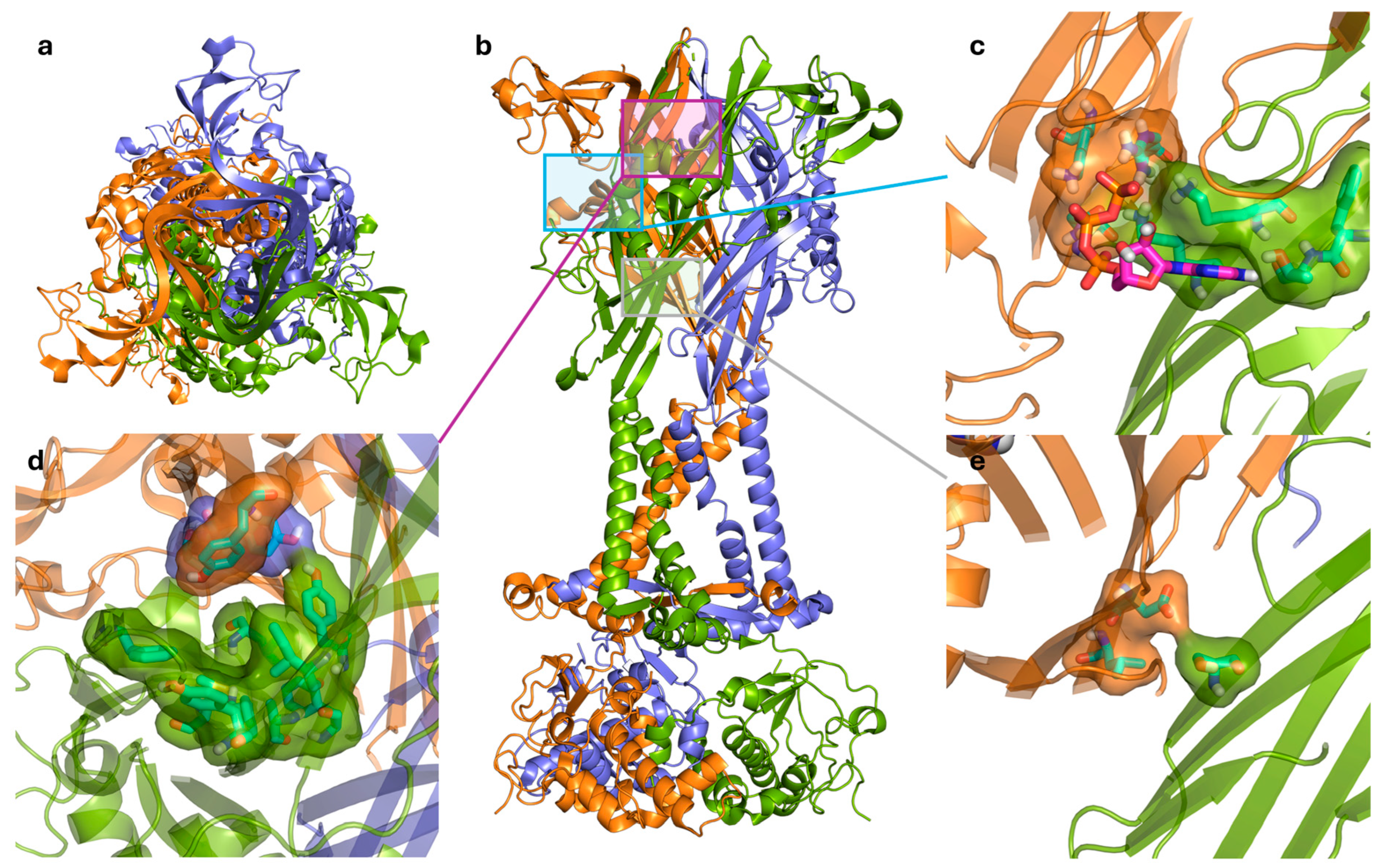
3. The ATP Binding Site
4. Allosteric Modulation and Binding Sites
4.1. Negative Allosteric Modulators
4.2. Positive Allosteric Modulators
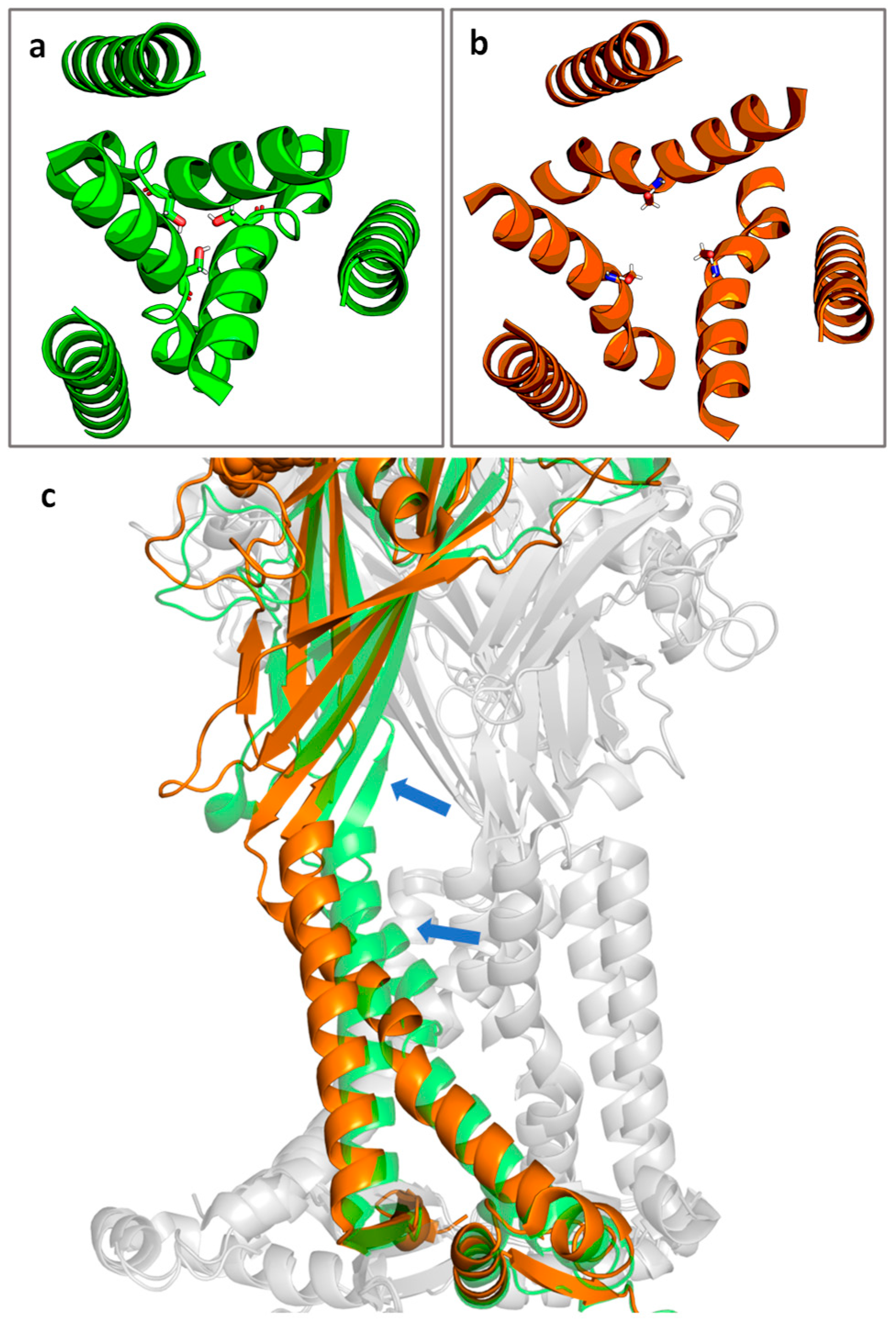
5. Membrane Composition and Receptor Function
The Lipid Environment Critically Impacts P2X7R Activity
6. Genetic Variability: Single Nucleotide Polymorphisms of the P2X7R
6.1. SNPs Complicate Drug Development
6.2. P2RX7 SNPs, Functional Effects and Disease Associations
6.3. P2RX7 SNPs in Mental Health Disorders
6.4. P2RX7 SNPs in Cancer
| rsID | MAF * | Amino Acid Change | Functional Effect [Reference] | Disease Association [Reference] | Lack of Significant Disease Association # [Reference] |
|---|---|---|---|---|---|
| rs17525809 | 0.05 (0.02–0.07) | V76A | Partial loss of pore function [82] Partial loss of ion channel and pore function [123] | Decreased risk of pneumonia and sepsis [88] Increased risk of gout [86] and MS a [87] | Anxiety [124], IS b [125], MM c [126], pancreatic cancer [110] |
| rs28360447 | 0.01 (0.00–0.02) | G150R | Complete loss of pore function [82] Complete loss of ion channel and pore function [92,123] Complete loss of ion channel function and significantly reduced pore function [110] | Decreased risk of pancreatic cancer [110] Decreased BMD d [127] | MM c [126], schizophrenia [128] |
| rs208294 | 0.46 (0.35–0.71) | H155Y | Gain of pore function [82] Gain of ion channel function and increased rate of dye uptake [123] Gain of ion channel function but no significant effect on pore function [92] | Potential protective effect against Alzheimer’s disease [129] Increased risk of alcoholism [90], anxiety [90], chronic pain as PMP e [93], HHV-6A infection f [130], MDD g [90], SLE h with a history of pericarditis [131] | BMD d [132], chronic pain in OA i [93], IS b [125], MM c [126], PTC j [112], RA k [133], schizophrenia [128], SLE h [133], TB l [99] |
| rs28360451 | <0.01 (<0.01) | E186K | Complete loss of ion channel and pore function [123] | Hypertrophic cardiomyopathy [84] | - |
| rs7958311 | 0.29 (0.05–0.45) | R270H | Loss of pore function [82] No significant change in pore function [123] Gain of ion channel function but loss of pore function [92] | Decreased risk of chronic pain [93] Increased risk of chronic pelvic pain [92], fibromyalgia [92], IBS m [92], MDD g with previous stress exposure [91] | Pancreatic cancer [110], TB l [99] |
| rs7958316 | 0.01 (0.00–0.02) | R276H | Complete loss of pore function [82] Normal channel function but reduced pore function [110] Loss of ion channel and pore function [92] | Increased risk of gout [86], pancreatic cancer [110] | Anxiety [124] |
| rs28360457 | <0.01 (0.00–0.01) | R307Q | Complete loss of ion channel and pore function [83,92] | Decreased risk of MS a [85] Higher rate of bone loss in post-menopausal women [132,134] Increased risk of hepatocellular cancer [96] | BMD d [127], MM c [126], pancreatic cancer [110], RA k [133], schizophrenia [128], SLE h [133], disease severity in MS a [135] |
| rs1718119 | 0.31 (0.10–0.46) | A348T | Gain of ion channel and pore function [123] No significant effect on ion channel and pore function [92] | Decreased risk of hepatocellular cancer [96] Increased risk of gout [95], toxoplasmosis [136] Synergistic effect with Q460R, causing increased disease severity in relapse-remitting MS a [135] | Anxiety [124], BD n [107], chronic pain as PMP e and OA i [93], IS b [125], MDD g [104], MM g [126], pancreatic cancer [110], Schizophrenia [128], TB l [99] |
| rs2230911 | 0.14 (0.08–0.31) | T357S | Partial loss of ion channel and pore function [92,123] | Increased risk of pneumonia [88] | Hepatocellular cancer [96], MDD g [104], MM c [126], pancreatic cancer [110], RA k [133], Schizophrenia [128], SLE h [133], TB l [99], toxoplasmosis [136], disease severity in MS a [135] |
| rs2230912 | 0.07 (0.00–0.18) | Q460R | Partial loss of pore function [82] No effect [123] Gain of ion channel function but no effect on pore function [92] | Increased risk of anxiety [90], BD n development [101,105], MDD g [90,103] Synergistic effect with A348T, causing increased disease severity in relapse-remitting MS a [135] | BD n [106,107,108,109], chronic pain in PMP e and OA i [93], MDD g [102,106,107,108], MM c [126], ocular toxoplasmosis [136,137], pancreatic cancer [110], schizophrenia [128] |
| rs3751143 | 0.19 (0.10–0.29) | E496A | Partial loss of ion channel and pore function [92,123] | Decreased BMD d [127] Decreased risk of IS b [125] Increased risk of BD n [138], breast cancer [111], CLL o [113], follicular subtype of PTC j [112], hepatocellular cancer [96], ocular toxoplasmosis [137], Parkinson’s disease [139], TB l [97,98,140] Synergistic protective effect with H155Y against Alzheimer’s disease [129] Increased survival in CLL o [114] | Anxiety [124], BMD d [132], cancer [120], chronic pain in PMP e and OA i [93], CLL o [117,118], MDD g [104], MM c [126], pancreatic cancer [110], PTC j [112], RA k [133], Schizophrenia [128], SLE h [133], toxoplasmosis [136], disease severity in MS a [135] |
