Pathologic Signaling and Disease Implications of Insulin-like Growth Factor Binding Proteins in Cancer, Cardiovascular Disease, and Fibrosis
Abstract
1. Introduction
2. IGFBP Structure and Function
2.1. Canonical Functions and Maintenance of Homeostasis
2.2. Non-Canonical Functions and Pathological Pathways
3. IGFBP Signaling Pathways in Cancer
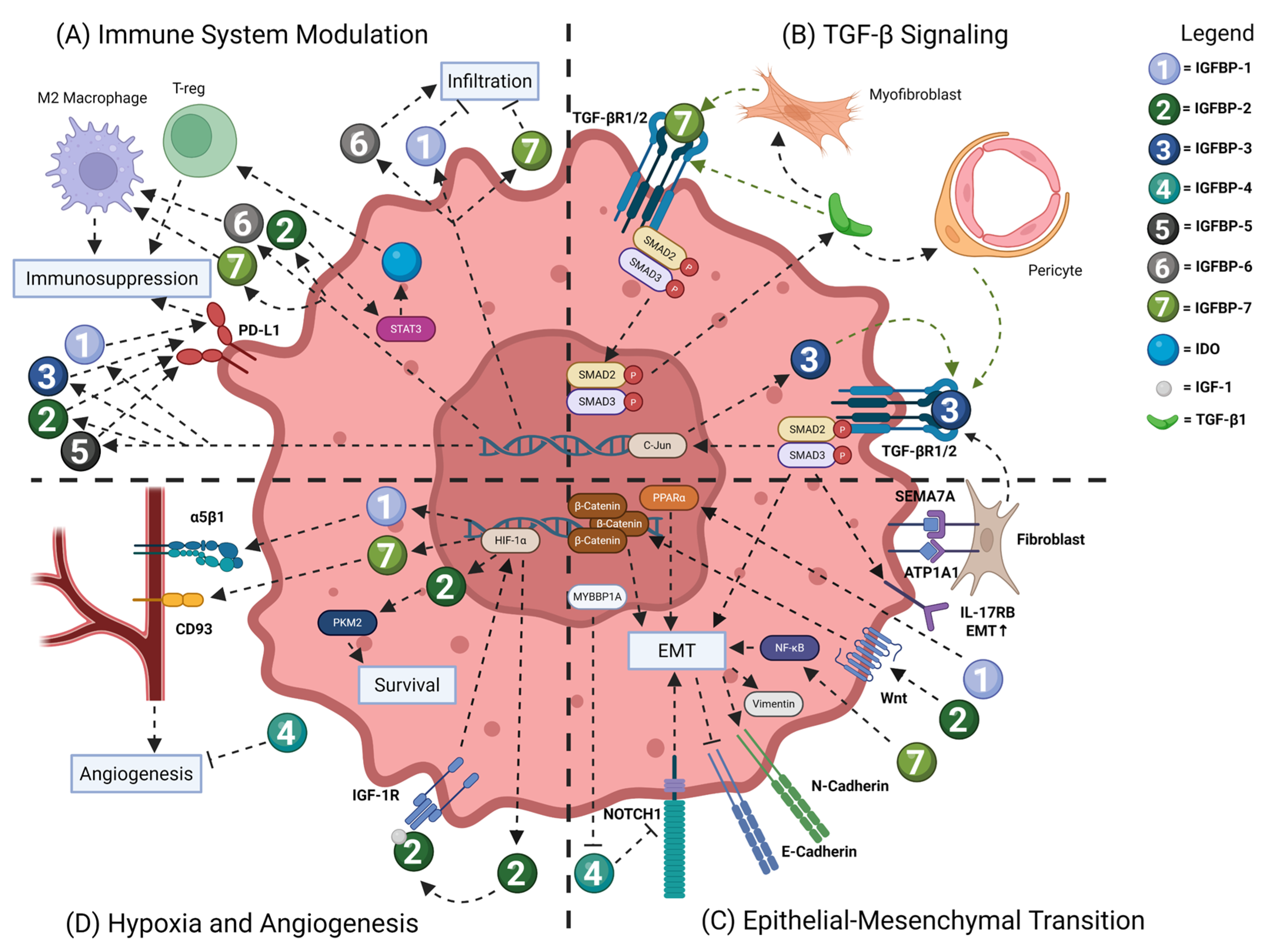
3.1. Immune System Modulation
3.2. TGF-β Signaling
3.3. Epithelial-to-Mesenchymal Transition
3.4. Hypoxia and Angiogenesis
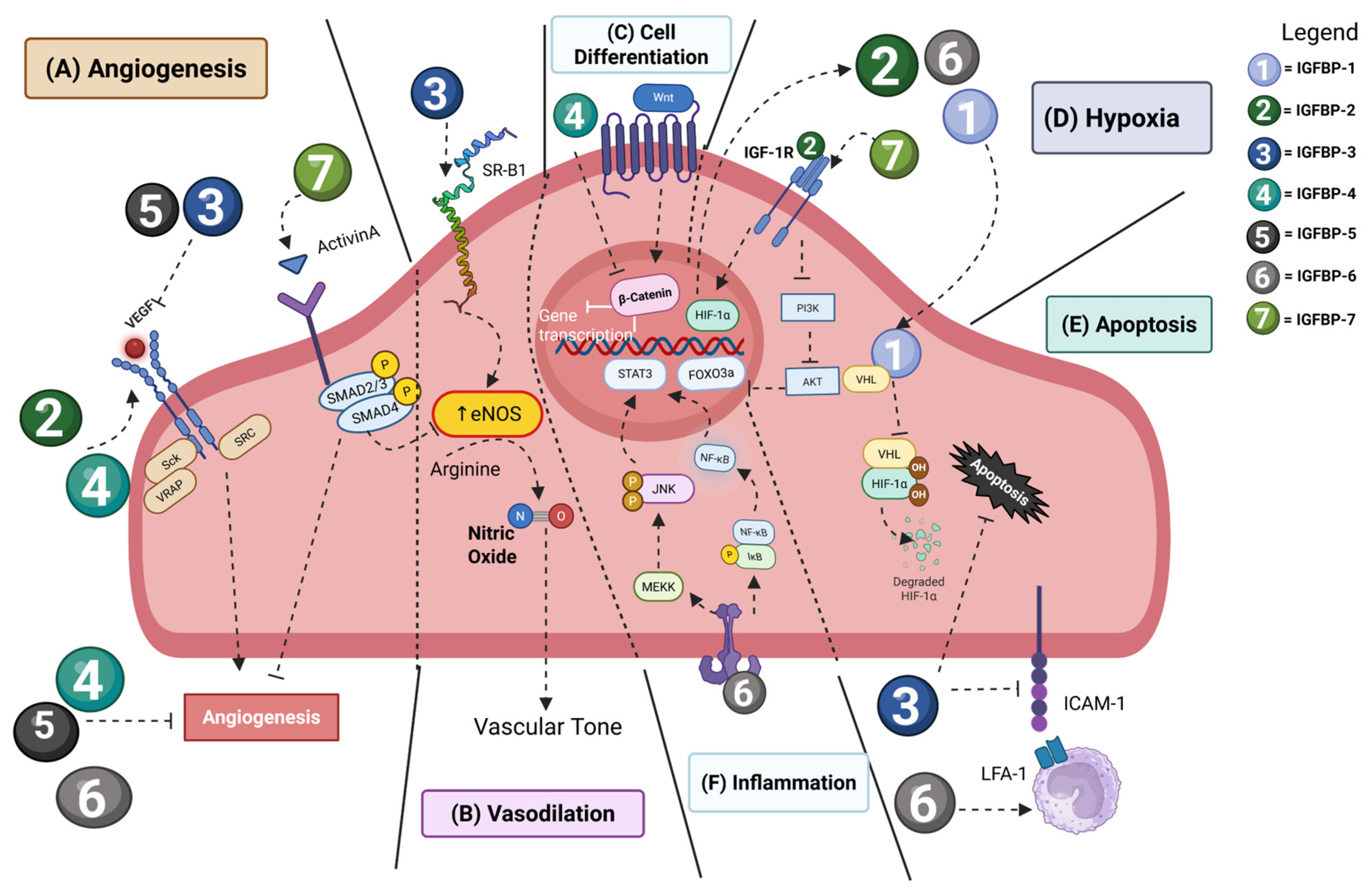
4. IGFBP Signaling Pathways in Vascular Homeostasis and Pathology
4.1. Hypoxia and Angiogenesis
4.2. Cardiovascular Disease
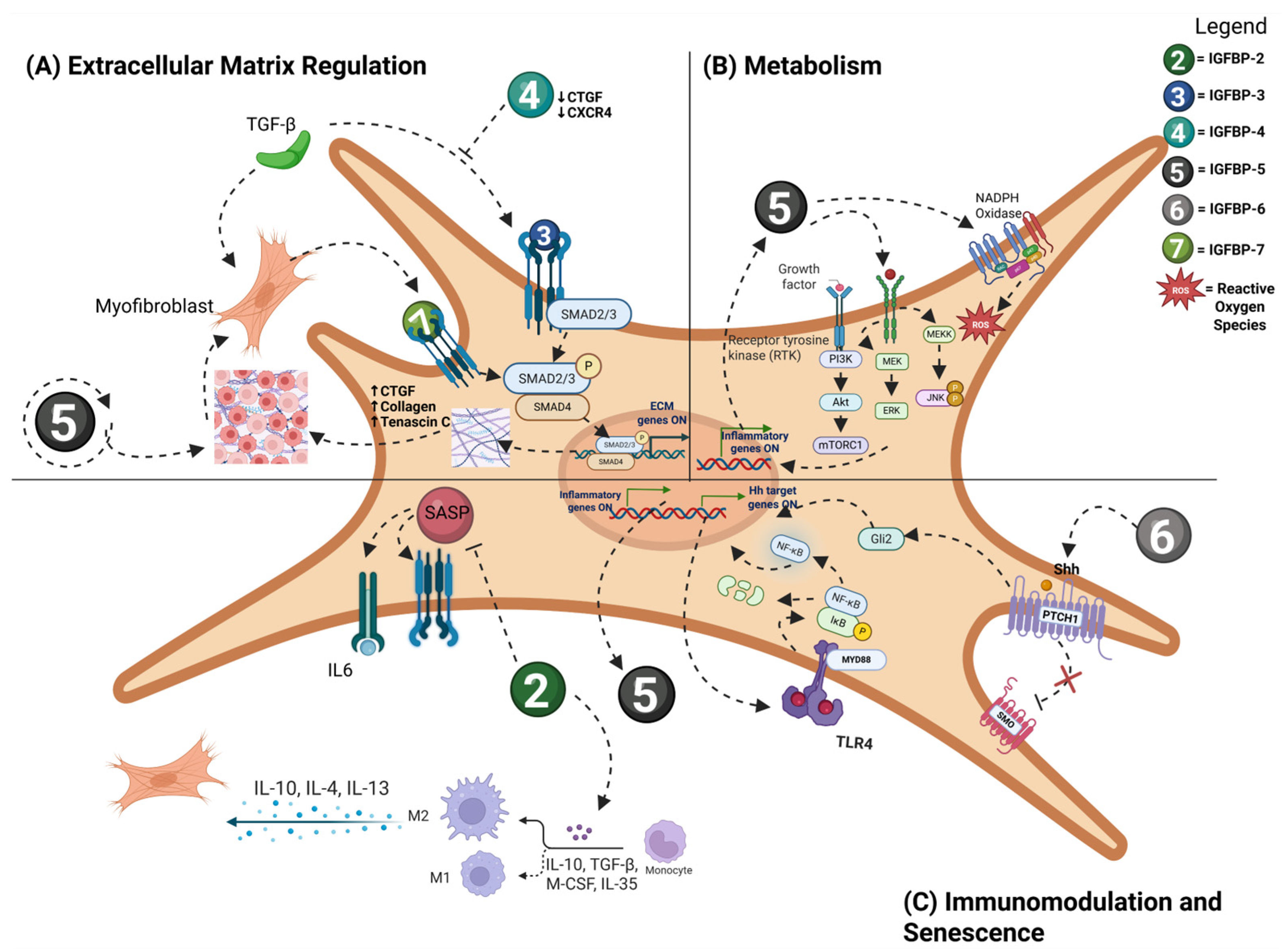
5. IGFBP Signaling Pathways in Fibrosis
6. IGFBPs as Biomarkers of Disease
7. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEC2 | Alveolar Epithelial Type 2 |
| ALS | Acid Labile Subunit |
| ARTA | All-Trans Retinoic Acid |
| ATP1A1 | ATPase Na+/K+-Transporting Subunit Alpha 1 |
| CAF | Cancer-Associated Fibroblast |
| CDK2 | Cyclin-Dependent Kinase 2 |
| CHF | Congestive Heart Failure |
| Clec4nhi | C-Type Lectin Domain Family 4 |
| CRC | Colorectal Cancer |
| CTGF | Connective Tissue Growth Factor |
| EC | Endothelial Cell |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-To-Mesenchymal Transition |
| ERK | Extracellular Signal-Regulated Kinase |
| FGF2 | Fibroblast Growth Factor 2 |
| FOXO3a | Fork-Head Box O3 |
| Fra-1 | Fos-Related Antigen 1 |
| HCC | Hepatocellular Carcinoma |
| HDF | Human Dermal Fibroblast |
| HIF | Hypoxia-Inducible Factor |
| HSC | Hepatic Stellate Cell |
| Htra3 | High-Temperature Requirement A3 |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IDO | Indoleamine 2, 3-Dioxygenase |
| IGF | Insulin-Like Growth Factor |
| IGFBP | Insulin-Like Growth Factor Binding Protein |
| IGF-1R | Insulin-Like Growth Factor Receptor 1 |
| IGFBP-rP | Insulin-like Growth Factor Binding Protein-Related Protein |
| IL-17RB | Interleukin 17 Receptor B |
| ImpL2 | Ecdysone-Inducible Gene L2 |
| IPF | Idiopathic Pulmonary Fibrosis |
| JNK | c-Jun N-Terminal Kinase |
| MACE | Major Adverse Cardiovascular Events |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase 1 |
| METTL3 | Methyltransferase-Like 3 |
| MFAP5 | Microfibril-Associated Protein 5 |
| MMP | Matrix Metalloproteinases |
| MRSS | Modified Rodnan Skin Score |
| mTOR | Mechanistic Targeting of Rapamycin |
| MYBBP1A | MYB Binding Protein 1a |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (Reduced) |
| NAFLD | Non-alcoholic Fatty Liver Disease |
| NFAT4 | Nuclear Factor of Activated T cells 4 |
| NLS | Nuclear Localization Sequence |
| NO | Nitric Oxide |
| NOTCH1 | Neurogenic Locus Notch Homolog Protein 1 |
| NPC | Nuclear Pore Complex |
| NYHA | New York Heart Association |
| PAH | Pulmonary Arterial Hypertension |
| PAPP-A | Pregnancy-Associated Plasma Protein A |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3K | Phosphoinositide 3-Kinase |
| PKM2 | Pyruvate Kinase |
| PPAR-λ | Peroxisome Proliferator-Activated Receptor-λ |
| ROCK | Rho-Associated Protein Kinase |
| ROS | Reactive Oxygen Species |
| RXR-α | Retinoid X Receptor Alpha |
| SASP | Senescence-Associated Secretory Phenotype |
| SEMA7A | Semaphorin 7A |
| SHH | Sonic Hedgehog |
| SiRNA | Small Interfering Ribonucleic Acid |
| SMAD | Suppressor of Mothers Against Decapentaplegic |
| SREBP2 | Sterol Regulatory Element-Binding Protein 2 |
| SSc | Systemic Sclerosis |
| SSc-ILD | Systemic Sclerosis-Associated Interstitial Lung Disease |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAM | Tumor-Associated Macrophages |
| TCGA | The Cancer Genome Atlas Program |
| TGF-β | Transforming Growth Factor |
| TIMP-2 | Tissue Inhibitor of Metalloproteinases 2 |
| TLR4 | Toll-Like Receptor 4 |
| TME | Tumor Microenvironment |
| TRα1 | Thyroid Hormone Receptor Alpha 1 |
| VDR | Vitamin D Receptor |
| VEGF | Vascular Endothelial Growth Factor |
| VEGF-A | Vascular Endothelial Growth Factor-A |
| VEGFR | Vascular Endothelial Growth Factor receptor |
References
- Siraj, Y.; Aprile, D.; Alessio, N.; Peluso, G.; Di Bernardo, G.; Galderisi, U. IGFBP7 is a key component of the senescence-associated secretory phenotype (SASP) that induces senescence in healthy cells by modulating the insulin, IGF, and activin A pathways. Cell Commun. Signal. 2024, 22, 540. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef]
- Chen, L.; Hui, L.; Li, J. The multifaceted role of insulin-like growth factor binding protein 7. Front. Cell Dev. Biol. 2024, 1, 1420862. [Google Scholar] [CrossRef]
- Song, F.; Zhou, X.X.; Hu, Y.; Li, G.; Wang, Y. The Roles of Insulin-Like Growth Factor Binding Protein Family in Development and Diseases. Adv. Ther. 2021, 38, 885–903. [Google Scholar] [CrossRef]
- Baxter, R.C. Signaling Pathways of the Insulin-like Growth Factor Binding Proteins. Endocr. Rev. 2023, 44, 753–778. [Google Scholar] [CrossRef]
- Forbes, B.E.; Blyth, A.J.; Wit, J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol. Cell. Endocrinol. 2020, 518, 111035. [Google Scholar] [CrossRef]
- Werner, H. The IGF1 Signaling Pathway: From Basic Concepts to Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 14882. [Google Scholar] [CrossRef]
- Miller, B.S.; Rogol, A.D.; Rosenfeld, R.G. The History of the Insulin-Like Growth Factor System. Horm. Res. Paediatr. 2022, 95, 619–630. [Google Scholar] [CrossRef]
- Kim, H.; Fu, Y.; Hong, H.J.; Lee, S.-G.; Lee, D.S.; Kim, H.M. Structural basis for assembly and disassembly of the IGF/IGFBP/ALS ternary complex. Nat. Commun. 2022, 13, 4434. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Oxvig, C. The IGF System and Aging. Endocr. Rev. 2025, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Squillaro, T.; Di Bernardo, G.; Galano, G.; De Rosa, R.; Melone, M.A.B.; Peluso, G.; Galderisi, U. Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence. Elife 2020, 9, e54523, Erratum in eLife 2022, 11, e80871. [Google Scholar] [CrossRef]
