Bioengineered Skin from a Platelet-Derived Hydrogel Repairs Full Thickness Wounds in a Pre-Clinical Mouse Model
Abstract
1. Introduction
2. Results
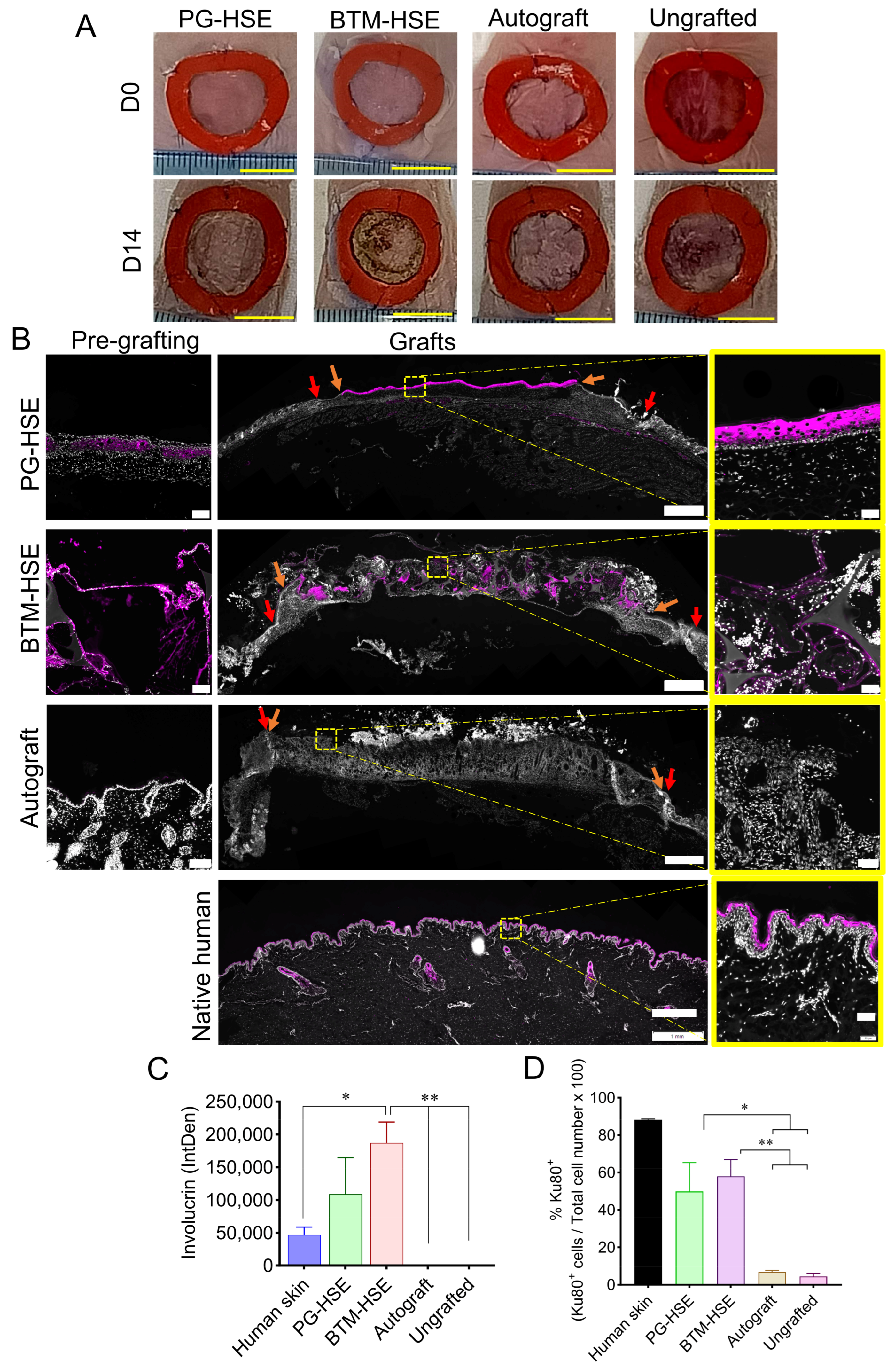
2.1. Platelet-Derived Hydrogel Is an Effective Scaffold for Constructing a PG-HSE Graft and Closing Wounds in a Mouse Model
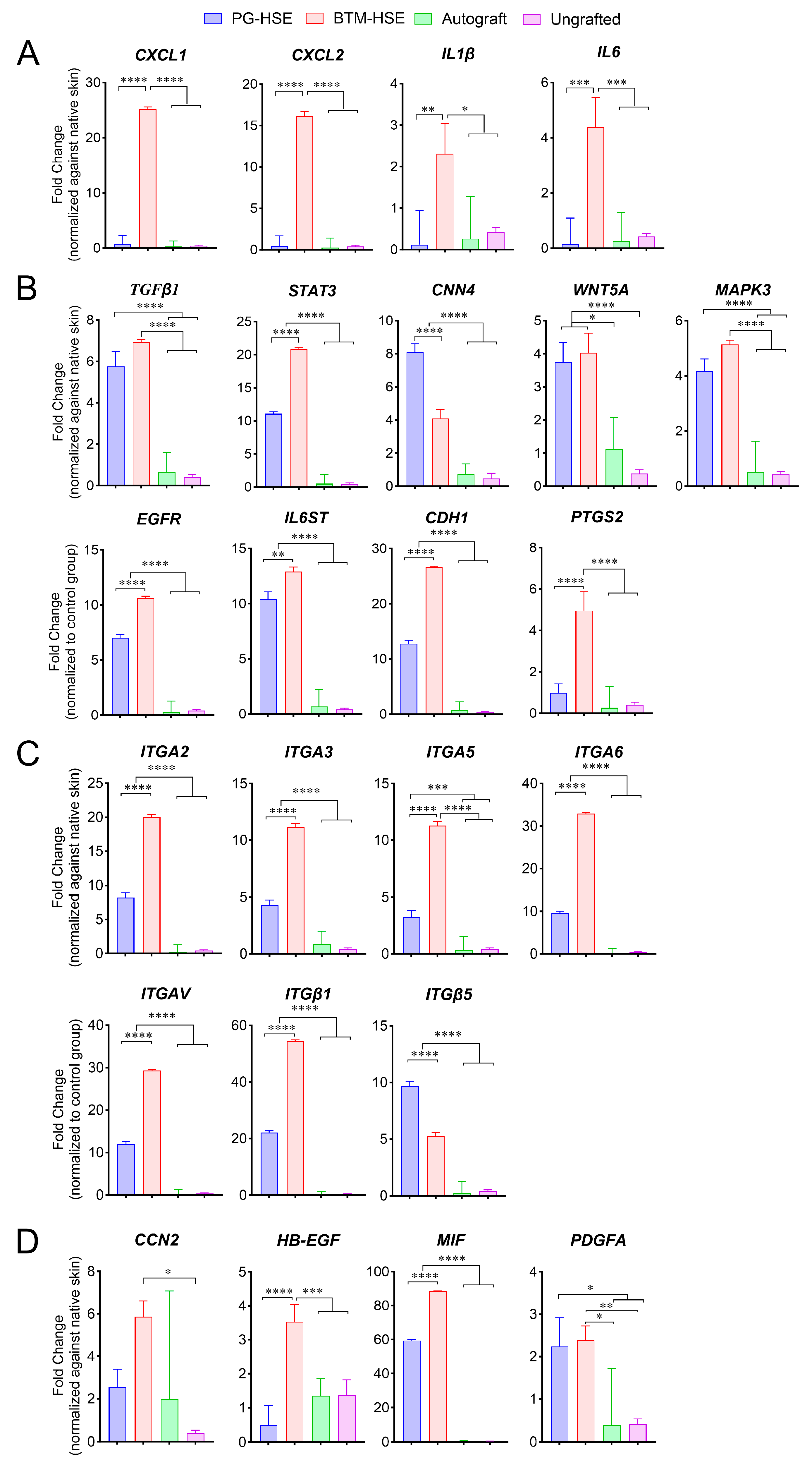
2.2. PG-HSE Grafts Show Lower Transcription Levels of Inflammatory Markers, Compared to BTM-HSE Grafts
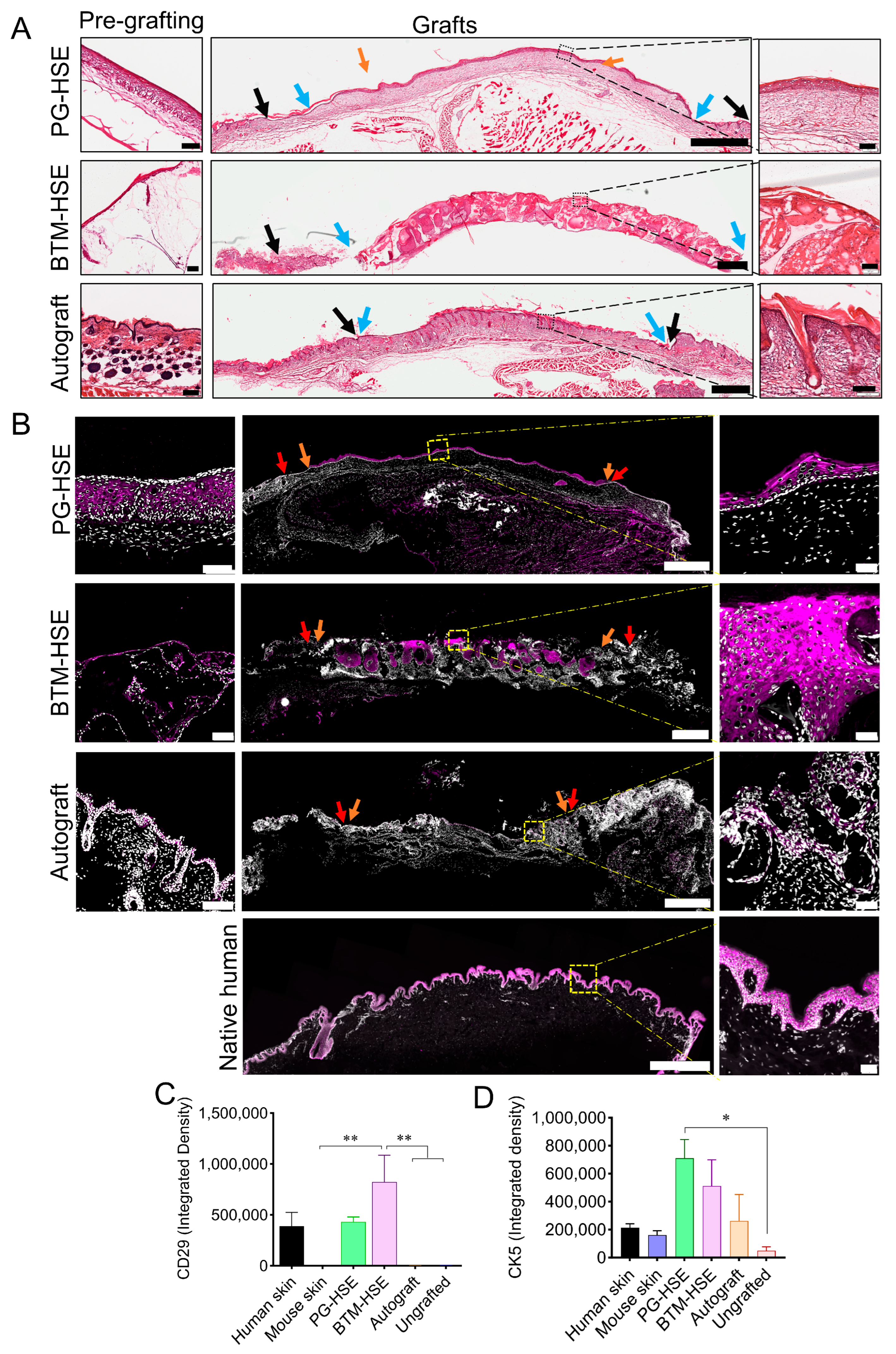
2.3. Basal Keratinocytes Are Sustained in Both PG-HSE and BTM-HSE Grafts
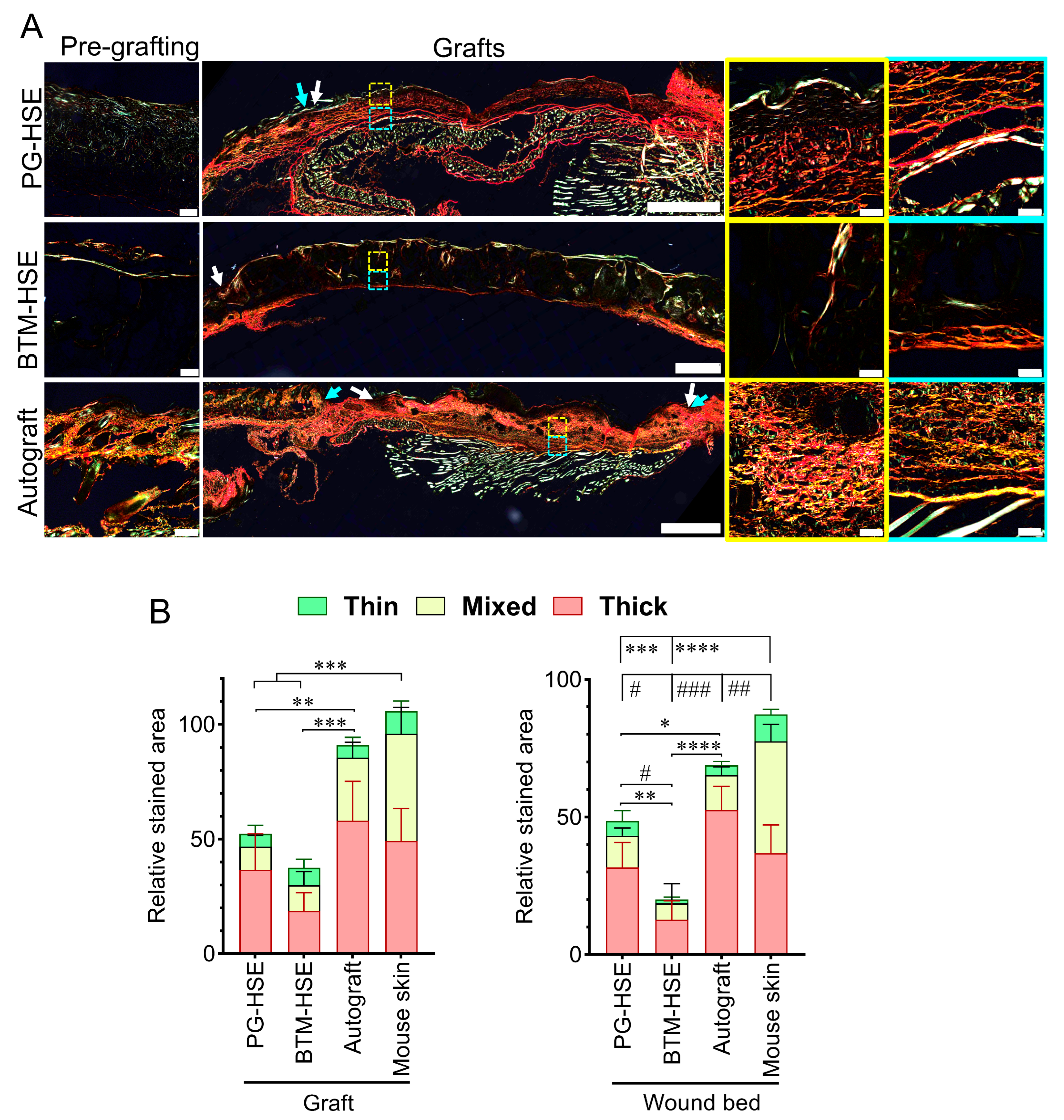
2.4. PG-HSE Grafts Enhance Neo-Dermis Formation by Influencing Collagen Synthesis and Degradation
2.5. PG-HSE Grafting Restores Skin Barrier Function
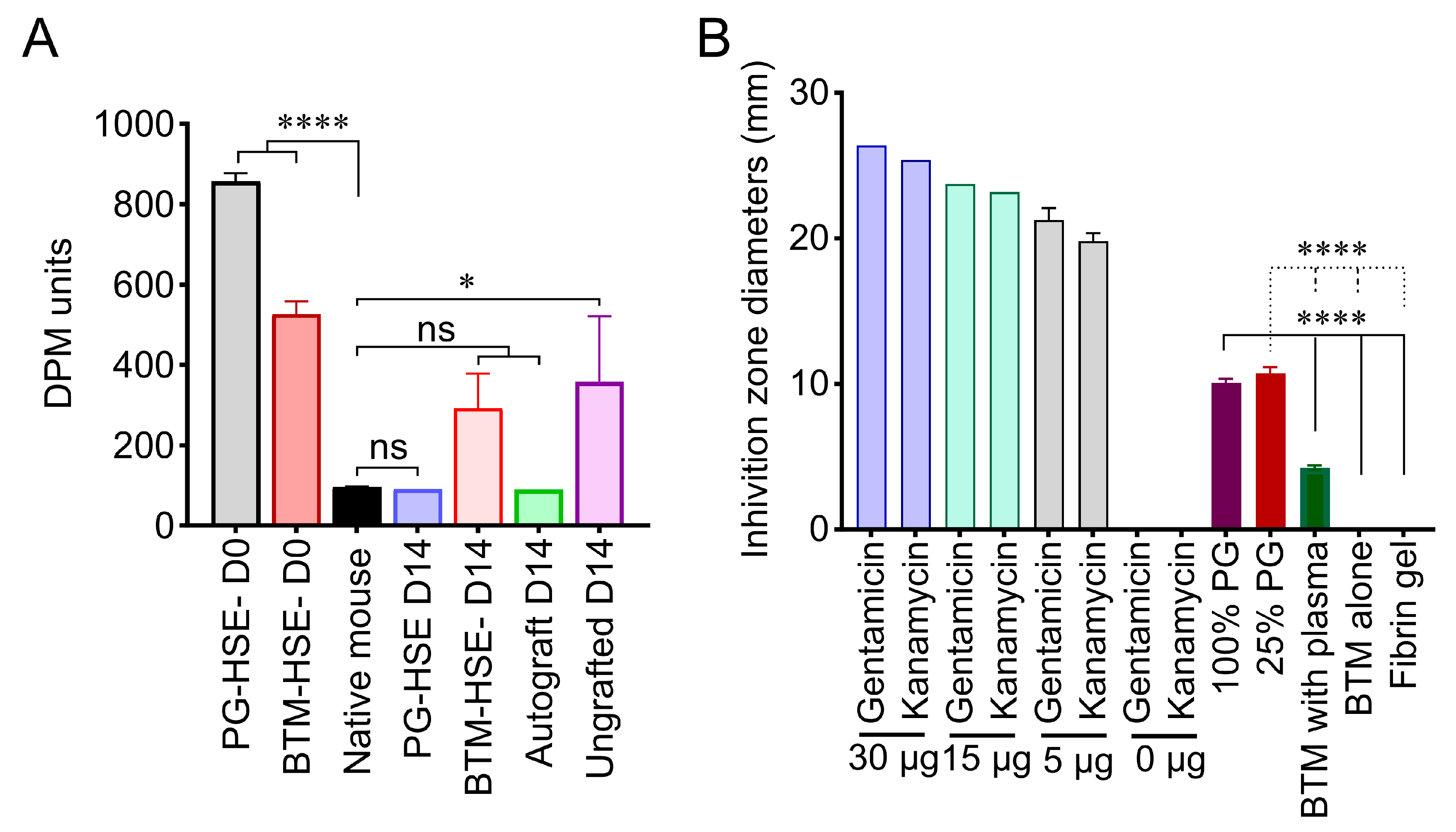
2.6. PG-HSE Carries Intrinsic Anti-Microbial Properties That May Influence Graft Survival
3. Discussion
4. Materials and Methods
4.1. Access to Human-Derived Material
4.2. Isolation and Expansion of Primary Adult Fibroblasts and Keratinocytes
4.3. Fibroblast Proliferation Assay
4.4. PG-HSE and BTM-HSE Construction
4.5. Mouse Surgery
4.6. Histological Analysis
4.7. Immunofluorescent Staining
4.8. Wound Contraction
4.9. Skin Barrier Function
4.10. RT2 Profiler PCR Array
4.11. Disc Diffusion Antibacterial Test
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PG | Platelet-derived hydrogel |
| BTM | Biodegradable temporising matrix |
| PG-HSE | Platelet gel derived human skin equivalent |
| BTM-HSE | Biodegradable temporising matrix derived human skin equivalent |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| TGF | Transforming growth factor |