| rs1653624 | 0.01 (0.00–0.02) | I568N | Complete loss of ion channel and pore function [123] Partial loss of ion channel and pore function [92] | Higher rate of bone loss in post-menopausal women [134] Increased risk of gout [86] | BMD d [127,132], MM c [126], pancreatic cancer [110], schizophrenia [128] |
7. Haplotypes and Functional Predictions
Haplotypes May Better Explain Disease Associations
8. Splice Variants and Alternatively Spliced Heterotrimers
8.1. Splice Variants
| Variant Name | Suggested Effects [Reference] a | NCBI Accession Number |
|---|---|---|
| P2X7A | Normal function [149,155] | Y09561.1 (RefSeq: NM_002562.6) |
| P2X7B | Reduced agonist sensitivity, but similar channel function to P2X7A homotrimers [149,156] Significant reduction in pore formation [149,156] Little change to antagonist sensitivity [149,156] Heterotrimer formation with P2X7A [156] | AY847298.1 |
| P2X7C | N/I [149] | AY847299.1 |
| P2X7D | N/I [149] | AY847300.1 |
| P2X7E | Deletion of ATP-binding site No surface expression, leading to a non-functional receptor [149,150] | AY847301.1 |
| P2X7F | N/I [149] | AY847302.1 |
| P2X7G | N/I [149] | AY847303.1 |
| P2X7H | Non-functional receptor [149] | AY847304.1 |
| P2X7J | Deficient pore formation Reduced channel function Heterotrimer formation with P2X7A [155] | DQ399293.1 |
| P2X7L | Loss of channel and pore function due to deletion of ATP-binding site Heterotrimer formation with P2X7A [150] | MK465687.1 |
| P2X7M b (ΔE2) | N/I [89] | - |
| P2X7N | N/I [150] | MK465688.1 |
| P2X7O | N/I [150] | MK465689.1 |
| P2X7P | N/I [150] | MK465690.1 |
| P2X7Q | N/I [150] | MK465691.1 |
| Variant 4 (V4) | N/I [157] | - (RefSeq [146]: NR_033950.2) |
| Variant 7 (V7) | N/I [157] | - (RefSeq [146]: NR_033953.2) |
8.2. P2X7B and Alternative Splicing Heterotrimerisation
8.3. Splice Variants Are Not Well Characterised
9. Clinical Failures and Therapeutic Barriers
9.1. Clinical Trials and the Polymorphous P2X7R
9.2. The P2X7R Has Potential as a Diagnostic or Prognostic Marker
10. Forward-Looking: Potential of In Silico Studies in the P2X7R
New Structural Insights Now Enable Computational Investigations
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, N.-L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered Cytokine Production in Mice Lacking P2X7 Receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef]
- Sluyter, R.; Shemon, A.N.; Wiley, J.S. Glu496 to Ala Polymorphism in the P2X7 Receptor Impairs ATP-Induced IL-1β Release from Human Monocytes. J. Immunol. 2004, 172, 3399–3405. [Google Scholar] [CrossRef]
- Mehta, V.B.; Hart, J.; Wewers, M.D. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J. Biol. Chem. 2001, 276, 3820–3826. [Google Scholar] [CrossRef]
- Sluyter, R.; Dalitz, J.G.; Wiley, J.S. P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004, 5, 588–591. [Google Scholar] [CrossRef]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 Interactions and Signaling—Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef]
- Pelegrin, P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem. Pharmacol. 2021, 187, 114385. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020, 10, 28. [Google Scholar] [CrossRef]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, C.; Jia, F.; Wu, H.; Hao, H.; Jing, S. P2X7 receptor as a key player in pathological pain: Insights into Neuropathic, inflammatory, and cancer pain. Front. Pharmacol. 2025, 16, 1585545. [Google Scholar] [CrossRef]
- Wei, S.; Song, X.; Mou, Y.; Yang, T.; Wang, Y.; Wang, H.; Ren, C.; Song, X. New insights into pathogenisis and therapies of P2X7R in Parkinson’s disease. npj Park. Dis. 2025, 11, 108. [Google Scholar] [CrossRef]
- Ribeiro, D.E.; Oliveira-Giacomelli, Á.; Glaser, T.; Arnaud-Sampaio, V.F.; Andrejew, R.; Dieckmann, L.; Baranova, J.; Lameu, C.; Ratajczak, M.Z.; Ulrich, H. Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol. Psychiatry 2021, 26, 1044–1059. [Google Scholar] [CrossRef]
- Garcia-Villalba, J.; Hurtado-Navarro, L.; Penin-Franch, A.; Molina-Lopez, C.; Martinez-Alarcon, L.; Angosto-Bazarra, D.; Baroja-Mazo, A.; Pelegrin, P. Soluble P2X7 Receptor Is Elevated in the Plasma of COVID-19 Patients and Correlates with Disease Severity. Front. Immunol. 2022, 13, 894470. [Google Scholar] [CrossRef]
- Korb, V.G.; Schultz, I.C.; Beckenkamp, L.R.; Wink, M.R. A Systematic Review of the Role of Purinergic Signalling Pathway in the Treatment of COVID-19. Int. J. Mol. Sci. 2023, 24, 7865. [Google Scholar] [CrossRef]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Peverini, L.; Beudez, J.; Dunning, K.; Chataigneau, T.; Grutter, T. New Insights Into Permeation of Large Cations Through ATP-Gated P2X Receptors. Front. Mol. Neurosci. 2018, 11, 265. [Google Scholar] [CrossRef]
- Khalafalla, M.G.; Woods, L.T.; Camden, J.M.; Khan, A.A.; Limesand, K.H.; Petris, M.J.; Erb, L.; Weisman, G.A. P2X7 receptor antagonism prevents IL-1β release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathy. J. Biol. Chem. 2017, 292, 16626–16637. [Google Scholar] [CrossRef]
