Chronic Stress and Autoimmunity: The Role of HPA Axis and Cortisol Dysregulation
Abstract
1. Introduction
2. The Physiology of the HPA Axis and Cortisol
3. Chronic Stress and HPA Axis Dysregulation
4. Immunological Consequences of HPA Axis Dysfunction
5. Clinical and Experimental Evidence
| Study/Authors | Population/Model | HPA Axis Findings | Cytokine/Immune Findings | Autoimmune/Clinical Outcome |
|---|---|---|---|---|
| Montero-López et al. (2017) [14] | 35 women with SLE, Sjögren’s, systemic sclerosis vs. 30 controls | Elevated hair and salivary cortisol | Altered stress biomarkers; higher somatization and lower anxiety scores | Supports hyperactivation of the HPA axis in autoimmune disease |
| Song et al. (2018) [33] | Population-based cohort (>100,000) with stress-related disorders | Stress-related disorders are used as a proxy for chronic HPA dysregulation | Association with pro-inflammatory signaling pathways | Higher risk of autoimmune diseases (HR ≈ 1.1–1.5 by disease) |
| Zhang et al. (2025) [34] | Lupus-prone MRL-lpr vs. MRL/MPJ mice under predator stress | Heightened HPA sensitivity; stress reactivity increased | ↑ IL-6, ↑ Th17, ↓ Treg; ↑ anti-dsDNA antibodies; proteinuria | Accelerated lupus progression in predisposed mice |
| Lei et al. (2025) [20] | Narrative mini-review—depressive disorders with chronic stress | HPA dysregulation; hippocampal GR downregulation/feedback impairment | Neuroinflammation; reduced glucocorticoid signaling efficacy | Increased vulnerability to inflammatory and autoimmune conditions |
| Hannibal & Bishop (2014) [24] | Clinical populations with chronic pain | Cortisol dysfunction in chronic stress contexts | Altered cytokine regulation and sensitization to pain | Rationale for stress-management to restore HPA–immune balance |
| Breunig et al. (2025) [27] | Genetic/psychiatric comorbidity analysis | Shared stress–immune regulatory architecture | Overlap of inflammatory pathways across disorders | Supports clustering of psychiatric and autoimmune diseases |
6. Molecular and Cellular Mechanisms
| Immune Cell Type | Normal (Acute Stress) | Chronic Stress/HPA Dysregulation | Key References |
|---|---|---|---|
| Macrophages | Balanced cytokine production; tissue repair support | ↑ TNF-α, ↑ IL-1β, ↑ IL-6; pro-inflammatory phenotype | [23,25] |
| Dendritic Cells | Tolerogenic programming is maintained by cortisol; balanced antigen presentation | Reduced tolerogenicity and impaired maintenance of self-tolerance | [13,26] |
| T Regulatory Cells (Tregs) | ↑ IL-10; suppression of autoreactive responses | ↓ Treg number/function; ↓ IL-10; impaired tolerance | [12,21,34] |
| Th17 Cells | Limited activation under immune homeostasis | ↑ IL-17; promotes autoimmunity and chronic inflammation | [20,34] |
| B Cells | Controlled antibody production | ↑ Autoantibody production (e.g., anti-dsDNA) | [13,34] |
| Natural Killer (NK) Cells | Cytolytic activity and immune surveillance | ↓ NK activity; reduced cytotoxicity and cytokine output | [41,42] |
| Microglia/Astrocytes | CNS homeostasis; controlled cytokine milieu | ↑ Pro-inflammatory cytokines; impaired GR sensitivity; neuroinflammation | [20,43] |
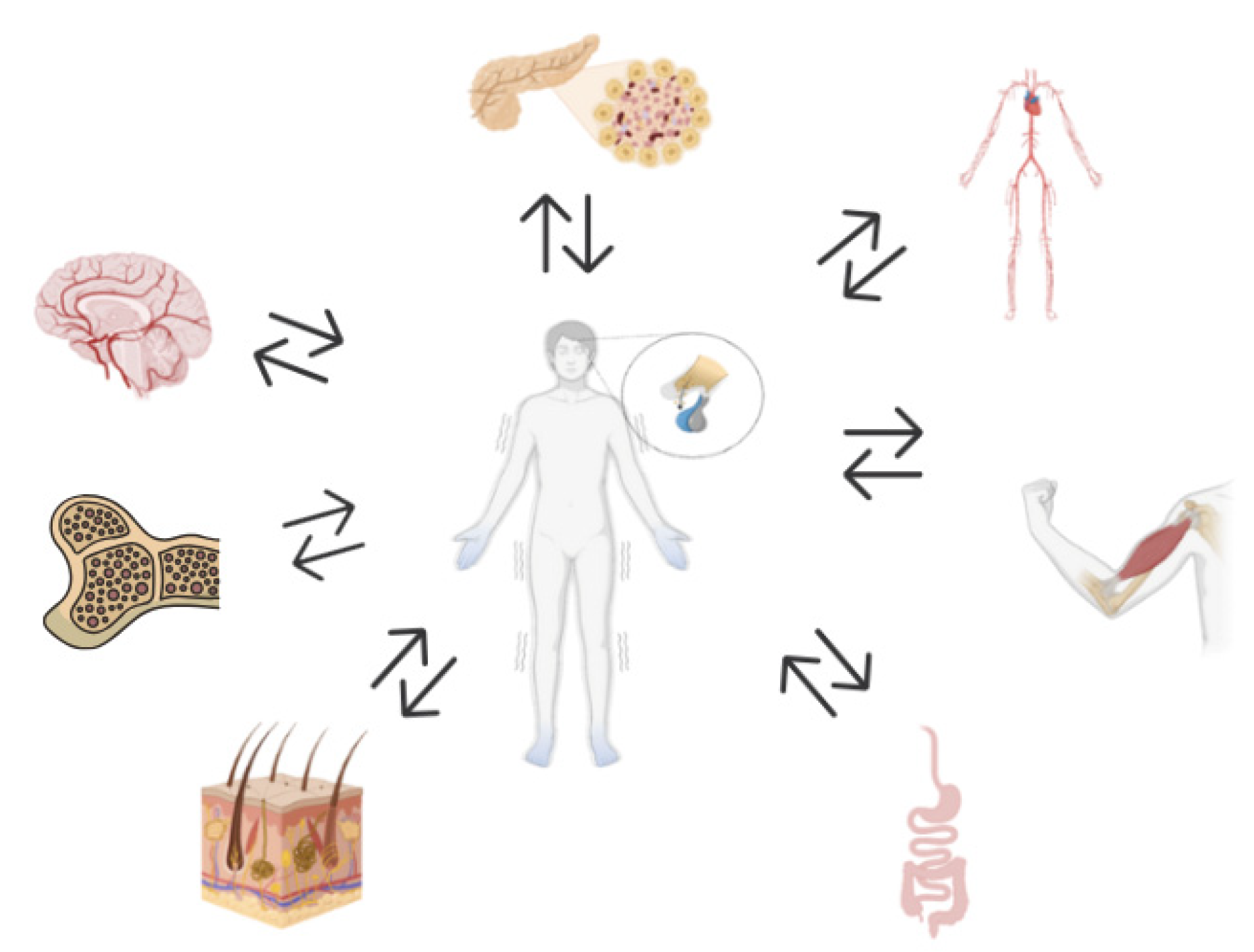
6.1. Neuroimmune Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
6.2. Endocrine Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
6.3. Cardiovascular Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