| PDGF | Platelet-derived growth factor |
| ITG | Integrin |
| IGF | Insulin-like growth factor |
| HB-EGF | Heparin-binding epidermal growth factor-like growth factor |
| CXCL | Chemokine C-X-C ligand |
| IL | Interleukins |
| CDH | Cadherin or E-cadherin |
| EGFR | Epidermal growth factor receptor |
| COL | Collagen |
| PTGS | Prostaglandin-endoperoxide synthase |
| COX | Cyclooxygenase |
| STAT | Signal transducer and activator of transcription |
| CCN | Cellular communication network factor |
| MMP | Matrix metalloproteinase |
| CTS | Cathepsin |
| PLAU | Plasminogen activator, urokinase |
| SERPINE1 | Serine protease inhibitor clade E member |
| uPA/tPA | Tissue-type plasminogen activator |
| PF | Platelet factor |
| PAI | Plasminogen activator inhibitor |
| CTAP | Connective tissue activating peptide |
| RANTES | Regulated on activation, normal T-cell expressed and secreted |
| PRP | Platelet rich plasma |
| DPM | Dermal phase meter |
References
- Palackic, A.; Duggan, R.P.; Campbell, M.S.; Walters, E.; Branski, L.K.; Ayadi, A.E.; Wolf, S.E. The Role of Skin Substitutes in Acute Burn and Reconstructive Burn Surgery: An Updated Comprehensive Review. Semin. Plast. Surg. 2022, 36, 33–42. [Google Scholar] [CrossRef]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Advances in Skin Tissue Bioengineering and the Challenges of Clinical Translation. Front. Surg. 2021, 8, 640879. [Google Scholar] [CrossRef]
- Sierra-Sanchez, A.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 35. [Google Scholar] [CrossRef]