- Bergamin, L.S.; Braganhol, E.; Figueiró, F.; Casali, E.A.; Zanin, R.F.; Sévigny, J.; Battastini, A.M. Involvement of purinergic system in the release of cytokines by macrophages exposed to glioma-conditioned medium. J. Cell. Biochem. 2015, 116, 721–729. [Google Scholar] [CrossRef]
- Kan, L.K.; Drill, M.; Jayakrishnan, P.C.; Sequeira, R.P.; Galea, E.; Todaro, M.; Sanfilippo, P.G.; Hunn, M.; Williams, D.A.; O’Brien, T.J.; et al. P2X7 receptor antagonism by AZ10606120 significantly reduced in vitro tumour growth in human glioblastoma. Sci. Rep. 2023, 13, 8435. [Google Scholar] [CrossRef]
- Amoroso, F.; Capece, M.; Rotondo, A.; Cangelosi, D.; Ferracin, M.; Franceschini, A.; Raffaghello, L.; Pistoia, V.; Varesio, L.; Adinolfi, E. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: Evidence in experimental neuroblastoma. Oncogene 2015, 34, 5240–5251. [Google Scholar] [CrossRef]
- Giannuzzo, A.; Saccomano, M.; Napp, J.; Ellegaard, M.; Alves, F.; Novak, I. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int. J. Cancer 2016, 139, 2540–2552. [Google Scholar] [CrossRef]
- Agrawal, A.; Buckley, K.A.; Bowers, K.; Furber, M.; Gallagher, J.A.; Gartland, A. The effects of P2X7 receptor antagonists on the formation and function of human osteoclasts in vitro. Purinergic Signal. 2010, 6, 307–315. [Google Scholar] [CrossRef]
- Carmo, M.R.; Menezes, A.P.; Nunes, A.C.; Pliássova, A.; Rolo, A.P.; Palmeira, C.M.; Cunha, R.A.; Canas, P.M.; Andrade, G.M. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014, 81, 142–152. [Google Scholar] [CrossRef]
- Engel, T.; Gomez-Villafuertes, R.; Tanaka, K.; Mesuret, G.; Sanz-Rodriguez, A.; Garcia-Huerta, P.; Miras-Portugal, M.T.; Henshall, D.C.; Diaz-Hernandez, M. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012, 26, 1616–1628. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Li, X.-Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.T.; Hsieh, G.; Zhu, C.Z.; Mikusa, J.P.; Hernandez, G.; Zhong, C.; Gauvin, D.M.; Chandran, P.; et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a Novel and Selective P2X7 Receptor Antagonist, Dose-Dependently Reduces Neuropathic Pain in the Rat. J. Pharmacol. Exp. Ther. 2006, 319, 1376–1385. [Google Scholar] [CrossRef]
- Andó, R.D.; Méhész, B.; Gyires, K.; Illes, P.; Sperlágh, B. A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br. J. Pharmacol. 2010, 159, 1106–1117. [Google Scholar] [CrossRef]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Oken, A.C.; Turcu, A.L.; Tzortzini, E.; Georgiou, K.; Nagel, J.; Westermann, F.G.; Barniol-Xicota, M.; Seidler, J.; Kim, G.-R.; Lee, S.-D.; et al. A polycyclic scaffold identified by structure-based drug design effectively inhibits the human P2X7 receptor. Nat. Commun. 2025, 16, 8283. [Google Scholar] [CrossRef] [PubMed]
- Shinotsuka, N.; Shimizu, H.; Komatsu, T.; Ito, A.; Yoshikawa, S.; Takashima, T.; Sugiura, A.; Moriguchi, Y.; Ohno, Y.; Yamasawa, M.; et al. AK1780, a selective P2X7 receptor antagonist with high central nervous system penetration, exhibits analgesic effect in rat neuropathic pain model. J. Pain. 2025, 31, 105380. [Google Scholar] [CrossRef]
- Arcangeli, A.; Becchetti, A. New Trends in Cancer Therapy: Targeting Ion Channels and Transporters. Pharmaceuticals 2010, 3, 1202–1224. [Google Scholar] [CrossRef]
- Karasawa, A.; Kawate, T. Structural basis for subtype-specific inhibition of the P2X7 receptor. eLife 2016, 5, e22153. [Google Scholar] [CrossRef]
- Jiang, L.H. Inhibition of P2X(7) receptors by divalent cations: Old action and new insight. Eur. Biophys. J. 2009, 38, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A. The P2X7 Receptor: Methods and Protocols, 1st ed.; Humana: New York, NY, USA, 2022; Volume 2510, p. 394. [Google Scholar]
- Fuller, S.J.; Stokes, L.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Genetics of the P2X7 receptor and human disease. Purinergic Signal. 2009, 5, 257–262. [Google Scholar] [CrossRef] [PubMed]
- De Salis, S.K.F.; Li, L.; Chen, Z.; Lam, K.W.; Skarratt, K.K.; Balle, T.; Fuller, S.J. Alternatively Spliced Isoforms of the P2X7 Receptor: Structure, Function and Disease Associations. Int. J. Mol. Sci. 2022, 23, 8174. [Google Scholar] [CrossRef]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670.e613. [Google Scholar] [CrossRef]
- Oken, A.C.; Lisi, N.E.; Krishnamurthy, I.; McCarthy, A.E.; Godsey, M.H.; Glasfeld, A.; Mansoor, S.E. High-affinity agonism at the P2X7 receptor is mediated by three residues outside the orthosteric pocket. Nat. Commun. 2024, 15, 6662. [Google Scholar] [CrossRef]
- Oken, A.C.; Ditter, I.A.; Lisi, N.E.; Krishnamurthy, I.; Godsey, M.H.; Mansoor, S.E. P2X7 receptors exhibit at least three modes of allosteric antagonism. Sci. Adv. 2024, 10, eado5084. [Google Scholar] [CrossRef]
- Habermacher, C.; Dunning, K.; Chataigneau, T.; Grutter, T. Molecular structure and function of P2X receptors. Neuropharmacology 2016, 104, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef]
- Schrödinger, L. Maestro, Schrödinger Release 2024-2; Schrödinger, LLC: New York, NY, USA, 2024.
- Schrödinger, L. The PyMOL Molecular Graphics System, Version 2.3; Schrödinger, LLC: New York, NY, USA, 2019.