- Ng, E.F.Y.; Kaida, A.; Nojima, H.; Miura, M. Roles of IGFBP-3 in cell migration and growth in an endophytic tongue squamous cell carcinoma cell line. Sci. Rep. 2022, 12, 11503. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ku, S.-Y.; Jee, B.-C.; Suh, C.-S.; Choi, Y.M.; Kim, J.G.; Moon, S.Y.; Kim, S.H. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum. Reprod. 2006, 21, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Forbes, M.E.; Fuller, G.N.; Li, J.; Yang, X.; Zhang, W. IGFBP2: Integrative hub of developmental and oncogenic signaling network. Oncogene 2020, 39, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Span, M.; Rayhanna, M.H.; Perera, M.; Day, M.L. Insulin-like Growth Factor Binding Protein 3 Increases Mouse Preimplantation Embryo Cleavage Rate by Activation of IGF1R and EGFR Independent of IGF1 Signalling. Cells 2022, 11, 3762. [Google Scholar]
- Gupta, M.B. The role and regulation of IGFBP-1 phosphorylation in fetal growth restriction. J. Cell Commun. Signal. 2015, 9, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Aida, K.; Duan, C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc. Natl. Acad. Sci. USA 2005, 102, 1240–1245. [Google Scholar] [CrossRef]
- Camacho de Gutiérrez, A.R.; Calisici, O.; Wrenzycki, C.; Gutiérrez-Añez, J.C.; Hoeflich, C.; Hoeflich, A.; Bajcsy, Á.C.; Schmicke, M. Effect of IGFBP-4 during In Vitro Maturation on Developmental Competence of Bovine Cumulus Oocyte Complexes. Animals 2024, 14, 673. [Google Scholar] [CrossRef]
- Wei, L.F.; Weng, X.F.; Huang, X.C.; Peng, Y.H.; Guo, H.P.; Xu, Y.W. IGFBP2 in cancer: Pathological role and clinical significance (Review). Oncol. Rep. 2021, 45, 427–438. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Yang, Y.T.; Xu, X.D.; Chen, J.S.; Chen, T.L.; Chen, H.J.; Zhu, Y.B.; Lin, J.Y.; Li, Y.; et al. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene 2019, 38, 1815–1831. [Google Scholar] [CrossRef]
- Yaqoob, U.; Luo, F.; Greuter, T.; Jalan Sakrikar, N.; Sehrawat, T.S.; Lu, J.; Hu, X.; Gao, J.; Kostallari, E.; Chen, J.; et al. GIPC-Regulated IGFBP-3 Promotes HSC Migration In Vitro and Portal Hypertension In Vivo Through a β1-Integrin Pathway. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 545–559. [Google Scholar] [CrossRef]
- Varma Shrivastav, S.; Bhardwaj, A.; Pathak, K.A.; Shrivastav, A. Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3): Unraveling the Role in Mediating IGF-Independent Effects Within the Cell. Front. Cell Dev. Biol. 2020, 8, 286. [Google Scholar] [CrossRef]
- Poreba, E.; Durzynska, J. Nuclear localization and actions of the insulin-like growth factor 1 (IGF-1) system components: Transcriptional regulation and DNA damage response. Mutation Res. Rev. Mutat. Res. 2020, 784, 108307. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Aprile, D.; Peluso, G.; Mazzone, V.; Patrone, D.; Di Bernardo, G.; Galderisi, U. IGFBP5 is released by senescent cells and is internalized by healthy cells, promoting their senescence through interaction with retinoic receptors. Cell Commun. Signal. 2024, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Renna, F.; Ballarò, R.; Costelli, P. The Redox Balance: A Target for Interventions Against Muscle Wasting in Cancer Cachexia? Antioxid. Redox Signal. 2020, 33, 542–558. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Murthy, G.G.; Wang, G.; Rahman, T.; Korman, B. Insulin-like Growth Factor Binding Protein-7 (IGFBP7) Plays a Pathogenic Role in Dermal Fibrosis and Is Increased in Systemic Sclerosis [Abstract]. Arthritis Rheumatol. 2024, 76 (Suppl. S9). Available online: https://acrabstracts.org/abstract/insulin-like-growth-factor-binding-protein-7-igfbp7-plays-a-pathogenic-role-in-dermal-fibrosis-and-is-increased-in-systemic-sclerosis/ (accessed on 16 October 2025).
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Yan, Z.; Yan, L.; Ding, H.; Wang, A.; Xu, Q.; Sun, L.; Yuan, Y. Expression characteristics and their functional role of IGFBP gene family in pan-cancer. BMC Cancer 2023, 23, 371. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Wei, Y.-Q.; Wei, X.-W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef]
- Yao, Z.; Han, J.; Wu, J.; Li, M.; Chen, R.; Jian, M.; Yang, Z.; Wang, X.; Zhang, Y.; Hu, J.; et al. Deciphering the multidimensional impact of IGFBP1 expression on cancer prognosis, genetic alterations, and cellular functionality: A comprehensive Pan-cancer analysis. Heliyon 2024, 10, e37402. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Zhang, Y.P.; Zheng, F.; Zhou, L. Multiple bioinformatics analysis identifies IGFBP1 as associated with the prognosis of stomach adenocarcinoma. Medicine 2023, 102, e33346. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, S.; Liu, J.; Huang, Y.; Chen, K.; Zhang, X. MMP9 and IGFBP1 Regulate Tumor Immune and Drive Tumor Progression in Clear Cell Renal Cell Carcinoma. J. Cancer 2021, 12, 2243–2257. [Google Scholar] [CrossRef]
- Lu, H.; Yu, X.; Xu, Z.; Deng, J.; Zhang, M.J.; Zhang, Y.; Sun, S. Prognostic Value of IGFBP6 in Breast Cancer: Focus on Glucometabolism. Technol. Cancer Res. Treat. 2024, 23, 15330338241271998. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xu, L.; Zhang, J. Co-amplified with PDGFRA, IGFBP7 is a prognostic biomarker correlated with the immune infiltrations of glioma. Cancer Med. 2023, 12, 4951–4967. [Google Scholar] [CrossRef]
- Yi, X.; Zheng, X.; Xu, H.; Li, J.; Zhang, T.; Ge, P.; Liao, D.; Li, H.; Lyu, X.; Ai, J. IGFBP7 and the Tumor Immune Landscape: A Novel Target for Immunotherapy in Bladder Cancer. Front. Immunol. 2022, 13, 898493. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, R.; Song, C.; Wang, H.; Rong, J.; Wang, F.; Yan, L.; Song, Y.; Xie, Y. Increased IGFBP7 Expression Correlates with Poor Prognosis and Immune Infiltration in Gastric Cancer. J. Cancer 2021, 12, 1343–1355. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Yang, T.; Chen, J.; Zhang, X.; Liang, X. IGFBP2 Drives Regulatory T Cell Differentiation through STAT3/IDO Signaling Pathway in Pancreatic Cancer. J. Pers. Med. 2022, 12, 2005. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Song, Q.; Liu, L.; Forbes, E.; Tian, W.; Zhang, Z.; Kang, Y.; Wang, H.; Fleming, J.B.; et al. IGFBP2 promotes tumor progression by inducing alternative polarization of macrophages in pancreatic ductal adenocarcinoma through the STAT3 pathway. Cancer Lett. 2021, 500, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Walterskirchen, N.; Müller, C.; Ramos, C.; Zeindl, S.; Stang, S.; Herzog, D.; Sachet, M.; Schimek, V.; Unger, L.; Gerakopoulos, V.; et al. Metastatic colorectal carcinoma-associated fibroblasts have immunosuppressive properties related to increased IGFBP2 expression. Cancer Lett. 2022, 540, 215737. [Google Scholar] [CrossRef]