- Chogan, F.; Chen, Y.; Wood, F.; Jeschke, M.G. Skin Tissue Engineering Advances in Burns: A Brief Introduction to the Past, the Present, and the Future Potential. J. Burn Care Res. 2023, 44 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef]
- Paul, M.; Kaur, P.; Herson, M.; Cheshire, P.; Cleland, H.; Akbarzadeh, S. Use of Clotted Human Plasma and Aprotinin in Skin Tissue Engineering: A Novel Approach to Engineering Composite Skin on a Porous Scaffold. Tissue Eng. Part C Methods 2015, 21, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Llames, S.G.; Del Rio, M.; Larcher, F.; Garcia, E.; Garcia, M.; Escamez, M.J.; Jorcano, J.L.; Holguin, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 2004, 77, 350–355. [Google Scholar] [CrossRef]
- Monfort, A.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Izeta, A. Production of human tissue-engineered skin trilayer on a plasma-based hypodermis. J. Tissue Eng. Regen. Med. 2013, 7, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Alexaline, M.M.; Trouillas, M.; Nivet, M.; Bourreau, E.; Leclerc, T.; Duhamel, P.; Martin, M.T.; Doucet, C.; Fortunel, N.O.; Lataillade, J.J. Bioengineering a human plasma-based epidermal substitute with efficient grafting capacity and high content in clonogenic cells. Stem Cells Transl. Med. 2015, 4, 643–654. [Google Scholar] [CrossRef]
- Dearman, B.L.; Stefani, K.; Li, A.; Greenwood, J.E. “Take” of a polymer-based autologous cultured composite "skin" on an integrated temporizing dermal matrix: Proof of concept. J. Burn Care Res. 2013, 34, 151–160. [Google Scholar] [CrossRef]