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Cevoli, F.; Arnould, B.; Peralta, F.A.; Grutter, T. Untangling Macropore Formation and Current Facilitation in P2X7. Int. J. Mol. Sci. 2023, 24, 10896. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The Cytolytic P2z Receptor for Extracellular ATP Identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- Jursik, C.; Sluyter, R.; Georgiou, J.G.; Fuller, S.J.; Wiley, J.S.; Gu, B.J. A quantitative method for routine measurement of cell surface P2X7 receptor function in leucocyte subsets by two-colour time-resolved flow cytometry. J. Immunol. Methods 2007, 325, 67–77. [Google Scholar] [CrossRef]
- Gu, B.J.; Avula, P.; Wiley, J.S. Assays to Measure Purinoceptor Pore Dilation. In Purinergic Signaling: Methods and Protocols, 1st ed.; Pelegrín, P., Ed.; Humana: New York, NY, USA, 2020; pp. 323–334. [Google Scholar]
- Steinberg, T.H.; Newman, A.S.; Swanson, J.A.; Silverstein, S.C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J. Biol. Chem. 1987, 262, 8884–8888. [Google Scholar] [CrossRef] [PubMed]
- Lammas, D.A.; Stober, C.; Harvey, C.J.; Kendrick, N.; Panchalingam, S.; Kumararatne, D.S. ATP-Induced Killing of Mycobacteria by Human Macrophages Is Mediated by Purinergic P2Z(P2X7) Receptors. Immunity 1997, 7, 433–444. [Google Scholar] [CrossRef]
- Li, M.; Toombes, G.E.S.; Silberberg, S.D.; Swartz, K.J. Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nat. Neurosci. 2015, 18, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Harkat, M.; Peverini, L.; Cerdan, A.H.; Dunning, K.; Beudez, J.; Martz, A.; Calimet, N.; Specht, A.; Cecchini, M.; Chataigneau, T.; et al. On the permeation of large organic cations through the pore of ATP-gated P2X receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E3786–E3795. [Google Scholar] [CrossRef]
- Karasawa, A.; Michalski, K.; Mikhelzon, P.; Kawate, T. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. eLife 2017, 6, e31186. [Google Scholar] [CrossRef]
- Alves, L.A.; de Melo Reis, R.A.; de Souza, C.A.M.; de Freitas, M.S.; Teixeira, P.C.N.; Neto Moreira Ferreira, D.; Xavier, R.F. The P2X7 receptor: Shifting from a low- to a high-conductance channel—An enigmatic phenomenon? Biochim. Biophys. Acta Biomembr. 2014, 1838, 2578–2587. [Google Scholar] [CrossRef]
- Ugur, M.; Ugur, Ö. A Mechanism-Based Approach to P2X7 Receptor Action. Mol. Pharmacol. 2019, 95, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-H.; Caseley, E.A.; Muench, S.P.; Roger, S. Structural basis for the functional properties of the P2X7 receptor for extracellular ATP. Purinergic Signal. 2021, 17, 331–344. [Google Scholar] [CrossRef]
- Browne, L.E.; Jiang, L.H.; North, R.A. New structure enlivens interest in P2X receptors. Trends Pharmacol. Sci. 2010, 31, 229–237. [Google Scholar] [CrossRef]
- Wilkinson, W.J.; Jiang, L.-H.; Surprenant, A.; North, R.A. Role of Ectodomain Lysines in the Subunits of the Heteromeric P2X2/3 Receptor. Mol. Pharmacol. 2006, 70, 1159–1163. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Bin Dayel, A.; Evans, R.J.; Schmid, R. Mapping the Site of Action of Human P2X7 Receptor Antagonists AZ11645373, Brilliant Blue G, KN-62, Calmidazolium, and ZINC58368839 to the Intersubunit Allosteric Pocket. Mol. Pharmacol. 2019, 96, 355–363. [Google Scholar] [CrossRef]
- Caseley, E.A.; Muench, S.P.; Baldwin, S.A.; Simmons, K.; Fishwick, C.W.; Jiang, L.H. Docking of competitive inhibitors to the P2X7 receptor family reveals key differences responsible for changes in response between rat and human. Bioorg. Med. Chem. Lett. 2015, 25, 3164–3167. [Google Scholar] [CrossRef]
- Stokes, L.; Bidula, S.; Bibič, L.; Allum, E. To Inhibit or Enhance? Is There a Benefit to Positive Allosteric Modulation of P2X Receptors? Front. Pharmacol. 2020, 11, 627. [Google Scholar] [CrossRef]
- Darville, T.; Welter-Stahl, L.; Cruz, C.; Sater, A.A.; Andrews, C.W., Jr.; Ojcius, D.M. Effect of the Purinergic Receptor P2X7 on Chlamydia Infection in Cervical Epithelial Cells and Vaginally Infected Mice. J. Immunol. 2007, 179, 3707–3714. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, V.; Orecchioni, S.; Talarico, G.; Reggiani, F.; Mazzetti, C.; Bertolini, F.; Orioli, E.; Adinolfi, E.; Di Virgilio, F.; Pezzi, A.; et al. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget 2016, 8, 5895–5908. [Google Scholar] [CrossRef]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef]
- Roger, S.; Jelassi, B.; Couillin, I.; Pelegrin, P.; Besson, P.; Jiang, L.H. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2584–2602. [Google Scholar] [CrossRef]
- Bidula, S.M.; Cromer, B.A.; Walpole, S.; Angulo, J.; Stokes, L. Mapping a novel positive allosteric modulator binding site in the central vestibule region of human P2X7. Sci. Rep. 2019, 9, 3231. [Google Scholar] [CrossRef]
- Silberberg, S.D.; Li, M.; Swartz, K.J. Ivermectin Interaction with Transmembrane Helices Reveals Widespread Rearrangements during Opening of P2X Receptor Channels. Neuron 2007, 54, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trudell, J.; Li, K.; Alkana, R.; Davies, D.; Asatryan, L. Tryptophan 46 is a site for ethanol and ivermectin action in P2X4 receptors. Purinergic Signal. 2013, 9, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Nörenberg, W.; Sobottka, H.; Hempel, C.; Plötz, T.; Fischer, W.; Schmalzing, G.; Schaefer, M. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. Br. J. Pharmacol. 2012, 167, 48–66. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Michel, V.; Bakovic, M. Lipid rafts in health and disease. Biol. Cell 2007, 99, 129–140. [Google Scholar] [CrossRef]
- Savio, L.E.B.; de Andrade Mello, P.; Santos, S.A.C.S.; de Sousa, J.C.; Oliveira, S.D.S.; Minshall, R.D.; Kurtenbach, E.; Wu, Y.; Longhi, M.S.; Robson, S.C.; et al. P2X7 receptor activation increases expression of caveolin-1 and formation of macrophage lipid rafts, thereby boosting CD39 activity. J. Cell Sci. 2020, 133, jcs237560. [Google Scholar] [CrossRef]
- Robinson, L.E.; Shridar, M.; Smith, P.; Murrell-Lagnado, R.D. Plasma Membrane Cholesterol as a Regulator of Human and Rodent P2X7 Receptor Activation and Sensitization. J. Biol. Chem. 2014, 289, 31983–31994. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Evans, R.J. Contribution of the Juxtatransmembrane Intracellular Regions to the Time Course and Permeation of ATP-gated P2X7 Receptor Ion Channels. J. Biol. Chem. 2015, 290, 14556–14566. [Google Scholar] [CrossRef]
- Gonnord, P.; Delarasse, C.; Auger, R.; Benihoud, K.; Prigent, M.; Cuif, M.H.; Lamaze, C.; Kanellopoulos, J.M. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 2009, 23, 795–805. [Google Scholar] [CrossRef]
- Schäfer, W.; Stähler, T.; Pinto Espinoza, C.; Danquah, W.; Knop, J.H.; Rissiek, B.; Haag, F.; Koch-Nolte, F. Origin, distribution, and function of three frequent coding polymorphisms in the gene for the human P2X7 ion channel. Front. Pharmacol. 2022, 13, 1033135. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Fuller, S.J.; Sluyter, R.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1β secretion. FASEB J. 2010, 24, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Sluyter, R.; Skarratt, K.K.; Shemon, A.N.; Dao-Ung, L.P.; Fuller, S.J.; Barden, J.A.; Clarke, A.L.; Petrou, S.; Wiley, J.S. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J. Biol. Chem. 2004, 279, 31287–31295. [Google Scholar] [CrossRef]
