Fenofibrate as a Modulator of the Renin–Angiotensin System in Su/Hx-Induced Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Results
2.1. Validation of the Su/Hx-Induced PAH Model and the Protective Effects of Fenofibrate
2.2. Fenofibrate Administration Attenuates Medial Wall Thickening in Pulmonary Arterioles in a Su/Hx-Induced PAH Model
2.3. Fenofibrate Administration Improves Pulmonary Hemodynamics in the Su/Hx-Induced PAH Model
2.4. Effect of Fenofibrate Administration on Renin–Angiotensin System Components in Lung Tissue in the Su/Hx-Induced PAH Model
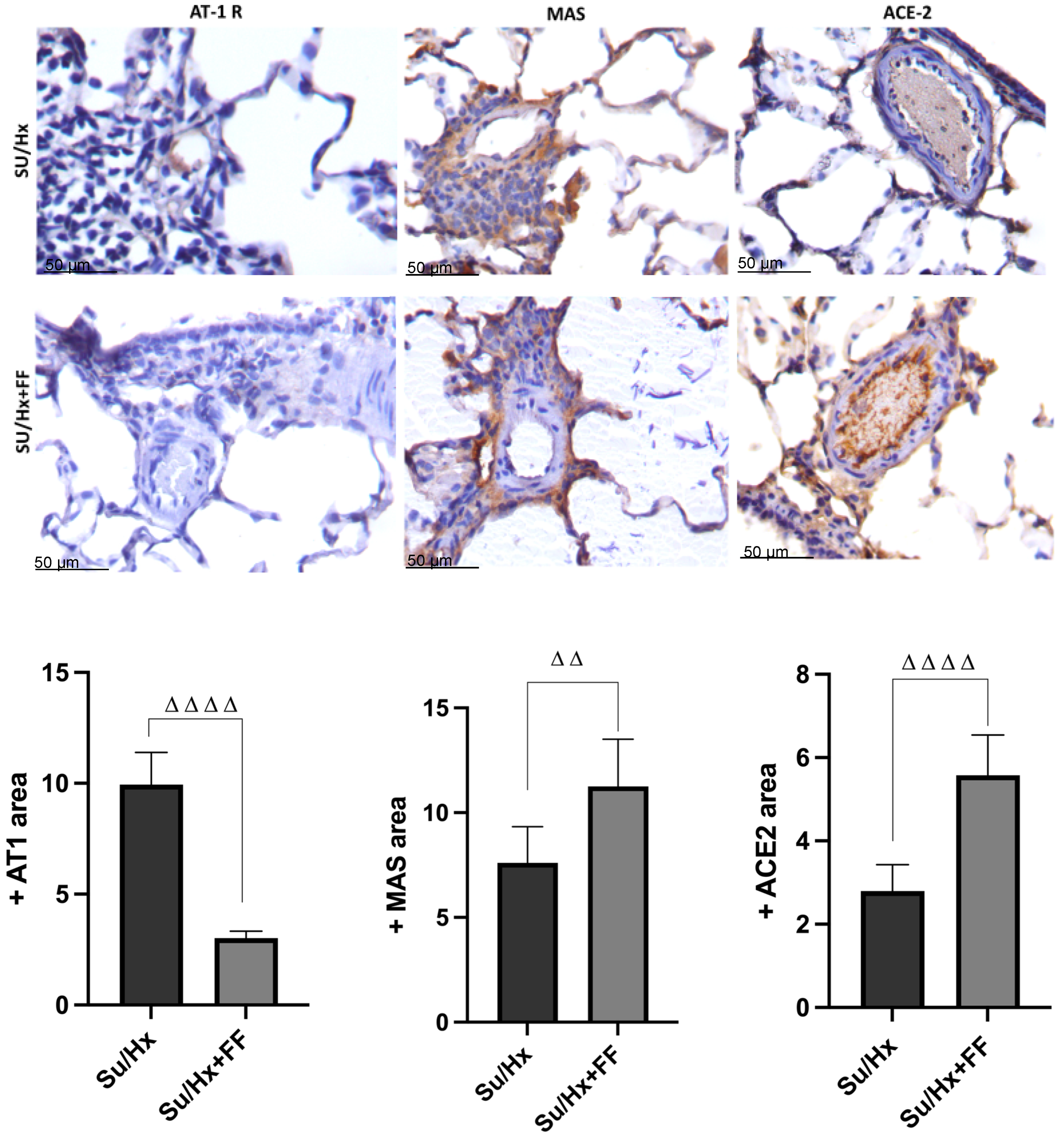
2.5. Effect of Fenofibrate Administration on Pulmonary Expression of AT1R, MAS Receptor, and ACE2 in Lung Tissue in the Su/Hx-Induced PAH Model
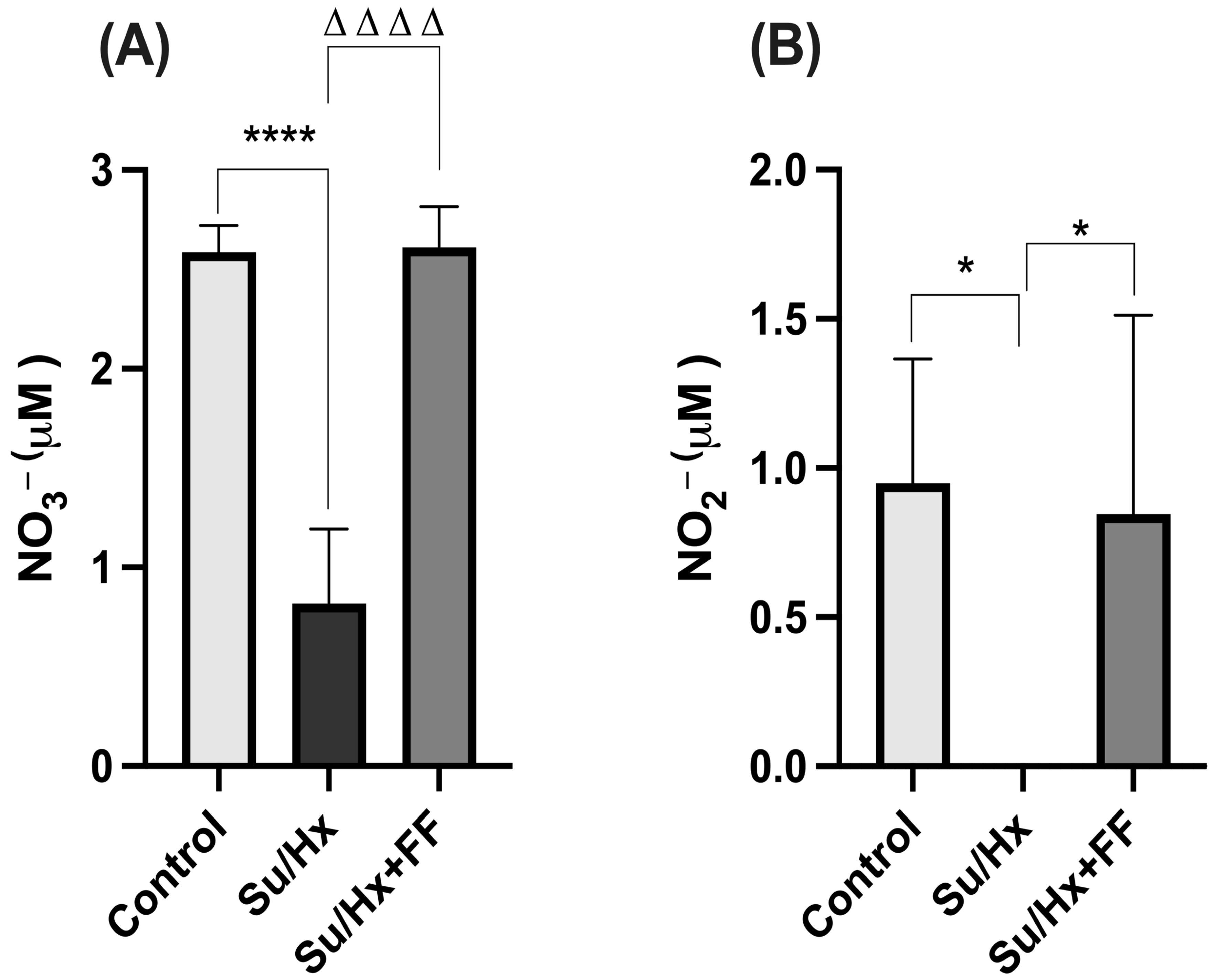
2.6. Effect of Fenofibrate Administration on Nitrate (NO3−) and Nitrite (NO2−) Concentrations in Lung Tissue in a Su/Hx-Induced PAH Model
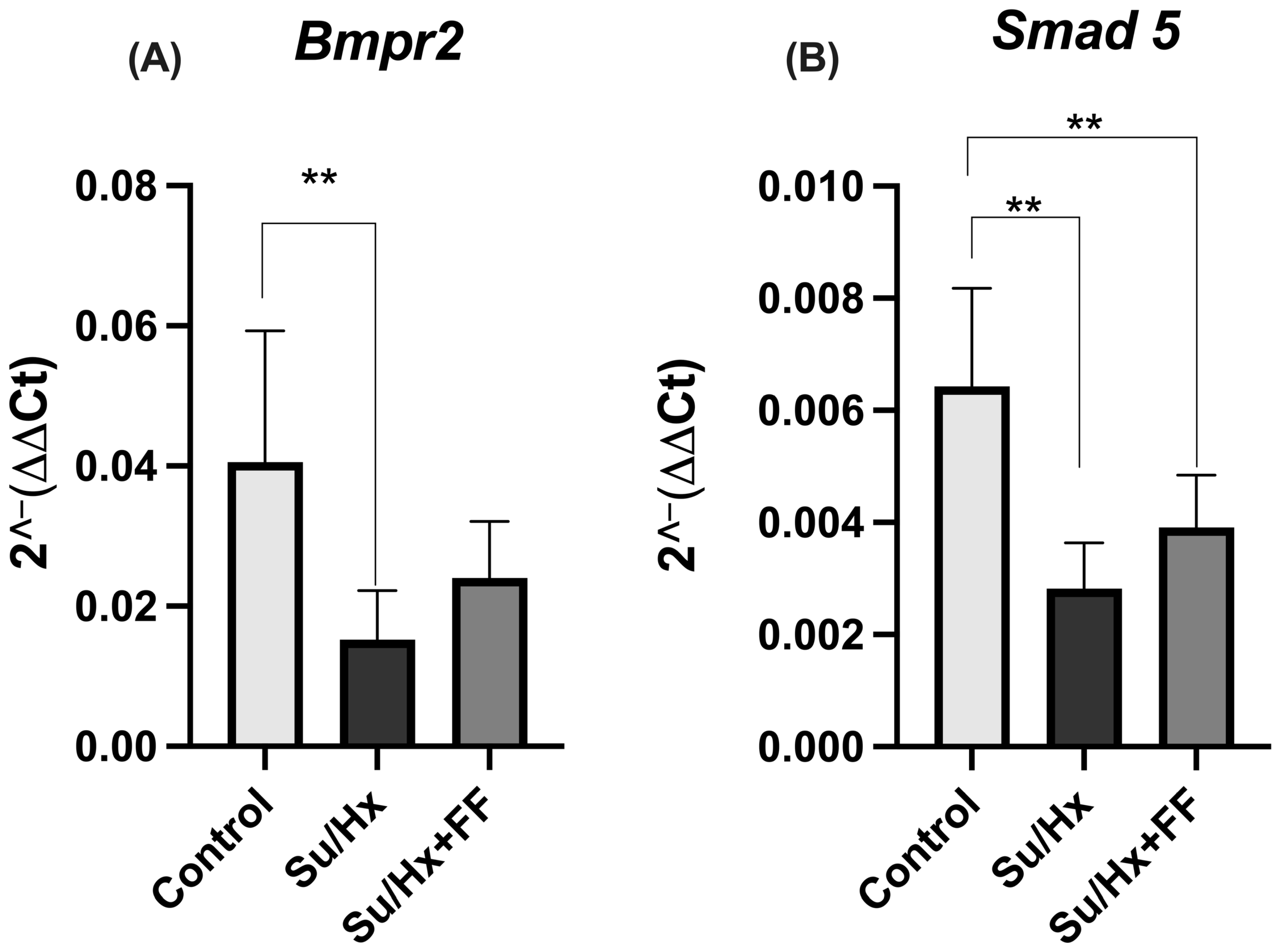
2.7. Effect of Fenofibrate Administration on Bmpr2 and Smad5 Expression in Lung Tissue in the Su/Hx-Induced PAH Model
2.8. Effect of Fenofibrate Administration on Cardiac Hemodynamics in the Su/Hx-Induced PAH Model
2.9. Effect of Fenofibrate Administration on Renin–Angiotensin System Components in the Right Ventricle in the Su/Hx-Induced PAH Model
2.10. Effect of Fenofibrate Administration on Nitrate (NO3−) and Nitrite (NO2−) Concentrations in the Right Ventricle in a Su/Hx-Induced PAH Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. SU5416 and Fenofibrate Preparation
4.3. Induction of Pulmonary Arterial Hypertension
4.4. Hemodynamic Parameters
4.5. Evaluation of Right Ventricular Hypertrophy
4.6. Immunohistochemistry Analysis of Wall Thickness
4.7. Concentrations of Angiotensin II and Angiotensin 1–7 in Lung and Right Ventricle
4.8. Determination of Angiotensin-Converting Enzyme 2 by ELISA
4.9. Immunohistochemistry
4.10. Determination of Nitrate (NO3−) and Nitrite (NO2−) Levels by Colorimetric Assay Kit
4.11. Bmpr2 and Smad5 mRNA Expression by RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guignabert, C.; Dorfmüller, P. Pathology and pathobiology of pulmonary hypertension. Semin. Respir. Crit. Care Med. 2013, 34, 551–559. [Google Scholar] [CrossRef]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; Savale, L.; Boucly, A.; Thuillet, R.; Tu, L.; Ottaviani, M.; Rhodes, C.J.; De Groote, P.; Prévot, G.; Bergot, E.; et al. Serum and Pulmonary Expression Profiles of the Activin Signaling System in Pulmonary Arterial Hypertension. Circulation 2023, 147, 1809–1822. