The Immune System in Antarctic and Subantarctic Fish of the Genus Harpagifer Is Affected by the Effects of Combined Microplastics and Thermal Increase
Abstract
1. Introduction
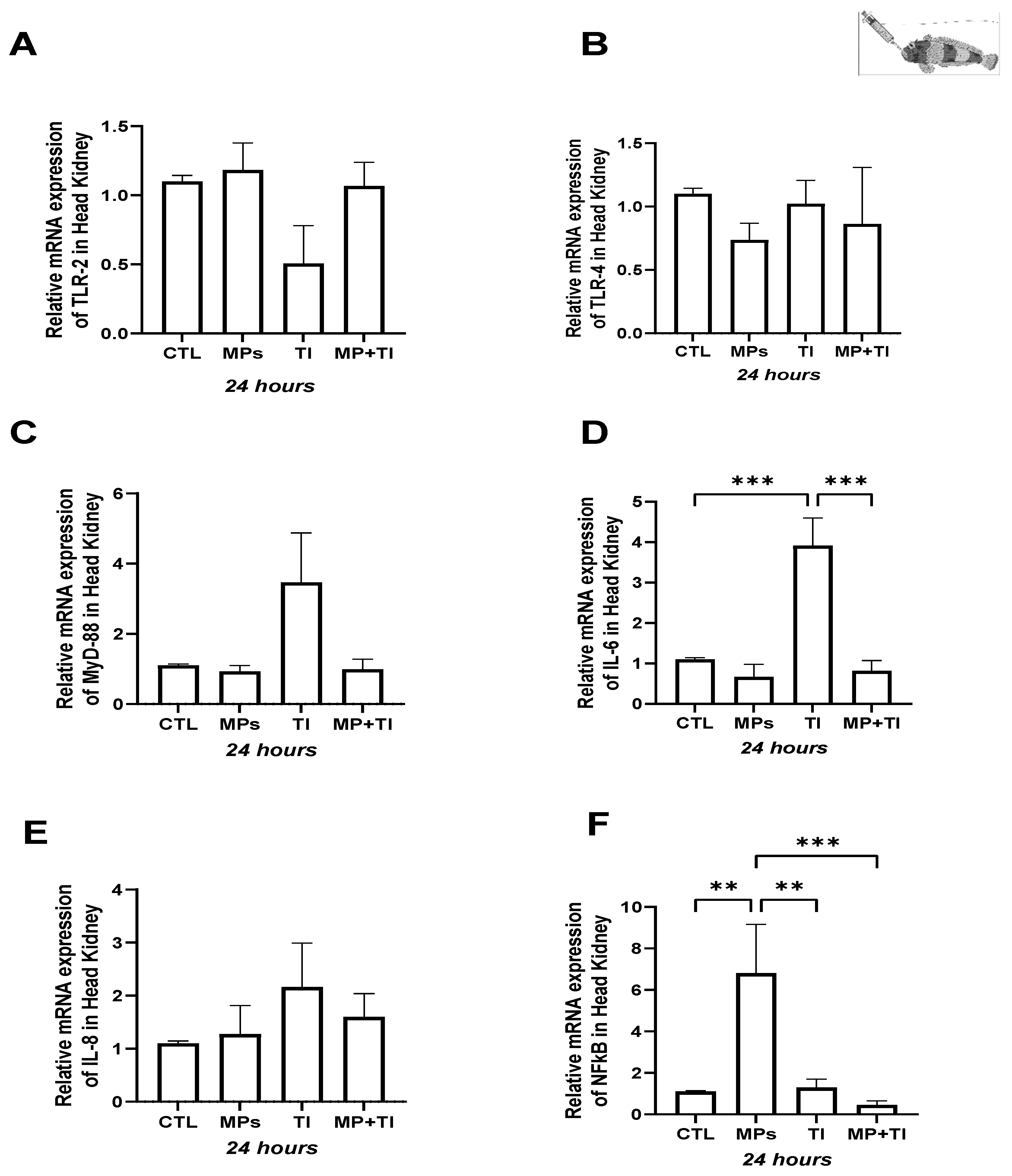
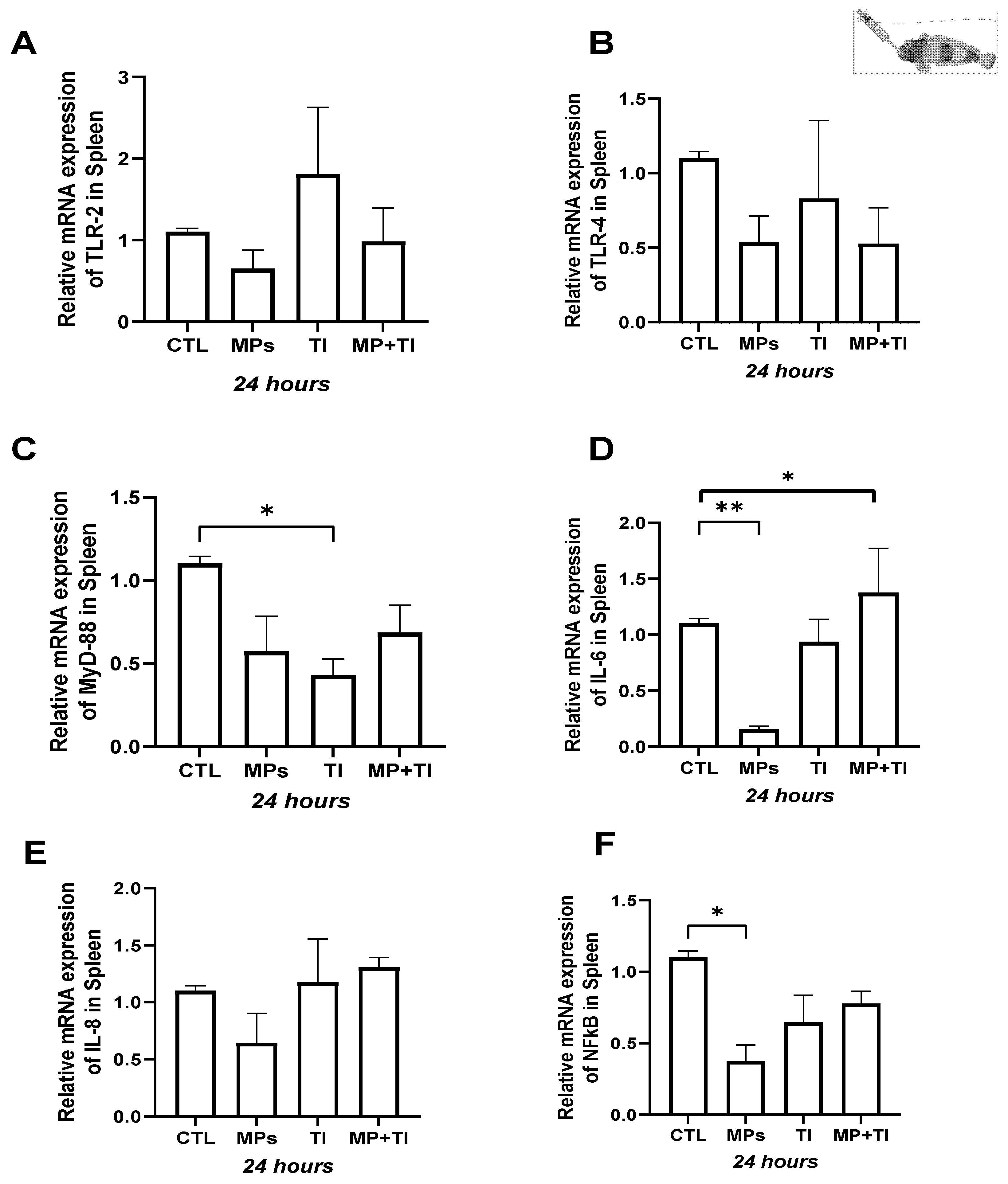
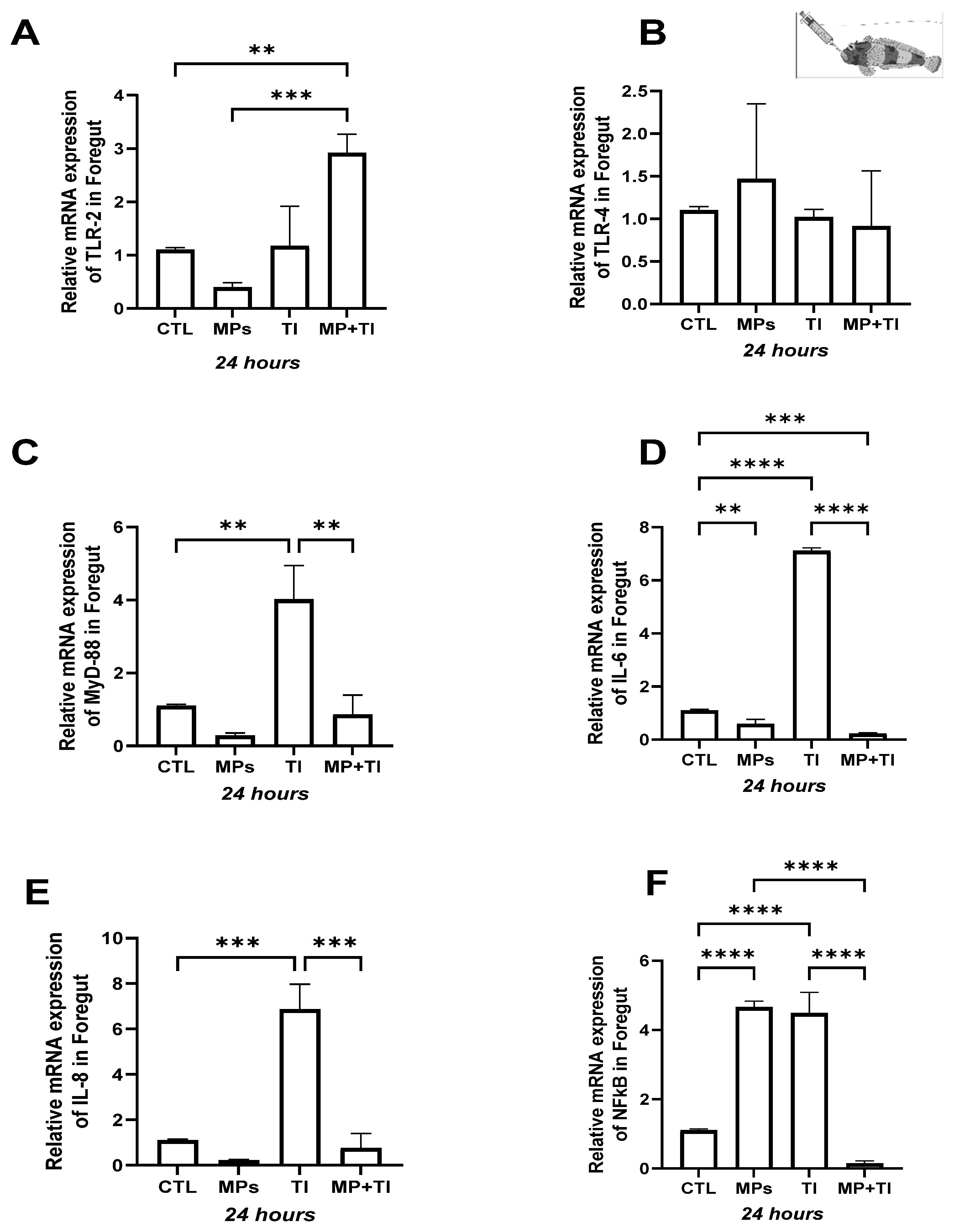
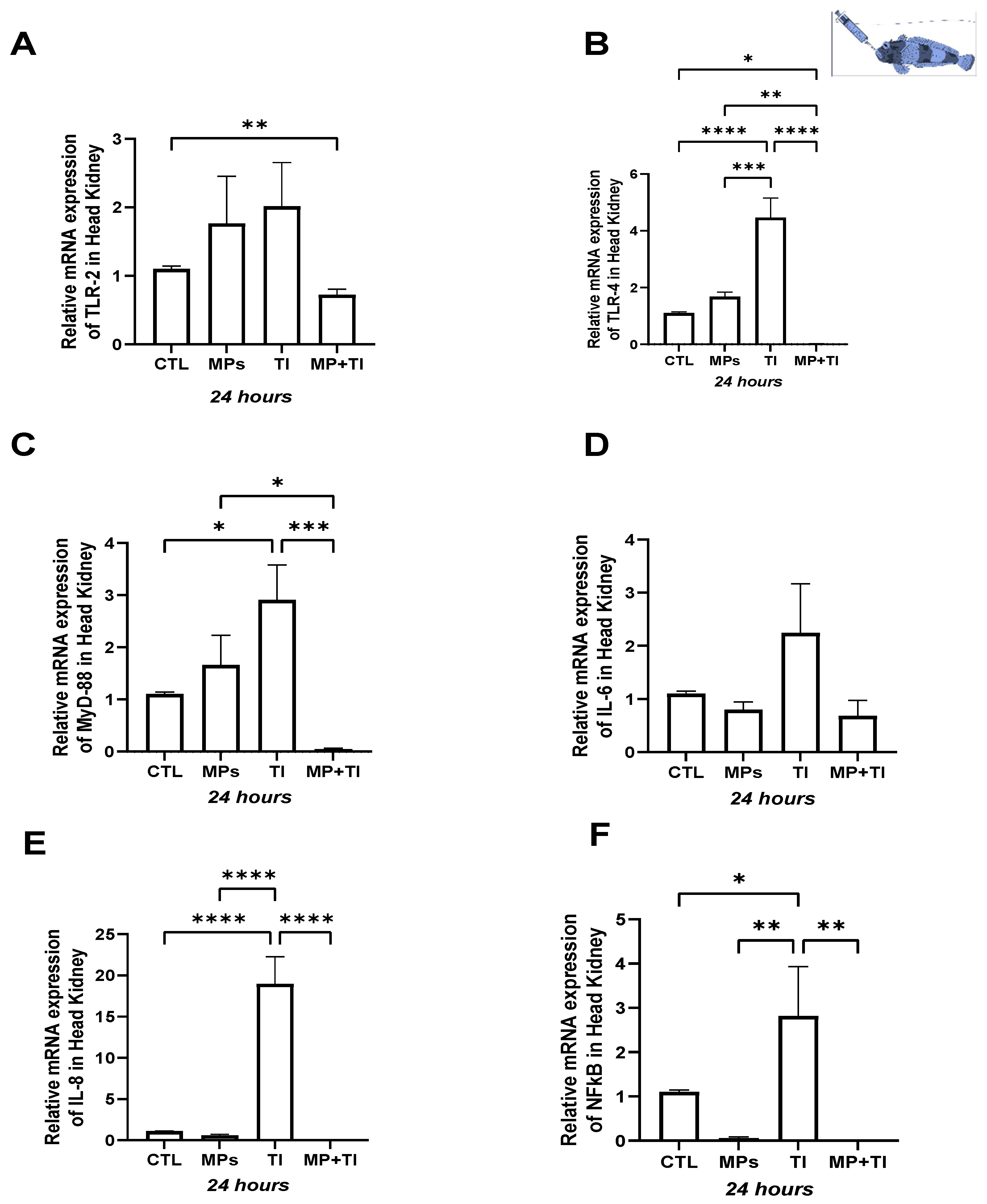
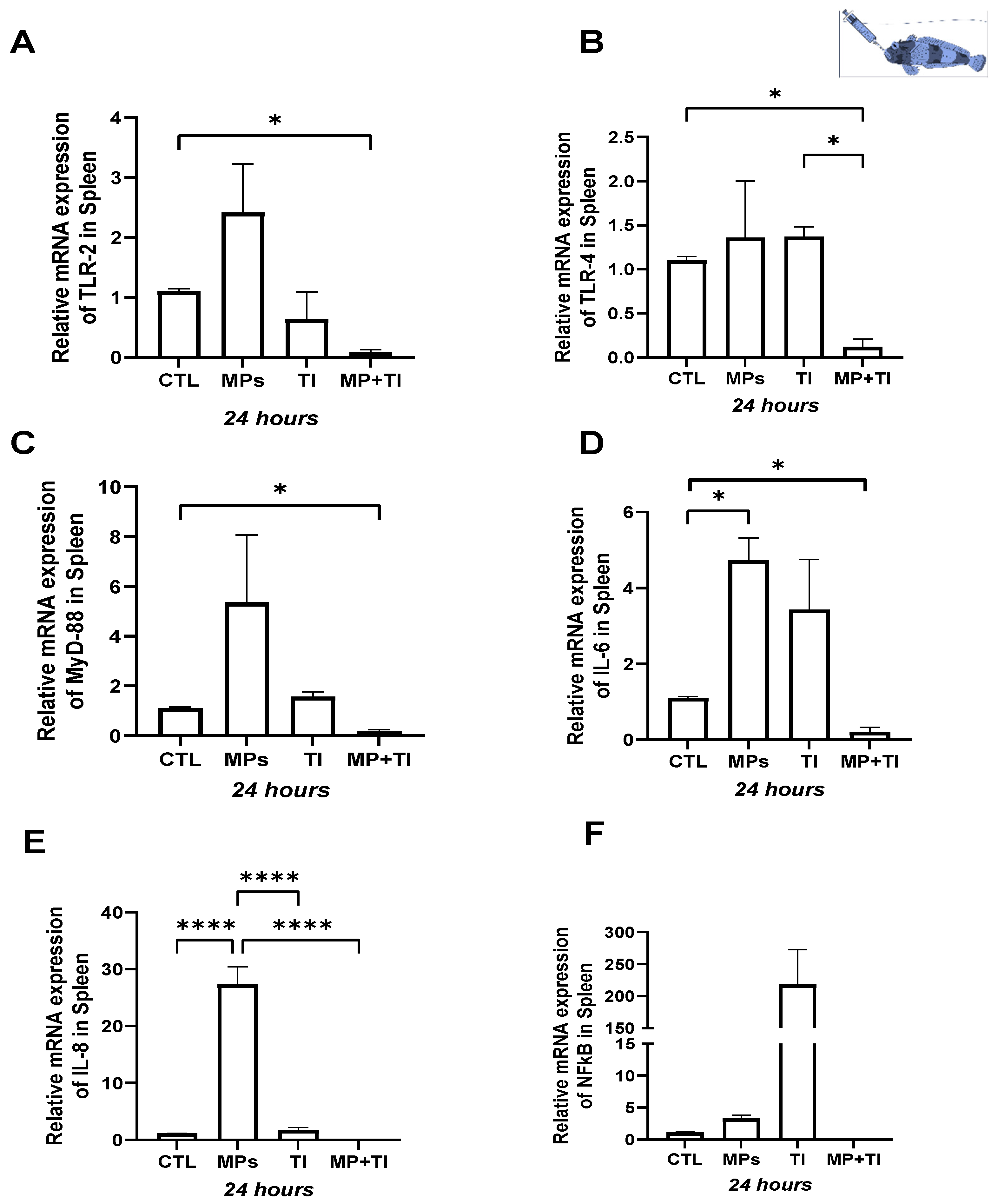
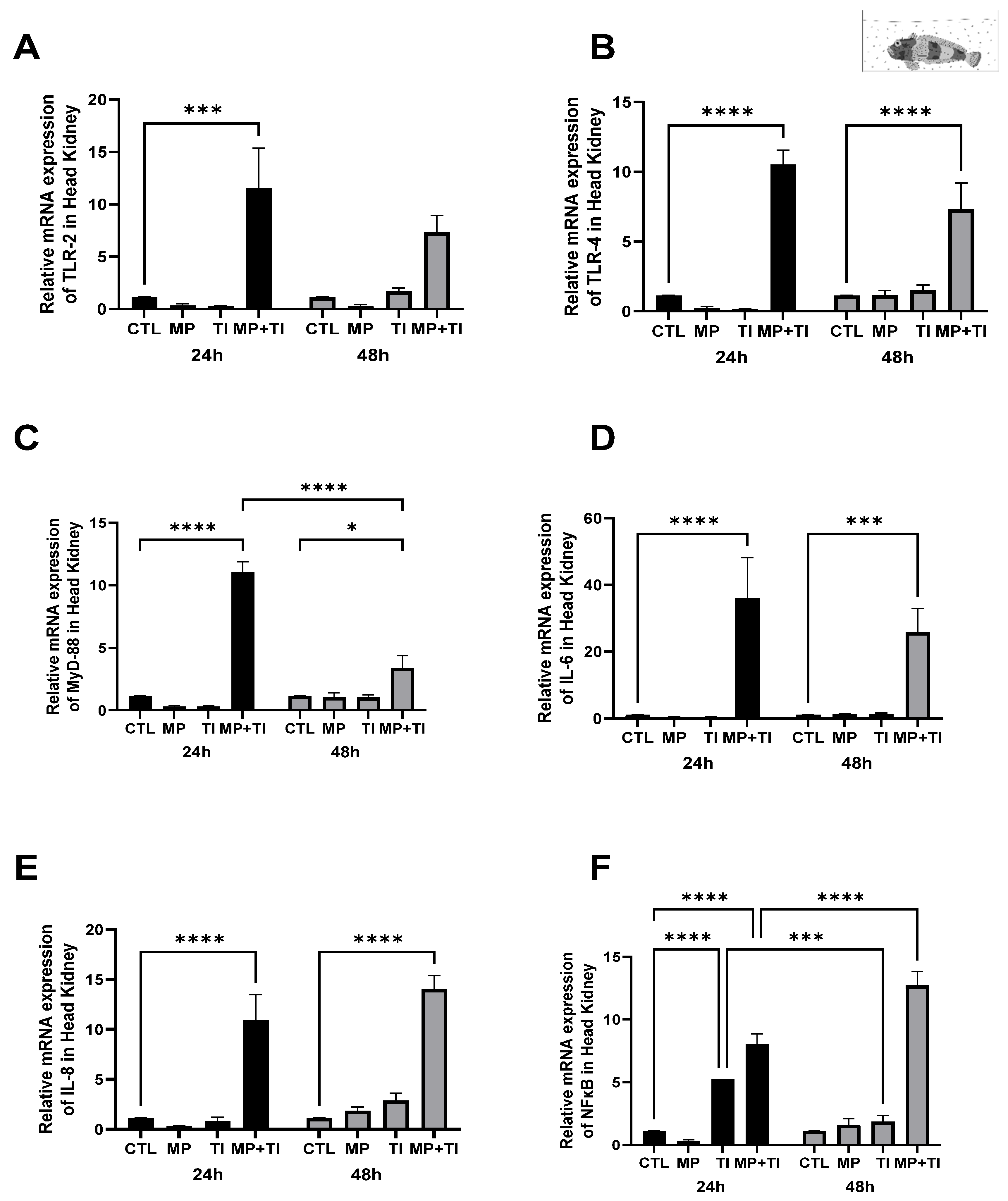
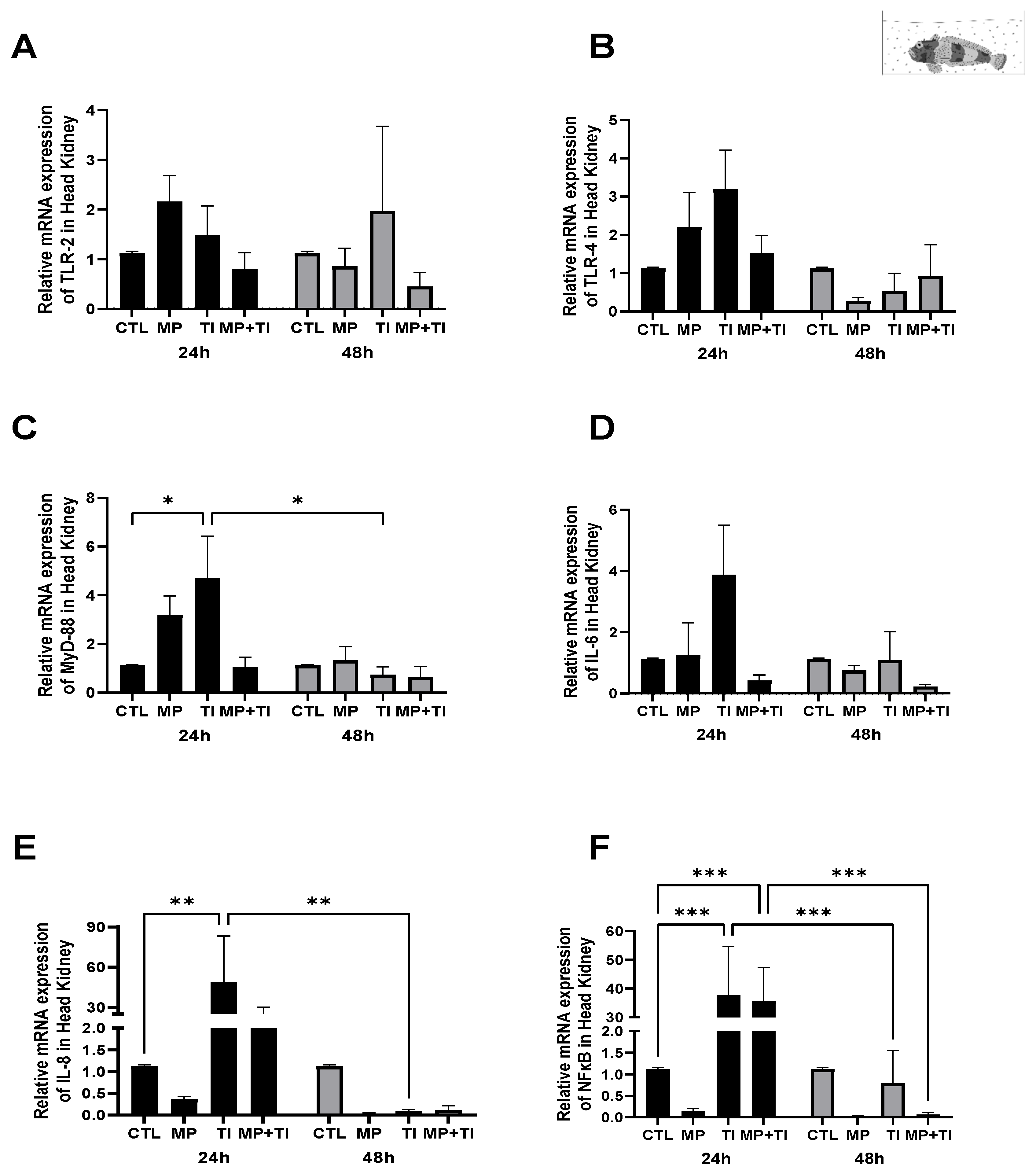
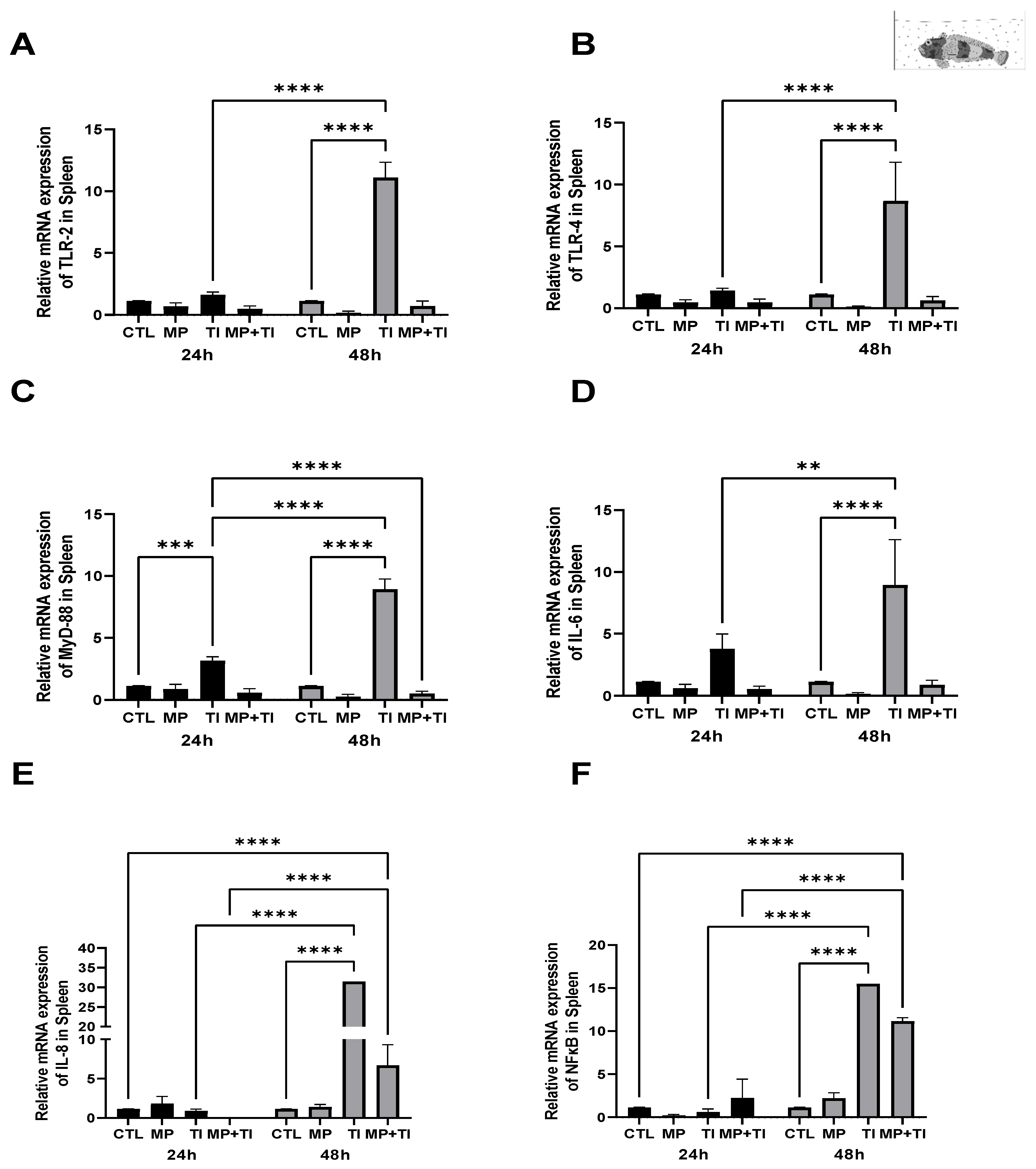
2. Results
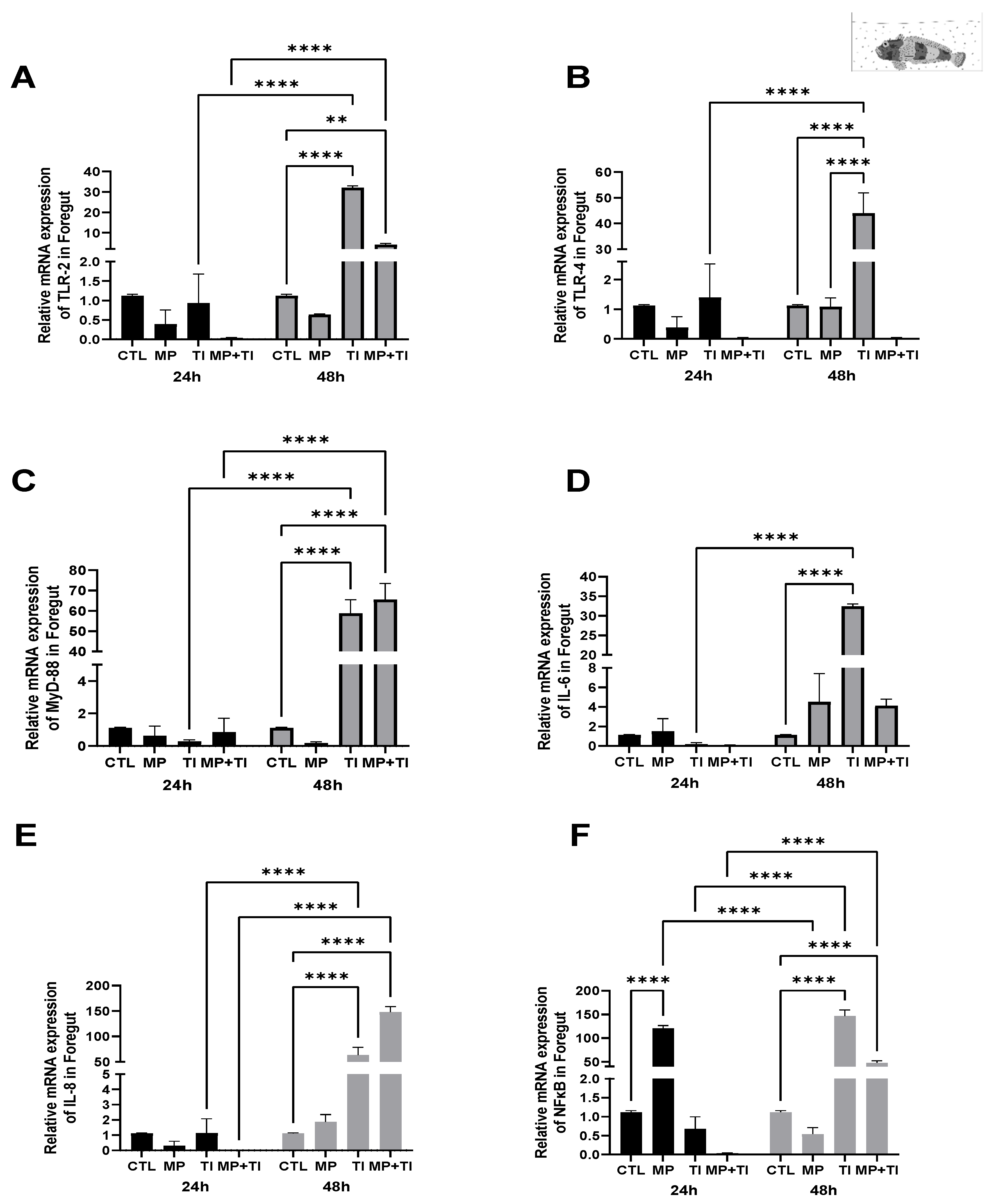
- (A)
- Oral (cannula) MP delivery groups
- (B)
- Exposure to waterborne MPs
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palacios, M. Macroalgas submareales de los canales interiores del Parque Nacional Bernardo O’Higgins (~49°–51° S), región de Magallanes, Chile. An. Inst. Patagon. 2018, 46, 41–50. [Google Scholar] [CrossRef]
- Hou, D.; Hong, M.; Wang, Y.; Dong, P.; Cheng, H.; Yan, H.; Yao, Z.; Li, D.; Wang, K.; Zhang, D. Assessing the risks of potential bacterial pathogens attaching to different microplastics during the summer–autumn period in a mariculture cage. Microorganisms 2021, 9, 1909. [Google Scholar] [CrossRef]
- Hinojosa, I.A.; Thiel, M. Floating marine debris in fjords, gulfs and channels of southern Chile. Mar. Pollut. Bull. 2009, 58, 341–350. [Google Scholar] [CrossRef]
- Jambeck, J.; Moss, E.; Dubey, B.; Arifin, Z.; Godfrey, L.; Hardesty, B.D.; Hendrawan, I.G.; Hien, T.T.; Liu, J.; Matlock, M.; et al. Leveraging multi-target strategies to address plastic pollution in the context of an already stressed ocean. In The Blue Compendium: From Knowledge to Action for a Sustainable Ocean Economy; Springer International Publishing: Cham, Switzerland, 2023; pp. 141–184. [Google Scholar]
- Jorquera, A.; Castillo, C.; Murillo, V.; Araya, J.; Pinochet, J.; Narváez, D.; Pantoja-Gutiérrez, S.; Urbina, M.A. Physical and anthropogenic drivers shaping the spatial distribution of microplastics in the marine sediments of Chilean fjords. Sci. Total Environ. 2022, 814, 152506. [Google Scholar] [PubMed]
- Lacerda, A.L.D.F.; Rodrigues, L.D.S.; van Sebille, E.; Rodrigues, F.L.; Ribeiro, L.; Secchi, E.R.; Kessler, F. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 2019, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Bessa, F.; Ratcliffe, N.; Otero, V.; Sobral, P.; Marques, J.C.; Waluda, C.M.; Trathan, P.N. Xavier Microplastics in gentoo penguins from the Antarctic region. Sci. Rep. 2019, 9, 14191. [Google Scholar] [CrossRef]