- Biswas, A.; Raza, A.; Das, S.; Kapoor, M.; Jayarajan, R.; Verma, A.; Shamsudheen, K.V.; Murry, B.; Seth, S.; Bhargava, B.; et al. Loss of function mutation in the P2X7, a ligand-gated ion channel gene associated with hypertrophic cardiomyopathy. Purinergic Signal. 2019, 15, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Field, J.; Dutertre, S.; Ou, A.; Kilpatrick, T.J.; Lechner-Scott, J.; Scott, R.; Lea, R.; Taylor, B.V.; Stankovich, J.; et al. A rare P2X7 variant Arg307Gln with absent pore formation function protects against neuroinflammation in multiple sclerosis. Hum. Mol. Genet. 2015, 24, 5644–5654. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.H.; Cheng, M.; Tang, J.P.; Dai, X.J.; Zhang, Y.; Li, X.P.; Liu, Q.; Wang, Y.L. Single nucleotide polymorphisms associated with P2X7R function regulate the onset of gouty arthritis. PLoS ONE 2017, 12, e0181685. [Google Scholar] [CrossRef]
- Oyanguren-Desez, O.; Rodríguez-Antigüedad, A.; Villoslada, P.; Domercq, M.; Alberdi, E.; Matute, C. Gain-of-function of P2X7 receptor gene variants in multiple sclerosis. Cell Calcium 2011, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Guggemos, J.; Fuller, S.J.; Skarratt, K.K.; Mayer, B.; Schneider, E.M. Loss-of-function/gain-of-function polymorphisms of the ATP sensitive P2X7R influence sepsis, septic shock, pneumonia, and survival outcomes. Front. Immunol. 2024, 15, 1352789. [Google Scholar] [CrossRef]
- Sun, C.; Chu, J.; Singh, S.; Salter, R.D. Identification and characterization of a novel variant of the human P2X7 receptor resulting in gain of function. Purinergic Signal. 2010, 6, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Soronen, P.; Mantere, O.; Melartin, T.; Suominen, K.; Vuorilehto, M.; Rytsälä, H.; Arvilommi, P.; Holma, I.; Holma, M.; Jylhä, P.; et al. P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156, 435–447. [Google Scholar] [CrossRef]
- Gonda, X.; Hullam, G.; Antal, P.; Eszlari, N.; Petschner, P.; Hökfelt, T.G.; Anderson, I.M.; Deakin, J.F.W.; Juhasz, G.; Bagdy, G. Significance of risk polymorphisms for depression depends on stress exposure. Sci. Rep. 2018, 8, 3946. [Google Scholar] [CrossRef]
- Zorina-Lichtenwalter, K.; Ase, A.R.; Verma, V.; Parra, A.I.M.; Komarova, S.; Khadra, A.; Séguéla, P.; Diatchenko, L. Characterization of Common Genetic Variants in P2RX7 and Their Contribution to Chronic Pain Conditions. J. Pain 2024, 25, 545–556. [Google Scholar] [CrossRef]
- Sorge, R.E.; Trang, T.; Dorfman, R.; Smith, S.B.; Beggs, S.; Ritchie, J.; Austin, J.S.; Zaykin, D.V.; Vander Meulen, H.; Costigan, M.; et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012, 18, 595–599. [Google Scholar] [CrossRef]
- Bradley, H.J.; Baldwin, J.M.; Goli, G.R.; Johnson, B.; Zou, J.; Sivaprasadarao, A.; Baldwin, S.A.; Jiang, L.-H. Residues 155 and 348 Contribute to the Determination of P2X7 Receptor Function via Distinct Mechanisms Revealed by Single-nucleotide Polymorphisms. J. Biol. Chem. 2011, 286, 8176–8187. [Google Scholar] [CrossRef]
- Li, M.-Y.; Fang, X.; Ma, Y.; Pan, X.-Y.; Dai, X.-J.; Li, X.-M.; Li, X.-L.; Wang, Y.-P.; Tao, J.-H.; Li, X.-P. The functional change of the P2X7R containing the Ala348 to Thr polymorphism is associated with the pathogenesis of gout. Sci. Rep. 2023, 13, 5603. [Google Scholar] [CrossRef]
- Duan, S.; Yu, J.; Han, Z.; Cheng, Z.; Liang, P. Association Between P2RX7 Gene and Hepatocellular Carcinoma Susceptibility: A Case-Control Study in a Chinese Han Population. Med. Sci. Monit. 2016, 22, 1916–1923. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, L.; Yan, H.; Jiao, W.; Miao, Q.; Feng, W.; Wu, X.; Gu, Y.; Jiao, A.; Guo, Y.; et al. Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol. Med. Microbiol. 2010, 60, 165–170. [Google Scholar] [CrossRef]
- Areeshi, M.Y.; Mandal, R.K.; Panda, A.K.; Haque, S. Association of P2X7 A1513C (rs3751143) Gene Polymorphism with Risk of Tuberculosis: Evidence from a Meta-Analysis. Genet. Test. Mol. Biomarkers 2013, 17, 662–668. [Google Scholar] [CrossRef]
- Taheri, M.; Sarani, H.; Moazeni-Roodi, A.; Naderi, M.; Hashemi, M. Association between P2X7 Polymorphisms and Susceptibility to Tuberculosis: An Updated Meta-Analysis of Case-Control Studies. Medicina 2019, 55, 298. [Google Scholar] [CrossRef]
- Lucae, S.; Salyakina, D.; Barden, N.; Harvey, M.; Gagne, B.; Labbe, M.; Binder, E.B.; Uhr, M.; Paez-Pereda, M.; Sillaber, I.; et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 2006, 15, 2438–2445. [Google Scholar] [CrossRef]
- Barden, N.; Harvey, M.; Gagné, B.; Shink, E.; Tremblay, M.; Raymond, C.; Labbé, M.; Villeneuve, A.; Rochette, D.; Bordeleau, L.; et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 374–382. [Google Scholar] [CrossRef]
- Feng, W.P.; Zhang, B.; Li, W.; Liu, J. Lack of Association of P2RX7 Gene rs2230912 Polymorphism with Mood Disorders: A Meta-Analysis. PLoS ONE 2014, 9, e88575. [Google Scholar] [CrossRef]
- Czamara, D.; Müller-Myhsok, B.; Lucae, S. The P2RX7 polymorphism rs2230912 is associated with depression: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Vereczkei, A.; Abdul-Rahman, O.; Halmai, Z.; Nagy, G.; Szekely, A.; Somogyi, A.; Faludi, G.; Nemoda, Z. Association of purinergic receptor P2RX7 gene polymorphisms with depression symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 207–216. [Google Scholar] [CrossRef]
- McQuillin, A.; Bass, N.J.; Choudhury, K.; Puri, V.; Kosmin, M.; Lawrence, J.; Curtis, D.; Gurling, H.M. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol. Psychiatry 2009, 14, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Grigoroiu-Serbanescu, M.; Herms, S.; Mühleisen, T.W.; Georgi, A.; Diaconu, C.C.; Strohmaier, J.; Czerski, P.; Hauser, J.; Leszczynska-Rodziewicz, A.; Jamra, R.A.; et al. Variation in P2RX7 candidate gene (rs2230912) is not associated with bipolar I disorder and unipolar major depression in four European samples. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 1017–1021. [Google Scholar] [CrossRef]
- Green, E.K.; Grozeva, D.; Raybould, R.; Elvidge, G.; Macgregor, S.; Craig, I.; Farmer, A.; McGuffin, P.; Forty, L.; Jones, L.; et al. P2RX7: A bipolar and unipolar disorder candidate susceptibility gene? Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 1063–1069. [Google Scholar] [CrossRef]
- Halmai, Z.; Dome, P.; Vereczkei, A.; Abdul-Rahman, O.; Szekely, A.; Gonda, X.; Faludi, G.; Sasvari-Szekely, M.; Nemoda, Z. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J. Affect. Disord. 2013, 150, 104–109. [Google Scholar] [CrossRef]
- Backlund, L.; Nikamo, P.; Hukic, D.S.; Ek, I.R.; Träskman-Bendz, L.; Landén, M.; Edman, G.; Schalling, M.; Frisén, L.; Osby, U. Cognitive manic symptoms associated with the P2RX7 gene in bipolar disorder. Bipolar Disord. 2011, 13, 500–508. [Google Scholar] [CrossRef]
- Magni, L.; Yu, H.; Christensen, N.M.; Poulsen, M.H.; Frueh, A.; Deshar, G.; Johansen, A.Z.; Johansen, J.S.; Pless, S.A.; Jørgensen, N.R.; et al. Human P2X7 receptor variants Gly150Arg and Arg276His polymorphisms have differential effects on risk association and cellular functions in pancreatic cancer. Cancer Cell Int. 2024, 24, 148. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1Β-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Dardano, A.; Falzoni, S.; Caraccio, N.; Polini, A.; Tognini, S.; Solini, A.; Berti, P.; Di Virgilio, F.; Monzani, F. 1513A>C Polymorphism in the P2X7 Receptor Gene in Patients with Papillary Thyroid Cancer: Correlation with Histological Variants and Clinical Parameters. J. Clin. Endocrinol. Metab. 2009, 94, 695–698. [Google Scholar] [CrossRef]