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gloria, J.L.; Martínez-Olivares, C.E.; Rojas-Morales, P.; Hernández-Pando, R.; Carbó, R.; Rubio-Gayosso, I.; Arellano-Buendía, A.S.; Rada, K.M.; Sánchez-Muñoz, F.; Osorio-Alonso, H. Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 8600. [Google Scholar] [CrossRef]
- Galhotra, P.; Prabhakar, P.; Meghwani, H.; Mohammed, S.A.; Banerjee, S.K.; Seth, S.; Hote, M.P.; Reeta, K.H.; Ray, R.; Maulik, S.K. Beneficial effects of fenofibrate in pulmonary hypertension in rats. Mol. Cell. Biochem. 2018, 449, 185–194. [Google Scholar] [CrossRef]
- Mocumbi, A.; Humbert, M.; Saxena, A.; Jing, Z.C.; Sliwa, K.; Thienemann, F.; Archer, S.L.; Stewart, S. Pulmonary hypertension. Nat. Rev. Dis. Primers 2024, 10, 1. [Google Scholar] [CrossRef]
- de Man, F.S.; Tu, L.; Handoko, M.L.; Rain, S.; Ruiter, G.; François, C.; Schalij, I.; Dorfmüller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 780–789. [Google Scholar] [CrossRef]
- Sandoval, J.; Del Valle-Mondragón, L.; Masso, F.; Zayas, N.; Pulido, T.; Teijeiro, R.; Gonzalez-Pacheco, H.; Olmedo-Ocampo, R.; Sisniega, C.; Paez-Arenas, A.; et al. Angiotensin converting enzyme 2 and angiotensin (1–7) axis in pulmonary arterial hypertension. Eur. Respir. J. 2020, 56, 1902416. [Google Scholar] [CrossRef]
- Cuthbertson, I.; Morrell, N.W.; Caruso, P. BMPR2 Mutation and Metabolic Reprogramming in Pulmonary Arterial Hypertension. Circ. Res. 2023, 132, 109–126. [Google Scholar] [CrossRef]
- Wang, L.; Moonen, J.R.; Cao, A.; Isobe, S.; Li, C.G.; Tojais, N.F.; Taylor, S.; Marciano, D.P.; Chen, P.I.; Gu, M.; et al. Dysregulated Smooth Muscle Cell BMPR2-ARRB2 Axis Causes Pulmonary Hypertension. Circ. Res. 2023, 132, 545–564. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.E.; Lai, Y.C.; Gladwin, M.T. Nitrate-nitrite-nitric oxide pathway in pulmonary arterial hypertension therapeutics. Circulation 2012, 125, 2824–2826. [Google Scholar] [CrossRef] [PubMed]
- Christou, H.; Michael, Z.; Spyropoulos, F.; Chen, Y.; Rong, D.; Khalil, R.A. Carbonic anhydrase inhibition improves pulmonary artery reactivity and nitric oxide-mediated relaxation in sugen-hypoxia model of pulmonary hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R835–R850. [Google Scholar] [CrossRef]