- Laura, W.J.; Elisa, B.; Emily, R.; Clara, M. Organic or junk food? Microplastic contamination in Antarctic krill and salps. R. Soc. Open Sci. 2023, 10, 221421. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, N.; Liu, W.; Guo, R.; Jin, H. Occurrence of microplastics in Antarctic fishes: Abundance, size, shape, and polymer composition. Sci. Total Environ. 2023, 903, 166186. [Google Scholar]
- Santillán, L.; Forero López, A.D.; Colombo, C.V.; Rimondino, G.N.; Malanca, F.E.; De-la-Torre, G.E. Microplastics in Antarctic penguins and seals in Admiralty Bay, King George Island, Antarctica. Arct. Antarct. Alp. Res. 2025, 57, 2507463. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Bo, J.; Zheng, R.; Hong, F.; Gao, F.; Miao, X.; Li, H.; Fang, C. First Evidence of Microplastic Contamination in Antarctic Fish (Actinopterygii, Perciformes). Water 2022, 14, 3070. [Google Scholar] [CrossRef]
- Ergas, M.; Figueroa, D.; Paschke, K.; Urbina, M.A.; Navarro, J.M.; Vargas-Chacoff, L. Cellulosic and microplastic fibers in the Antarctic fish Harpagifer antarcticus and Sub-Antarctic Harpagifer bispinis. Mar. Pollut. Bull. 2023, 194, 115380. [Google Scholar] [CrossRef]
- Mancuso, M.; Conti Nibali, V.; Porcino, N.; Branca, C.; Natale, S.; Smedile, F.; Azzaro, M.; D’Angelo, G.; Bottari, T. Monitoring of anthropogenic microplastic pollution in antarctic fish (Emerald rockcod) from the Terranova Bay after a quarter of century. Sci. Total Environ. 2023, 904, 167244. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Ryan, P.G.; Pichegru, L.; Perolod, V.; Moloney, C.L. Monitoring marine plastics-will we know if we are making a difference? S. Afr. J. Sci. 2020, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, L.; Li, D. Characterization of small plastic debris on tourism beaches around the South China Sea. Reg. Stud. Mar. Sci. 2015, 1, 55–62. [Google Scholar] [CrossRef]
- Lusher, A. Microplastics in the marine environment: Distribution, interactions and effects. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 245–307. [Google Scholar]
- Watts, A.J.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef]
- Nelms, S.E.; Clark, B.L.; Duncan, E.M.; Germanov, E.; Godley, B.J.; Parton, K.J.; Pham, C.K.; Rodríguez, Y. Plastic pollution and marine megafauna: Recent advances and future directions. In Plastic Pollution in the Global Ocean; World Scientific: Singapore, 2023; pp. 97–138. [Google Scholar] [CrossRef]