6.4. Gastrointestinal Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
6.5. Tegumentar Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
6.6. Musculoskeletal Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
6.7. Hematopoietic Consequences of HPA Axis Dysfunction in Chronic Stress and Autoimmunity
7. Therapeutic Implications and Interventions
8. Future Directions and Gaps
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 11β-HSD1 | 11β-hydroxysteroid dehydrogenase 1; |
| ACTH | Adrenocorticotropic Hormone |
| AP-1 | Activator Protein-1 |
| ATG16L1 | Autophagy Related 16 Like 1 |
| CARD9 | Caspase Recruitment Domain Family Member 9 |
| CAR | Cortisol Awakening Response |
| CDH1 | Cadherin 1 |
| COVID-19 | Coronavirus Disease 2019 |
| CRH | Corticotropin-Releasing Hormone |
| CTLA-4 | Cytotoxic T-Lymphocyte Associated Protein 4 |
| CYP11B | Cytochrome P450 Family 11 Subfamily B |
| DNA | Deoxyribonucleic Acid |
| dsDNA | Double-Stranded Deoxyribonucleic Acid |
| EULAR | European League Against Rheumatism |
| GC | Glucocorticoid |
| GI | Gastrointestinal |
| GNA12 | Guanine nucleotide-binding protein subunit alpha-12 |
| GR | Glucocorticoid Receptor |
| GWAS | Genome-Wide Association Studies |
| H3K27 | Histone H3 Lysine 27 |
| HLA | Human Leukocyte Antigen |
| HNF4A | Hepatocyte Nuclear Factor 4 Alpha |
| HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| HR | Hazard Ratio |
| HSC | hematopoietic stem cells |
| ICOSLG | Inducible T Cell Costimulator Ligand |
| IFN-γ | Interferon Gamma |
| IL | Interleukin |
| IL1B | Interleukin 1 Beta |
| IL6 | Interleukin 6 |
| IL10 | Interleukin 10 |
| IL12B | Interleukin 12 Beta |
| IL23R | Interleukin 23 Receptor |
| IBD | Inflammatory Bowel Diseases |
| IRGM | Immunity-Related GTPase Family M Protein |
| JAK2 | Janus Kinase 2 |
| LAMB | Laminin Subunit Beta 1 |
| MBSR | Mindfulness-Based Stress Reduction |
| MR | Mineralocorticoid Receptor |
| mRNA | Messenger Ribonucleic Acid |
| MRL/MPJ | Mouse Strain Control for MRL-lpr |
| MS | Multiple Sclerosis |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NK cells | Natural Killer Cells |
| NKX2.3 | NK2 Homeobox 3 |
| NOD2 | Nucleotide-Binding Oligomerization Domain Containing 2 |
| OTS | Overtraining Syndrome |
| ORMDL3 | ORMDL Sphingolipid Biosynthesis Regulator 3 |
| PD-1 | Programmed Cell Death Protein 1 |
| PTPN22 | Protein Tyrosine Phosphatase Non-Receptor Type 22 |
| PRDM1 | PR/SET Domain 1 |
| PVN | Paraventricular Nucleus |
| RA | Rheumatoid Arthritis |
| REL | Proto-Oncogene, NF-κB Subunit |
| SCL-90-R | Symptom Checklist-90-Revised |
| SLE | Systemic Lupus Erythematosus |
| SMAD3 | Mothers Against Decapentaplegic Homolog 3 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| T1DM | Type 1 Diabetes Mellitus |
| TGF-β | Transforming Growth Factor Beta |
| Th1 | T Helper Cell Subset 1 |
| Th2 | T Helper Cell Subset 2 |
| Th17 | T Helper Cell Subset 17 |
| TLA | Three letter acronyms |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TNFa | Tumor Necrosis Factor Alpha (gene symbol) |
| Tregs | Regulatory T Cells |
| TYK2 | Tyrosine Kinase 2 |
| UV | Ultraviolet |
References