- Christou, H.; Hudalla, H.; Michael, Z.; Filatava, E.J.; Li, J.; Zhu, M.; Possomato-Vieira, J.S.; Dias-Junior, C.; Kourembanas, S.; Khalil, R.A. Impaired pulmonary arterial vasoconstriction and nitric oxide-mediated relaxation underlie severe pulmonary hypertension in the Sugen-hypoxia rat model. J. Pharmacol. Exp. Ther. 2018, 364, 258–274. [Google Scholar] [CrossRef]
- Klinger, J.R. Plasma nitrite/nitrate levels: A new biomarker for pulmonary arterial hypertension? Eur. Respir. J. 2016, 48, 1265–1267. [Google Scholar] [CrossRef]
- Mandras, S.; Mehta, S.; Vaidya, A. Combination Therapy in Pulmonary Arterial Hypertension—Targeting the Nitric Oxide and Prostacyclin Pathways. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Archer, S.L.; Dorfmüller, P.; Erzurum, S.C.; Guignabert, C.; Michelakis, E.; Rabinovitch, M.; Schermuly, R.; Stenmark, K.R.; Morrell, N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D4–D12. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; de Man, F.; Lombès, M. ACE2 as therapy for pulmonary arterial hypertension: The good outweighs the bad. Eur. Respir. J. 2018, 51, 1800848. [Google Scholar] [CrossRef]
- Yamazato, Y.; Ferreira, A.J.; Hong, K.H.; Sriramula, S.; Francis, J.; Yamazato, M.; Yuan, L.; Bradford, C.N.; Shenoy, V.; Oh, S.P.; et al. Prevention of pulmonary hypertension by Angiotensin-converting enzyme 2 gene transfer. Hypertension 2009, 54, 365–371. [Google Scholar] [CrossRef]
- Shenoy, V.; Ferreira, A.J.; Qi, Y.; Fraga-Silva, R.A.; Díez-Freire, C.; Dooies, A.; Jun, J.Y.; Sriramula, S.; Mariappan, N.; Pourang, D.; et al. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, A.; Pan, Y.; Wang, X.; Xu, Y.; Desai, A.A.; Tang, H.; Han, Y. Research Progress on Pulmonary Arterial Hypertension and the Role of the Angiotensin Converting Enzyme 2-Angiotensin-(1–7)-Mas Axis in Pulmonary Arterial Hypertension. Cardiovasc. Drugs Ther. 2022, 36, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Danilov, S.M.; Satyan, K.B.; Morris, K.G.; Stenmark, K.R. Right ventricular angiotensin converting enzyme activity and expression is increased during hypoxic pulmonary hypertension. Cardiovasc. Res. 1997, 34, 393–403. [Google Scholar] [CrossRef]
- Fried, N.D.; Morris, T.M.; Whitehead, A.; Lazartigues, E.; Yue, X.; Gardner, J.D. Angiotensin II type 1 receptor mediates pulmonary hypertension and right ventricular remodeling induced by inhaled nicotine. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1526–H1534. [Google Scholar] [CrossRef]
- Ma, Z.; Viswanathan, G.; Sellig, M.; Jassal, C.; Choi, I.; Garikipati, A.; Xiong, X.; Nazo, N.; Rajagopal, S. β-Arrestin-Mediated Angiotensin II Type 1 Receptor Activation Promotes Pulmonary Vascular Remodeling in Pulmonary Hypertension. JACC Basic Transl. Sci. 2021, 6, 854–869. [Google Scholar] [CrossRef]