- Bakir, A.; Desender, M.; Wilkinson, T.; Van Hoytema, N.; Amos, R.; Airahui, S.; Jennifer Graham Maes, T. Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. Pollut. Bull. 2020, 160, 111572. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, H.J.; Marciano, K.; Downs, C.A. Migration of nonylphenol from food-grade plastic is toxic to the coral reef fish species Pseudochromis fridmani. Chemosphere 2015, 139, 223–228. [Google Scholar] [CrossRef]
- Hu, D.; Shen, M.; Zhang, Y.; Li, H.; Zeng, G. Microplastics and nanoplastics: Would they affect global biodiversity change? Environ. Sci. Pollut. Res. 2019, 26, 19997–20002. [Google Scholar] [CrossRef]
- Gallagher, A.; Rees, A.; Rowe, R.; Stevens, J.; Wright, P. Microplastics in the Solent estuarine complex, UK: An initial assessment. Mar. Pollut. Bull. 2016, 102, 243–249. [Google Scholar] [CrossRef]
- Meng, W.; Xing, B.; Cheng, S.; Nie, Y.; Zeng, H.; Qu, X.; Xu, B.; Zhang, C.; Yu, J.; Hong, S.W. Preparation of high quality carbon nanotubes by catalytic pyrolysis of waste plastics using FeNi-based catalyst. Waste Manag. 2024, 189, 11–22. [Google Scholar]
- Strungaru, S.A.; Jijie, R.; Nicoara, M.; Plavan, G.; Faggio, C. Micro-(nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. TrAC Trends Anal. Chem. 2019, 110, 116–128. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bulletin. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Kaloyianni, M.; Bobori, D.C.; Xanthopoulou, D.; Malioufa, G.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Kastrinaki, G.; Dimitriadi, A.; Koumoundouros, G.; et al. Toxicity and functional tissue responses of two freshwater fish after exposure to polystyrene microplastics. Toxics 2021, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Tvarijonaviciute, A.; Pereira, M.; Martins, M.; Tort, L.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics 2018, 110, 435–441. [Google Scholar] [CrossRef]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar]
- Sousa, C.S.V.; Power, D.M.; Guerreiro, P.M.; Louro, B.; Chen, L.; Canário, A.V.M. Transcriptomic Down-Regulation of Immune System Components in Barrier and Hematopoietic Tissues after Lipopolysaccharide Injection in Antarctic Notothenia coriiceps. Fishes 2022, 7, 171. [Google Scholar] [CrossRef]
- Sousa, C.S.V.; Peng, M.; Guerreiro, P.M.; Cardoso, J.C.R.; Chen, L.; Canário, A.V.M.; Power, D.M. Differential tissue immune stimulation through immersion in bacterial and viral agonists in the Antarctic Notothenia rossii. Fish Shellfish Immunol. 2024, 148, 109516. [Google Scholar] [CrossRef] [PubMed]
