Prediction of Hyperinflammatory Phenotypes in Critically Ill Patients via Routine Clinical Data and IL-6: Towards Personalized Anti-Inflammatory Therapy
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics, Survival Analysis, and Multivariate Analysis on Mortality
2.2. Association of IL-6 Plasma Levels with Clinical Variables and Neutropenia
2.3. Bayesian Logistic Regression and Analysis of Inflammatory Phenotypes
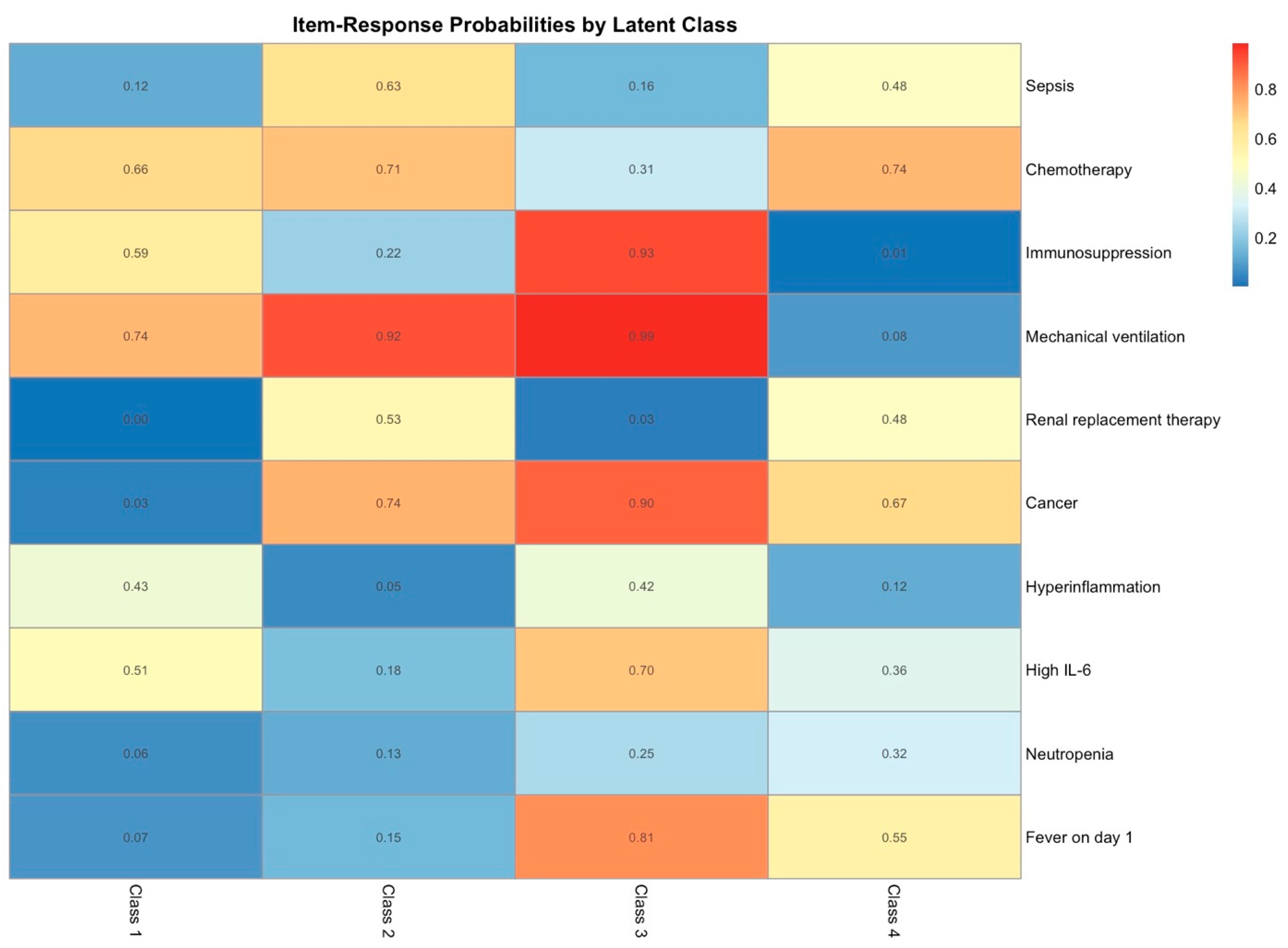
2.4. Latent Class Analysis
3. Discussion
3.1. Study Rationale
3.2. Cohort Characteristics and Initial Inflammatory Observations
3.3. Neutropenia as a Driver of IL-6 Dysregulation and Distinct Outcomes
3.4. IL-6 Prognostic Limitations and the Need for Multi-Cytokine Approaches
3.5. Uncovering Hyperinflammatory Phenotypes and Clinical Subgroups
3.6. Translational Implications for Targeted Anti-Cytokine Therapy
3.7. Limitations, Unresolved Mechanisms, and Future Validation Needs
4. Materials and Methods
4.1. Patient Population and Study Design
4.2. IL-6 Measurement and Quality Control
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| ANOVA | Analysis of variance |
| ARDS | Acute respiratory distress syndrome |
| aBIC | (Sample size–) adjusted Bayesian information criterion |
| BIC | Bayesian information criterion |
| CAR | Chimeric antigen receptor |
| CI | Confidence interval |
| CRP | C-reactive protein |
| CRS | Cytokine release syndrome |
| DNN | Deep Neural Network |
| HLH | Hemophagocytic lymphohistiocytosis |
| HSCT | Hematopoietic stem cell transplantation |
| ICU | Intensive care unit |
| IL-6 | Interleukin-6 |
| IQR | Interquartile range |
| LCA | Latent class analysis |
| LOS | Length of stay |
| LRT | Likelihood ratio test |
| MICU | Medical intensive care unit |
| Npar | Number of estimated parameters |
| OR | Odds ratio |
| PCT | Procalcitonin |
| SAPS | Simplified Acute Physiology Score II |
| SD | Standard deviation |
| SLE | Systemic lupus erythematosus |
| TISS | Therapeutic Intervention Scoring System |
| XGB | eXtreme Gradient Boosting |
References
- Rousseau, A.-F.; Martindale, R. Nutritional and metabolic modulation of inflammation in critically ill patients: A narrative review of rationale, evidence and grey areas. Ann. Intensive Care 2024, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Kotch, C.; Barrett, D.; Teachey, D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert. Rev. Clin. Immunol. 2019, 15, 813–822. [Google Scholar] [CrossRef]
- Vilbert, M.; Hein, E.C.K.; Madeira, T.; Michelon, I.; Priantti, J.N.; Lobo, M.; Dacoregio, M.I.; Castro, C.; Cavalcante, L.; Wu, C.-Y.; et al. Safety and efficacy of interleukin-6 blockade for immune-related adverse events: A systematic review and meta-analysis. J. Clin. Oncol. 2024, 42, e24149. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Burrell, A.; Boyle, A.J.; Gordon, A.C.; McAuley, D.F.; Silversides, J. Sepsis subphenotypes, theragnostics and personalized sepsis care. Intensive Care Med. 2025, 51, 756–768. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Daud, M.; Khan, M.B.; Qudrat, Q.U.; Ullah, I.; Khan, S.; Khan, M.Z.; Yousuf, I.; Ahmad, F. Role of C-reactive Protein and Procalcitonin in Early Diagnostic Accuracy and Their Prognostic Significance in Sepsis. Cureus 2024, 16, e70358. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Syed Khaja, A.S.; Binsaleh, N.K.; Beg, M.M.A.; Ashfaq, F.; Khan, M.I.; Almutairi, M.G.; Qanash, H.; Saleem, M.; Ginawi, I.A.M. Clinical importance of cytokine (IL-6, IL-8, and IL-10) and vitamin D levels among patients with Type-1 diabetes. Sci. Rep. 2024, 14, 24225. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Umur, E.; Bulut, S.B.; Yiğit, P.; Bayrak, E.; Arkan, Y.; Arslan, F.; Baysoy, E.; Kaleli-Can, G.; Ayan, B. Exploring the Role of Hormones and Cytokines in Osteoporosis Development. Biomedicines 2024, 12, 1830. [Google Scholar] [CrossRef]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.d.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Soler, M.F.; Abaurrea, A.; Azcoaga, P.; Araujo, A.M.; Caffarel, M.M. New perspectives in cancer immunotherapy: Targeting IL-6 cytokine family. J. Immunother. Cancer 2023, 11, e007530. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Mehmi, I.; Goldberg, J.D.; Dimitrova, M.; Arriola, P.; Puranik, A.; Mizutani, T.; Idga, S.; Li, X.; Levinson, B.A.; et al. A phase II study of the interleukin-6 (IL-6) receptor blocking antibody sarilumab (Sari) in combination with ipilimumab (Ipi), nivolumab (Nivo) and relatlimab (Rela) in patients with unresectable stage III or stage IV melanoma. J. Clin. Oncol. 2025, 43, 9510. [Google Scholar] [CrossRef]
- Chertow, G.M.; Chang, A.M.; Felker, G.M.; Heise, M.; Velkoska, E.; Fellström, B.; Charytan, D.M.; Clementi, R.; Gibson, C.M.; Goodman, S.G.; et al. IL-6 inhibition with clazakizumab in patients receiving maintenance dialysis: A randomized phase 2b trial. Nat. Med. 2024, 30, 2328–2336, Correction in: Nat. Med. 2024, 30, 2373. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D. IL-6 Blockade in Cytokine Storm Syndromes. Adv. Exp. Med. Biol. 2024, 1448, 565–572. [Google Scholar]
- Brunkhorst, F.M.; Adamzik, M.; Axer, H.; Bauer, M.; Bode, C.; Bone, H.-G.; Brenner, T.; Bucher, M.; David, S.; Dietrich, M.; et al. S3-Leitlinie Sepsis—Prävention, Diagnose, Therapie und Nachsorge—Update 2025. Med. Klin. Intensiv. 2025. [Google Scholar] [CrossRef]