- Yuan, Y.M.; Luo, L.; Guo, Z.; Yang, M.; Ye, R.S.; Luo, C. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 249–253. [Google Scholar] [CrossRef]
- Maron, B.A.; Leopold, J.A. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series). Pulm. Circ. 2014, 4, 200–210. [Google Scholar] [CrossRef]
- Chang, H.; Chang, C.Y.; Lee, H.J.; Chou, C.Y.; Chou, T.C. Magnolol ameliorates pneumonectomy and monocrotaline-induced pulmonary arterial hypertension in rats through inhibition of angiotensin II and endothelin-1 expression. Phytomedicine 2018, 51, 205–213. [Google Scholar] [CrossRef]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Mei, D.; Wong, W.F. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 2018, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Balakumar, P.; Sambathkumar, R.; Mahadevan, N.; Muhsinah, A.B.; Alsayari, A.; Venkateswaramurthy, N.; Dhanaraj, S.A. Molecular targets of fenofibrate in the cardiovascular-renal axis: A unifying perspective of its pleiotropic benefits. Pharmacol. Res. 2019, 144, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Noonan, J.E.; Jenkins, A.J.; Ma, J.X.; Keech, A.C.; Wang, J.J.; Lamoureux, E.L. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013, 62, 3968–3975. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Amiri, F.; Benkirane, K.; Iglarz, M.; Diep, Q.N. Peroxisome proliferator-activated receptors: Vascular and cardiac effects in hypertension. Hypertension 2003, 42, 664–668. [Google Scholar] [CrossRef]
- Ibarra-Lara, L.; Sánchez-Aguilar, M.; Sánchez-Mendoza, A.; Del Valle-Mondragón, L.; Soria-Castro, E.; Carreón-Torres, E.; Díaz-Díaz, E.; Vázquez-Meza, H.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Fenofibrate Therapy Restores Antioxidant Protection and Improves Myocardial Insulin Resistance in a Rat Model of Metabolic Syndrome and Myocardial Ischemia: The Role of Angiotensin II. Molecules 2016, 22, 31. [Google Scholar] [CrossRef]
- Won, T.W. Fenofibrate, a peroxisome proliferator-activated receptor α-agonist, blocks lipopolysaccharide-induced inflammatory pathways in mouse liver. Korean J. Hepatobiliary Pancreat. Surg. 2013, 17, 89–108. [Google Scholar] [CrossRef]
- Balakumar, P.; Rohilla, A.; Mahadevan, N. Pleiotropic actions of fenofibrate on the heart. Pharmacol. Res. 2011, 63, 8–12. [Google Scholar] [CrossRef]