- Saravia, J.; Paschke, K.; Pontigo, J.P.; Nualart, D.; Navarro, J.M.; Vargas-Chacoff, L. Effects of temperature on the innate immune response on Antarctic and sub-Antarctic fish Harpagifer antarcticus and Harpagifer bispinis challenged with two immunostimulants, LPS and Poly I: C: In vivo and in vitro approach. Fish Shellfish Immunol. 2022, 130, 391–408. [Google Scholar] [CrossRef]
- Matzinger, P. Friendly and dangerous signals: Is the tissue in control? Nat. Immunol. 2007, 8, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Pradeu, T.; Pichlmair, A.; Wray, K.B.; Mikkelsen, J.G.; Olagnier, D.; Mogensen, T.H. Early host defense against virus infections. Cell Rep. 2024, 43, 115070. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Niepmann, S.; Knolle, P.A.; Hornung, V. Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J. Immunol. 2016, 197, 2900–2908. [Google Scholar] [CrossRef]
- Bilyk, K.T.; Vargas-Chacoff, L.; Cheng, C.H. Evolution in chronic cold: Varied loss of cellular response to heat in Antarctic notothenioid fish. BMC Evol. Biol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Sousa, C.; Fernandes, S.A.; Cardoso, J.C.; Wang, Y.; Zhai, W.; Guerreiro, P.M.; Chen, L.; Canário, A.V.M.; Power, D.M. Toll-like receptor evolution: Does temperature matter? Front. Immunol. 2022, 13, 812890. [Google Scholar] [CrossRef]
- Martínez, D.; De Lázaro, O.; Cortés, P.; Oyarzún-Salazar, R.; Paschke, K.; Vargas-Chacoff, L. Hypoxia modulates the transcriptional immunological response in Oncorhynchus kisutch. Fish Shellfish Immunol. 2022, 106, 1042–1051. [Google Scholar] [CrossRef]
- Sousa, C.R.; Yamasaki, S.; Brown, G.D. Myeloid C-type lectin receptors in innate immune recognition. Immunity 2024, 57, 700–717. [Google Scholar] [CrossRef]
- Urbina, M.; Luna-Jorquera, G.; Thiel, M.; Acuña-Ruz, T.; Cristi, M.A.; Andrade, C.; Ahrendt, C.; Castillo, C.; Chevallier, A.; Cornejo-D’Ottone, M.; et al. A country’s response to tackling plastic pollution in aquatic ecosystems: The Chilean way. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 31, 420–440. [Google Scholar] [CrossRef]
- Nualart, D.P.; Paschke, K.; Guerreiro, P.M.; McCormick, S.D.; González-Wevar, C.; Cheng, C.C.; Chacoff, L.V. Combined effects of PVC microplastics and thermal rise alter the oxidative stress response in Antarctic fish Harpagifer antarcticus and Sub-Antarctic Harpagifer bispinis. Mar. Pollut. Bull. 2025, 220, 18438. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Lloris, D.; Pequeño, G.; Rucabado, J.; Lamilla, J. El género Harpagifer Richardson, 1844, en el extremo sur de América (Pisces, Harpagiferidae). Ser. Cient. 1996, 46, 41–58. [Google Scholar]