- Ramos, C.; Walterskirchen, N.; Knöbl, V.; Zotter, C.; Müller, C.; Gerakopoulos, V.; Rauch, A.; Falk, L.; Sachet, M.; D’Angelo, E.; et al. Colorectal cancer peritoneal metastasis is promoted by tissue-specific fibroblasts that can arise in response to various local disorders. Cancer Lett. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, L.; Huang, P.; Wang, Z.; Guo, Q.; Huang, C.; Leng, W.; Qin, S. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discov. 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Guo, C.; Li, J.; Liang, G. Cancer-associated fibroblast-associated gene IGFBP2 promotes glioma progression through induction of M2 macrophage polarization. Am. J. Physiol. Cell Physiol. 2024, 326, C252–C268. [Google Scholar] [CrossRef]
- Longhitano, L.; Vicario, N.; Forte, S.; Giallongo, C.; Broggi, G.; Caltabiano, R.; Barbagallo, G.M.V.; Altieri, R.; Raciti, G.; Di Rosa, M.; et al. Lactate modulates microglia polarization via IGFBP6 expression and remodels tumor microenvironment in glioblastoma. Cancer Immunol. Immunother. 2023, 72, 1–20, Erratum in Cancer Immunol. Immunother. 2023, 72, 21. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Liu, M.; Yan, W.; Chen, D.; Luo, J.; Dai, L.; Chen, H.; Chen, K.N. IGFBP1(hi)WNT3A(lo) Subtype in Esophageal Cancer Predicts Response and Prolonged Survival with PD-(L)1 Inhibitor. Biology 2022, 11, 1575. [Google Scholar] [CrossRef]
- Wen, Z.; Sun, H.; Zhang, Z.; Zheng, Y.; Zheng, S.; Bin, J.; Liao, Y.; Shi, M.; Zhou, R.; Liao, W. High baseline tumor burden-associated macrophages promote an immunosuppressive microenvironment and reduce the efficacy of immune checkpoint inhibitors through the IGFBP2-STAT3-PD-L1 pathway. Cancer Commun. 2023, 43, 562–581. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Mu, P.; Zhang, X.; Qi, R.; Zhang, Y.; Zhang, H.; Zhu, X.; Dong, Z.; Dong, Y. IGFBP3 induces PD-L1 expression to promote glioblastoma immune evasion. Cancer Cell Int. 2024, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, G.; Zeng, Q.; Zhang, R.; Lin, Y.; Li, Y.; Huang, B.; Pan, H. IGFBP5, as a Prognostic Indicator Promotes Tumor Progression and Correlates with Immune Microenvironment in Glioma. J. Cancer 2024, 15, 232–250. [Google Scholar] [CrossRef]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gong, Y.; Zhao, L.; Song, P.; Zhang, H.; Zhang, Y.; Ju, H.; Wang, X.; Wang, B.; et al. IGFBP3 induced by the TGF-β/EGFRvIII transactivation contributes to the malignant phenotype of glioblastoma. iScience 2023, 26, 106639. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Wu, Y.; Ma, C.; Wei, D.; Pan, L.; Cai, L. IGFBP7 remodels the tumor microenvironment of esophageal squamous cell carcinoma by activating the TGFβ1/SMAD signaling pathway. Oncol. Lett. 2022, 24, 251. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Tapia-Galisteo, A.; Martín-García, L.; Tarín, C.; Corbacho, C.; Gómez-López, G.; Sánchez-Tirado, E.; Campuzano, S.; González-Cortés, A.; Yáñez-Sedeño, P.; et al. TGF-β-induced IGFBP-3 is a key paracrine factor from activated pericytes that promotes colorectal cancer cell migration and invasion. Mol. Oncol. 2020, 14, 2609–2628. [Google Scholar] [CrossRef]
- Hong, Z.; Xie, W.; Zhuo, H.; Wei, X.; Wang, K.; Cheng, J.; Lin, L.; Hou, J.; Chen, X.; Cai, J. Crosstalk between Cancer Cells and Cancer-Associated Fibroblasts Mediated by TGF-β1-IGFBP7 Signaling Promotes the Progression of Infiltrative Gastric Cancer. Cancers 2023, 15, 3965. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352, Erratum in Nat. Rev. Mol. Cell Biol. 2021, 22, 834. [Google Scholar] [CrossRef] [PubMed]
- Celià-Terrassa, T.; Kang, Y. How important is EMT for cancer metastasis? PLoS Biol. 2024, 22, e3002487. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Han, T.; Zhou, J.; Liu, Y.; Guo, J.; Liu, Z.; Bai, Y.; Xing, Y.; Ding, X.; et al. IGFBP1 promotes the proliferation and migration of lung adenocarcinoma cells through the PPARα pathway. Transl. Oncol. 2024, 49, 102095. [Google Scholar] [CrossRef]
- Alicea, G.M.; Patel, P.; Portuallo, M.E.; Fane, M.E.; Wei, M.; Chhabra, Y.; Dixit, A.; Carey, A.E.; Wang, V.; Rocha, M.R.; et al. Age-Related Increases in IGFBP2 Increase Melanoma Cell Invasion and Lipid Synthesis. Cancer Res. Commun. 2024, 4, 1908–1918. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, W.; Huang, Y.; Cheng, H. Exosomal IGFBP2 derived from highly metastatic promotes hepatocellular carcinoma metastasis by inducing epithelial mesenchymal transition. Gene 2024, 913, 148374. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhang, P.; Wei, M.; Tian, T.; Guan, Y.; Han, C.; Wei, W.; Ma, Y. IGFBP2 drives epithelial-mesenchymal transition in hepatocellular carcinoma via activating the Wnt/β-catenin pathway. Infect. Agent Cancer 2023, 18, 73. [Google Scholar] [CrossRef]
- Dar, A.A.; Majid, S.; Nosrati, M.; de Semir, D.; Federman, S.; Kashani-Sabet, M. Functional modulation of IGF-binding protein-3 expression in melanoma. J. Investig. Dermatol. 2010, 130, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Naspi, A.; Zingariello, M.; Sancillo, L.; Panasiti, V.; Polinari, D.; Martella, M.; Rosa Alba, R.; Londei, P. IGFBP-3 inhibits Wnt signaling in metastatic melanoma cells. Mol. Carcinog. 2017, 56, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Nakajima, G.; Hamil, T.; Fodstad, O.; Riker, A.; Ju, J. Association of insulin-like growth factor binding protein-3 expression with melanoma progression. Mol. Cancer Ther. 2006, 5, 3078–3084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.I.; Tien, S.C.; Ko, Y.L.; Chang, C.C.; Hsu, M.F.; Chien, H.J.; Peng, H.Y.; Jeng, Y.M.; Tien, Y.W.; Chang, Y.T.; et al. SEMA7A-mediated juxtacrine stimulation of IGFBP-3 upregulates IL-17RB at pancreatic cancer invasive front. Cancer Gene Ther. 2024, 31, 1840–1855. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, Z.; Li, S.; Yin, W. IGFBP7 promotes gastric cancer by facilitating epithelial-mesenchymal transition of gastric cells. Heliyon 2024, 10, e30986. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Jin, Y.; Qi, M.; Si, L.; Shi, X.; Cai, M.; Fu, H.; Liu, Y.; Guo, R. IGFBP2 Promotes Proliferation and Glycolysis of Endometrial Cancer by Regulating PKM2/HIF-1α Axis. Cancer Sci. 2025, 116, 656–672. [Google Scholar] [CrossRef]