- Sánchez-Aguilar, M.; Ibarra-Lara, L.; Del Valle-Mondragón, L.; Soria-Castro, E.; Torres-Narváez, J.C.; Carreón-Torres, E.; Sánchez-Mendoza, A.; Rubio-Ruíz, M.E. Nonclassical Axis of the Renin-Angiotensin System and Neprilysin: Key Mediators That Underlie the Cardioprotective Effect of PPAR-Alpha Activation during Myocardial Ischemia in a Metabolic Syndrome Model. PPAR Res. 2020, 2020, 8894525. [Google Scholar] [CrossRef]
- Vargas, R.A.V.; Varela Millán, J.M.; Fajardo Bonilla, E. Renin-angiotensin system: Basic and clinical aspects—A general perspective. Endocrinol. Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Vera, T.; Taylor, M.; Bohman, Q.; Flasch, A.; Roman, R.J.; Stec, D.E. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension 2005, 45, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sitapara, R.; Sugarragchaa, C.; Zisman, L.S. SU5416 plus hypoxia but not selective VEGFR2 inhibition with cabozantinib plus hypoxia induces pulmonary hypertension in rats: Potential role of BMPR2 signaling. Pulm. Circ. 2021, 11, 20458940211021528. [Google Scholar] [CrossRef] [PubMed]
- Poble, P.B.; Phan, C.; Quatremare, T.; Bordenave, J.; Thuillet, R.; Cumont, A.; Huertas, A.; Tu, L.; Dorfmüller, P.; Humbert, M.; et al. Therapeutic effect of pirfenidone in the sugen/hypoxia rat model of severe pulmonary hypertension. FASEB J. 2019, 33, 3670–3679. [Google Scholar] [CrossRef]
- Williams, T.L.; Nyimanu, D.; Kuc, R.E.; Foster, R.; Glen, R.C.; Maguire, J.J.; Davenport, A.P. The biased apelin receptor agonist, MM07, reverses Sugen/hypoxia-induced pulmonary arterial hypertension as effectively as the endothelin antagonist macitentan. Front. Pharmacol. 2024, 15, 1369489. [Google Scholar] [CrossRef]
- Tu, L.; Thuillet, R.; Perrot, J.; Ottaviani, M.; Ponsardin, E.; Kolkhof, P.; Humbert, M.; Viengchareun, S.; Lombès, M.; Guignabert, C. Mineralocorticoid Receptor Antagonism by Finerenone Attenuates Established Pulmonary Hypertension in Rats. Hypertension 2022, 79, 2262–2273. [Google Scholar] [CrossRef]
- Sztuka, K.; Jasińska-Stroschein, M. Systematic Review and Meta-Analysis of Interventions Tested in Animal Models of Pulmonary Hypertension. Vasc. Pharmacol. 2018, 110, 55–63. [Google Scholar] [CrossRef]
- de Raaf, M.A.; Schalij, I.; Gomez-Arroyo, J.; Rol, N.; Happé, C.; de Man, F.S.; Vonk-Noordegraaf, A.; Westerhof, N.; Voelkel, N.F.; Bogaard, H.J. SuHx Rat Model: Partly Reversible Pulmonary Hypertension and Progressive Intima Obstruction. Eur. Respir. J. 2014, 44, 160–168. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef]