- Barre, M.; Behnes, M.; Hamed, S.; Pauly, D.; Lepiorz, D.; Lang, S.; Akin, I.; Borggrefe, M.; Bertsch, T.; Hoffmann, U. Revisiting the prognostic value of monocyte chemotactic protein 1 and interleukin-6 in the sepsis-3 era. J. Crit. Care 2018, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Spittler, A.; Razenberger, M.; Kupper, H.; Kaul, M.; Hackl, W.; Boltz-Nitulescu, G.; Függer, R.; Roth, E. Relationship between interleukin-6 plasma concentration in patients with sepsis, monocyte phenotype, monocyte phagocytic properties, and cytokine production. Clin. Infect. Dis. 2000, 31, 1338–1342. [Google Scholar] [CrossRef]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L. Impact of interleukin 6 levels on acute lung injury risk and disease severity in critically ill sepsis patients. World J. Clin. Cases 2024, 12, 5374–5381. [Google Scholar] [CrossRef]
- Molano Franco, D.; Arevalo-Rodriguez, I.; Roqué, I.F.M.; Montero Oleas, N.G.; Nuvials, X.; Zamora, J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst. Rev. 2019, 4, Cd011811. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.J.; Crooks, C.; Naja, M.; Ledlie, A.; Goulden, B.; Liddle, T.; Khan, E.; Mehta, P.; Martin-Gutierrez, L.; Waddington, K.E.; et al. COVID-19-associated hyperinflammation and escalation of patient care: A retrospective longitudinal cohort study. Lancet Rheumatol. 2020, 2, e594–e602. [Google Scholar] [CrossRef] [PubMed]
- Opoka-Winiarska, V.; Grywalska, E.; Roliński, J. Could hemophagocytic lymphohistiocytosis be the core issue of severe COVID-19 cases? BMC Med. 2020, 18, 214. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ruiz-Botella, M.; Martín-Loeches, I.; Jimenez Herrera, M.; Solé-Violan, J.; Gómez, J.; Bodí, M.; Trefler, S.; Papiol, E.; Díaz, E.; et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit. Care 2021, 25, 63. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355, Erratum in: JAMA Intern. Med. 2021, 181, 1021. [Google Scholar] [CrossRef]
- Kalın, B.S.; Özçaylak, S.; Solmaz, İ.; Kılıç, J. Assessment of Risk Factors for Mortality in Patients in Medical Intensive Care Unit of a Tertiary Hospital. Indian J. Crit. Care Med. 2022, 26, 49–52. [Google Scholar] [CrossRef]
- Wei, M.; Huang, M.; Duan, Y.; Wang, D.; Xing, X.; Quan, R.; Zhang, G.; Liu, K.; Zhu, B.; Ye, Y.; et al. Prognostic and risk factor analysis of cancer patients after unplanned ICU admission: A real-world multicenter study. Sci. Rep. 2023, 13, 22340. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ren, Y. Prognostic value of SAPS II score for 28-day mortality in ICU patients with acute pulmonary embolism. Int. J. Cardiol. 2025, 430, 133201. [Google Scholar] [CrossRef]
- Basiri, R.; Ahmadi Hekmatikar, H.; Najafzadeh, M.J.; Baniasad, A. Evaluation of the performance of disease severity indices (SOFA, SAPS III, and MPM II) for the prediction of mortality rate in COVID-19 patients admitted to the intensive care units: A retrospective cross-sectional study. BMC Infect. Dis. 2025, 25, 637. [Google Scholar] [CrossRef]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.P.; Anderson, B.J.; Hudock, K.M.; Dunn, T.G.; Kazi, A.; Tommasini, A.; Charles, D.; Shashaty, M.G.S.; Mikkelsen, M.E.; Christie, J.D.; et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: A cohort study of severe sepsis. Crit. Care 2016, 20, 222. [Google Scholar] [CrossRef] [PubMed]
- Omert, L.; Tsukada, K.; Hierholzer, C.; Lyons, V.A.; Carlos, T.M.; Peitzman, A.B.; Billiar, T.R. A role of neutrophils in the down-regulation of IL-6 and CD14 following hemorrhagic shock. Shock 1998, 9, 391–396. [Google Scholar] [CrossRef]
- Kopf, M.; Baumann, H.; Freer, G.; Freudenberg, M.; Lamers, M.; Kishimoto, T.; Zinkernagel, R.; Bluethmann, H.; Köhler, G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 1994, 368, 339–342. [Google Scholar]
- Wang, X.S.; Williams, L.A.; Krishnan, S.; Liao, Z.; Liu, P.; Mao, L.; Shi, Q.; Mobley, G.M.; Woodruff, J.F.; Cleeland, C.S. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav. Immun. 2012, 26, 699–705. [Google Scholar]
- Weymann, K.B.; Wood, L.J.; Zhu, X.; Marks, D.L. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav. Immun. 2014, 37, 84–94. [Google Scholar]
- Wood, L.J.; Nail, L.M.; Perrin, N.A.; Elsea, C.R.; Fischer, A.; Druker, B.J. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol. Res. Nurs. 2006, 8, 157–169. [Google Scholar] [CrossRef]
- Ammirati, E.; Cannistraci, C.V.; Cristell, N.A.; Vecchio, V.; Palini, A.G.; Tornvall, P.; Paganoni, A.M.; Miendlarzewska, E.A.; Sangalli, L.M.; Monello, A.; et al. Identification and Predictive Value of Interleukin-6+ Interleukin-10+ and Interleukin-6− Interleukin-10+ Cytokine Patterns in ST-Elevation Acute Myocardial Infarction. Circ. Res. 2012, 111, 1336–1348. [Google Scholar] [CrossRef]
- Araújo, R.; Von Rekowski, C.P.; Fonseca, T.A.H.; Calado, C.R.C.; Ramalhete, L.; Bento, L. Multiplex Targeted Proteomic Analysis of Cytokine Ratios for ICU Mortality in Severe COVID-19. Proteomes 2025, 13, 35. [Google Scholar] [CrossRef]