- Chen, J.Q.; Szodoray, P.; Zeher, M. Toll-Like Receptor Pathways in Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2015, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Shedlock, A.M.; Langefeld, C.D. Genetics of autoimmune diseases: Insights from population genetics. J. Hum. Genet. 2015, 60, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Vitlic, A.; Lord, J.M.; Phillips, A.C. Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. Age 2014, 36, 9631. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic–Pituitary–Adrenocortical Stress Response. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2016; pp. 603–621. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic–Pituitary–Adrenal Axis: Development, Programming Actions of Hormones, and Maternal–Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Barrett, K.E.; Barman, S.M.; Brooks, H.L.; Yuan, J.J. The Adrenal Medulla & Adrenal Cortex. In Ganong’s Review of Medical Physiology, 27th ed.; McGraw Hill: Columbus, OH, USA, 2025. [Google Scholar]
- Ince, L.M.; Weber, J.; Scheiermann, C. Control of leukocyte trafficking by stress-associated hormones. Front. Immunol. 2019, 10, 3143. [Google Scholar] [CrossRef]
- Lockett, J.; Inder, W.J.; Clifton, V.L. The Glucocorticoid Receptor: Isoforms, Functions, and Contribution to Glucocorticoid Sensitivity. Endocr. Rev. 2024, 45, 593–624. [Google Scholar] [CrossRef]
- Madden, K.S. Catecholamines, sympathetic innervation, and immunity. Brain Behav. Immun. 2003, 17, 5–10. [Google Scholar] [CrossRef]
- Ring, M. An Integrative Approach to HPA Axis Dysfunction: From Recognition to Recovery. Am. J. Med. 2025, 138, 1451–1463. [Google Scholar] [CrossRef]
- Karin, O.; Raz, M.; Tendler, A.; Bar, A.; Korem Kohanim, Y.; Milo, T.; Alon, U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol. Syst. Biol. 2020, 16, e9510. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA–Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Montero-López, E.; Santos-Ruiz, A.; González, R.; Navarrete-Navarrete, N.; Ortego-Centeno, N.; Martínez-Augustín, O.; Rodríguez-Blázquez, M.; Peralta-Ramírez, M.I. Analyses of hair and salivary cortisol for evaluating hypothalamic–pituitary–adrenal axis activation in patients with autoimmune disease. Stress 2017, 20, 541–548. [Google Scholar] [CrossRef]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and circadian regulation of cortisol: A short review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef]
- Hakamata, Y.; Komi, S.; Moriguchi, Y.; Izawa, S.; Motomura, Y.; Sato, E.; Mizukami, S.; Kim, Y.; Hanakawa, T.; Inoue, Y.; et al. Amygdala-centred functional connectivity affects daily cortisol concentrations: A putative link with anxiety. Sci. Rep. 2017, 7, 8313. [Google Scholar] [CrossRef] [PubMed]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Haegeman, G. The Interplay between the Glucocorticoid Receptor and Nuclear Factor-κB or Activator Protein-1: Molecular Mechanisms for Gene Repression. Endocr. Rev. 2003, 24, 488–522. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Heck, A.L.; Miller, A.M.; Sheng, J.A.; Stover, S.A.; Daniels, R.M.; Bales, N.J.; Fleury, T.K.; Handa, R.J. Chronic variable stress alters hypothalamic–pituitary–adrenal axis function in the female mouse. Physiol. Behav. 2019, 209, 112613. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.A.; Phang, V.W.X.; Lee, Y.Z.; Kow, A.S.F.; Tham, C.L.; Ho, Y.C.; Lee, M.T. Chronic Stress-Associated Depressive Disorders: The Impact of HPA Axis Dysregulation and Neuroinflammation on the Hippocampus—A Mini Review. Int. J. Mol. Sci. 2025, 26, 2940. [Google Scholar] [CrossRef]