- Nicolls, M.R.; Mizuno, S.; Taraseviciene-Stewart, L.; Farkas, L.; Drake, J.I.; Alvira, C.M.; Cool, C.D.; Voelkel, N.F. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm. Circ. 2012, 2, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Hahn, R.T. Right ventricular-pulmonary arterial coupling and outcomes in heart failure and valvular heart disease. Struct. Heart 2021, 5, 128–139. [Google Scholar] [CrossRef]
- Chemla, D.; Castelain, V.; Humbert, M.; Hébert, J.L.; Simonneau, G.; Lecarpentier, Y.; Hervé, P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 2004, 126, 1313–1317. [Google Scholar] [CrossRef]
- Ren, X.; Johns, R.A.; Gao, W.D. Right heart in pulmonary hypertension: From adaptation to failure. Pulm. Circ. 2019, 9, 2045894019845611. [Google Scholar] [CrossRef]
- Ogilvie, L.M.; Edgett, B.A.; Gray, S.; Al-Mufty, S.; Huber, J.S.; Brunt, K.R.; Simpson, J.A. A new approach to improve the hemodynamic assessment of cardiac function independent of respiratory influence. Sci. Rep. 2021, 11, 17223. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Howard, L.S.; Gomberg-Maitland, M.; Hoeper, M.M. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation 2020, 141, 678–693. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lin, Y.; Zhu, Y.; Gao, L.; Ji, M.; Zhang, L.; Xie, M.; Li, Y. Clinical usefulness of right ventricle-pulmonary artery coupling in cardiovascular disease. J. Clin. Med. 2023, 12, 2526. [Google Scholar] [CrossRef]
- Dayer, N.; Ltaief, Z.; Liaudet, L.; Lechartier, B.; Aubert, J.D.; Yerly, P. Pressure overload and right ventricular failure: From pathophysiology to treatment. J. Clin. Med. 2023, 12, 4722. [Google Scholar] [CrossRef]
- Tornling, G.; Batta, R.; Salvail, D.; Raud, J.; Denton, C.P. Effects of the oral angiotensin II type 2 receptor agonist C21 in Sugen-hypoxia induced pulmonary hypertension in rats. Int. J. Mol. Sci. 2023, 24, 7478. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Hanrott, K.; Budd, D.C.; Torres, F.; Grünig, E.; Escribano-Subias, P.; Meseguer, M.L.; Halank, M.; Opitz, C.; Hall, D.A.; et al. An open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single doses of GSK2586881 in participants with pulmonary arterial hypertension. Pulm. Circ. 2022, 12, e12024. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Rathinasabapathy, A.; Austin, E.A.; Brittain, E.L.; Carrier, E.J.; Chen, X.; Fessel, J.P.; Fike, C.D.; Fong, P.; Fortune, N.; et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1702638. [Google Scholar] [CrossRef]