- Banakh, I.; Cheshire, P.; Rahman, M.; Carmichael, I.; Jagadeesan, P.; Cameron, N.R.; Cleland, H.; Akbarzadeh, S. A Comparative Study of Engineered Dermal Templates for Skin Wound Repair in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 4508. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dearman, B.L.; Crompton, K.E.; Moore, T.G.; Greenwood, J.E. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J. Burn Care Res. 2009, 30, 717–728. [Google Scholar] [CrossRef]
- Lo, C.H.; Brown, J.N.; Dantzer, E.J.G.; Maitz, P.K.M.; Vandervord, J.G.; Wagstaff, M.J.D.; Barker, T.M.; Cleland, H. Wound healing and dermal regeneration in severe burn patients treated with NovoSorb(R) Biodegradable Temporising Matrix: A prospective clinical study. Burns 2022, 48, 529–538. [Google Scholar] [CrossRef]
- Greenwood, J.E.; Damkat-Thomas, L.; Schmitt, B.; Dearman, B. Successful proof of the ‘two-stage strategy’ for major burn wound repair. Burn. Open 2020, 4, 10. [Google Scholar] [CrossRef]
- Rybarczyk, B.J.; Lawrence, S.O.; Simpson-Haidaris, P.J. Matrix-fibrinogen enhances wound closure by increasing both cell proliferation and migration. Blood 2003, 102, 4035–4043. [Google Scholar] [CrossRef]
- Laurens, N.; Koolwijk, P.; de Maat, M.P. Fibrin structure and wound healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.A.; Schwarzbauer, J.E. Requirements for alpha(5)beta(1) integrin-mediated retraction of fibronectin-fibrin matrices. J. Biol. Chem. 1999, 274, 20943–20948. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Garcia, N.; Loh, Y.S.; Marks, D.C.; Banakh, I.; Jagadeesan, P.; Cameron, N.R.; Yung-Chih, C.; Costa, M.; Peter, K.; et al. A platelet-derived hydrogel improves neovascularisation in full thickness wounds. Acta Biomater. 2021, 136, 199–209. [Google Scholar] [CrossRef]