- Liu, Y.; Nelson, M.V.; Bailey, C.; Zhang, P.; Zheng, P.; Dome, J.S.; Liu, Y.; Wang, Y. Targeting the HIF-1α-IGFBP2 axis therapeutically reduces IGF1-AKT signaling and blocks the growth and metastasis of relapsed anaplastic Wilms tumor. Oncogene 2021, 40, 4809–4819. [Google Scholar] [CrossRef]
- Xiao, G.; Li, Y.; Hu, Y.; Tan, K.; Wang, M.; Zhu, K.; San, M.; Cheng, Q.; Tayier, D.; Hu, T.; et al. Intratumor HIF-1α modulates production of a cachectic ligand to cause host wasting. Cell Insight 2025, 4, 100247. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Iwamoto, H.; Seki, T.; Nakamura, T.; Masuda, A.; Sakaue, T.; Tanaka, T.; Imamura, Y.; Niizeki, T.; Nakano, M.; et al. Tumor-derived insulin-like growth factor-binding protein-1 contributes to resistance of hepatocellular carcinoma to tyrosine kinase inhibitors. Cancer Commun. 2023, 43, 415–434. [Google Scholar] [CrossRef]
- Das, S.K.; Bhutia, S.K.; Azab, B.; Kegelman, T.P.; Peachy, L.; Santhekadur, P.K.; Dasgupta, S.; Dash, R.; Dent, P.; Grant, S.; et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013, 73, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Y.; Zhu, Y.; Song, G. Structural insight into CD93 recognition by IGFBP7. Structure 2024, 32, 282–291.e284. [Google Scholar] [CrossRef]
- Furusaka, Y.; Inoue, S.; Mizoguchi, I.; Hasegawa, H.; Katahira, Y.; Watanabe, A.; Sakamoto, E.; Sekine, A.; Miyakawa, S.; Umezu, T.; et al. Potent antitumor effects of the conditioned medium of bone marrow-derived mesenchymal stem cells via IGFBP-4. Cancer Sci. 2023, 114, 2499–2514. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cavallero, S.; Gu, Y.; Chen, T.H.; Hughes, J.; Hassan, A.B.; Bruning, J.C.; Pashmforoush, M.; Sucov, H.M. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development 2011, 138, 1795–1805. [Google Scholar] [CrossRef]
- Zhu, W.; Fan, Y.; Frenzel, T.; Gasmi, M.; Bartus, R.T.; Young, W.L.; Yang, G.Y.; Chen, Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 2008, 39, 1254–1261. [Google Scholar] [CrossRef]
- Bach, L.A. Endothelial cells and the IGF system. J. Mol. Endocrinol. 2015, 54, R1–R13. [Google Scholar] [CrossRef]
- Dallinga, M.G.; Habani, Y.I.; Kayser, R.P.; Van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. IGF-binding proteins 3 and 4 are regulators of sprouting angiogenesis. Mol. Biol. Rep. 2020, 47, 2561–2572. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, H.; Lin, P.; Zhang, Z.; Chen, M.; Zhang, Y.; Mo, J.; Zhu, Y.; Liu, N.; Chen, X. Insulin-like growth factor binding protein-1 regulates HIF-1alpha degradation to inhibit apoptosis in hypoxic cardiomyocytes. Cell Death Discov. 2021, 7, 242. [Google Scholar] [CrossRef]
- Haywood, N.J.; Slater, T.A.; Drozd, M.; Warmke, N.; Matthews, C.; Cordell, P.A.; Smith, J.; Rainford, J.; Cheema, H.; Maher, C.; et al. IGFBP-1 in Cardiometabolic Pathophysiology-Insights From Loss-of-Function and Gain-of-Function Studies in Male Mice. J. Endocr. Soc. 2020, 4, bvz006. [Google Scholar] [CrossRef]
- Li, B.; Shaikh, F.; Younes, H.; Abuhalimeh, B.; Zamzam, A.; Abdin, R.; Qadura, M. The Prognostic Potential of Insulin-like Growth Factor-Binding Protein 1 for Cardiovascular Complications in Peripheral Artery Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, B.C.; Quanz, K.; Baldauf, J.; Petrovic, A.; Ruppert, C.; Guenther, A.; Gall, H.; Tello, K.; Grimminger, F.; Ghofrani, H.A.; et al. The diverging roles of insulin-like growth factor binding proteins in pulmonary arterial hypertension. Vascul. Pharmacol. 2024, 155, 107379. [Google Scholar] [CrossRef]
- Barutaut, M.; Fournier, P.; Peacock, W.F.; Evaristi, M.F.; Caubere, C.; Turkieh, A.; Desmoulin, F.; Eurlings, L.W.M.; van Wijk, S.; Rocca, H.B.; et al. Insulin-like Growth Factor Binding Protein 2 predicts mortality risk in heart failure. Int. J. Cardiol. 2020, 300, 245–251. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Zhong, Y.; Ge, Y.; Li, C.; Qiao, Z.; Zhu, J. Insulin-like growth factor-binding protein 3 inhibits angiotensin II-induced aortic smooth muscle cell phenotypic switch and matrix metalloproteinase expression. Exp. Physiol. 2020, 105, 1827–1839. [Google Scholar] [CrossRef]
- Chang, K.H.; Chan-Ling, T.; McFarland, E.L.; Afzal, A.; Pan, H.; Baxter, L.C.; Shaw, L.C.; Caballero, S.; Sengupta, N.; Li Calzi, S.; et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc. Natl. Acad. Sci. USA 2007, 104, 10595–10600. [Google Scholar] [CrossRef] [PubMed]
- Kielczewski, J.L.; Jarajapu, Y.P.; McFarland, E.L.; Cai, J.; Afzal, A.; Li Calzi, S.; Chang, K.H.; Lydic, T.; Shaw, L.C.; Busik, J.; et al. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circ. Res. 2009, 105, 897–905. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Miller, M.J.; Peng, B.; Liu, L.; Soderland, C.; Tang, J.; Kern, T.S.; Pintar, J.; Steinle, J.J. IGFBP-3 and TNF-alpha regulate retinal endothelial cell apoptosis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Wise, J.; Raudsepp, S.D.; Paton, L.N.; Powell, J.; Jina, K.; Aldous, S.; Troughton, R.W.; Adamson, P.D.; Richards, A.M.; et al. Insulin-like growth factor binding protein-3 (IGFBP-3): A biomarker of coronary artery disease induced myocardial ischaemia. Eur. Heart J. Open 2025, 5, oeaf028. [Google Scholar] [CrossRef]
- Zhu, W.; Shiojima, I.; Ito, Y.; Li, Z.; Ikeda, H.; Yoshida, M.; Naito, A.T.; Nishi, J.; Ueno, H.; Umezawa, A.; et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 2008, 454, 345–349, Erratum in Nature 2014, 506, 254. [Google Scholar] [CrossRef]
- Wo, D.; Chen, J.; Li, Q.; Ma, E.; Yan, H.; Peng, J.; Zhu, W.; Fang, Y.; Ren, D.N. IGFBP-4 enhances VEGF-induced angiogenesis in a mouse model of myocardial infarction. J. Cell Mol. Med. 2020, 24, 9466–9471. [Google Scholar] [CrossRef]
- Adasheva, D.A.; Lebedeva, O.S.; Goliusova, D.V.; Postnikov, A.B.; Teriakova, M.V.; Kopylova, I.V.; Lagarkova, M.A.; Katrukha, A.G.; Serebryanaya, D.V. PAPP-A-Specific IGFBP-4 Proteolysis in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Int. J. Mol. Sci. 2023, 24, 8420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Lu, X.; Chen, M.; Zhang, T.; Shi, M.; Yao, W.; Zhang, H.; Gao, R.; Li, X.; Zhou, Y.; et al. IGFBP5 affects cardiomyocyte survival and functional recovery in mice following myocardial ischemia. Commun. Biol. 2024, 7, 1594. [Google Scholar] [CrossRef]