- Andrijevic, I.; Matijasevic, J.; Andrijevic, L.; Kovacevic, T.; Zaric, B. Interleukin-6 and procalcitonin as biomarkers in mortality prediction of hospitalized patients with community acquired pneumonia. Ann. Thorac. Med. 2014, 9, 162–167. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Shi, S.; Xiao, J. Association between IL-6 and severe disease and mortality in COVID-19 disease: A systematic review and meta-analysis. Postgrad. Med. J. 2022, 98, 871–879. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Li, L.; Lai, T.; Peng, H.; Gui, L.; He, W. Interleukin-6 is better than C-reactive protein for the prediction of infected pancreatic necrosis and mortality in patients with acute pancreatitis. Front. Cell. Infect. Microbiol. 2022, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Swaroopa, D.; Bhaskar, K.; Mahathi, T.; Katkam, S.; Raju, Y.S.; Chandra, N.; Kutala, V.K. Association of serum interleukin-6, interleukin-8, and Acute Physiology and Chronic Health Evaluation II score with clinical outcome in patients with acute respiratory distress syndrome. Indian J. Crit. Care Med. 2016, 20, 518–525. [Google Scholar] [CrossRef]
- Luporini, R.L.; Rodolpho, J.M.d.A.; Kubota, L.T.; Martin, A.C.B.M.; Cominetti, M.R.; Anibal, F.d.F.; Pott-Junior, H. IL-6 and IL-10 are associated with disease severity and higher comorbidity in adults with COVID-19. Cytokine 2021, 143, 155507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Cao, L.; Wu, T.; Zhang, Z.; Han, M.; Liu, H.; Huang, S.; Bai, Z.; Wu, S. The evaluation of cytokines in predicting the organ injury of critically pediatric patients: A retrospective study. Transl. Pediatr. 2024, 13, 1169–1178. [Google Scholar] [CrossRef]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.A.; Samara, O.; Monzon, A.; Montalbano, G.; Scherger, S.; DeSanto, K.; Chastain, D.B.; Sillau, S.; Montoya, J.G.; Franco-Paredes, C.; et al. Proinflammatory cytokines levels in sepsis and healthy volunteers, and tumor necrosis factor-alpha associated sepsis mortality: A systematic review and meta-analysis. Cytokine 2022, 158, 156006. [Google Scholar] [CrossRef]
- dos Santos Medeiros, S.M.d.F.R.; Sousa Lino, B.M.N.; Perez, V.P.; Sousa, E.S.S.; Campana, E.H.; Miyajima, F.; Carvalho-Silva, W.H.V.; Dejani, N.N.; de Sousa Fernandes, M.S.; Yagin, F.H.; et al. Predictive biomarkers of mortality in patients with severe COVID-19 hospitalized in intensive care unit. Front. Immunol. 2024, 15, 2024. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.J.; Botero, L.E.; Isaza, J.P.; Cano, L.E.; López, L.; Hoyos, L.M.; Correa, E.; Torres, A. Cytokine levels as predictors of mortality in critically ill patients with severe COVID-19 pneumonia: Case-control study nested within a cohort in Colombia. Front. Med. 2022, 9, 1005636. [Google Scholar] [CrossRef]
- Azaiz, M.B.; Jemaa, A.B.; Sellami, W.; Romdhani, C.; Ouslati, R.; Gharsallah, H.; Ghazouani, E.; Ferjani, M. Deciphering the balance of IL-6/IL-10 cytokines in severe to critical COVID-19 patients. Immunobiology 2022, 227, 152236. [Google Scholar] [CrossRef]
- Tsurumi, A.; Que, Y.A.; Ryan, C.M.; Tompkins, R.G.; Rahme, L.G. TNF-α/IL-10 Ratio Correlates with Burn Severity and May Serve as a Risk Predictor of Increased Susceptibility to Infections. Front. Public Health 2016, 4, 216. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, S.; Ahmad, M.K.; Singh, R.; Pradhan, A.; Chandra, S.; Kumar, S. TNF-α/IL-10 ratio: An independent predictor for coronary artery disease in North Indian population. Diabetes Metab. Syndr. 2018, 12, 221–225. [Google Scholar] [CrossRef]
- Olvera, O.I.A.; Silva, J.A.V.; López, G.L.A.; Gomez, G.A.A.; Velázquez, G.L.; Chaves, J.C.M. Prognostic value of IL-6/lymphocyte ratio to predict mortality in severe pneumonia in an intensive care unit of a northeast hospital in Mexico. J. Crit. Care 2024, 81, 154729. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Pang, X.; Huang, Y.; Yang, B.; Yang, Y.; Chen, K.; Liu, X.; Mao, P.; Li, Y. Interleukin-10/lymphocyte ratio predicts mortality in severe septic patients. PLoS ONE 2017, 12, e0179050. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.S.; Das, B.K.; Tripathy, R.; Prusty, B.K.; Parida, M.K.; Tripathy, S.R.; Panda, A.K.; Ravindran, B.; Mukherjee, R. Machine learning identifies cytokine signatures of disease severity and autoantibody profiles in systemic lupus erythematosus—A pilot study. Sci. Rep. 2024, 14, 28765. [Google Scholar] [CrossRef]
- Lee, N.; Jeon, K.; Park, M.J.; Song, W.; Jeong, S. Predicting survival in patients with SARS-CoV-2 based on cytokines and soluble immune checkpoint regulators. Front. Cell. Infect. Microbiol. 2024, 14, 1397297. [Google Scholar] [CrossRef]
- Araújo, R.; Ramalhete, L.; Von Rekowski, C.P.; Fonseca, T.A.H.; Calado, C.R.C.; Bento, L. Cytokine-Based Insights into Bloodstream Infections and Bacterial Gram Typing in ICU COVID-19 Patients. Metabolites 2025, 15, 204. [Google Scholar] [CrossRef]
- Mi, Y.; Burnham, K.L.; Charles, P.D.; Heilig, R.; Vendrell, I.; Whalley, J.; Torrance, H.D.; Antcliffe, D.B.; May, S.M.; Neville, M.J.; et al. High-throughput mass spectrometry maps the sepsis plasma proteome and differences in patient response. Sci. Transl. Med. 2024, 16, eadh0185. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Mi, Y.; Santhakumaran, S.; Burnham, K.L.; Prevost, A.T.; Ward, J.K.; Marshall, T.J.; Bradley, C.; Al-Beidh, F.; Hutton, P.; et al. Patient stratification using plasma cytokines and their regulators in sepsis: Relationship to outcomes, treatment effect and leucocyte transcriptomic subphenotypes. Thorax 2024, 79, 515. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Thair, S.; Hsu, J.L.; Walley, K.R.; Russell, J.A.; Boyd, J. Cytokines and Signaling Molecules Predict Clinical Outcomes in Sepsis. PLoS ONE 2013, 8, e79207. [Google Scholar]