- Miller, G.E.; Cohen, S.; Ritchey, A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002, 21, 531–541. [Google Scholar] [CrossRef]
- Guilliams, T.G.; Edwards, L. The Stress Response System: Chronic Stress and the HPA Axis—Clinical Assessment and Therapeutic Considerations; Point Institute: Stevens Point, WI, USA, 2010. [Google Scholar]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The Stressed CNS: When Glucocorticoids Aggravate Inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Fraccarollo, D.; Geffers, R.; Galuppo, P.; Bauersachs, J. Mineralocorticoid receptor promotes cardiac macrophage inflammaging. Basic Res. Cardiol. 2024, 119, 243. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; del Rey, A. Physiology of psychoneuroimmunology: A personal view. Brain Behav. Immun. 2007, 21, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Breunig, S.; Lee, Y.H.; Karlson, E.W.; Krishnan, A.; Lawrence, J.M.; Schaffer, L.S.; Grotzinger, A.D. Examining the genetic links between clusters of immune-mediated diseases and psychiatric disorders. Transl. Psychiatry 2025, 15, 252. [Google Scholar] [CrossRef]
- Ueda, H.; Howson, J.M.; Esposito, L.; Heward, J.; Snook, H.; Chamberlain, G.; Rainbow, D.B.; Hunter, K.M.; Smith, A.N.; Di Genova, G.; et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003, 423, 506–511. [Google Scholar] [CrossRef]
- Lin, S.C.; Yen, J.H.; Tsai, J.J.; Tsai, W.C.; Ou, T.T.; Liu, H.W.; Chen, C.J. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004, 50, 770–775. [Google Scholar] [CrossRef]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.M.T.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A.C. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef]
- Prokunina, L.; Padyukov, L.; Bennet, A.; de Faire, U.; Wiman, B.; Prince, J.; Alfredsson, L.; Klareskog, L.; Alarcón-Riquelme, M. Association of the PD-1.3A allele of the PDCD1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum. 2004, 50, 1770–1773. [Google Scholar] [CrossRef]
- Criswell, L.A.; Pfeiffer, K.A.; Lum, R.F.; Gonzales, B.; Novitzke, J.; Kern, M.; Moser, K.L.; Begovich, A.B.; Carlton, V.E.; Li, W.; et al. Analysis of families in the Multiple Autoimmune Disease Genetics Consortium (MADGC) collection: The PTPN22 620W allele associates with multiple autoimmune phenotypes. Am. J. Hum. Genet. 2005, 76, 561–571. [Google Scholar] [CrossRef]
- Song, H.; Fang, F.; Tomasson, G.; Arnberg, F.K.; Mataix-Cols, D.; Fernández de la Cruz, L.F.; Almqvist, C.; Fall, K.; Valdimarsdóttir, U.A. Association of Stress-Related Disorders with Subsequent Autoimmune Disease. JAMA 2018, 319, 2388–2400. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Dang, R.N.; Tian, X.X.; Li, L.J.; Zhou, Q.M.; Li, X.Y.; Wu, Y.S.; Zou, H.M. Effects of environmentally-induced anxiety on autoimmunity in the MRL/lpr mouse. Lupus Sci. Med. 2025, 12, e001528. [Google Scholar] [CrossRef]
- Begovich, A.B.; Caillier, S.J.; Alexander, H.C.; Penko, J.M.; Hauser, S.L.; Barcellos, L.F.; Oksenberg, J.R. The R620W polymorphism of the protein tyrosine phosphatase PTPN22 is not associated with multiple sclerosis. Am. J. Hum. Genet. 2005, 76, 184–187. [Google Scholar] [CrossRef]