- Eastman, J.T. Antarctic Fish Biology: Evolution in a Unique Environment; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Hüne, M.; González-Wevar, C.; Poulin, E.; Mansilla, A.; Fernández, D.A.; Barrera-Oro, E. Low level of genetic divergence between Harpagifer fish species (Perciformes: Notothenioidei) suggests a Quaternary colonization of Patagonia from the Antarctic Peninsula. Polar Biol. 2015, 38, 607–617. [Google Scholar] [CrossRef]
- Mohyuddin, S.G.; Khan, I.; Zada, A.; Qamar, A.; Arbab, A.A.I.; Ma, X.; Yu, Z.; Liu, X.; Yong, Y.; Ju, X.H.; et al. Influence of Heat Stress on Intestinal Epithelial Barrier Function, Tight Junction Protein, and Immune and Reproductive Physiology. BioMed Res. Int. 2022, 2022, 8547379. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhu, S.; Yang, H.; Cui, H.; Guo, H.; Deng, J.; Ren, Z.; Geng, Y.; Ouyang, P.; Xu, Z.; et al. The dysregulation of inflammatory pathways triggered by copper exposure. Biol. Trace Elem. Res. 2023, 201, 539–548. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, W.; Hu, F.; Song, X.; Cheng, Z.; Zhou, J. Prolonged oral ingestion of microplastics induced inflammation in the liver tissues of C57BL/6J mice through polarization of macrophages and increased infiltration of natural killer cells. Ecotoxicol. Environ. Saf. 2021, 227, 112882. [Google Scholar] [CrossRef]
- Espinosa, C.; Beltrán, J.M.G.; Esteban, M.A.; Cuesta, A. In vitro effects of virgin microplastics on fish head-kidney leucocyte activities. Environ. Pollut. 2018, 235, 30–38. [Google Scholar] [CrossRef]
- González-Fernández, C.; Cuesta, A. Nanoplastics Increase Fish Susceptibility to Nodavirus Infection and Reduce Antiviral Immune Responses. Int. J. Mol. Sci. 2022, 23, 1483. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 117–140. [Google Scholar]
- Ben-Hasan, A.; Vahabnezhad, A.; Burt, J.A.; Alrushaid, T.; Walters, C.J. Fishery implications of smaller asymptotic body size: Insights from fish in an extreme environment. Fish. Res. 2024, 271, 106918. [Google Scholar]
- Somero, G.N. Adaptation of enzymes to temperature: Searching for basic “strategies”. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 321–333. [Google Scholar]
- Dowd, W.W.; Kültz, D. Lost in translation? Evidence for a muted proteomic response to thermal stress in a stenothermal Antarctic fish and possible evolutionary mechanisms. Physiol. Genom. 2024, 56, 721–740. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Buckley, B.A.; Airaksinen, S.E.K.J.; Somero, G.N. Heat-shock protein expression is absent in the Antarctic fish Trematomus bernacchii (family Nototheniidae). J. Exp. Biol. 2000, 203, 2331–2339. [Google Scholar] [CrossRef]