- Sinha, P.; Kerchberger, V.E.; Willmore, A.; Chambers, J.; Zhuo, H.; Abbott, J.; Jones, C.; Wickersham, N.; Wu, N.; Neyton, L.; et al. Identifying molecular phenotypes in sepsis: An analysis of two prospective observational cohorts and secondary analysis of two randomised controlled trials. Lancet Respir. Med. 2023, 11, 965–974. [Google Scholar] [CrossRef]
- Reddy, K.; O’Kane, C.M.; Antcliffe, D.B.; McDowell, C.; Bradley, P.A.; Black, L.; Murphy, L.; Shankar-Hari, M.; Gordon, A.C.; Shyamsundar, M.; et al. Inflammatory Phenotypes Can Be Prospectively Identified at the Bedside in Patients With the Acute Respiratory Distress Syndrome; Results From a Multicenter, Prospective, Observational Cohort Study. Am. J. Respir. Crit. Care Med. 2025, 211, A3130. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Sinha, P.; Delucchi, K.L.; Thompson, B.T.; McAuley, D.F.; Matthay, M.A.; Calfee, C.S.; for the NHLBI ARDS Network. Latent class analysis of ARDS subphenotypes: A secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care. Med. 2018, 44, 1859–1869. [Google Scholar] [PubMed]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am. J. Respir. Crit. Care. Med. 2017, 195, 331–338, Erratum in: Am. J. Respir. Crit. Care. Med. 2018, 198, 1590 and Am. J. Respir. Crit. Care. Med. 2019, 200, 649. [Google Scholar] [CrossRef]
- Sinha, P.; Delucchi, K.L.; Chen, Y.; Zhuo, H.; Abbott, J.; Wang, C.; Wickersham, N.; McNeil, J.B.; Jauregui, A.; Ke, S.; et al. Latent class analysis-derived subphenotypes are generalisable to observational cohorts of acute respiratory distress syndrome: A prospective study. Thorax 2022, 77, 13. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Furfaro, D.; Cummings, M.J.; Abrams, D.; Delucchi, K.; Maddali, M.V.; He, J.; Thompson, A.; Murn, M.; Fountain, J.; et al. Latent Class Analysis Reveals COVID-19-related Acute Respiratory Distress Syndrome Subgroups with Differential Responses to Corticosteroids. Am. J. Respir. Crit. Care. Med. 2021, 204, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, M.B. Management of neutropenia in cancer patients. Clin. Adv. Hematol. Oncol. 2012, 10, 825–826. [Google Scholar]
- DeMerle, K.M.; Kennedy, J.N.; Chang, C.H.; Delucchi, K.; Huang, D.T.; Kravitz, M.S.; Shapiro, N.I.; Yealy, D.M.; Angus, D.C.; Calfee, C.S.; et al. Identification of a hyperinflammatory sepsis phenotype using protein biomarker and clinical data in the ProCESS randomized trial. Sci. Rep. 2024, 14, 6234. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- MacGregor, F.; Oprey, A.; Caulfield, C.; MacTavish, P.; Lowrie, R.; Henderson, P. Does timing of tocilizumab administration affect mortality in COVID-19? A Scottish multicentre retrospective cohort study. BMJ Open Respir. Res. 2024, 11, e002264. [Google Scholar] [CrossRef]
- Qiu, X.; Nair, M.G.; Jaroszewski, L.; Godzik, A. Deciphering Abnormal Platelet Subpopulations in COVID-19, Sepsis and Systemic Lupus Erythematosus through Machine Learning and Single-Cell Transcriptomics. Int. J. Mol. Sci. 2024, 25, 5941. [Google Scholar] [CrossRef]
- Webb, C.E.; Vautrinot, J.; Hers, I. IL-6 as a Mediator of Platelet Hyper-Responsiveness. Cells 2025, 14, 766. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Li, Y.; Wang, Z.; Ma, F.; Luo, R.; Xu, X.; Zhou, G.; Wang, J.; Niu, J.; et al. Platelets mediate inflammatory monocyte activation by SARS-CoV-2 spike protein. J. Clin. Invest. 2022, 132, e150101. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care. Med. 2017, 43, 304–377. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.A.; El-Mekkawy, M.S.; Shehata, A.M.; Whdan, N.A. Clinical study of serum interleukin-6 in children with community-acquired pneumonia. Egypt J. Pediatr. 2018, 66, 43–48. [Google Scholar]
- Geppert, A.; Dorninger, A.; Delle-Karth, G.; Zorn, G.; Heinz, G.; Huber, K. Plasma concentrations of interleukin-6, organ failure, vasopressor support, and successful coronary revascularization in predicting 30-day mortality of patients with cardiogenic shock complicating acute myocardial infarction*. Crit. Care. Med. 2006, 34, 2035–2042. [Google Scholar]
- Geppert, A.; Steiner, A.; Zorn, G.; Delle-Karth, G.; Koreny, M.; Haumer, M.; Siostrzonek, P.; Huber, K.; Heinz, G. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit. Care Med. 2002, 30, 1987–1994. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Harwood, P.J.; Loughenbury, P.; Van Griensven, M.; Krettek, C.; Pape, H.C. Correlation between IL-6 levels and the systemic inflammatory response score: Can an IL-6 cutoff predict a SIRS state? J. Trauma 2008, 65, 646–652. [Google Scholar]
- Lazarsfeld, P.F.; Henry, N.W. Latent Structure Analysis; Houghton Mifflin: Boston, MA, USA, 1968. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Sclove, S.L. Application of Model-Selection Criteria to Some Problems in Multivariate Analysis. Psychometrika 1987, 52, 333–343. [Google Scholar] [CrossRef]
- Celeux, G.; Soromenho, G. An entropy criterion for assessing the number of clusters in a mixture model. J. Classif. 1996, 13, 195–212. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 8 October 2025).