- Homer, C.R.; Richmond, A.L.; Rebert, N.A.; Achkar, J.P.; McDonald, C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology 2010, 139, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Barrett, J.C.; Parkes, M.; Satsangi, J. New IBD genetics: Common pathways with other diseases. Gut 2011, 60, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P. Epigenetics: Principles and practice. Dig. Dis. 2011, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- El Hadad, J.; Schreiner, P.; Vavricka, S.R.; Greuter, T. The Genetics of Inflammatory Bowel Disease. Mol. Diagn. Ther. 2024, 28, 27–35. [Google Scholar] [CrossRef]
- Richard-Miceli, C.; Criswell, L.A. Emerging patterns of genetic overlap across autoimmune disorders. Genome Med. 2012, 4, 6. [Google Scholar] [CrossRef]
- Sanders, V.M.; Straub, R.H. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav. Immun. 2002, 16, 290–332. [Google Scholar] [CrossRef]
- Muscari, I.; Fierabracci, A.; Adorisio, S.; Moretti, M.; Cannarile, L.; Hong, V.T.M.; Ayroldi, E.; Delfino, D.V. Glucocorticoids and natural killer cells: A suppressive relationship. Front. Immunol. 2021, 12, 744215. [Google Scholar] [CrossRef]
- Johnson, J.D.; Barnard, D.F.; Kulp, A.C.; Mehta, D.M. Neuroendocrine Regulation of Brain Cytokines After Psychological Stress. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef]
- Solomon, G.F. Emotions, stress, the central nervous system, and immunity. Ann. N. Y. Acad. Sci. 1969, 164, 335–343. [Google Scholar] [CrossRef]
- Webster Marketon, J.I.; Glaser, R. Stress hormones and immune function. Cell. Immunol. 2008, 252, 16–26. [Google Scholar] [CrossRef]
- Carvalho, L.A.; Urbanova, L.; Hamer, M.; Hackett, R.A.; Lazzarino, A.I.; Steptoe, A. Blunted glucocorticoid and mineralocorticoid sensitivity to stress in people with diabetes. Brain Behav. Immun. 2015, 48, 209–218. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Int. J. Mol. Sci. 2022, 23, 12345. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; de Jonge, P.; Beekman, A.T.; Penninx, B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Psychoneuroendocrinology 2013, 38, 3515–3526. [Google Scholar] [CrossRef]
- Anisman, H. Cascading effects of stressors and inflammatory immune system activation: Implications for major depressive disorder. J. Psychiatry Neurosci. 2009, 34, 4–20. [Google Scholar] [CrossRef]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.B.; Prestia, D.; Mondelli, V.; Pariante, C.; Patti, S.; Olivieri, B.; Arzani, C.; Masotti, M.; Respino, M.; Antonioli, M.; et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology 2016, 63, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Glover, V.; O’Connor, T.G.; O’Donnell, K. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 2010, 35, 17–22. [Google Scholar] [CrossRef]
- Gaffey, A.E.; Bergeman, C.S.; Clark, L.A.; Wirth, M.M. Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 2016, 68, 928–945. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Crosstalk of Alzheimer’s disease-phenotype, HPA axis, splenic oxidative stress and frailty in late-stages of dementia: Effects of social isolation. Front. Aging Neurosci. 2020, 12, 12. [Google Scholar] [CrossRef]
- Agorastos, A.; Chrousos, G.P. Vagal effects of endocrine HPA axis challenges on resting autonomic activity assessed by heart rate variability measures in healthy humans. Psychoneuroendocrinology 2019, 102, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; Kater, C.E. Hypothalamic–Pituitary–Adrenal (HPA) Axis Functioning in Overtraining Syndrome: Findings from Endocrine and Metabolic Responses. Clin. Endocrinol. 2019, 91, 293–302. [Google Scholar] [CrossRef]