- Song, F.; Hu, Y.; Hong, Y.X.; Sun, H.; Han, Y.; Mao, Y.J.; Wu, W.Y.; Li, G.; Wang, Y. Deletion of endothelial IGFBP5 protects against ischaemic hindlimb injury by promoting angiogenesis. Clin. Transl. Med. 2024, 14, e1725. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Y.; Wang, R.; Wu, W.; Li, Z.; Wang, M.; Liu, R.; Zhang, C.; Li, W.; Wang, S. IGFBP6 regulates vascular smooth muscle cell proliferation and morphology via cyclin E-CDK2. J. Cell Physiol. 2020, 235, 9538–9556. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhao, W.; Jiang, H.; Zhao, Y.; Liao, Z.; Liu, Z.; Xu, M.; Jiang, S.; Wu, L.; Yang, Y.; et al. Endothelial IGFBP6 suppresses vascular inflammation and atherosclerosis. Nat. Cardiovasc. Res. 2025, 4, 145–162. [Google Scholar] [CrossRef]
- Hooper, A.T.; Shmelkov, S.V.; Gupta, S.; Milde, T.; Bambino, K.; Gillen, K.; Goetz, M.; Chavala, S.; Baljevic, M.; Murphy, A.J.; et al. Angiomodulin is a specific marker of vasculature and regulates vascular endothelial growth factor-A-dependent neoangiogenesis. Circ. Res. 2009, 105, 201–208. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, W.; Torphy, R.J.; Yao, S.; Zhu, G.; Lin, R.; Lugano, R.; Miller, E.N.; Fujiwara, Y.; Bian, L.; et al. Blockade of the CD93 pathway normalizes tumor vasculature to facilitate drug delivery and immunotherapy. Sci. Transl. Med. 2021, 13, eabc8922. [Google Scholar] [CrossRef] [PubMed]
- Bracun, V.; van Essen, B.; Voors, A.A.; van Veldhuisen, D.J.; Dickstein, K.; Zannad, F.; Metra, M.; Anker, S.; Samani, N.J.; Ponikowski, P.; et al. Insulin-like growth factor binding protein 7 (IGFBP7), a link between heart failure and senescence. ESC Heart Fail. 2022, 9, 4167–4176. [Google Scholar] [CrossRef]
- Zhang, L.; Smyth, D.; Al-Khalaf, M.; Blet, A.; Du, Q.; Bernick, J.; Gong, M.; Chi, X.; Oh, Y.; Roba-Oshin, M.; et al. Insulin-like growth factor-binding protein-7 (IGFBP7) links senescence to heart failure. Nat. Cardiovasc. Res. 2022, 1, 1195–1214. [Google Scholar] [CrossRef]
- Katoh, M.; Nomura, S.; Yamada, S.; Ito, M.; Hayashi, H.; Katagiri, M.; Heryed, T.; Fujiwara, T.; Takeda, N.; Nishida, M.; et al. Vaccine Therapy for Heart Failure Targeting the Inflammatory Cytokine Igfbp7. Circulation 2024, 150, 374–389. [Google Scholar] [CrossRef]
- Yu, L.C.; He, R.; Wang, D.X.; Qi, D. Activated Clec4n(hi) Neutrophils Aggravate Lung Injury in an Endothelial IGFBP7-Dependent Manner. Am. J. Respir. Cell Mol. Biol. 2024, 71, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Cen, L.; Zhou, T.; Yu, M.; Chen, X.; Jiang, W.; Li, Y.; Yu, C.; Shen, Z. Insulin-like growth factor binding protein 1 ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021, 36, 3438–3447. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, H.; Stal, P.; Hultcrantz, R.; Brismar, K.; Ansurudeen, I. IGFBP-1 and IGF-I as markers for advanced fibrosis in NAFLD—A pilot study. Scand. J. Gastroenterol. 2017, 52, 1427–1434. [Google Scholar] [CrossRef]
- Domvri, K.; Organtzis, I.; Apostolopoulos, A.; Fouka, E.; Kontakiotis, T.; Papakosta, D. Prognostic Value of Serum Biomarkers in Patients with Idiopathic Pulmonary Fibrosis in Relation to Disease Progression. J. Pers. Med. 2023, 13, 1307. [Google Scholar] [CrossRef]
- Guiot, J.; Bondue, B.; Henket, M.; Corhay, J.L.; Louis, R. Raised serum levels of IGFBP-1 and IGFBP-2 in idiopathic pulmonary fibrosis. BMC Pulm. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Njock, M.S.; Andre, B.; Gester, F.; Henket, M.; de Seny, D.; Moermans, C.; Malaise, M.G.; Louis, R. Serum IGFBP-2 in systemic sclerosis as a prognostic factor of lung dysfunction. Sci. Rep. 2021, 11, 10882. [Google Scholar] [CrossRef]
- Guiot, J.; Henket, M.; Corhay, J.L.; Moermans, C.; Louis, R. Sputum biomarkers in IPF: Evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS ONE 2017, 12, e0171344. [Google Scholar] [CrossRef]
- Ding, J.F.; Sun, H.; Song, K.; Zhou, Y.; Tu, B.; Shi, K.H.; Lu, D.; Xu, S.S.; Tao, H. IGFBP3 epigenetic promotion induced by METTL3 boosts cardiac fibroblast activation and fibrosis. Eur. J. Pharmacol. 2023, 942, 175494. [Google Scholar] [CrossRef] [PubMed]
- Brissett, M.; Veraldi, K.L.; Pilewski, J.M.; Medsger, T.A., Jr.; Feghali-Bostwick, C.A. Localized expression of tenascin in systemic sclerosis-associated pulmonary fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheumatol. 2012, 64, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Identification of multiple, differentially expressed messenger RNAs in dermal fibroblasts from patients with systemic sclerosis. Arthritis Rheumatol. 1999, 42, 1451–1457. [Google Scholar] [CrossRef]
- Aston, C.; Jagirdar, J.; Lee, T.C.; Hur, T.; Hintz, R.L.; Rom, W.N. Enhanced insulin-like growth factor molecules in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, 1597–1603. [Google Scholar] [CrossRef]
- Su, Y.; Nishimoto, T.; Hoffman, S.; Nguyen, X.X.; Pilewski, J.M.; Feghali-Bostwick, C. Insulin-like growth factor binding protein-4 exerts antifibrotic activity by reducing levels of connective tissue growth factor and the C-X-C chemokine receptor 4. FASEB Bioadv. 2019, 1, 167–179. [Google Scholar] [CrossRef]
- Zhao, Q.; Shao, T.; Huang, S.; Zhang, J.; Zong, G.; Zhuo, L.; Xu, Y.; Hong, W. The insulin-like growth factor binding protein-microfibrillar associated protein-sterol regulatory element binding protein axis regulates fibroblast-myofibroblast transition and cardiac fibrosis. Br. J. Pharmacol. 2024, 181, 2492–2508. [Google Scholar] [CrossRef]
- Nguyen, X.X.; Muhammad, L.; Nietert, P.J.; Feghali-Bostwick, C. IGFBP-5 Promotes Fibrosis via Increasing Its Own Expression and That of Other Pro-fibrotic Mediators. Front. Endocrinol. 2018, 9, 601. [Google Scholar] [CrossRef]
- Yasuoka, H.; Garrett, S.M.; Nguyen, X.X.; Artlett, C.M.; Feghali-Bostwick, C.A. NADPH oxidase-mediated induction of reactive oxygen species and extracellular matrix deposition by insulin-like growth factor binding protein-5. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L644–L655. [Google Scholar] [CrossRef]
- Longhitano, L.; Tibullo, D.; Vicario, N.; Giallongo, C.; La Spina, E.; Romano, A.; Lombardo, S.; Moretti, M.; Masia, F.; Coda, A.R.D.; et al. IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis. Aging 2021, 13, 25055–25071. [Google Scholar] [CrossRef]
- Liso, A.; Venuto, S.; Coda, A.R.D.; Giallongo, C.; Palumbo, G.A.; Tibullo, D. IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis. Int. J. Mol. Sci. 2022, 23, 4358. [Google Scholar] [CrossRef]