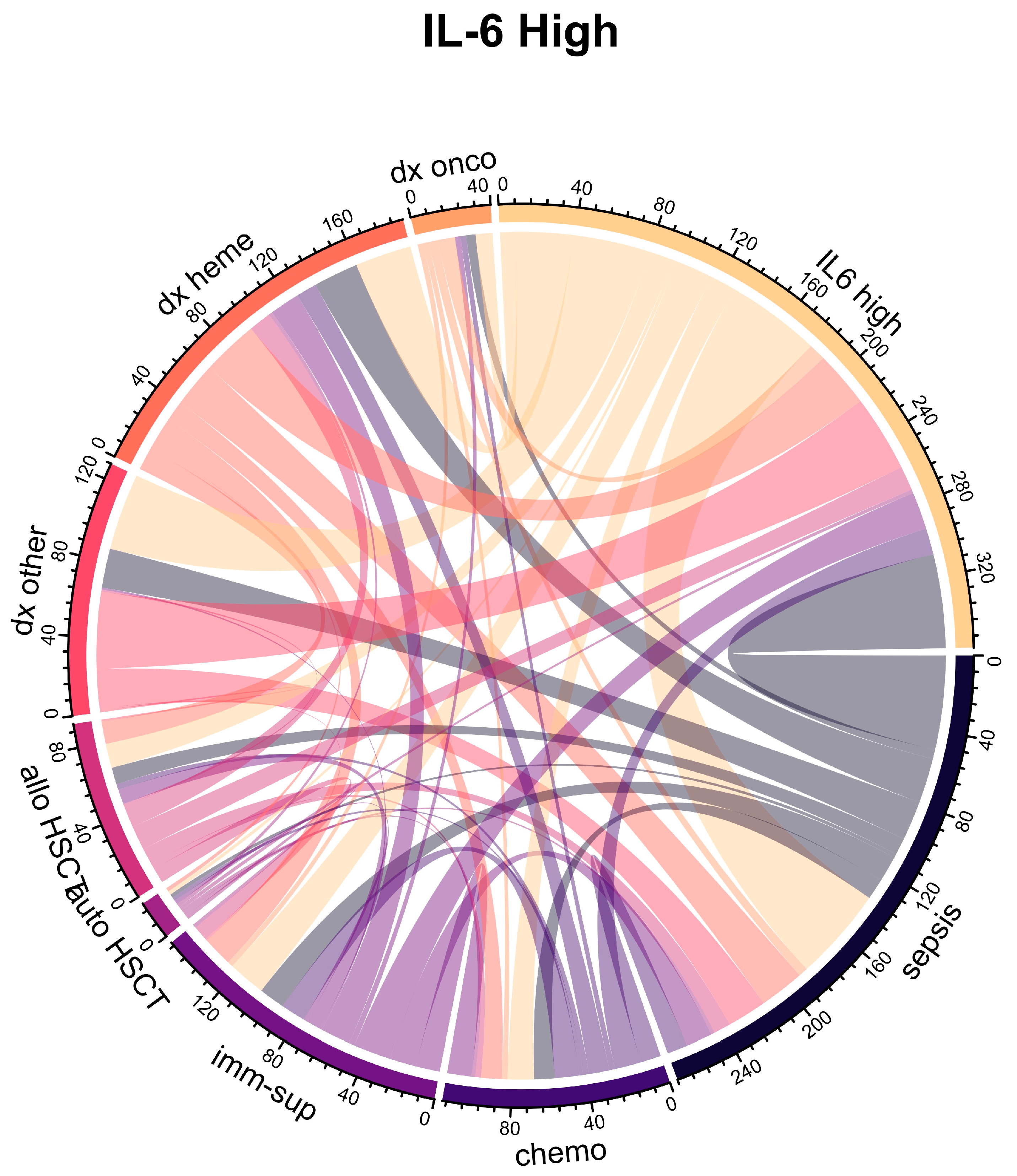
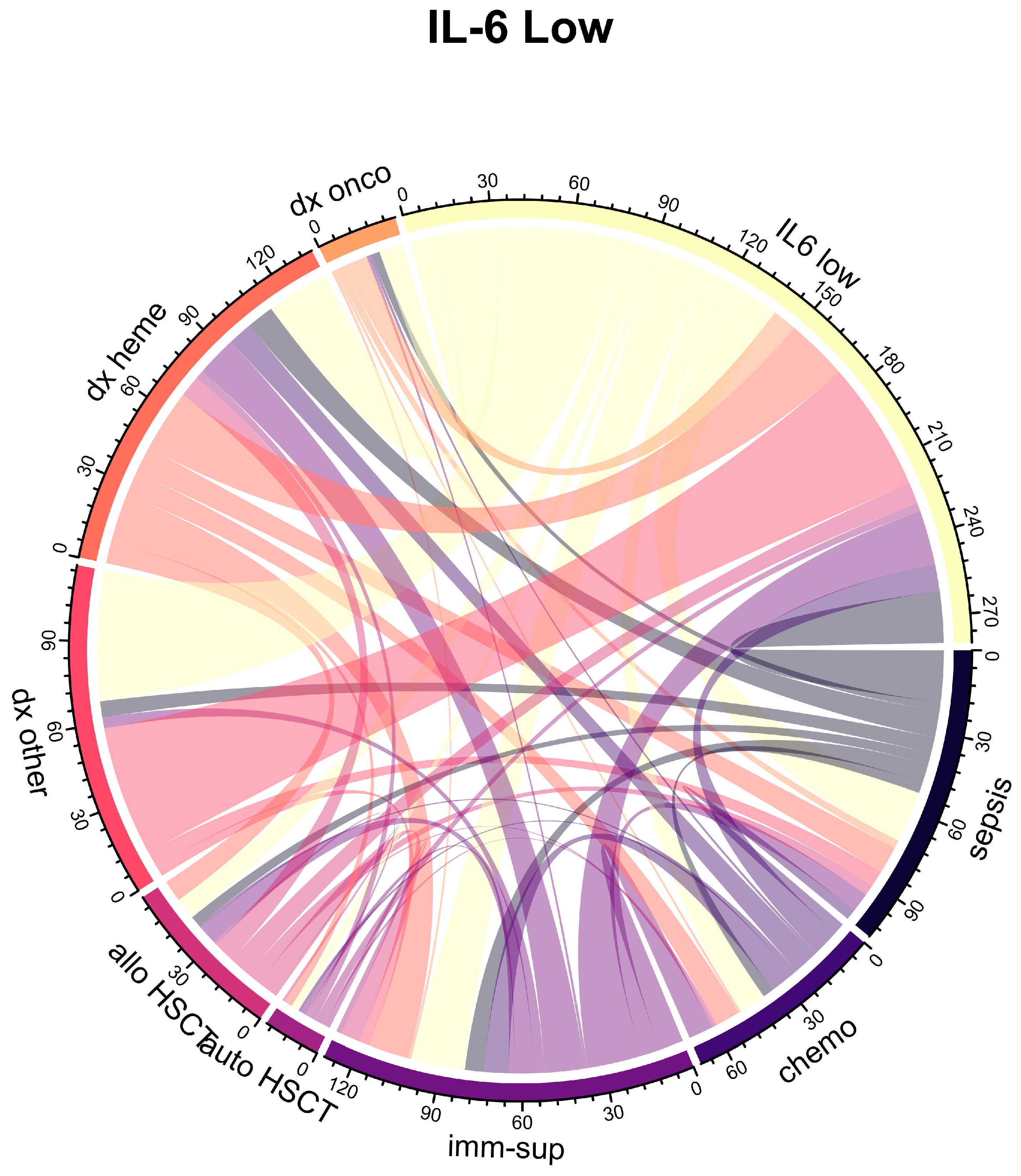
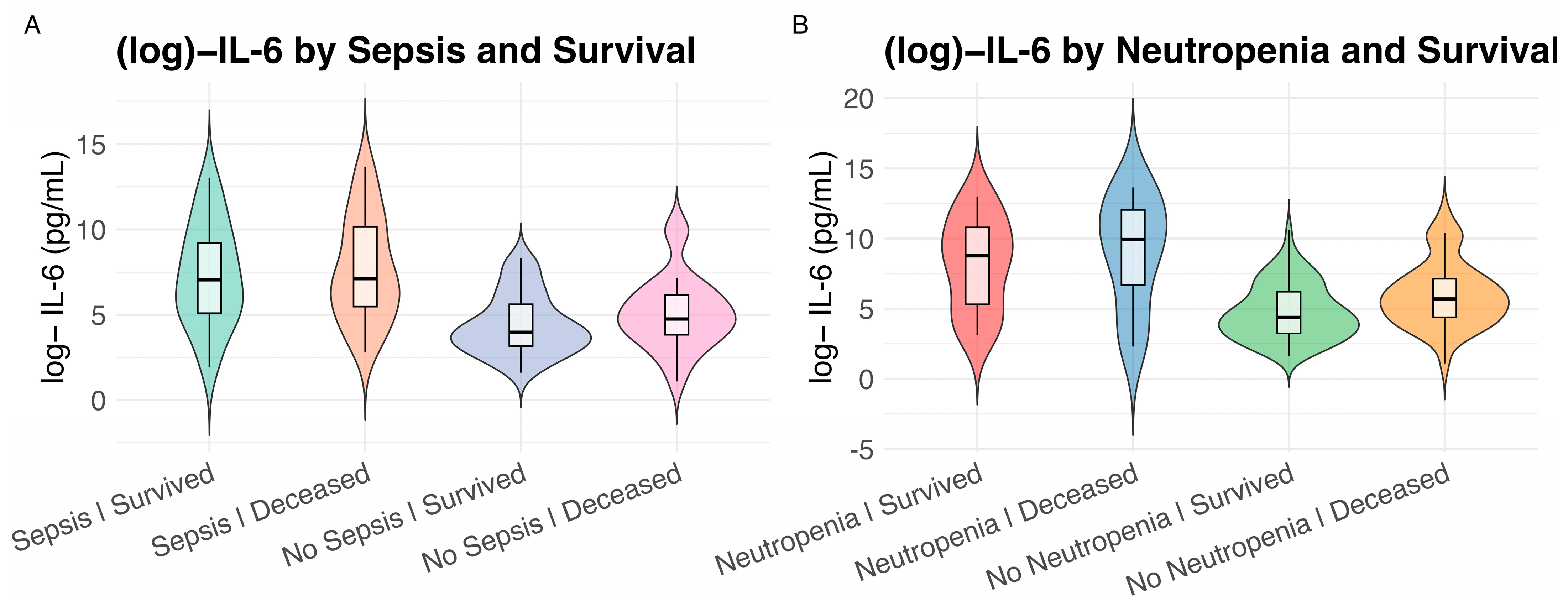
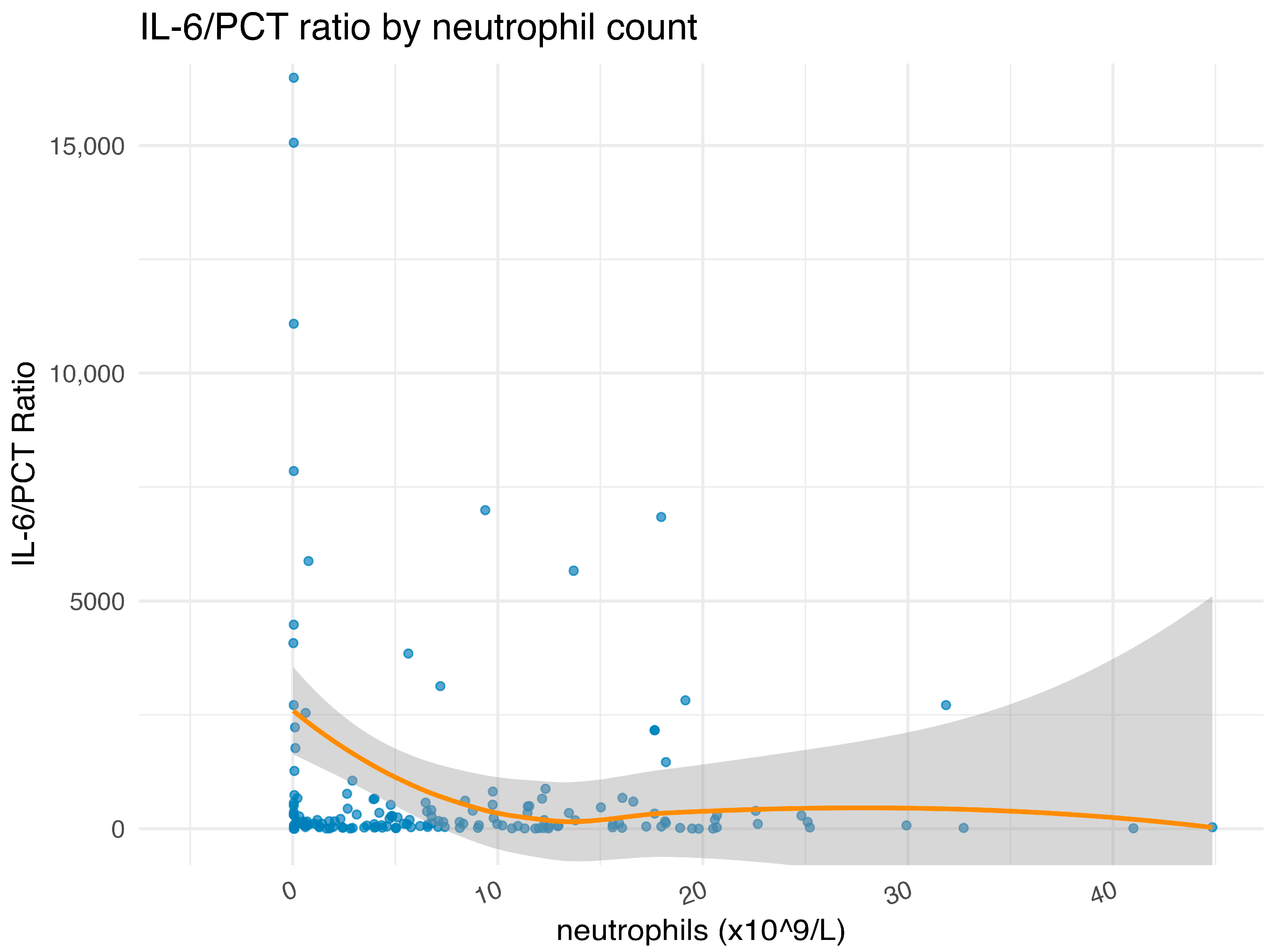



| Characteristic | Overall 1 n = 160 | IL-6 High 1 n = 80 | IL-6 Low 1 n = 80 | p Value 2 |
|---|---|---|---|---|
| Sex: female | 52 (33%) | 25 (31%) | 27 (34%) | 0.7 |
| Age [years] | 59 [44, 69] | 60 [44, 69] | 58 [45, 70] | 0.8 |
| Diagnosis | 0.5 | |||
| Other | 89 (56%) | 41 (51%) | 48 (60%) | |
| Hematologic | 53 (33%) | 30 (38%) | 23 (29%) | |
| Oncologic | 18 (11%) | 9 (11%) | 9 (11%) | |
| Chemotherapy | 24 (15%) | 14 (18%) | 10 (13%) | 0.4 |
| Immunosuppression | 40 (25%) | 20 (25%) | 20 (25%) | >0.9 |
| Autologous HSCT | 5 (3.1%) | 2 (2.5%) | 3 (3.8%) | >0.9 |
| Allogeneic HSCT | 21 (13%) | 13 (16%) | 8 (10%) | 0.2 |
| Sepsis | 69 (43%) | 50 (63%) | 19 (24%) | <0.001 |
| Mechanical ventilation | 85 (53%) | 59 (74%) | 26 (33%) | <0.001 |
| Renal replacement therapy | 18 (11%) | 13 (16%) | 5 (6.3%) | 0.045 |
| Noradrenaline day 1 [µg /kg/min] | 0 [0, 16] | 10 [0, 66] | 0 [0, 1] | <0.001 |
| TISS | 15 [9, 19] | 18 [11, 25] | 10 [5, 15] | <0.001 |
| SAPS | 46 [33, 64] | 59 [43, 71] | 34 [27, 53] | <0.001 |
| Fever on day 1 | 44 (28%) | 35 (44%) | 9 (11%) | <0.001 |
| IL-6 [pg/mL] | 200 [42, 1576] | 1576 [569, 15,748] | 42 [19, 83] | <0.001 |
| Lactate [mmol/L] | 2.1 [1.3, 3.9] | 2.4 [1.4, 5.8] | 1.8 [1.2, 2.8] | 0.011 |
| CRP [mg/L] | 105 [27, 220] | 158 [87, 303] | 41 [11, 139] | <0.001 |
| PCT [ng/mL] | 1 [0, 16] | 9 [1, 43] | 0 [0, 1] | <0.001 |
| Cortisol [nmol/L] | 328 [200, 513] | 436 [307, 651] | 232 [130, 391] | <0.001 |
| Neutrophils [×109/L] | 6 [1, 12] | 6 [0, 16] | 7 [2, 12] | 0.6 |
| Neutropenia [<1.0 × 109/L] | 31 (19%) | 23 (29%) | 8 (10%) | 0.003 |
| Leukocytes [×109/L] | 9 [3, 17] | 7 [1, 18] | 9 [5, 16] | 0.083 |
| ICU LOS [days] | 5 [3, 14] | 8 [3, 17] | 4 [3, 9] | 0.007 |
| Survival | 101 (63%) | 41 (51%) | 60 (75%) | 0.002 |
| Characteristic | Beta | 95% CI | p Value | Exp (Beta) | Exp (95% CI) |
|---|---|---|---|---|---|
| Mechanical ventilation | 0.80 | 0.02, 1.6 | 0.046 | 2.22 | 1.02–4.88 |
| SAPS | 0.04 | 0.02, 0.06 | <0.001 | 1.04 | 1.02–1.06 |
| Lactate [mmol/L] | 0.16 | 0.08, 0.24 | <0.001 | 1.18 | 1.08–1.28 |