- Domenig, O.; Manzel, A.; Grobe, N.; Kaltenecker, C.C.; Kovarik, J.J.; Stegbauer, J.; Gurley, S.B.; Le Thuc, O.; Bader, M.; Linker, R.A.; et al. Neprilysin is a Mediator of Alternative Renin-Angiotensin-System Activation in the Murine and Human Kidney. Sci. Rep. 2016, 6, 33678. [Google Scholar] [CrossRef]
- Sánchez-Gloria, J.L.; Martínez-Olivares, C.E.; Del Valle-Mondragón, L.; Cortés-Camacho, F.; Zambrano-Vásquez, O.R.; Hernández-Pando, R.; Sánchez-Muñoz, F.; Sánchez-Lozada, L.G.; Osorio-Alonso, H. Allicin, an Emerging Treatment for Pulmonary Arterial Hypertension: An Experimental Study. Int. J. Mol. Sci. 2023, 24, 12959. [Google Scholar] [CrossRef]
- Devendran, A.; Kar, S.; Bailey, R.; Trivieri, M.G. The Role of Bone Morphogenetic Protein Receptor Type 2 (BMPR2) and the Prospects of Utilizing Induced Pluripotent Stem Cells (iPSCs) in Pulmonary Arterial Hypertension Disease Modeling. Cells 2022, 11, 3823. [Google Scholar] [CrossRef]
- Zuckerbraun, B.S.; George, P.; Gladwin, M.T. Nitrite in pulmonary arterial hypertension: Therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovasc. Res. 2011, 89, 542–552. [Google Scholar] [CrossRef]
- Rubio-Ruíz, M.E.; Plata-Corona, J.C.; Soria-Castro, E.; Díaz-Juárez, J.A.; Sánchez-Aguilar, M. Pleiotropic Effects of Peroxisome Proliferator-Activated Receptor Alpha and Gamma Agonists on Myocardial Damage: Molecular Mechanisms and Clinical Evidence—A Narrative Review. Cells 2024, 13, 1488. [Google Scholar] [CrossRef]
- Chakkarwar, V.A.; Kawtikwar, P. Fenofibrate Prevents Nicotine-Induced Acute Kidney Injury: Possible Involvement of Endothelial Nitric Oxide Synthase. Indian J. Nephrol. 2021, 31, 435–441. [Google Scholar] [CrossRef]
- Balsa, A.; Pérez-Ternero, C.; Sassi, Y.; Bodega, G.; Lillo-Moya, J.; Soria, F.N.; de Frutos, S.; Muñoz-Chápuli, R.; Muñoz, C.; Zaragoza, C.; et al. Therapeutic Approaches in Pulmonary Arterial Hypertension with Beneficial Effects on Right Ventricular Function—Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 15539. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.); Institute for Laboratory Animal Research (U.S.). Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; 220p. [Google Scholar]
- Leary, S.L.; American Veterinary Medical Association (AVMA). AVMA Guidelines for the Euthanasia of Animals, 2020 ed.; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; 121p. [Google Scholar]
- Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 16 October 2025).