- Bilyk, K.T.; Zhuang, X.; Vargas-Chacoff, L.; Cheng, C.H. Evolution of chaperome gene expression and regulatory elements in the antarctic notothenioid fishes. Heredity 2021, 126, 424–441. [Google Scholar] [CrossRef]
- Saravia, J.; Nualart, D.; Paschke, K.; Pontigo, J.P.; Navarro, J.M.; Vargas-Chacoff, L. Temperature and immune challenges modulate the transcription of genes of the ubiquitin and apoptosis pathways in two high-latitude Notothenioid fish across the Antarctic Polar Front. Fish Physiol. Biochem. 2024, 50, 1429–1443. [Google Scholar] [CrossRef]
- Campos-Sánchez, J.C.; Esteban, M.Á. Review of inflammation in fish and value of the zebrafish model. J. Fish Dis. 2020, 44, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Niklitschek, E.J.; Soto, D.; Lafon, A.; Molinet, C.; Toledo, P. Southward expansion of the Chilean salmon industry in the Patagonian Fjords: Main environmental challenges. Rev. Aquac. 2013, 5, 172–195. [Google Scholar] [CrossRef]
- Basu, M.; Paichha, M.; Swain, B.; Lenka, S.S.; Singh, S.; Chakrabarti, R.; Samanta, M. Modulation of TLR2, TLR4, TLR5, NOD1 and NOD2 receptor gene expressions and their downstream signaling molecules following thermal stress in the Indian major carp catla (Catla catla). 3 Biotech 2015, 5, 1021–1030. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.H.; Park, J.W.; Lee, J.H.; Kim, J.H. High water temperature-mediated immune gene expression of olive flounder, Paralichthys olivaceus according to pre-stimulation at high temperatures. Environ. Toxicol. Pharmacol. 2023, 101, 104159. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, H.; Zhou, Y.; You, Y.; Lei, D.; Li, Y.; Wang, Y. Aged fragmented-polypropylene microplastics induced ageing statues-dependent bioenergetic imbalance and reductive stress: In vivo and liver organoids-based in vitro study. Environ. Int. 2024, 191, 108949. [Google Scholar]
- Giménez, J.; Fiori, S.; Torroglosa, M.E.; Celentano, E.; Masello, A.; Defeo, O.; Lomovasky, B.J. Environmentally-driven variations in reproductive traits in a sandy beach bivalve throughout its geographic range. Estuar. Coast. Shelf Sci. 2024, 303, 108816. [Google Scholar] [CrossRef]
- Piersma, T.; Drent, J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003, 18, 228–233. [Google Scholar] [CrossRef]
- Petzel, D. Drinking in Antarctic fishes. Polar Biol. 2005, 28, 763–768. [Google Scholar] [CrossRef]