| CRP [mg/L] | 0.01 | 0.00, 0.01 | <0.001 | 1.01 | 1–1.01 |
| Neutrophils [×109/L] | −0.05 | −0.09, 0.00 | 0.045 | 0.95 | 0.91–1 |
| Parameter | log(OR) Mean | log(OR) Median | OR Mean | OR 2.5% | OR 97.5% |
|---|---|---|---|---|---|
| Sex: male | 0.255 | 0.255 | 1.291 | 0.557 | 2.873 |
| Age [years] | 0.002 | 0.002 | 1.002 | 0.980 | 1.025 |
| (log) IL-6 | 0.067 | 0.067 | 1.070 | 0.909 | 1.261 |
| Neutropenia | −0.298 | −0.302 | 0.742 | 0.262 | 2.143 |
| Fever on day 1 | −0.033 | −0.034 | 0.967 | 0.407 | 2.289 |
| Mechanical ventilation | 1.980 | 1.968 | 7.246 | 3.140 | 17.000 |
| Characteristic | Overall 1 n = 160 | Hyperinflammatory Phenotype 1 n = 67 | Non-Hyperinflammatory Phenotype 1 n = 93 | p Value 2 |
|---|---|---|---|---|
| Sepsis | 69 (43%) | 49 (73%) | 20 (22%) | <0.001 |
| Mechanical ventilation | 85 (53%) | 67 (100%) | 18 (19%) | <0.001 |
| Noradrenaline day 1 [µg/kg/min] | 0 [0, 16] | 17 [6, 97] | 0 [0, 0] | <0.001 |
| TISS | 15 [9, 19] | 19 [15, 26] | 10 [5, 15] | <0.001 |
| SAPS | 46 [33, 64] | 61 [46, 71] | 35 [28, 52] | <0.001 |
| Fever on day 1 | 44 (28%) | 32 (48%) | 12 (13%) | <0.001 |
| IL-6 [pg/mL] | 200 [42, 1576] | 1452 [400, 20,659] | 51 [22, 148] | <0.001 |
| Lactate day 1 [mmol/L] | 2.1 [1.3, 3.9] | 2.5 [1.5, 6.4] | 1.7 [1.1, 2.6] | <0.001 |
| CRP day 1 [mg/L] | 105 [27, 220] | 172 [79, 303] | 64 [13, 143] | <0.001 |
| PCT day 1 [ng/mL] | 1 [0, 16] | 11 [1, 45] | 0 [0, 3] | <0.001 |
| Cortisol day 1 [nmol/L] | 328 [200, 513] | 485 [322, 710] | 248 [142, 402] | <0.001 |
| Allogeneic HSCT | 21 (13%) | 14 (21%) | 7 (7.5%) | 0.013 |
| ICU LOS [days] | 5 [3, 14] | 12 [6, 21] | 3 [3, 6] | <0.001 |
| Mortality | 59 (37%) | 39 (58%) | 20 (22%) | <0.001 |
| Predicted Mortality Probability [%] | 48 [15, 57] | 57 [54, 61] | 16 [14, 19] | <0.001 |
| No. of Latent Classes (k) | No. of Patients per Class | npar | AIC | aBIC | G2 | p Value LRT | Entropy |
|---|---|---|---|---|---|---|---|
| 1 | 160 | 10 | 1892.395 | 1891.490 | 657.90 | - | - |
| 2 | 67, 93 | 21 | 1625.818 | 1623.919 | 369.32 | <0.001 | 1.00 |
| 3 | 74, 38, 48 | 32 | 1550.623 | 1547.729 | 272.13 | <0.001 | 0.95 |
| 4 | 30, 66, 22, 42 | 43 | 1495.672 | 1491.782 | 195.17 | <0.001 | 0.94 |
| 5 | 19, 69, 22, 9, 41 | 54 | 1498.887 | 1494.003 | 176.40 | 0.07 | 0.96 |