- Banakh, I.; Rahman, M.M.; Arellano, C.L.; Marks, D.C.; Mukherjee, S.; Gargett, C.E.; Cleland, H.; Akbarzadeh, S. Platelet lysate can support the development of a 3D-engineered skin for clinical application. Cell Tissue Res. 2023, 391, 173–188. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, M.; Nizamoglu, M.; Kaper, H.J.; Brouwer, L.A.; Borghuis, T.; Burgess, J.K.; Harmsen, M.C.; Sharma, P.K. Fibroblast alignment and matrix remodeling induced by a stiffness gradient in a skin-derived extracellular matrix hydrogel. Acta Biomater. 2024, 182, 67–80. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, M.; Choi, K.Y. Approaches for Regenerative Healing of Cutaneous Wound with an Emphasis on Strategies Activating the Wnt/beta-Catenin Pathway. Adv. Wound Care 2022, 11, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Bergdall, V.K.; Tober, K.L.; Hill, K.J.; Mitra, S.; Flavahan, N.A.; Oberyszyn, T.M. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am. J. Pathol. 2004, 165, 753–761. [Google Scholar] [CrossRef]
- Ghaffari, A.; Kilani, R.T.; Ghahary, A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J. Investig. Dermatol. 2009, 129, 340–347. [Google Scholar] [CrossRef]