- Jin, Z.; Jin, Z.; Liu, Z.; Yin, Y.; Zhang, Y.; Zhang, Y.; Kang, J.; Fang, Y.; Jiang, W.; Ning, B. Pantothenic acid ameliorates hepatic fibrosis by targeting IGFBP6 to regulate the TGF-beta/SMADs pathway. Commun. Biol. 2025, 8, 1127. [Google Scholar] [CrossRef]
- Raykha, C.; Crawford, J.; Gan, B.S.; Fu, P.; Bach, L.A.; O’Gorman, D.B. IGF-II and IGFBP-6 regulate cellular contractility and proliferation in Dupuytren’s disease. Biochim. Biophys. Acta 2013, 1832, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Sendur, H.N.; Babayeva, A.; Cerit, M.N.; Cerit, E.T.; Yalcin, M.M.; Altinova, A.E.; Akturk, M.; Karakoc, M.A.; Toruner, F.B. Quantitative ultrasound techniques and biochemical markers to assess liver steatosis and fibrosis in newly diagnosed acromegaly. J. Endocrinol. Investig. 2024, 47, 2823–2833. [Google Scholar] [CrossRef]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, K.E.; Chung, R.T.; Lee, H.; Kleiner, D.E.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2020, 106, e520–e533. [Google Scholar] [CrossRef]
- Schanz, M.; Kimmel, M.; Alscher, M.D.; Amann, K.; Daniel, C. TIMP-2 and IGFBP7 in human kidney biopsies in renal disease. Clin. Kidney J. 2023, 16, 1434–1446. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhao, Z.; Meng, Y.; Bian, J.; Bao, R.; Zhu, K.; Yang, T. IGFBP7 regulates sepsis-induced epithelial-mesenchymal transition through ERK1/2 signaling. Acta Biochim. Biophys. Sin. 2019, 51, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Xie, S.S.; Shen, X.Y.; Li, Z.; Hu, X.W.; Zhang, Y.; Dong, Z.H.; Wang, J.N.; Li, X.Y.; Dong, Y.H.; et al. Renal tubular epithelial IGFBP7 interacts with PKM2 to drive renal lipid accumulation and fibrosis. Mol. Ther. 2025, 33, 3757–3777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bo, J.; Zhao, Z.; Han, Y.; Zhang, Q.; Liu, L. Depletion of Igfbp7 alleviates zebrafish NAFLD progression through inhibiting hepatic ferroptosis. Life Sci. 2023, 332, 122086. [Google Scholar] [CrossRef]
- Ko, T.; Nomura, S.; Yamada, S.; Fujita, K.; Fujita, T.; Satoh, M.; Oka, C.; Katoh, M.; Ito, M.; Katagiri, M.; et al. Cardiac fibroblasts regulate the development of heart failure via Htra3-TGF-beta-IGFBP7 axis. Nat. Commun. 2022, 13, 3275. [Google Scholar] [CrossRef]
- Zhu, T.; Mu, B.R.; Li, B.; Ran, Z.; Wang, D.M.; Zhou, Y.; Liu, L.; Wu, Q.L.; Lu, M.H.; Xiong, D.Q. IGFBP7: A novel biomarker involved in a positive feedback loop with TGF-beta1 in idiopathic pulmonary fibrosis. Cell Signal. 2025, 133, 111867. [Google Scholar] [CrossRef]
- Yan, Y.M.; Zheng, J.N.; Li, Y.; Yang, Q.R.; Shao, W.Q.; Wang, Q. Insulin-like growth factor binding protein 7 as a candidate biomarker for systemic sclerosis. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S131), 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hwa, V.; Oh, Y.; Rosenfeld, R.G. The Insulin-Like Growth Factor-Binding Protein (IGFBP) Superfamily*. Endocr. Rev. 1999, 20, 761–787. [Google Scholar] [CrossRef]
- Han, S.; Li, Z.; Master, L.M.; Master, Z.W.; Wu, A. Exogenous IGFBP-2 promotes proliferation, invasion, and chemoresistance to temozolomide in glioma cells via the integrin β1-ERK pathway. Br. J. Cancer 2014, 111, 1400–1409. [Google Scholar] [CrossRef]
- Yau, S.W.; Azar, W.J.; Sabin, M.A.; Werther, G.A.; Russo, V.C. IGFBP-2—Taking the lead in growth, metabolism and cancer. J. Cell Commun. Signal. 2015, 9, 125–142. [Google Scholar] [CrossRef]
- LeRoith, D.; Roberts, C.T. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Liu, B.; Ma, L.; Li, H.; Bang, P.; Koeffler, H.P.; Cohen, P. Cellular Internalization of Insulin-like Growth Factor Binding Protein-3: Distinct Endocytic Pathways Facilitate Re-Uptake and Nuclear Localization. J. Biol. Chem. 2004, 279, 469–476. [Google Scholar] [CrossRef]
- Walker, G.; MacLeod, K.; Williams, A.R.W.; Cameron, D.A.; Smyth, J.F.; Langdon, S.P. Insulin-like Growth Factor Binding Proteins IGFBP3, IGFBP4, and IGFBP5 Predict Endocrine Responsiveness in Patients with Ovarian Cancer. Clin. Cancer Res. 2007, 13, 1438–1444. [Google Scholar] [CrossRef]
- Waters, J.A.; Urbano, I.; Robinson, M.; House, C.D. Insulin-like growth factor binding protein 5: Diverse roles in cancer. Front. Oncol. 2022, 12, 1052457. [Google Scholar] [CrossRef]
- Voutilainen, R.; Franks, S.; Mason, H.D.; Martikainen, H. Expression of insulin-like growth factor (IGF), IGF-binding protein, and IGF receptor messenger ribonucleic acids in normal and polycystic ovaries. J. Clin. Endocrinol. Metab. 1996, 81, 1003–1008. [Google Scholar] [CrossRef]
- Jin, L.; Shen, F.; Weinfeld, M.; Sergi, C. Insulin Growth Factor Binding Protein 7 (IGFBP7)-Related Cancer and IGFBP3 and IGFBP7 Crosstalk. Front. Oncol. 2020, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Wajapeyee, N.; Kapoor, V.; Mahalingam, M.; Green, M.R. Efficacy of IGFBP7 for treatment of metastatic melanoma and other cancers in mouse models and human cell lines. Mol. Cancer Ther. 2009, 8, 3009–3014. [Google Scholar] [CrossRef]
- Huang, B.-L.; Wei, L.-F.; Lin, Y.-W.; Huang, L.-S.; Qu, Q.-Q.; Li, X.-H.; Chu, L.-Y.; Xu, Y.-W.; Wang, W.-D.; Peng, Y.-H.; et al. Serum IGFBP-1 as a promising diagnostic and prognostic biomarker for colorectal cancer. Sci. Rep. 2024, 14, 1839. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-W.; Chen, H.; Hong, C.-Q.; Chu, L.-Y.; Yang, S.-H.; Huang, L.-S.; Guo, H.; Chen, L.-Y.; Liu, C.-T.; Huang, X.-Y.; et al. Serum IGFBP-1 as a potential biomarker for diagnosis of early-stage upper gastrointestinal tumour. EBioMedicine 2020, 51, 102566, Erratum in EBioMedicine 2021, 66, 103295. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, J.; Zhang, X.; Zhang, M.; Fu, Y. Comprehensive Analysis of IGFBPs as Biomarkers in Gastric Cancer. Front. Oncol. 2021, 11, 723131. [Google Scholar] [CrossRef]
- Pohlman, A.W.; Moudgalya, H.; Jordano, L.; Lobato, G.C.; Gerard, D.; Liptay, M.J.; Seder, C.W.; Borgia, J.A. The role of IGF-pathway biomarkers in determining risks, screening, and prognosis in lung cancer. Oncotarget 2022, 13, 393–407. [Google Scholar] [CrossRef]
- Klimczak-Tomaniak, D.; de Bakker, M.; Bouwens, E.; Akkerhuis, K.M.; Baart, S.; Rizopoulos, D.; Mouthaan, H.; van Ramshorst, J.; Germans, T.; Constantinescu, A.; et al. Dynamic personalized risk prediction in chronic heart failure patients: A longitudinal, clinical investigation of 92 biomarkers (Bio-SHiFT study). Sci. Rep. 2022, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hong, C.-Q.; Luo, Y.-H.; Wei, L.-F.; Luo, Y.; Peng, Y.-H.; Xu, Y.-W. Prognostic value of IGFBP2 in various cancers: A systematic review and meta-analysis. Cancer Med. 2022, 11, 3035–3047. [Google Scholar] [CrossRef]