- Xia, L.P.; Shen, L.; Kou, H.; Zhang, B.J.; Zhang, L.; Wu, Y.; Li, X.J.; Xiong, J.; Yu, Y.; Wang, H. Prenatal Ethanol Exposure Enhances the Susceptibility to Metabolic Syndrome in Offspring Rats by HPA Axis-Associated Neuroendocrine Metabolic Programming. Int. J. Mol. Sci. 2016, 17, 1859. [Google Scholar] [CrossRef] [PubMed]
- Cozma, D.; Siatra, P.; Oikonomakos, I.; Kalra, D.; Friedrich, U.A.; Dahl, A.; Bornstein, S.R.; Zeissig, S.; Andoniadou, C.L.; Steenblock, C. Insulin Signaling in Hyperactivation of the HPA Axis in Metabolic Diseases. Ann. Rheum. Dis. 2022, 81, SAT272. [Google Scholar] [CrossRef]
- Jokinen, J. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J. Affect. Disord. 2009, 116, 88–92. [Google Scholar] [CrossRef]
- Girod, J.P.; Brotman, D.J. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiol. Rev. 2004, 12, 129–138. [Google Scholar] [CrossRef]
- Vodička, M.; Vavřínová, A.; Mikulecká, A.; Zicha, J.; Behuliak, M. Hyper-reactivity of HPA axis in Fischer 344 rats is associated with impaired cardiovascular and behavioral adaptation to repeated restraint stress. Physiol. Res. 2018, 67, 505–514. [Google Scholar] [CrossRef]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Jones, M.P.; Dilley, J.B.; Drossman, D.; Crowell, M.D. Brain–gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterol. Motil. 2006, 18, 91–103. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Quigley, E.M.M.; Dinan, T.G.; Clarke, G. A sustained hypothalamic–pituitary–adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol. Med. 2014, 44, 3123–3134. [Google Scholar] [CrossRef]
- Bonaz, B.L. Brain–Gut Interactions in Inflammatory Bowel Disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota–gut–brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Tuckey, R.C.; Paus, R. Differential expression of HPA axis homolog in the skin. Trends Endocrinol. Metab. 2007, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Jozic, I.; Stojadinovic, O.; Kirsner, R.S.F.; Tomic-Canic, M. Skin under the (Spot)-Light: Cross-Talk with the Central Hypothalamic-Pituitary-Adrenal (HPA) Axis. J. Investig. Dermatol. 2015, 135, 1469–1471. [Google Scholar] [CrossRef]
- Vilela, D.D.C.; Chamusca, F.V.; Andrade, J.C.S.; Vallve, M.L.F.; Gonzalez, A.C.; Andrade, Z.A.; Medrado, A.R.A.P.; Reis, S.R.A. Influence of the HPA axis on the inflammatory response in cutaneous wounds with the use of 670-nm laser photobiomodulation. Lasers Med. Sci. 2016, 3, 114–120. [Google Scholar] [CrossRef]
- Lim, H.S. Early stage ultraviolet irradiation damage to skin collagen can be suppressed by HPA axis control via controlled CYP11B. Biomed. Pharmacother. 2022, 155, 113716. [Google Scholar] [CrossRef]
- Han, M.; Ban, J.J.; Bae, J.S.; Shin, C.Y.; Lee, D.H.; Chung, J.H. UV irradiation to mouse skin decreases hippocampal neurogenesis and synaptic protein expression via HPA axis activation. Sci. Rep. 2017, 7, 42338. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Association between Stress and the HPA Axis in Atopic Dermatitis. Int. J. Mol. Sci. 2017, 18, 2131. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Alvarez, P.; Levine, J.D. Sexual dimorphic role of the glucocorticoid receptor in chronic muscle pain produced by early-life stress. Pain 2013, 154, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Zhu, X.; Szumowski, M.; Scott, G.D.; Grossberg, A.J.; Levasseur, P.R.; Graham, K.; Khan, S.; Damaraju, S.; Colmers, W.F.; et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. Brain Behav. Immun. 