- Wiley, J.S.; Dao-Ung, L.P.; Gu, B.J.; Sluyter, R.; Shemon, A.N.; Li, C.; Taper, J.; Gallo, J.; Manoharan, A. A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: A molecular study. Lancet 2002, 359, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Thunberg, U.; Tobin, G.; Johnson, A.; Söderberg, O.; Padyukov, L.; Hultdin, M.; Klareskog, L.; Enblad, G.; Sundström, C.; Roos, G.; et al. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet 2002, 360, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, A.; Grignolo, M.; Ruo, L.; Ricci, L.; Adinolfi, E. P2X7 Variants in Pathophysiology. Int. J. Mol. Sci. 2024, 25, 6673. [Google Scholar] [CrossRef]
- De Marchi, E.; Pegoraro, A.; Adinolfi, E. P2X7 Receptor in Hematological Malignancies. Front. Cell Dev. Biol. 2021, 9, 645605. [Google Scholar] [CrossRef]
- Starczynski, J.; Pepper, C.; Pratt, G.; Hooper, L.; Thomas, A.; Hoy, T.; Milligan, D.; Bentley, P.; Fegan, C. The P2X7 receptor gene polymorphism 1513 A-->C has no effect on clinical prognostic markers, in vitro sensitivity to fludarabine, Bcl-2 family protein expression or survival in B-cell chronic lymphocytic leukaemia. Br. J. Haematol. 2003, 123, 66–71. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ibbotson, R.E.; Orchard, J.A.; Gardiner, A.C.; Seear, R.V.; Chase, A.J.; Oscier, D.G.; Cross, N.C. P2X7 polymorphism and chronic lymphocytic leukaemia: Lack of correlation with incidence, survival and abnormalities of chromosome 12. Leukemia 2003, 17, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Nückel, H.; Frey, U.H.; Dürig, J.; Dührsen, U.; Siffert, W. 1513A/C polymorphism in the P2X7 receptor gene in chronic lymphocytic leukemia: Absence of correlation with clinical outcome. Eur. J. Haematol. 2004, 72, 259–263. [Google Scholar] [CrossRef]
- Wang, B.J.; Chen, J.Y.; Guan, Y.; Liu, D.C.; Cao, Z.C.; Kong, J.; Wu, Z.S.; Wu, W.Y. Predicted the P2RX7 rs3751143 polymorphism is associated with cancer risk: A meta-analysis and systematic review. Biosci. Rep. 2021, 41, BSR20193877. [Google Scholar] [CrossRef]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (synonymous) SNPs: Should we care about them? Methods Mol. Biol. 2009, 578, 23–39. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, C.; Cui, Q. A Large-Scale Analysis of the Relationship of Synonymous SNPs Changing MicroRNA Regulation with Functionality and Disease. Int. J. Mol. Sci. 2015, 16, 23545–23555. [Google Scholar] [CrossRef]
- Roger, S.; Mei, Z.Z.; Baldwin, J.M.; Dong, L.; Bradley, H.; Baldwin, S.A.; Surprenant, A.; Jiang, L.H. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J. Psychiatr. Res. 2010, 44, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, A.; Lucae, S.; Unschuld, P.G.; Ising, M.; Kern, N.; Salyakina, D.; Lieb, R.; Uhr, M.; Binder, E.B.; Keck, M.E.; et al. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders. J. Affect. Disord. 2007, 101, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Gidlöf, O.; Smith, J.G.; Melander, O.; Lövkvist, H.; Hedblad, B.; Engström, G.; Nilsson, P.; Carlson, J.; Berglund, G.; Olsson, S.; et al. A Common Missense Variant in the ATP Receptor P2X7 Is Associated with Reduced Risk of Cardiovascular Events. PLoS ONE 2012, 7, e37491. [Google Scholar] [CrossRef]
- Vangsted, A.J.; Klausen, T.W.; Gimsing, P.; Abildgaard, N.; Andersen, N.F.; Gang, A.O.; Holmström, M.; Gregersen, H.; Vogel, U.; Schwarz, P.; et al. Genetic variants in the P2RX7 gene are associated with risk of multiple myeloma. Eur. J. Haematol. 2014, 93, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Husted, L.B.; Harsløf, T.; Stenkjær, L.; Carstens, M.; Jørgensen, N.R.; Langdahl, B.L. Functional polymorphisms in the P2X7 receptor gene are associated with osteoporosis. Osteoporos. Int. 2013, 24, 949–959. [Google Scholar] [CrossRef]
- Hansen, T.; Jakobsen, K.D.; Fenger, M.; Nielsen, J.; Krane, K.; Fink-Jensen, A.; Lublin, H.; Ullum, H.; Timm, S.; Wang, A.G.; et al. Variation in the purinergic P2RX7 receptor gene and schizophrenia. Schizophr. Res. 2008, 104, 146–152. [Google Scholar] [CrossRef]
- Sanz, J.M.; Falzoni, S.; Rizzo, R.; Cipollone, F.; Zuliani, G.; Di Virgilio, F. Possible protective role of the 489C>T P2X7R polymorphism in Alzheimer’s disease. Exp. Gerontol. 2014, 60, 117–119. [Google Scholar] [CrossRef]
- Pegoraro, A.; Bortolotti, D.; Marci, R.; Caselli, E.; Falzoni, S.; De Marchi, E.; Di Virgilio, F.; Rizzo, R.; Adinolfi, E. The P2X7 Receptor 489C>T Gain of Function Polymorphism Favors HHV-6A Infection and Associates with Female Idiopathic Infertility. Front. Pharmacol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Hu, S.; Yu, F.; Ye, C.; Huang, X.; Lei, X.; Dai, Y.; Xu, H.; Wang, Y.; Yu, Y. The presence of P2RX7 single nuclear polymorphism is associated with a gain of function in P2X7 receptor and inflammasome activation in SLE complicated with pericarditis. Clin. Exp. Rheumatol. 2020, 38, 442–449. [Google Scholar] [PubMed]
- Gartland, A.; Skarratt, K.K.; Hocking, L.J.; Parsons, C.; Stokes, L.; Jørgensen, N.R.; Fraser, W.D.; Reid, D.M.; Gallagher, J.A.; Wiley, J.S. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur. J. Hum. Genet. 2012, 20, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Portales-Cervantes, L.; Niño-Moreno, P.; Salgado-Bustamante, M.; García-Hernández, M.H.; Baranda-Candido, L.; Reynaga-Hernández, E.; Barajas-López, C.; González-Amaro, R.; Portales-Pérez, D.P. The His155Tyr (489C>T) single nucleotide polymorphism of P2RX7 gene confers an enhanced function of P2X7 receptor in immune cells from patients with rheumatoid arthritis. Cell. Immunol. 2012, 276, 168–175. [Google Scholar] [CrossRef]
- Jørgensen, N.R.; Husted, L.B.; Skarratt, K.K.; Stokes, L.; Tofteng, C.L.; Kvist, T.; Jensen, J.-E.B.; Eiken, P.; Brixen, K.; Fuller, S.; et al. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur. J. Hum. Genet. 2012, 20, 675–681. [Google Scholar] [CrossRef]
- Guerini, F.R.; Agliardi, C.; Bolognesi, E.; Zanzottera, M.; Caputo, D.; Pasanisi, M.B.; Rovaris, M.; Clerici, M. Two Single Nucleotide Polymorphisms in the Purinergic Receptor P2X7 Gene Are Associated with Disease Severity in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 15381. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.E.; Peixoto-Rangel, A.L.; Hargrave, A.C.; Roubaix, L.A.; Mui, E.J.; Boulter, N.R.; Miller, E.N.; Fuller, S.J.; Wiley, J.S.; Castellucci, L.; et al. Evidence for associations between the purinergic receptor P2X7 (P2RX7) and toxoplasmosis. Genes Immun. 2010, 11, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Galvis, C.A.; McLeod, R.; Gómez-Marín, J.E.; de-la-Torre, A.; Rocha-Roa, C.; Cardona, N.; Sepúlveda-Arias, J.C. Genetic Variations in the Purinergic P2X7 Receptor Are Associated with the Immune Response to Ocular Toxoplasmosis in Colombia. Microorganisms 2023, 11, 2508. [Google Scholar] [CrossRef]
- Gubert, C.; Andrejew, R.; Jacintho Moritz, C.E.; Dietrich, F.; Vasconcelos-Moreno, M.P.; dos Santos, B.T.M.Q.; Fijtman, A.; Kauer-Sant’Anna, M.; Kapczinski, F.; da Silva Magalhães, P.V.; et al. Bipolar disorder and 1513A>C P2RX7 polymorphism frequency. Neurosci. Lett. 2019, 694, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, X.; Li, Y.; Zou, H.; Xie, A. Association of P2X7 receptor gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurosci. Lett. 2013, 546, 42–45. [Google Scholar] [CrossRef]
- Miller, C.M.; Boulter, N.R.; Fuller, S.J.; Zakrzewski, A.M.; Lees, M.P.; Saunders, B.M.; Wiley, J.S.; Smith, N.C. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 2011, 7, e1002212. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information; U.S. National Library of Medicine. ALFA: Allele Frequency Aggregator. Available online: https://www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed on 16 April 2025).