| 6 | 38, 23, 12, 9, 20, 58 | 65 | 1509.001 | 1503.122 | 164.50 | 0.372 | 0.97 |
| Characteristic |
Class 1 1 n = 66 |
Class 2 1 n = 42 |
Class 3 1 n = 30 |
Class 4 1 n = 22 | p Value 2 |
|---|---|---|---|---|---|
| Sex | 0.466 | ||||
| Female | 22 (33.3) | 11 (26.2) | 9 (30.0) | 10 (45.5) | |
| Male | 44 (66.7) | 31 (73.8) | 21 (70.0) | 12 (54.5) | |
| Age [years] | 56.35 (18.01) | 58.69 (14.54) | 55.70 (17.66) | 48.55 (16.81) | 0.154 |
| Diagnosis | <0.001 | ||||
| Other | 57 (86.4) | 32 (76.2) | 0 (0.0) | 0 (0.0) | |
| Hematologic | 4 (6.1) | 4 (9.5) | 25 (83.3) | 20 (90.9) | |
| Oncologic | 5 (7.6) | 6 (14.3) | 5 (16.7) | 2 (9.1) | |
| Chemotherapy | 0 (0.0) | 0 (0.0) | 13 (43.3) | 11 (50.0) | <0.001 |
| Immunosuppression | 4 (6.1) | 1 (2.4) | 20 (66.7) | 15 (68.2) | <0.001 |
| Autologous HSCT | 1 (1.5) | 0 (0.0) | 3 (10.0) | 1 (4.5) | 0.080 |
| Allogeneic HSCT | 0 (0.0) | 3 (7.1) | 10 (33.3) | 8 (36.4) | <0.001 |
| Sepsis | 5 (7.6) | 29 (69.0) | 16 (53.3) | 19 (86.4) | <0.001 |
| Mechanical ventilation | 13 (19.7) | 42 (100.0) | 8 (26.7) | 22 (100.0) | <0.001 |
| Renal replacement therapy | 3 (4.5) | 8 (19.0) | 3 (10.0) | 4 (18.2) | 0.084 |
| Noradrenaline day 1 [µg/kg/min] | 2.38 (7.35) | 88.55 (153.43) | 3.13 (7.50) | 41.50 (42.89) | <0.001 |
| Fever day 1 | 8 (12.1) | 16 (38.1) | 4 (13.3) | 16 (72.7) | <0.001 |
| TISS | 11.20 (7.76) | 20.95 (7.69) | 12.00 (9.86) | 19.23 (7.49) | <0.001 |
| SAPS | 37.18 (17.45) | 58.88 (15.58) | 46.57 (21.26) | 63.64 (19.81) | <0.001 |
| IL-6 day 1 [pg/mL] | 274.11 (658.52) | 11,418.14 (33,729.84) | 22,157.07 (88,397.60) | 126,147.59 (245,161.65) | <0.001 |
| IL-6 level | <0.001 | ||||
| High | 15 (22.7) | 37 (88.1) | 6 (20.0) | 22 (100.0) | |
| Low | 51 (77.3) | 5 (11.9) | 24 (80.0) | 0 (0.0) | |
| Lactate day 1 [mmol/L] | 2.45 (2.76) | 5.34 (5.14) | 2.59 (2.23) | 4.72 (6.31) | 0.001 |
| Lactate day 2 [mmol/L] | 2.15 (2.22) | 4.80 (3.79) | 2.62 (2.79) | 4.31 (5.09) | <0.001 |
| CRP day 1 [mg/L] | 78.10 (82.51) | 163.56 (125.65) | 124.32 (102.37) | 257.41 (127.23) | <0.001 |
| CRP day 2 [mg/L] | 88.46 (84.73) | 191.00 (110.94) | 134.55 (105.46) | 282.04 (115.57) | <0.001 |
| PCT day 1 [ng/mL] | 3.74 (10.30) | 42.00 (75.62) | 23.25 (60.50) | 41.72 (57.58) | 0.001 |