- Medina, A.; Ghaffari, A.; Kilani, R.T.; Ghahary, A. The role of stratifin in fibroblast-keratinocyte interaction. Mol. Cell. Biochem. 2007, 305, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Simone, T.M.; Higgins, C.E.; Czekay, R.P.; Law, B.K.; Higgins, S.P.; Archambeault, J.; Kutz, S.M.; Higgins, P.J. SERPINE1: A Molecular Switch in the Proliferation-Migration Dichotomy in Wound-"Activated" Keratinocytes. Adv. Wound Care 2014, 3, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.F.; Ventre, M.; Netti, P.A. Tuning the material-cytoskeleton crosstalk via nanoconfinement of focal adhesions. Biomaterials 2014, 35, 2743–2751. [Google Scholar] [CrossRef]
- Peyton, S.R.; Kim, P.D.; Ghajar, C.M.; Seliktar, D.; Putnam, A.J. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials 2008, 29, 2597–2607. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; McKenzie, M.B.; Rahman, M.M.; Cleland, H. Allogeneic Platelet-Rich Plasma: Is It Safe and Effective for Wound Repair? Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2021, 62, 1–12. [Google Scholar] [CrossRef]
- Garcia, N.; Rahman, M.M.; Arellano, C.L.; Banakh, I.; Yung-Chih, C.; Peter, K.; Cleland, H.; Lo, C.H.; Akbarzadeh, S. Graft-Host Interaction and Its Effect on Wound Repair Using Mouse Models. Int. J. Mol. Sci. 2023, 24, 16277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lawrence, D.A.; Zhang, L. Sequences within domain II of the urokinase receptor critical for differential ligand recognition. J. Biol. Chem. 2003, 278, 29925–29932. [Google Scholar] [CrossRef]
- Chandra, P.; Faizan, M.; Porwal, M.; Sharma, H.; Sachan, N. An Overview and Review of Growth Factors in Wound Healing: Emerging Trends and Innovations. Curr. Diabetes Rev. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Herbert, S.P.; Stainier, D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 551–564. [Google Scholar] [CrossRef]