- Rodrigues, D.; Pequeno, J.; Pais, J.; Antunes, J.; Sobral, P.; Costa, M.H. Comparing microplastics ingested by the bogue Boops boops (Teleostei: Sparidae) to those available in its feeding areas: A case study on the Portuguese west coast. Reg. Stud. Mar. Sci. 2023, 65, 103093. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Arjona, F.J.; Ruiz-Jarabo, I.; García-Lopez, A.; Flik, G.; Mancera, J.M. Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J. Therm. Biol. 2020, 88, 102526. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Abelli, L.; Fossi, M.C.; Panti, C. Effects of microplastics on head-kidney gene expression and biochemical biomarkers in adult zebrafish. In MICRO2020 Fate and Impacts of Microplastics: Knowledge and Responsibilities; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Olabuenaga, S.E. Sistema inmune en peces. Gayana 2000, 64, 205–215. [Google Scholar] [CrossRef]
- Somero, G.N.; DeVries, A.L. Temperature tolerance of some Antarctic fishes. Science 1967, 156, 257–258. [Google Scholar] [CrossRef]
- Martínez, D.; Vargas-Lagos, C.; Oyarzún, R.; Loncoman, C.A.; Pontigo, J.P.; Yáñez, A.Y.; Vargas-Chacoff, L. Temperature modulates the immunological response of the sub-antarctic notothenioid fish Eleginops maclovinus injected with Piscirickettsia salmonis. Fish Shellfish Immunol. 2018, 82, 492–503. [Google Scholar] [PubMed]
- Navarro, J.M.; Paschke, K.; Ortiz, A.; Vargas-Chacoff, L.; Pardo, L.M.; Valdivia, N. The Antarctic fish Harpagifer antarcticus under current temperatures and salinities and future scenarios of climate change. Prog. Oceanogr. 2019, 174, 37–43. [Google Scholar] [CrossRef]
- Gunaalan, K.; Almeda, R.; Lorenz, C.; Vianello, A.; Iordachescu, L.; Papacharalampos, K.; Kiær, C.M.R.; Vollertsen, J.; Nielsen, T.G. Abundance and distribution of microplastics in surface waters of the Kattegat/Skagerrak (Denmark). Environ. Pollut. 2022, 318, 120853. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Chacoff, L.; Martínez, D.; Oyarzún-Salazar, R.; Paschke, K.; Navarro, J.M. The osmotic response capacity of the Antarctic fish Harpagifer antarcticus is insufficient to cope with projected temperature and salinity under climate change. J. Therm. Biol. 2021, 96, 102835. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [PubMed]



| Primer | Nucleotide Sequences (5′→3′) | Efficiency (%) Head Kidney | Efficiency (%) Spleen | Efficiency (%) Foregut | Reference |
|---|---|---|---|---|---|