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
- Phan, L.; Zhang, H.; Wang, Q.; Villamarin, R.; Hefferon, T.; Ramanathan, A.; Kattman, B. The evolution of dbSNP: 25 years of impact in genomic research. Nucleic Acids Res. 2025, 53, D925–D931. [Google Scholar] [CrossRef]
- Bethesda (MD): National Library of Medicine (US); National Center for Biotechnology Information. dbSNP. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 16 April 2025).
- Jørgensen, N.R. Role of the purinergic P2X receptors in osteoclast pathophysiology. Curr. Opin. Pharmacol. 2019, 47, 97–101. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Bethesda (MD): National Library of Medicine (US); National Center for Biotechnology Information. RefSeq. Available online: https://www.ncbi.nlm.nih.gov/refseq/ (accessed on 8 September 2025).
- De Salis, S.K.F.; Chen, J.Z.; Skarratt, K.K.; Fuller, S.J.; Balle, T. Deep learning structural insights into heterotrimeric alternatively spliced P2X7 receptors. Purinergic Signal. 2024, 20, 431–447. [Google Scholar] [CrossRef]
- Cheewatrakoolpong, B.; Gilchrest, H.; Anthes, J.C.; Greenfeder, S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem. Biophys. Res. Commun. 2005, 332, 17–27. [Google Scholar] [CrossRef]
- Skarratt, K.K.; Gu, B.J.; Lovelace, M.D.; Milligan, C.J.; Stokes, L.; Glover, R.; Petrou, S.; Wiley, J.S.; Fuller, S.J. A P2RX7 single nucleotide polymorphism haplotype promotes exon 7 and 8 skipping and disrupts receptor function. FASEB J. 2020, 34, 3884–3901. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Bethesda (MD): National Library of Medicine (US); National Center for Biotechnology Information. GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 8 September 2025).
- Sluyter, R.; Stokes, L. Significance of P2X7 Receptor Variants to Human Health and Disease. Recent Pat. DNA Gene Seq. 2011, 5, 41–54. [Google Scholar] [CrossRef]
- Nicke, A.; Kuan, Y.H.; Masin, M.; Rettinger, J.; Marquez-Klaka, B.; Bender, O.; Górecki, D.C.; Murrell-Lagnado, R.D.; Soto, F. A Functional P2X7 Splice Variant with an Alternative Transmembrane Domain 1 Escapes Gene Inactivation in P2X7 Knock-out Mice. J. Biol. Chem. 2009, 284, 25813–25822. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Li, X.; Wang, L.; Zhou, L.; Gorodeski, G.I. A Truncated P2X7 Receptor Variant (P2X7-j) Endogenously Expressed in Cervical Cancer Cells Antagonizes the Full-length P2X7 Receptor through Hetero-oligomerization. J. Biol. Chem. 2006, 281, 17228–17237. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandonà, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ni, H.; Zhang, L.; Xing, Y.; Fan, J.; Li, P.; Li, T.; Jia, R.; Ge, S.; Zhang, H.; et al. P2RX7-V3 is a novel oncogene that promotes tumorigenesis in uveal melanoma. Tumour Biol. 2016, 37, 13533–13543. [Google Scholar] [CrossRef]
- Pegoraro, A.; Orioli, E.; De Marchi, E.; Salvestrini, V.; Milani, A.; Di Virgilio, F.; Curti, A.; Adinolfi, E. Differential sensitivity of acute myeloid leukemia cells to daunorubicin depends on P2X7A versus P2X7B receptor expression. Cell Death Dis. 2020, 11, 876. [Google Scholar] [CrossRef]
- Liao, V.W.Y.; Chua, H.C.; Kowal, N.M.; Chebib, M.; Balle, T.; Ahring, P.K. Concatenated γ-aminobutyric acid type A receptors revisited: Finding order in chaos. J. Gen. Physiol. 2019, 151, 798–819. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.W.Y.; Kusay, A.S.; Balle, T.; Ahring, P.K. Heterologous expression of concatenated nicotinic ACh receptors: Pros and cons of subunit concatenation and recommendations for construct designs. Br. J. Pharmacol. 2020, 177, 4275–4295. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef]
- Eser, A.; Colombel, J.-F.; Rutgeerts, P.; Vermeire, S.; Vogelsang, H.; Braddock, M.; Persson, T.; Reinisch, W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn’s Disease: A Randomized Placebo-controlled, Double-blind, Phase IIa Study. Inflamm. Bowel Dis. 2015, 21, 2247–2253. [Google Scholar] [CrossRef]
- Recourt, K.; de Boer, P.; van der Ark, P.; Benes, H.; van Gerven, J.M.A.; Ceusters, M.; van Nueten, L.; Drevets, W.C.; Bhatacharya, A.; Browning, M.; et al. Characterization of the central nervous system penetrant and selective purine P2X7 receptor antagonist JNJ-54175446 in patients with major depressive disorder. Transl. Psychiatry 2023, 13, 266. [Google Scholar] [CrossRef]
- Recourt, K.; van der Aart, J.; Jacobs, G.; de Kam, M.; Drevets, W.; van Nueten, L.; Kanhai, K.; Siebenga, P.; Zuiker, R.; Ravenstijn, P.; et al. Characterisation of the pharmacodynamic effects of the P2X7 receptor antagonist JNJ-54175446 using an oral dexamphetamine challenge model in healthy males in a randomised, double-blind, placebo-controlled, multiple ascending dose trial. J. Psychopharmacol. 2020, 34, 1030–1042. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Gidley Baird, A.; Glazer, S.; Barden, J.A.; Glazer, A.; Teh, L.C.; King, J. A phase I clinical trial demonstrates that nfP2X7-targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br. J. Dermatol. 2017, 177, 117–124. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Sanz, J.M.; Amico, A.; Violi, A.; Ghisellini, S.; Pizzicotti, S.; Passaro, A.; Papi, A.; Libanore, M.; Di Virgilio, F.; et al. The shed P2X7 receptor is an index of adverse clinical outcome in COVID-19 patients. Front. Immunol. 2023, 14, 1182454. [Google Scholar] [CrossRef]
- Amaral, E.P.; Ribeiro, S.C.M.; Lanes, V.R.; Almeida, F.M.; de Andrade, M.R.M.; Bomfim, C.C.B.; Salles, É.M.; Bortoluci, K.R.; Coutinho-Silva, R.; Hirata, M.H.; et al. Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis. PLoS Pathog. 2014, 10, e1004188. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.; Danieletto, S.; Gidley-Baird, A.; Teh, L.C.; Barden, J.A. Early prostate cancer detected using expression of non-functional cytolytic P2X7 receptors. Histopathology 2004, 44, 206–215. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Xu, L.; An, H.; Chang, Y.; Yang, Y.; Zhang, W.; Xu, J. P2X7 receptor predicts postoperative cancer-specific survival of patients with clear-cell renal cell carcinoma. Cancer Sci. 2015, 106, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.M.; Oliphant, C.J.; Hassan, S.; Peille, A.L.; Bronsert, P.; Falzoni, S.; Di Virgilio, F.; McNulty, S.; Lara, R. ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef]
- Schmidt, S.; Isaak, A.; Junker, A. Spotlight on P2X7 Receptor PET Imaging: A Bright Target or a Failing Star? Int. J. Mol. Sci. 2023, 24, 1374. [Google Scholar] [CrossRef]
- Van Weehaeghe, D.; Koole, M.; Schmidt, M.E.; Deman, S.; Jacobs, A.H.; Souche, E.; Serdons, K.; Sunaert, S.; Bormans, G.; Vandenberghe, W.; et al. [11C]JNJ54173717, a novel P2X7 receptor radioligand as marker for neuroinflammation: Human biodistribution, dosimetry, brain kinetic modelling and quantification of brain P2X7 receptors in patients with Parkinson’s disease and healthy volunteers. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2051–2064. [Google Scholar] [CrossRef]