- Gong, L.; Liu, Q.; Jia, M.; Sun, X. Systematic analysis of IGF2BP family members in non-small-cell lung cancer. Hum. Genom. 2024, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ai, J.; Zheng, Y.; Zhou, W.; Zhang, L.; Zhu, J.; Zhang, H.; Wang, S. IGFBP2/ITGA5 promotes gefitinib resistance via activating STAT3/CXCL1 axis in non-small cell lung cancer. Cell Death Dis. 2024, 15, 447. [Google Scholar] [CrossRef]
- Venuto, S.; Coda, A.R.D.; González-Pérez, R.; Laselva, O.; Tolomeo, D.; Storlazzi, C.T.; Liso, A.; Conese, M. IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression. Int. J. Mol. Sci. 2023, 24, 4804. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Zhou, B.; Yuan, S.; Gao, S. Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer. Open Med. 2024, 19, 20230893. [Google Scholar] [CrossRef]
- Wang, W.; Yu, K.; Zhao, S.Y.; Mo, D.G.; Liu, J.H.; Han, L.J.; Li, T.; Yao, H.C. The impact of circulating IGF-1 and IGFBP-2 on cardiovascular prognosis in patients with acute coronary syndrome. Front. Cardiovasc. Med. 2023, 10, 1126093. [Google Scholar] [CrossRef]
- Yamamoto, N.; Oshima, T.; Yoshihara, K.; Aoyama, T.; Hayashi, T.; Yamada, T.; Sato, T.; Shiozawa, M.; Yoshikawa, T.; Morinaga, S.; et al. Clinicopathological significance and impact on outcomes of the gene expression levels of IGF-1, IGF-2 and IGF-1R, IGFBP-3 in patients with colorectal cancer: Overexpression of the IGFBP-3 gene is an effective predictor of outcomes in patients with colorectal cancer. Oncol. Lett. 2017, 13, 3958–3966. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, H.; Kutbi, E.H.; Tan, S.C.; Low, T.Y.; Zhang, C. Association of IGF-1 and IGFBP-3 levels with gastric cancer: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14764. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Frille, A.; Petersen, M.A.; Oberhuber-Kurth, J.; Hofmann, L.; Gläser, A.; Taubenheim, S.; Klagges, S.; Kraemer, S.; Broschewitz, J.; et al. IGFBP3 inhibits tumor growth and invasion of lung cancer cells and is associated with improved survival in lung cancer patients. Transl. Oncol. 2023, 27, 101566. [Google Scholar] [CrossRef] [PubMed]
- Arafat, H.M.; Al-Astani, T.A.D.; Shafii, N.; Muhamad, R.; Shamallakh, O.M.; Naser, I.; Al Laham, N.; Siddig, A. Impact of IGFBP-3 A-202C genetic variant on breast cancer susceptibility and serum biomarkers (IGFBP-3 and IGF-1) in Palestinian women. PLoS ONE 2025, 20, e0325289. [Google Scholar] [CrossRef]
- Monson, K.R.; Goldberg, M.; Wu, H.-C.; Santella, R.M.; Chung, W.K.; Terry, M.B. Circulating growth factor concentrations and breast cancer risk: A nested case-control study of IGF-1, IGFBP-3, and breast cancer in a family-based cohort. Breast Cancer Res. 2020, 22, 109. [Google Scholar] [CrossRef]
- Gheysarzadeh, A.; Bakhtiari, H.; Ansari, A.; Emami Razavi, A.; Emami, M.H.; Mofid, M.R. The insulin-like growth factor binding protein-3 and its death receptor in pancreatic ductal adenocarcinoma poor prognosis. J. Cell Physiol. 2019, 234, 23537–23546. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Huang, Z.-L.; Yang, J.-H.; Xu, Y.-H.; Sun, J.-S.; Zheng, Q.; Wei, C.; Song, W.; Yuan, Z. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J. Exp. Clin. Cancer Res. 2016, 35, 46, Erratum in J. Exp. Clin. Cancer Res. 2016, 35, 77. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Fujimoto, M.; Matsushita, T.; Hasegawa, M.; Takehara, K.; Sato, S. Elevated serum insulin-like growth factor (IGF-1) and IGF binding protein-3 levels in patients with systemic sclerosis: Possible role in development of fibrosis. J. Rheumatol. 2008, 35, 2363–2371. [Google Scholar] [CrossRef]
- Nur, S.I.; Ozturk, A.; Kavas, M.; Bulut, I.; Alparslan, S.; Aydogan, E.S.; Atinkaya, B.C.; Kolay, M.; Coskun, A. IGFBP-4: A promising biomarker for lung cancer. J. Med. Biochem. 2021, 40, 237–244. [Google Scholar] [CrossRef]
- Torres, G.; Yang, J.; Griffiths, M.; Brandal, S.; Damico, R.; Vaidya, D.; Simpson, C.E.; Pauciulo, M.W.; Nichols, W.C.; Ivy, D.D.; et al. Insulin-like growth factor binding Protein-4: A novel indicator of pulmonary arterial hypertension severity and survival. Pulm. Circ. 2023, 13, e12235. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Singh Sunar, P.; Shah, S.; Chamlagain, R.; Babu Pokhrel, N.; Khanal, P.; Kumar Sah, S.; Poudel, S.; Belbase, K.; Chand, S.; et al. CT-IGFBP-4 as a Predictive Novel Biomarker of Ischemic Cardiovascular Events and Mortality: A Systematic Review. J. Interv. Cardiol. 2022, 2022, 1816504. [Google Scholar] [CrossRef] [PubMed]
- Shersher, D.D.; Vercillo, M.S.; Fhied, C.; Basu, S.; Rouhi, O.; Mahon, B.; Coon, J.S.; Warren, W.H.; Faber, L.P.; Hong, E.; et al. Biomarkers of the Insulin-Like Growth Factor Pathway Predict Progression and Outcome in Lung Cancer. Ann. Thorac. Surg. 2011, 92, 1805–1811. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, X.; Hua, H.; Zhang, C. IGFBP5 is Upregulated and Associated with Poor Prognosis in Colorectal Cancer. Int. J. Gen. Med. 2022, 15, 6485–6497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, X.; Wang, G.; Wang, W.; Ju, S.; Wang, X. Decreased expression of IGFBP6 correlates with poor survival in colorectal cancer patients. Pathol. Res. Pract. 2020, 216, 152909. [Google Scholar] [CrossRef]
- Cai, D.; Xu, Y.; Ding, R.; Qiu, K.; Zhang, R.; Wang, H.; Huang, L.; Xie, X.; Yan, H.; Deng, Y.; et al. Extensive serum biomarker analysis in patients with non-small-cell lung carcinoma. Cytokine 2020, 126, 154868. [Google Scholar] [CrossRef]
- Godina, C.; Khazaei, S.; Tryggvadottir, H.; Visse, E.; Nodin, B.; Jirström, K.; Borgquist, S.; Bosch, A.; Isaksson, K.; Jernström, H. Prognostic impact of tumor-specific insulin-like growth factor binding protein 7 (IGFBP7) levels in breast cancer: A prospective cohort study. Carcinogenesis 2021, 42, 1314–1325. [Google Scholar] [CrossRef]
- Chen, C.; Tian, X.; Zhao, X.; Ren, L. Clinical study of serum IGFBP7 in predicting lymphatic metastasis in patients with lung adenocarcinoma. Curr. Probl. Cancer 2020, 44, 100584. [Google Scholar] [CrossRef]
- Shah, A.M.; Myhre, P.L.; Arthur, V.; Dorbala, P.; Rasheed, H.; Buckley, L.F.; Claggett, B.; Liu, G.; Ma, J.; Nguyen, N.Q.; et al. Large scale plasma proteomics identifies novel proteins and protein networks associated with heart failure development. Nat. Commun. 2024, 15, 528. [Google Scholar] [CrossRef]