2011, 25, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, S.G.; Ross, A.; Steele, M.E.; Saligan, L.N. Differential impacts of body composition indices on cortisol response and HPA axis activity. Psychoneuroendocrinology 2020, 120, 104798. [Google Scholar] [CrossRef]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Adamaki, M. Nutrient insufficiencies and deficiencies involved in the pathogenesis of bruxism (Review). Mol. Med. Rep. 2021, 24, 693. [Google Scholar] [CrossRef]
- Leimkühler, N.B.; Schneider, R.K. Inflammatory bone marrow microenvironment. Hematol. Am. Soc. Hematol. Educ. Program. 2019, 2019, 294–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014, 20, 754–758. [Google Scholar] [CrossRef]
- Hofmann, J.; Kokkaliaris, K.D. Bone marrow niches for hematopoietic stem cells: Life span dynamics and adaptation to acute stress. Blood 2024, 144, 21–34. [Google Scholar] [CrossRef]
- Gomes, A.C.; Saraiva, M.; Gomes, M.S. The bone marrow hematopoietic niche and its adaptation to infection. Semin. Cell Dev. Biol. 2021, 112, 37–48. [Google Scholar] [CrossRef]
- Bogeska, R.; Mikecin, A.M.; Kaschutnig, P.; Fawaz, M.; Büchler-Schäff, M.; Le, D.; Ganuza, M.; Vollmer, A.; Paffenholz, S.V.; Asada, N.; et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 2022, 29, 1273–1284.e8. [Google Scholar] [CrossRef]
- Fudulu, D.P.; Horn, G.; Hazell, G.; Lefrançois-Martinez, A.M.; Martinez, A.; Angelini, G.D.; Lightman, S.L.; Spiga, F. Co-culture of monocytes and zona fasciculata adrenal cells: An in vitro model to study the immune-adrenal cross-talk. Mol. Cell Endocrinol. 2021, 526, 111195. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Feng, X.; Wang, L.W.; Zhou, R.; Sun, H.; Chen, X.; Lu, R.B.; Huang, Y.; Guo, Q.; Luo, X.H. Bone marrow immune cells respond to fluctuating nutritional stress to constrain weight regain. Cell Metab. 2023, 35, 1915–1930.e8. [Google Scholar] [CrossRef]
- Horikawa, I.; Nagai, H.; Taniguchi, M.; Chen, G.; Shinohara, M.; Suzuki, T.; Ishii, S.; Katayama, Y.; Kitaoka, S.; Furuyashiki, T. Chronic stress alters lipid mediator profiles associated with immune-related gene expressions and cell compositions in mouse bone marrow and spleen. J. Pharmacol. Sci. 2024, 154, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Menardo, E.; Brondino, N.; Borsotti, M.; Porcelli, S.; Serafini, G.; Amore, M. Nature and Mindfulness to Cope with Work-Related Stress: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 10827. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chou, H.C.; Chen, M.L.; Lin, W.C.; Chu, C.H. The Relationships Between Stress, Coping Strategies, and Quality of Life Among Gynecologic Cancer Survivors. Oncol. Nurs. Forum 2014, 41, E255–E263. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, K.; Krajewska-Kułak, E.; Sobolewski, M. Strategies for Coping With Stress Used by Nurses in Poland and Belarus During the COVID-19 Pandemic. Front. Psychol. 2021, 12, 610255. [Google Scholar] [CrossRef]
- Van Kerschaver, E.; Camerlink, I.; Tuyttens, F.A.M. Reducing Weaning Stress in Piglets by Pre-Weaning Socialization and Gradual Separation from the Sow: A Review. Animals 2020, 10, 1829. [Google Scholar] [CrossRef]