- Koole, M.; Schmidt, M.E.; Hijzen, A.; Ravenstijn, P.; Vandermeulen, C.; Van Weehaeghe, D.; Serdons, K.; Celen, S.; Bormans, G.; Ceusters, M.; et al. 18F-JNJ-64413739, a Novel PET Ligand for the P2X7 Ion Channel: Radiation Dosimetry, Kinetic Modeling, Test-Retest Variability, and Occupancy of the P2X7 Antagonist JNJ-54175446. J. Nucl. Med. 2019, 60, 683–690. [Google Scholar] [CrossRef]
- Hagens, M.H.J.; Golla, S.S.V.; Janssen, B.; Vugts, D.J.; Beaino, W.; Windhorst, A.D.; O’Brien-Brown, J.; Kassiou, M.; Schuit, R.C.; Schwarte, L.A.; et al. The P2X7 receptor tracer [11C]SMW139 as an in vivo marker of neuroinflammation in multiple sclerosis: A first-in man study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Engel, T. The P2X7 Receptor as a Mechanistic Biomarker for Epilepsy. Int. J. Mol. Sci. 2023, 24, 5410. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kasuya, G.; Yamaura, T.; Ma, X.B.; Nakamura, R.; Takemoto, M.; Nagumo, H.; Tanaka, E.; Dohmae, N.; Nakane, T.; Yu, Y.; et al. Structural insights into the competitive inhibition of the ATP-gated P2X receptor channel. Nat. Commun. 2017, 8, 876. [Google Scholar] [CrossRef]
- Sheng, D.; Yue, C.-X.; Jin, F.; Wang, Y.; Ichikawa, M.; Yu, Y.; Guo, C.-R.; Hattori, M. Structural insights into the orthosteric inhibition of P2X receptors by non-ATP analog antagonists. eLife 2024, 12, RP92829. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, E.G.; Faria, R.X.; Rodrigues, C.R.; Bello, M.L. Molecular dynamic simulations of full-length human purinergic receptor subtype P2X7 bonded to potent inhibitors. Eur. J. Pharm. Sci. 2020, 152, 105454. [Google Scholar] [CrossRef] [PubMed]
- Frangos, Z.J.; Wilson, K.A.; Aitken, H.M.; Cantwell Chater, R.; Vandenberg, R.J.; O’Mara, M.L. Membrane cholesterol regulates inhibition and substrate transport by the glycine transporter, GlyT2. Life Sci. Alliance 2023, 6, e202201708. [Google Scholar] [CrossRef] [PubMed]
- Zakany, F.; Kovacs, T.; Panyi, G.; Varga, Z. Direct and indirect cholesterol effects on membrane proteins with special focus on potassium channels. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158706. [Google Scholar] [CrossRef]
- Nutho, B.; Samsri, S.; Pornsuwan, S. Structural Dynamics of the Precatalytic State of Human Cytochrome c upon T28C, G34C, and A50C Mutations: A Molecular Dynamics Simulation Perspective. ACS Omega 2023, 8, 15229–15238. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Lima, M.d.F.; Cornélio, M.L. Detailed Characterization of the Cooperative Binding of Piperine with Heat Shock Protein 70 by Molecular Biophysical Approaches. Biomedicines 2020, 8, 629. [Google Scholar] [CrossRef]


| Subtype | Frequency (%) | Amino Acid (Nucleotide) Position and Residue | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 76 (253) | 150 (474) | 155 (489) | 270 (835) | 276 (853) | 307 (946) | 348 (1068) | 357 (1096) | 460 (1405) | 496 (1513) | 568 (1729) | ||
| H1 | 16.20 | Val | Gly | His | His | Arg | Arg | Ala | Thr | Gln | Glu | Ile |
| H2 | 5.29 | Val | Gly | His | Arg | Arg | Arg | Ala | Thr | Gln | Glu | Ile |
| H3 | 4.43 | Val | Gly | Tyr | His | Arg | Arg | Ala | Thr | Gln | Glu | Ile |
| H4 | 2.57 | Val | Gly | His | Arg | Arg | Arg | Ala | Thr | Gln | Glu | Asn |
| H5 | 2.31 | Val | Gly | Tyr | Arg | Arg | Arg | Ala | Thr | Gln | Glu | Ile |
| H6 | 1.40 | Val | Gly | Tyr | His | His | Arg | Ala | Thr | Gln | Glu | Ile |
| H7 | 1.06 | Val | Gly | His | His | Arg | Gln | Ala | Thr | Gln | Glu | Ile |
| H8 | 15.34 | Val | Gly | His | Arg | Arg | Arg | Thr | Thr | Gln | Glu | Ile |
| H9 | 4.54 | Ala | Gly | His | Arg | Arg | Arg | Thr | Thr | Gln | Glu | Ile |
| H10 | 2.40 | Val | Gly | Tyr | Arg | Arg | Arg | Thr | Thr | Gln | Glu | Ile |
| H11 | 11.77 | Val | Gly | Tyr | Arg | Arg | Arg | Ala | Thr | Gln | Ala | Ile |
| H12 | 2.57 | Val | Gly | His | Arg | Arg | Arg | Ala | Thr | Gln | Ala | Ile |
| H13 | 1.03 | Val | Arg | Tyr | Arg | Arg | Arg | Ala | Thr | Gln | Ala | Ile |
| H14 | 13.94 | Val | Gly | Tyr | Arg | Arg | Arg | Thr | Thr | Arg | Glu | Ile |
| H15 | 1.23 | Val | Gly | His | Arg | Arg | Arg | Thr | Thr | Arg | Glu | Ile |
| H16 | 5.00 | Val | Gly | His | Arg | Arg | Arg | Ala | Ser | Gln | Glu | Ile |
| H17 | 3.43 | Val | Gly | Tyr | Arg | Arg | Arg | Ala | Ser | Gln | Glu | Ile |
| Species | Sequence Identity to Human (%) | Technique Used | PDB ID [Reference] | Resolution (Å) | Bound Ligand | Pharmacodynamic Class |
|---|---|---|---|---|---|---|
| Chicken | 45 | X-ray | 5XW6 [179] | 3.1 | TNP-ATP | Competitive antagonist |
| Panda | 85 | X-ray | 5U1L [34] | 3.4 | - | Apo state |
| 5U1U [34] | 3.6 | A740003 | Allosteric antagonist | |||
| 5U1V [34] | 3.4 | A804598 | Allosteric antagonist | |||
| 5U1W [34] | 3.5 | AZ10606120 | Allosteric antagonist | |||
| 5U1X [34] | 3.2 | JNJ47965567 | Allosteric antagonist | |||
| 5U1Y [34] | 3.3 | GW791343 | Allosteric antagonist | |||
| 5U2H [34] | 3.9 | ATP A804598 | Agonist Allosteric antagonist | |||
| Cryo-EM | 8JV7 [180] | 3.6 | PPADS | Competitive antagonist | ||
| 8JV8 [180] | 3.3 | PPNDS | Competitive antagonist | |||
| 8Z1D | 4 | PSFL1191 | Allosteric antagonist | |||
| 8Z0Z | 3.3 | JNJ-54175446 | Allosteric antagonist | |||
| Mouse | 81 | Cryo-EM | 9E3Q a [31] | 2.5 | - | Apo state |
| Rat | 80 | Cryo-EM | 6U9V a [39] | 2.9 | - | Apo state |
| 6U9W a [39] | 3.3 | ATP | Agonist | |||
| 8TR5 a [40] | 2.5 | - | Apo state | |||
| 8TR6 a [41] | 2.2 | A438079 | Allosteric antagonist | |||
| 8TR7 a [41] | 2.5 | A839977 | Allosteric antagonist | |||
| 8TR8 a [41] | 2.2 | AZD9056 | Allosteric antagonist | |||
| 8TRA a [41] | 2.4 | GSK1482160 | Allosteric antagonist | |||
| 8TRB a [41] | 2.4 | JNJ47965567 | Allosteric antagonist | |||
| 8TRJ a [40] | 2.8 | BzATP | Agonist | |||
| 8TRK a [41] | 2.7 | Methyl blue | Allosteric antagonist | |||
| 8V4S a [40] | 2.5 | - | Apo state | |||
| Human | 100 | Cryo-EM | 9E3M a [31] | 2.5 | - | Apo state |
| 9E3N a [31] | 3.0 | ATP | Agonist | |||
| 9E3O a [31] | 2.8 | UB-ALT-P30 | Allosteric antagonist | |||
| 9E3P a [31] | 2.5 | UB-MBX-46 | Allosteric antagonist |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheah, J.S.Y.; Skarratt, K.K.; Fuller, S.J.; Balle, T. Genetic Variations in the P2X7 Receptor: Opportunities and Challenges for Drug Development. Int. J. Mol. Sci. 2025, 26, 10265. https://doi.org/10.3390/ijms262110265
Cheah JSY, Skarratt KK, Fuller SJ, Balle T. Genetic Variations in the P2X7 Receptor: Opportunities and Challenges for Drug Development. International Journal of Molecular Sciences. 2025; 26(21):10265. https://doi.org/10.3390/ijms262110265
Chicago/Turabian StyleCheah, Justin S. Y., Kristen K. Skarratt, Stephen J. Fuller, and Thomas Balle. 2025. "Genetic Variations in the P2X7 Receptor: Opportunities and Challenges for Drug Development" International Journal of Molecular Sciences 26, no. 21: 10265. https://doi.org/10.3390/ijms262110265
APA StyleCheah, J. S. Y., Skarratt, K. K., Fuller, S. J., & Balle, T. (2025). Genetic Variations in the P2X7 Receptor: Opportunities and Challenges for Drug Development. International Journal of Molecular Sciences, 26(21), 10265. https://doi.org/10.3390/ijms262110265