| Disease | Tissue Type | Expression | Sample Size | Response to Treatment/Longitudinal Study Results | References | |
|---|---|---|---|---|---|---|
| IGFBP-1 | Colorectal Cancer | Serum and tumor tissue | Increase | 328 | Poor survival | [142] |
| Esophageal Cancer | Serum | Increase | 1064 | - | [143] | |
| Gastric Cancer | Tumor tissue | Increase | 11,084 | Poor survival | [144] | |
| Lung Cancer | Tumor Tissue | Increase | 9736 | Poor survival | [145] | |
| Idiopathic pulmonary fibrosis (IPF) | Serum | Increase | 72, 50 | Levels increased with time/severity, insensitive to anti-fibrotic treatments | [107,108] | |
| Non-alcoholic fatty liver disease (NAFLD) | Serum | Increase | 52 | More advanced fibrosis | [106] | |
| Heart failure with reduced ejection fraction | Serum | Increase | 250 | Presence of heart failure and likelihood of adverse event | [146] | |
| Peripheral arterial disease | Serum | Increase | 465 | Increased risk of major adverse cardiovascular events (MACE) | [84] | |
| IGFBP-2 | Colorectal Cancer | Tumor tissue | Increase | 5560 | Poor survival | [147] |
| Esophageal Cancer | Tumor tissue | Increase | Not reported | - | [19] | |
| Gastric Cancer | Serum and tumor tissue | Increase | 118 | Correlates with tumor stage and poor survival | [19] | |
| Lung Cancer | Serum and tumor tissue | Study-dependent | 30, 20, 201 | Poor survival in lung adenocarcinoma and better survival in lung squamous cell carcinoma | [145,147,148,149,150] | |
| Breast Cancer | Serum and tumor tissue | Increase | 412 | Poor survival and tamoxifen resistance | [19] | |
| Pancreatic Cancer | Serum | Increase | 165 | Correlates with tumor stage and poor survival | [151] | |
| IPF | Serum | Increase | 50, 15 | Elevated in patients and decreased with anti-fibrotic treatment | [108,110] | |
| Systemic Sclerosis (SSc) | Serum | Increase | 102 | Negatively correlates with pulmonary function and disease progression | [109] | |
| Acute coronary syndrome | Serum | Increase | 277 | Increased risk of MACE | [152] | |
| Heart failure | Serum | Increase | 870 | Increased risk of cardiovascular mortality | [86] | |
| IGFBP-3 | Colorectal Cancer | Tumor tissue | Increase | 202 | Poor survival | [153] |
| Gastric Cancer | Serum and tumor tissue | Study-dependent | 1541, 11,084 | - | [144,154] | |
| Lung Cancer | Serum and tumor tissue | Study-dependent | 131 | Inverse relationship to tumor stage in non-small cell lung cancer, and disease risk in lung adenocarcinoma | [145,150,155] | |
| Breast Cancer | Serum | Increase | 334,236 | Increased risk of developing disease | [156,157] | |
| Pancreatic Cancer | Serum and tumor tissue | Study-dependent | 88, 478 | Serum negatively correlates and tumor expression positively correlates with survival | [158,159] | |
| SSc | Serum | Increase | 92 | - | [160] | |
| SSc-ILD | Lung tissue | Increase | Not reported | - | ||
| IGFBP-4 | Gastric Cancer | Tumor tissue | Increase | 11,084 | - | [144] |
| Lung Cancer | Serum | Increase | 83 | - | [161] | |
| Breast Cancer | Tumor tissue | Decrease | 162 | Improved survival | [28] | |
| Pulmonary Arterial Hypertension (PAH) | Serum | Increase | 2579 | Shorter 6 min walk distance, worse NYHA functional classification, and decreased survival. | [162] | |
| Ischemic heart disease | Serum | Increase | 1417 | Increased risk of MACE and mortality | [163] | |
| IGFBP-5 | Lung Cancer | Serum | Decrease | 100 | Improved survival | [164] |
| Colorectal Cancer | Tumor tissue | Increase | 56 | Correlates with tumor stage and poor survival | [165] | |
| IGFBP-6 | Breast Cancer | Tumor tissue | Decrease | 1091 | Improved survival | [35] |
| Colorectal Cancer | Tumor tissue | Decrease | 130 | Improved survival | [166] | |
| Lung Cancer | Serum | Decrease | 31 | - | [167] | |
| Fibrosis in NAFLD | Serum and liver tissue | Increase | 61 | Reduced IGFBP-6 expression on anti-fibrotic tesamorelin | [124] | |
| Atherosclerosis | Serum and artery tissue | Decrease | 3 | - | [98] | |
| IGFBP-7 | Gastric Cancer | Tumor tissue | Increase | 16 | Poor survival | [38] |
| Breast Cancer | Tumor tissue | Increase | 878 | Increased disease risk and poor survival | [168] | |
| Lung Cancer | Serum | Increase | 90 | Correlates with tumor stage and metastasis | [169] | |
| Heart failure | Serum | Increase | 2250, 313 | Increased risk of all three main hospitalization and mortality causes | [101,102] | |
| Heart failure risk | Serum | Increase | 13,900 | Risk of future CHF development in healthy individuals | [170] | |
| Pulmonary hypertension | Serum | Increase | 2582 | Decreased six-minute walk distance, higher mean right atrial pressure, decreased survival | [162] | |
| SSc | Serum | Increase | 37 | Higher modified Rodnan skin score (MRSS) | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sechrist, Z.R.; Cortés, J.S.; Patel, N.R.; Pittman, Z.J.; Guru Murthy, G.; Zhu, G.; Cole, C.L.; Korman, B.D. Pathologic Signaling and Disease Implications of Insulin-like Growth Factor Binding Proteins in Cancer, Cardiovascular Disease, and Fibrosis. Int. J. Mol. Sci. 2025, 26, 10248. https://doi.org/10.3390/ijms262110248
Sechrist ZR, Cortés JS, Patel NR, Pittman ZJ, Guru Murthy G, Zhu G, Cole CL, Korman BD. Pathologic Signaling and Disease Implications of Insulin-like Growth Factor Binding Proteins in Cancer, Cardiovascular Disease, and Fibrosis. International Journal of Molecular Sciences. 2025; 26(21):10248. https://doi.org/10.3390/ijms262110248
Chicago/Turabian StyleSechrist, Zachary R., Jaeden S. Cortés, Nidhi R. Patel, Zoe J. Pittman, Gayathri Guru Murthy, Guangzhen Zhu, Calvin L. Cole, and Benjamin D. Korman. 2025. "Pathologic Signaling and Disease Implications of Insulin-like Growth Factor Binding Proteins in Cancer, Cardiovascular Disease, and Fibrosis" International Journal of Molecular Sciences 26, no. 21: 10248. https://doi.org/10.3390/ijms262110248
APA StyleSechrist, Z. R., Cortés, J. S., Patel, N. R., Pittman, Z. J., Guru Murthy, G., Zhu, G., Cole, C. L., & Korman, B. D. (2025). Pathologic Signaling and Disease Implications of Insulin-like Growth Factor Binding Proteins in Cancer, Cardiovascular Disease, and Fibrosis. International Journal of Molecular Sciences, 26(21), 10248. https://doi.org/10.3390/ijms262110248