| PCT day 2 [ng/mL] | 5.57 (12.31) | 40.93 (57.93) | 41.94 (99.63) | 91.31 (101.79) | <0.001 |
| Cortisol day 1 [nmol/L] | 316.96 (368.28) | 558.39 (450.90) | 337.91 (278.34) | 736.87 (609.39) | <0.001 |
| Neutrophils [×109/L] | 9.06 (6.09) | 13.94 (10.36) | 5.02 (7.22) | 0.68 (1.65) | <0.001 |
| Neutropenia | 0 (0.0) | 1 (2.4) | 12 (40.0) | 18 (81.8) | <0.001 |
| Leukocytes [×109/L] | 14.77 (30.63) | 17.92 (13.57) | 15.38 (30.12) | 3.84 (13.63) | 0.196 |
| ICU LOS [days] | 5.09 (6.12) | 14.36 (15.82) | 12.13 (12.71) | 15.91 (10.57) | <0.001 |
| Mortality | 11 (16.7) | 25 (59.5) | 12 (40) | 11 (50) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linz, C.; Shimabukuro-Vornhagen, A.; Hesse, N.; Probst, L.; Garcia Borrega, J.; Eichenauer, D.A.; Kochanek, M.; von Bergwelt-Baildon, M.; Böll, B. Prediction of Hyperinflammatory Phenotypes in Critically Ill Patients via Routine Clinical Data and IL-6: Towards Personalized Anti-Inflammatory Therapy. Int. J. Mol. Sci. 2025, 26, 9967. https://doi.org/10.3390/ijms26209967
Linz C, Shimabukuro-Vornhagen A, Hesse N, Probst L, Garcia Borrega J, Eichenauer DA, Kochanek M, von Bergwelt-Baildon M, Böll B. Prediction of Hyperinflammatory Phenotypes in Critically Ill Patients via Routine Clinical Data and IL-6: Towards Personalized Anti-Inflammatory Therapy. International Journal of Molecular Sciences. 2025; 26(20):9967. https://doi.org/10.3390/ijms26209967
Chicago/Turabian StyleLinz, Charlotte, Alexander Shimabukuro-Vornhagen, Nina Hesse, Lucie Probst, Jorge Garcia Borrega, Dennis A. Eichenauer, Matthias Kochanek, Michael von Bergwelt-Baildon, and Boris Böll. 2025. "Prediction of Hyperinflammatory Phenotypes in Critically Ill Patients via Routine Clinical Data and IL-6: Towards Personalized Anti-Inflammatory Therapy" International Journal of Molecular Sciences 26, no. 20: 9967. https://doi.org/10.3390/ijms26209967
APA StyleLinz, C., Shimabukuro-Vornhagen, A., Hesse, N., Probst, L., Garcia Borrega, J., Eichenauer, D. A., Kochanek, M., von Bergwelt-Baildon, M., & Böll, B. (2025). Prediction of Hyperinflammatory Phenotypes in Critically Ill Patients via Routine Clinical Data and IL-6: Towards Personalized Anti-Inflammatory Therapy. International Journal of Molecular Sciences, 26(20), 9967. https://doi.org/10.3390/ijms26209967