- Wang, X.; Banda, J.; Qi, H.; Chang, A.K.; Bwalya, C.; Chao, L.; Li, X. Scarless wound healing: Current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022, 66, 26–37, Erratum in Cytokine Growth Factor Rev. 2022, 68, 116. [Google Scholar] [CrossRef]
- Willenborg, S.; Schönborn, K.; Sawant, M.; Bornikoel, A.; Yamane, T.; Zeinert, I.; Eckes, B.; Eming, S.A.; Krieg, T. Fibroblast-Derived TGFβ1 Regulates Skin Repair and Fibrosis. Wound Repair Regen. 2025, 33, e70065. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Sziksz, E.; Pap, D.; Lippai, R.; Beres, N.J.; Fekete, A.; Szabo, A.J.; Vannay, A. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediat. Inflamm. 2015, 2015, 764641. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial peptides from human platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef]
- Chen, J.; Wan, Y.; Lin, Y.; Jiang, H. The application of platelet-rich plasma for skin graft enrichment: A meta-analysis. Int. Wound J. 2020, 17, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Cl, K.; Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Khanna, M.; Yadav, S. Antimicrobial Effects of Platelet-Rich Plasma and Platelet-Rich Fibrin: A Scoping Review. Cureus 2023, 15, e51360. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T. Methods for the Serum-Free Culture of Keratinocytes and Transplantation of Collagen-GAG-Based Skin Substitutes. Methods Mol. Med. 1999, 18, 365–389. [Google Scholar]