| Toll-like receptor-2 Fw | ATGGAGCTGTTGACCAACCT | 102.9 | 102.5 | 102.3 | Saravia et al. (2022) [35] |
| Toll-like receptor-2 Rv | GTCTGTTACCGTGGGAACAAG | ||||
| Toll-like receptor-4 Fw | CGTGCTCTTCCCTACATGCT | 101.6 | 101.7 | 101.8 | Saravia et al. (2022) [35] |
| Toll-like receptor-4 Rv | GCTGTTGGGCTGTGATGTCT | ||||
| Myeloid differentiation factor 88 Fw | ACTTCCCAAAACATGGCGTG | 100.1 | 100.2 | 100.3 | (Martínez et al., 2018) [75] |
| Myeloid differentiation factor 88 Rv | ACCGTGTTCTTCGGGTTCAG | ||||
| Nuclear factor-κB Fw | GAAGAAGATGGCGGGAGCTA | 105.5 | 102.6 | 101.7 | Saravia et al. (2022) [35] |
| Nuclear factor-κB Rv | TGATGTCGACTGGAGGAATGTAG | ||||
| Interleukin-6 Fw | TTCTCAGGCAAGTGGAGAAGGAGT | 102.9 | 101.1 | 101.5 | Saravia et al. (2022) [35] |
| Interleukin-6 Rv | CACCTGACCAGGGTTCCTCATTTT | ||||
| Interleukin-8 Fw | GTGAAGGGATGAGTCTGAGAAGTC | 102.9 | 101.1 | 101.5 | Saravia et al. (2022) [35] |
| Interleukin-8 Rv | TCACAGTGGGAGTTGGCAGAA | ||||
| β-actin Fw | AGGTCATCACCATCGGAAACGA | 102.11 | 101.1 | 101.5 | Saravia et al. (2022) [35] |
| β-actin Rv | ACAGCACGGTGTTGGCGTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nualart, D.P.; Guerreiro, P.M.; Paschke, K.; McCormick, S.D.; Cheng, C.-H.C.; Vargas-Chacoff, L. The Immune System in Antarctic and Subantarctic Fish of the Genus Harpagifer Is Affected by the Effects of Combined Microplastics and Thermal Increase. Int. J. Mol. Sci. 2025, 26, 9968. https://doi.org/10.3390/ijms26209968
Nualart DP, Guerreiro PM, Paschke K, McCormick SD, Cheng C-HC, Vargas-Chacoff L. The Immune System in Antarctic and Subantarctic Fish of the Genus Harpagifer Is Affected by the Effects of Combined Microplastics and Thermal Increase. International Journal of Molecular Sciences. 2025; 26(20):9968. https://doi.org/10.3390/ijms26209968
Chicago/Turabian StyleNualart, Daniela P., Pedro M. Guerreiro, Kurt Paschke, Stephen D. McCormick, Chi-Hing Christina Cheng, and Luis Vargas-Chacoff. 2025. "The Immune System in Antarctic and Subantarctic Fish of the Genus Harpagifer Is Affected by the Effects of Combined Microplastics and Thermal Increase" International Journal of Molecular Sciences 26, no. 20: 9968. https://doi.org/10.3390/ijms26209968
APA StyleNualart, D. P., Guerreiro, P. M., Paschke, K., McCormick, S. D., Cheng, C.-H. C., & Vargas-Chacoff, L. (2025). The Immune System in Antarctic and Subantarctic Fish of the Genus Harpagifer Is Affected by the Effects of Combined Microplastics and Thermal Increase. International Journal of Molecular Sciences, 26(20), 9968. https://doi.org/10.3390/ijms26209968