- Ojangba, T.; Wang, X.; Li, Y.; Hu, J.; Chen, Y. Comprehensive Effects of Lifestyle Reform, Adherence, and Related Factors on Hypertension Control: A Review. Nutrients 2022, 14, 1514. [Google Scholar] [CrossRef]
- Taub, R.; Horesh, D.; Rubin, N.; Glick, I.; Reem, O.; Shriqui, G.; Agmon-Levin, N. Mindfulness-Based Stress Reduction for Systemic Lupus Erythematosus: A Mixed-Methods Pilot Randomized Controlled Trial of an Adapted Protocol. Mindfulness 2021, 12, 1873–1886. [Google Scholar] [CrossRef]
- Shkodina, A.D.; Pavlov, A.; Demidova, T.; Mikhaylov, V.; Orlova, Y. Pharmacological and non-pharmacological approaches for the management of neuropathic pain in multiple sclerosis. CNS Drugs 2024, 38, 205–224. [Google Scholar] [CrossRef]
- Taylor, P.C.; Van de Laar, M.; Laster, A.; Fakhouri, W.; Quebe, A.; de la Torre, I.; Jain, S. Call for action: Incorporating wellness practices into a holistic management plan for rheumatoid arthritis—Going beyond treat to target. Ann. Rheum. Dis. 2021, 80, 1289–1294. [Google Scholar] [CrossRef]
- Balsamo, S.; dos Santos-Neto, L. Fatigue in systemic lupus erythematosus: An association with reduced physical fitness. Clin. Exp. Rheumatol. 2011, 29, 471–475. [Google Scholar] [CrossRef]
- Singh, J.; Singh, N.; Sharma, R. Tele-yoga in the management of ankylosing spondylitis amidst COVID pandemic: A prospective randomized controlled trial. Complement. Ther. Clin. Pract. 2023, 50, 101672. [Google Scholar] [CrossRef]
- Parodis, I.; Gomez, A.; Tsoi, A.; Chow, J.W.; Pezzella, D.; Girard, C.; A Stamm, T.; Boström, C. Systematic literature review informing the EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus and systemic sclerosis. RMD Open 2022, 8, e002550. [Google Scholar] [CrossRef]
- Gebremeskel, G.G.; Haile, T.G.; Gebrewahd, G.T.; Hailay, A.; Aberhe, W.; Mebrahtom, G.; Zereabruk, K.; Negash, A.I.; Gebrekidan, H.; Tadesse, D.B. Effectiveness of non-pharmacological therapies for chronic pain in people with autoimmune diseases in Africa: A protocol for a systematic review and meta-analysis. PLoS ONE 2021, 16, e0258634. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2018, 195, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Begemann, K.; Rawashdeh, O.; Olejniczak, I.; Pilorz, V.; Vinícius, L.; de Assis, M.; Osorio-Mendoza, J.; Oster, H. Endocrine regulation of circadian rhythms. Npj Biol. Timing Sleep 2025, 2, 10. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunez, S.G.; Rabelo, S.P.; Subotic, N.; Caruso, J.W.; Knezevic, N.N. Chronic Stress and Autoimmunity: The Role of HPA Axis and Cortisol Dysregulation. Int. J. Mol. Sci. 2025, 26, 9994. https://doi.org/10.3390/ijms26209994
Nunez SG, Rabelo SP, Subotic N, Caruso JW, Knezevic NN. Chronic Stress and Autoimmunity: The Role of HPA Axis and Cortisol Dysregulation. International Journal of Molecular Sciences. 2025; 26(20):9994. https://doi.org/10.3390/ijms26209994
Chicago/Turabian StyleNunez, Sergio Gutierrez, Sara Peixoto Rabelo, Nikola Subotic, James Wilson Caruso, and Nebojsa Nick Knezevic. 2025. "Chronic Stress and Autoimmunity: The Role of HPA Axis and Cortisol Dysregulation" International Journal of Molecular Sciences 26, no. 20: 9994. https://doi.org/10.3390/ijms26209994
APA StyleNunez, S. G., Rabelo, S. P., Subotic, N., Caruso, J. W., & Knezevic, N. N. (2025). Chronic Stress and Autoimmunity: The Role of HPA Axis and Cortisol Dysregulation. International Journal of Molecular Sciences, 26(20), 9994. https://doi.org/10.3390/ijms26209994