- Vennin, C.; Melenec, P.; Rouet, R.; Nobis, M.; Cazet, A.S.; Murphy, K.J.; Herrmann, D.; Reed, D.A.; Lucas, M.C.; Warren, S.C.; et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 2019, 10, 3637. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Arellano, C.L.; Banakh, I.; Marks, D.C.; Carmichael, I.; Arfuso, F.; Lo, C.H.; Cleland, H.; Akbarzadeh, S. Bioengineered Skin from a Platelet-Derived Hydrogel Repairs Full Thickness Wounds in a Pre-Clinical Mouse Model. Int. J. Mol. Sci. 2025, 26, 9988. https://doi.org/10.3390/ijms26209988
Rahman MM, Arellano CL, Banakh I, Marks DC, Carmichael I, Arfuso F, Lo CH, Cleland H, Akbarzadeh S. Bioengineered Skin from a Platelet-Derived Hydrogel Repairs Full Thickness Wounds in a Pre-Clinical Mouse Model. International Journal of Molecular Sciences. 2025; 26(20):9988. https://doi.org/10.3390/ijms26209988
Chicago/Turabian StyleRahman, Md. M., Carlos L. Arellano, Ilia Banakh, Denese C. Marks, Irena Carmichael, Frank Arfuso, Cheng Hean Lo, Heather Cleland, and Shiva Akbarzadeh. 2025. "Bioengineered Skin from a Platelet-Derived Hydrogel Repairs Full Thickness Wounds in a Pre-Clinical Mouse Model" International Journal of Molecular Sciences 26, no. 20: 9988. https://doi.org/10.3390/ijms26209988
APA StyleRahman, M. M., Arellano, C. L., Banakh, I., Marks, D. C., Carmichael, I., Arfuso, F., Lo, C. H., Cleland, H., & Akbarzadeh, S. (2025). Bioengineered Skin from a Platelet-Derived Hydrogel Repairs Full Thickness Wounds in a Pre-Clinical Mouse Model. International Journal of Molecular Sciences, 26(20), 9988. https://doi.org/10.3390/ijms26209988