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S.; et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, F.; Vitali, S.H.; Touma, M.; Rose, C.D.; Petty, C.R.; Levy, P.; Kourembanas, S.; Christou, H. Echocardiographic markers of pulmonary hemodynamics and right ventricular hypertrophy in rat models of pulmonary hypertension. Pulm. Circ. 2020, 10, 2045894020910976. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.; Ring, J.; Phillips, M.I. High-performance liquid chromatography for the separation of angiotensin and its metabolites in human plasma and sweat. J. Chromatogr. Sci. 1990, 28, 524–528. [Google Scholar] [CrossRef]








| Gene | Direction | Sequences |
|---|---|---|
| Bmpr2 | Forward | gagccctccctggacttg |
| Reverse | atatcgaccccgtccaatc | |
| Smad5 | Forward | gcctatggacacaagcaaca |
| Reverse | aggcaacaggctgaacatct | |
| GAPDH | Qiagen (Cat. No. PPM02946E) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rada-Pascual, K.M.; Zúniga-Muñoz, A.M.; Alvarez-Alvarez, Y.Q.; Del Valle-Mondragón, L.; Rubio-Gayosso, I.; Martínez-Olivares, C.E.; Hernández-Pando, R.; Osorio-Alonso, H.; Sánchez-Gloria, J.L.; Flores, P.L.; et al. Fenofibrate as a Modulator of the Renin–Angiotensin System in Su/Hx-Induced Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2025, 26, 10251. https://doi.org/10.3390/ijms262110251
Rada-Pascual KM, Zúniga-Muñoz AM, Alvarez-Alvarez YQ, Del Valle-Mondragón L, Rubio-Gayosso I, Martínez-Olivares CE, Hernández-Pando R, Osorio-Alonso H, Sánchez-Gloria JL, Flores PL, et al. Fenofibrate as a Modulator of the Renin–Angiotensin System in Su/Hx-Induced Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2025; 26(21):10251. https://doi.org/10.3390/ijms262110251
Chicago/Turabian StyleRada-Pascual, Karla M., Alejandra M. Zúniga-Muñoz, Yamnia Q. Alvarez-Alvarez, Leonardo Del Valle-Mondragón, Ivan Rubio-Gayosso, Constanza E. Martínez-Olivares, Rogelio Hernández-Pando, Horacio Osorio-Alonso, José L. Sánchez-Gloria, Pedro L. Flores, and et al. 2025. "Fenofibrate as a Modulator of the Renin–Angiotensin System in Su/Hx-Induced Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 26, no. 21: 10251. https://doi.org/10.3390/ijms262110251
APA StyleRada-Pascual, K. M., Zúniga-Muñoz, A. M., Alvarez-Alvarez, Y. Q., Del Valle-Mondragón, L., Rubio-Gayosso, I., Martínez-Olivares, C. E., Hernández-Pando, R., Osorio-Alonso, H., Sánchez-Gloria, J. L., Flores, P. L., Sandoval, J., Gómez-Zamudio, J. H., Carbó, R., & Sánchez-Muñoz, F. (2025). Fenofibrate as a Modulator of the Renin–Angiotensin System in Su/Hx-Induced Pulmonary Arterial Hypertension. International Journal of Molecular Sciences, 26(21), 10251. https://doi.org/10.3390/ijms262110251








